Abstract
Autophagy is conserved in nature from lower eukaryotes to mammals and is an important self-cannibalizing, degradative process that contributes to the elimination of superfluous materials. Cardiac hypertrophy is primarily characterized by excess protein synthesis, increased cardiomyocyte size, and thickened ventricular walls and is a major risk factor that promotes arrhythmia and heart failure. In recent years, cardiomyocyte autophagy has been considered to play a role in controlling the hypertrophic response. However, the beneficial or aggravating role of cardiomyocyte autophagy in cardiac hypertrophy remains controversial. The exact mechanism of cardiomyocyte autophagy in cardiac hypertrophy requires further study. In this review, we summarize the controversies associated with autophagy in cardiac hypertrophy and provide insights into the role of autophagy in the development of cardiac hypertrophy. We conclude that future studies should emphasize the relationship between autophagy and the different stages of cardiac hypertrophy, as well as the autophagic flux and selective autophagy. Autophagy will be a potential therapeutic target for cardiac hypertrophy.
Keywords: autophagy, cardiac hypertrophy, cardiac function, cell signaling, autophagic flux
Introduction
The heart responds to environmental stimuli through a series of responses, including changes in chamber volume, systolic contraction, diastolic relaxation, heart rate, and muscle mass. Physiologic hypertrophy typically occurs during normal responses to healthy exercise or pregnancy. However, pathologic hypertrophy is commonly stimulated by stress or induced by diseases, such as hypertension, valvular heart disease, myocardial infarction, and neurohormones. Pathologic cardiac hypertrophy plays a compensatory role by increasing cardiac overload during early stages. However, a sustained pathological hypertrophic response eventually leads to systolic dysfunction and heart failure [1].
As a treatment option, the inhibition of pathological cardiac hypertrophy has become a potential therapeutic target. In recent years, studies have focused on the mechanism of autophagy in cardiac hypertrophy [2,3]. Autophagy is conserved from lower eukaryotes to mammals and is a normal physiological degradation mechanism. It is an important catabolic degradative process that eliminates superfluous materials [4]. However, partially due to conflicting studies, the role of autophagy in cardiac hypertrophy remains controversial. The autophagic mechanism in cardiac hypertrophy is complicated and has been considered, on one hand as a cardiomyocyte-protective process, and on the other hand as a detrimental mechanism contributing to the pathological progression of cardiac hypertrophy. Here, we review the conflicting reports in the literature and try to clarify the role of cardiomyocyte autophagy as a protective and adaptive mechanism for relieving disease pathogenesis or as a maladaptive process enhancing disease progression [5,6]. We conclude that additional studies are required to determine whether cardiomyocyte autophagy is beneficial or harmful.
Autophagy Definition, Classification, and Formation
Autophagy, also called self-digestion, is a highly conserved process from lower eukaryotes to mammals. Intracellular waste macromolecules are delivered to lysosomes via an autophagic pathway. These waste materials are subsequently degraded into reusable, biologically active monomers, such as amino acids [7]. Furthermore, the process is not only limited to proteins and macromolecules but also includes organelles [8]. Basic autophagy plays important physiological roles in maintaining cellular homeostasis [9]. For example, autophagy-related gene 5 (Atg5)- or Atg7-knockout mice were unable to survive longer than 12 h after birth due to a lack of nutrients provided by autophagy.
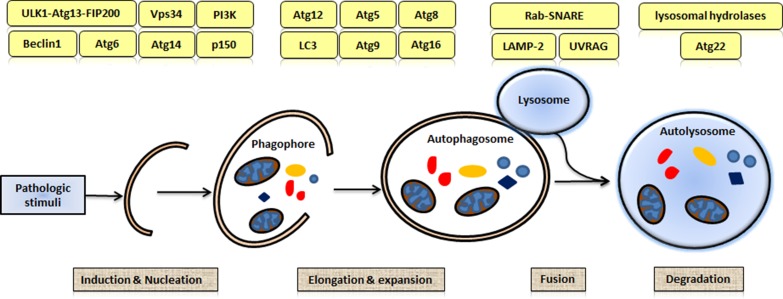
Three types of autophagy have been identified: macroautophagy, microautophagy, and chaperone-mediated autophagy [10]. Macroautophagy is the most active form and plays the most important role in intracellular degradation [11]. Macroautophagy is initiated through phagophore nucleation and expansion, subsequent autophagosome fusion with lysosomes, and finally the formation of autophagolysosomes [12,13]. Several Atg proteins are recruited to mediate this process [14]; however, the precise mechanisms remain unknown [15]. As shown in Fig. 1, the process of macroautophagy consists of at least four steps: induction and nucleation, elongation and expansion, fusion, and degradation. In the induction and nucleation step, a double-layered isolation membrane, or phagophore, is formed. The phagophore targets to and surrounds the cytosolic components (molecules and/or organelles) designated for degradation [16]. In the elongation and expansion step, the phagophore engulfs the cytosolic components and forms an autophagosome. Subsequently, the autophagosome fuses with a lysosome to form an autophagolysosome in which lysosomal hydrolases digest the macromolecules [17]. Once the digestion is completed, the degraded components are returned to the cytoplasm for reusing [18].
Figure 1.
Process of macroautophagy The process of macroautophagy consists of four steps: induction and nucleation, elongation and expansion, fusion, and degradation. Several autophagy-related (Atg) proteins are recruited to govern the processes of autophagy.
A few methods have been developed to study the autophagy process. Electron microscopy can be used to measure the formation of the double-membrane autophagosomes. A fluorescent dye, monodansylcadaverine, can be used to identify acidic autophagic vacuoles. The conversion of microtubule-associated protein light chain 3 (LC3) and the turnover of LC3-II which can be determined by western blot analysis are reliable markers for the formation of autophagosomes. Furthermore, LC3 transgenic mice are effective tools for the study of in vivo autophagic activity.
Regulators of Autophagy
Autophagy is often triggered by some extracellular and intracellular stimuli. Extracellular stimuli, such as hormones, therapeutic agents, amino acids depletion, and glucose starvation, affect the activity of the autophagic process [19]. Intracellular stimuli, such as the accumulation of misfolded proteins and the invasion of pathogens, also have similar effects that lead to an autophagic response. The protein kinase AKT is a central node linking growth factor signaling to the downstream pathways, that is related to the autophagy induction. In the presence of growth factors, AKT phosphorylates a wide array of substrates, including TSC2, PRAS40, GSK3, FoxO, and beclin-1, leading to the inactivation of these proteins. Conversely, growth factor restriction could result in the activation of these substrates, leading to subsequent initiation of autophagy [20]. Adenosine monophosphate (AMP)-activated protein kinase (AMPK) that is activated by an elevated AMP/ATP ratio initiates the metabolic process [21]. In some contexts, AMPK positively regulates autophagy by inhibiting the mTOR complex. Glucose deprivation in cardiac cells during ischemia stimulates cardiac autophagy via AMPK activation. RAB proteins, as members of the RAS GTPase superfamily, are key regulators of membrane trafficking and fusion events. RAB5 and RAB7 participate in several steps of the autophagic process, and both of them participate in the positive regulation of autophagy [22]. The FoxO family is a subclass of Forkhead transcription factors. FoxO factors were found to induce autophagy in muscle, neurons, and liver [23]. MicroRNAs (miRNAs) have been demonstrated to regulate the autophagy processes. Some miRNAs can directly target autophagy-associated proteins, such as BECN1 and RAB5A. Alternatively, miRNAs may indirectly modulate autophagy regulators, such as histone deacetylases (HDACs) and histone acetyl transferases (HATs) [24]. Numerous small molecules also have been found to positively regulate the autophagy process. For example, Amiodarone, Dexamethasone, Loperamide, Nitrendipine, Niclosamide, PP242, Rapamycin, Rottlerin, SMER28, Torin-1, Trehalose, and Valproic acid are autophagy activators [25–27].
Among the signaling pathways of the negative regulation of autophagy, mTOR signaling is a part of the central signaling pathway [28]. The activation of the mTOR signaling pathway suppresses autophagy. However, the inhibition of mTOR activity by starvation or rapamycin treatment leads to autophosphorylation, Atg1 activation, and an increase in the catabolic process of autophagy [29]. Various growth factors, such as insulin, cyclic guanosine monophosphate, and cyclic AMP, have been identified as physiological inhibitors of autophagy. A combination of leucine and other amino acids provides negative-feedback in autophagy, mainly because they are the final products of autophagic protein degradation. Inositol-1,4,5-trisphosphate (IP3) and its receptor are endogenous negative regulators of autophagy. Specific IP3R antagonists or the knockdown of IP3R isoforms with siRNAs leads to robust autophagy. When IP3R is inhibited, active autophagy is engaged in the preservation of energy homeostasis and promotes cell survival [30]. Class III and class I phosphatidylinositol 3-kinases (PI3K) are also involved in the regulation of autophagy. Class I PI3K has been shown to negatively regulate autophagy, whereas class III PI3K activates autophagy. Beclin-1/Atg6 forms a complex with Vps34, a member of class III PI3K. This complex is involved in the initiation of autophagy and the formation of autophagosomes. The inhibitors of class III PI3K, such as 3-methyladenine (3-MA), Wortmannin, and LY294002, inhibit autophagy [31]. Small molecules, such as 3-Methyleadenine, Nocodazole, Norclompiramine, Pepstatin A, Paclitaxel/Taxol, Quinacrin, Spautin, Vinblastine, and Wortmannin, are autophagy inhibitors [27].
The Emerging Role of Autophagy in Cardiac Hypertrophy
Stimuli-induced autophagy promotes cardiac hypertrophy
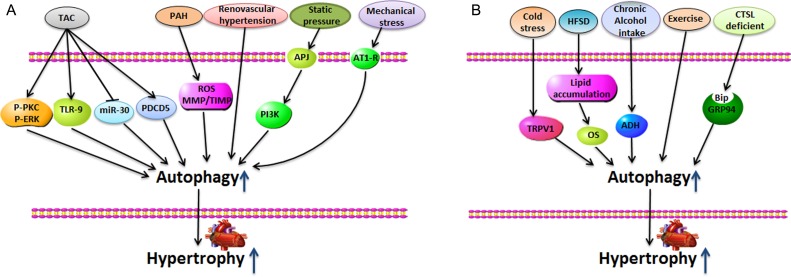
Cardiomyocyte autophagy has been associated with the development of cardiac hypertrophy. Many stresses that promote cardiac hypertrophy also induce autophagy in the heart. Here, we summarize the stimuli that promote cardiomyocyte autophagy and induce cardiac hypertrophy (Fig. 2).
Figure 2.
Stimuli-induced autophagy promotes cardiac hypertrophy (A) Transverse aortic constriction (TAC), pulmonary arterial hypertension (PAH), renovascular hypertension, static pressure, and mechanical stress induce marked heart hypertrophy, accompanied by high levels of autophagic responses in the heart. (B) Cold stress, high-fat/sucrose diet (HFSD), chronic alcohol intake, exercises, and cathepsin-L deficient appear to play roles in regulating autophagy during the development of cardiac hypertrophy.
Weng et al. [32] performed a transverse aortic constriction (TAC) surgery in mice. They found that TAC induced marked heart hypertrophy and promoted cardiac autophagy in the heart. Autophagic structures, Atg5, and Atg16 mRNA expression levels as well as LC3-II and beclin-1 protein levels are all increased in the TAC-treated group through the PKC and ERK1/2 pathways. Inflammation has been shown to contribute to the pathogenesis of cardiac hypertrophy. TAC also induced Toll-like receptor 9 (TLR9)-mediated inflammatory responses and autophagy in the heart. The deletion of TLR9 reversed the TAC-induced inflammation, autophagy, and cardiac dysfunction [33].
miRNAs play important roles in the regulation of cardiomyocyte autophagy and cardiac hypertrophy. The myocardial expression of miR-30a was found to be decreased in mouse hypertrophy models (thoracic aortic constriction) and in H9c2 cells treated with phenylephrine (PE). A miR-30a inhibitor markedly stimulated the level of cardiomyocyte autophagy and increased the cardiomyocyte cell size and the expressions of cardiac hypertrophy markers. The inhibition of autophagy suppressed the miR-30a inhibitor-induced cardiomyocyte hypertrophy. These results demonstrated that the down-regulated expression of miR-30a activated autophagy and increased the level of pressure overload-induced cardiomyocyte hypertrophy [34]. Treatment with angiotensin II (Ang II) inhibited miR-30 in cardiomyocytes and activated autophagy, resulting in myocardial hypertrophy. Furthermore, treatments with a miR-30a inhibitor aggravated the Ang II-induced cardiomyocyte hypertrophy. Conversely, treatments with mimics of miR-30a prevented the Ang II-induced hypertrophy [35].
Programmed cell death 5 (PDCD5) is critical for cardiac hypertrophy and remodeling. TAC and Ang II treatment significantly up-regulated the expression of PDCD5. PDCD5 transgenic mice showed dramatically increased autophagy and cardiac hypertrophy, and enlarged hearts were found in these dead mice. The p53 signaling pathway is involved in the PDCD5-induced activation of autophagy [36].
Pulmonary arterial hypertension (PAH) is a progressive disease associated with cardiac hypertrophy. The main characteristics of PAH include a marked elevation in pulmonary arterial pressure and right ventricular hypertrophy [37]. PAH was induced in an animal model by monocrotaline treatment. The induction of PAH was associated with the obvious upregulation in the expressions of the autophagy proteins LC3B-II, Atg5, and p62. Chloroquine has been shown to prevent the development of PAH, thus inhibiting autophagy [38]. In another study, the use of the Sugen5416/hypoxia/normoxia-induced PAH rat model showed that oxidative stress, metabolic maladaptation, and autophagy contributed to the pathogenesis of right ventricular hypertrophy [39]. Furthermore, renovascular hypertension has also been shown to alter cardiac structure and function. Zhang et al. [40] reported that renovascular hypertension promoted left ventricular hypertrophy and induced myocardial hypoxia through enhancing cellular autophagy and mitochondrial degradation. Valsartan has been shown to ameliorate renovascular hypertension by alleviating left ventricular hypertrophy via the inhibition of myocardial autophagy.
The angiotensin II receptor-like 1 (APJ) receptor is a G protein-coupled receptor (GPCR). The APJ system and its endogenous ligand, apelin, have important functions in cardiovascular systems [41–44]. Studies have shown that the APJ receptor could act as a sensor during static pressure-induced cardiomyocyte hypertrophy. Static pressure induces cardiomyocyte hypertrophy by up-regulating the APJ receptor and increasing the LC3-II/I ratio and beclin-1 expression. PI3K and other autophagy inhibitors have been shown to prevent static pressure-induced cardiomyocyte hypertrophy [44]. Lin et al. [45] reported that mechanical stretch induced cardiomyocyte autophagy and hypertrophy in rat neonatal cardiomyocytes and further showed that the AT1 receptor blocker Losartan significantly prevented mechanical stretch-induced cardiomyocyte autophagy and hypertrophy. Aortic banding (AB) increased the pressure overload, which promoted cardiac autophagy and induced heart failure. Beclin-1 is required for early autophagosome formation. The heterozygous disruption of the Beclin-1 gene decreased cardiomyocyte autophagy and alleviated pathological cardiac hypertrophy. However, beclin-1 overexpression enhanced autophagic activity and aggravated cardiac hypertrophy [46].
When adult male C57 mice were subject to abdominal aortic constriction and exposed to cold temperatures (4°C), the sustained cold stress promoted the expression of transient receptor potential vanilloid 1 (TRPV1) and AMPK phosphorylation, enhanced the ratio of LC3-II/LC3-I, and down-regulated p62. The sustained cold stress further triggered cardiac hypertrophy, the effects of which were reversed by TRPV1 antagonists and autophagy inhibitors. Finally, the cold exposure accentuated pressure overload-induced cardiac hypertrophy through a TRPV1 and autophagy-dependent mechanism [47]. Wild-type (WT) mice fed a high-fat/sucrose diet (HFSD)-developed metabolic dysfunction in the heart, leading to cardiac hypertrophy. HFSD increased cardiomyocyte autophagy and contributed to the development of HFSD-induced cardiac hypertrophy [48]. Chronic alcohol intake also led to the accumulation of autophagosomes (LC3-II and Atg7), resulting in cardiac hypertrophy. Chronic alcohol intake facilitated the production of acetaldehyde via the ADH pathway, triggered cardiac autophagosome formation, and impaired lysosomal degradation, leading to myocardial defects [49]. The carboxyl terminus of Hsp70-interacting protein (CHIP) plays an essential role in the maintenance of cardiac function. Cardiac CHIP regulates autophagy in cardiac hypertrophy. CHIP−/− mice have been shown to have aggravated cardiac hypertrophy and enhanced autophagic responses to exercise [50].
Cathepsin-L (CTSL) is a key member of the lysosomal protease family and plays an important role in the activation of the autophagy–lysosomal pathway. CTSL-deficient neonatal cardiomyocytes induced the accumulation of autophagosomes, impaired protein degradation, and decreased cell viability. PE-induced cardiac hypertrophy in vitro was more pronounced in CTSL-deficient neonatal cardiomyocytes than that in controls. An in vivo CTSL-deficiency markedly exacerbated AB-induced cardiac hypertrophy and deteriorated cardiac function. However, CTSL attenuated cardiac hypertrophy and preserved cardiac function through autophagy [51,52].
The autophagic pathway plays an essential role in cardiac hypertrophy and heart failure [53]. A patient transitioning from hypertrophic cardiomyopathy to heart failure was observed by ultrastructural analysis, which showed large, typical autophagic vacuoles in the hypertrophic cardiomyocytes. Those autophagic vacuoles contained glycogen granules, mitochondria, and degraded macromolecules. Autophagy contributed to the degeneration of myocardial cells and eventually resulted in cardiomyocyte death and loss, and finally heart failure [54].
Inhibited autophagy reverses cardiac hypertrophy
Adiponectin (APN), which is an adipokine, exerts its functions through the adiponectin receptors (AdipoR1 and AdipoR2) [55]. HFSD is known to induce cardiac hypertrophy and autophagy in the heart. However, AdipoR1-transgenic male mice fed with HFSD did not become obese or develop heart hypertrophy. AdipoR1 decreased the expressions of cardiac autophagic genes, exhibiting a positive effect on HFSD-induced cardiac hypertrophy [48].
After pulmonary artery constriction (PAC) surgery, mice were treated with folic acid (FA). A robust increase of reactive oxygen species (ROS) levels and the expression of autophagy protein LC3A/B were detected in the right ventricle (RV) of the PAC-treated mice. FA decreased the pressure in PAC mice, reduced the ROS level, and inhibited the expression of autophagy protein LC3A/B. FA also ameliorated mitochondrial dysfunction. These results indicated that FA reduced ROS production and improved right ventricular function by mitigating mitophagy in the right ventricle [56,57].
Renovascular hypertension has been shown to induce left ventricular hypertrophy, increase cellular autophagy and mitochondrial degradation, and inhibit mitochondrial biogenesis. Valsartan ameliorated myocardial autophagy and mitophagy and alleviated left ventricular hypertrophy [40]. HDACs are known to regulate cardiac plasticity. HDAC inhibitor trichostatin A (TSA) has beneficial effects against cardiac hypertrophy [58,59]. TSA prevents the activation of autophagy and alleviates both load- and agonist-induced hypertrophy. Furthermore, TSA also blocks PE-triggered autophagy and hypertrophy. The in vivo use of HDAC inhibitors in hypertrophic animals resulted in the return of ventricular masses and ventricular function to near-normal levels [60] (Fig. 3).
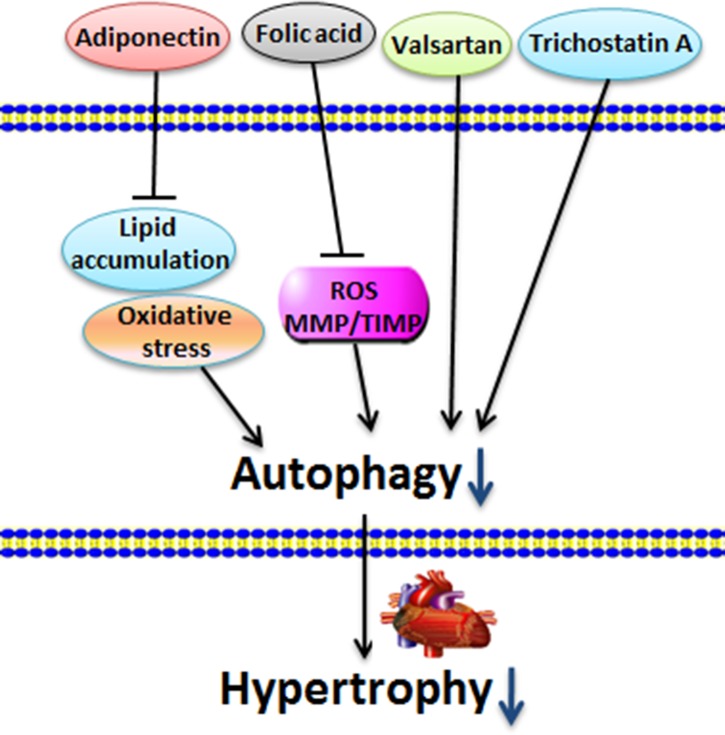
Figure 3.
Inhibited autophagy reverses cardiac hypertrophy Adiponectin receptor 1 (AdipoR1), FA, valsartan, and TSA decrease the cardiac autophagy level and exhibit positive effects on cardiac hypertrophy.
Several stimuli deteriorate cardiac hypertrophy by decreasing autophagy
Autophagy is an evolutionarily conserved process and plays an important role in cardiac hypertrophy. The interruption of autophagy may initiate ventricular dysfunction and cardiac hypertrophy and result in heart failure [61–63]. Cardiac aging is a complicated pathophysiological process that is characterized by enhanced remodeling and hypertrophy due to the interruption of autophagy. Aging decreases the levels of beclin-1 and Atg5 as well as the LC3-II/LC3-I ratio. Additionally, the autophagy inducer rapamycin reduces aging-induced cardiac remodeling and hypertrophy [64].
A high-fat diet induces cardiac hypertrophy and contractile dysfunction and is accompanied by the inhibition of autophagy. Rapamycin improves cardiac hypertrophy induced by a high-fat diet. APN is an important adipokine [65–67]. APN deficiency aggravates high-fat diet–induced cardiac hypertrophy by inhibiting myocardial autophagy. Rapamycin improves APN-deficiency-induced contractile dysfunction and cardiac hypertrophy [68].
miRNAs are endogenous small RNA molecules that regulate cardiac functions [69,70]. MiR-221 is involved in the development of cardiac hypertrophy. The cardiac-specific overexpression of miR-221 in mice impairs autophagy. In miR-221 transgenic mice, autophagic vesicle formation and autophagic flux are reduced. The overexpression of miR-221 eventually results in the dysfunction of the heart and evokes cardiac hypertrophy. The inactivation of mTOR reverses the miR-221-induced inhibition of autophagy and cardiac hypertrophy [71]. Furthermore, miR-212/132 participates in the regulation of cardiac hypertrophy and autophagy. Cardiomyocyte-specific miR-212/132 deficiency prevents pressure-overload-induced cardiac hypertrophy. However, the overexpression of miR-212/132 promotes pathological cardiac hypertrophy by inhibiting cardiac autophagy [72].
Atg5 is one of the most important proteins required for autophagy. A cardiac-specific Atg5 deficiency results in the development of cardiac hypertrophy [73,74]. A cardiomyocyte-specific deficiency of phosphatase and tensin homolog (PTEN) results in the interruption of autophagy and autophagic flux, and enhances the development of cardiac hypertrophy [75]. The administration of autophagy inducer rapamycin prevents PTEN deficiency-induced cardiac hypertrophy and interrupts autophagic flux in the heart [76,77].
Macrophage migration inhibitory factor (MIF) has a cardioprotective effect under various pathological conditions. MIF deficiency interrupts myocardial autophagy and overtly aggravates abdominal aorta constriction-induced cardiac hypertrophy. Rapamycin treatment alleviates MIF deficiency-induced cardiac hypertrophy in mice. In vitro, PE promotes H9c2 cell hypertrophy. MIF depletion in H9C2 reverses PE-induced mitophagy. Endogenous MIF regulates the mTOR signaling pathway to activate autophagy to protect against cardiac hypertrophy [78] (Fig. 4).
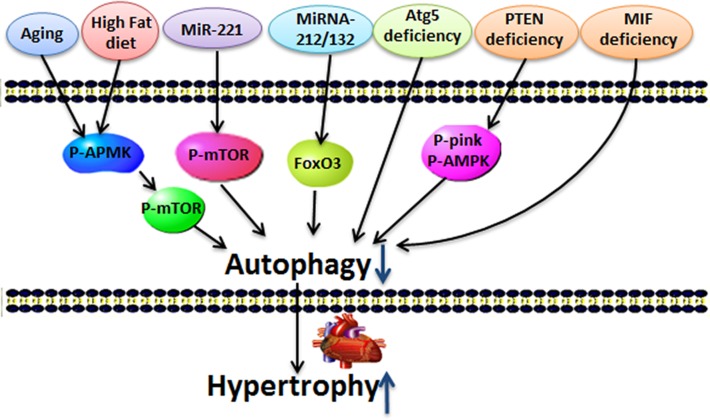
Figure 4.
Several stimuli deteriorate cardiac hypertrophy by decreasing autophagy Aging, high-fat diet, miRNA-221, miRNA-212/13, Atg5 deficiency, PTEN deletion, and MIF deficiency induce cardiac hypertrophy and down-regulate autophagy.
High level of autophagy ameliorates cardiac hypertrophy
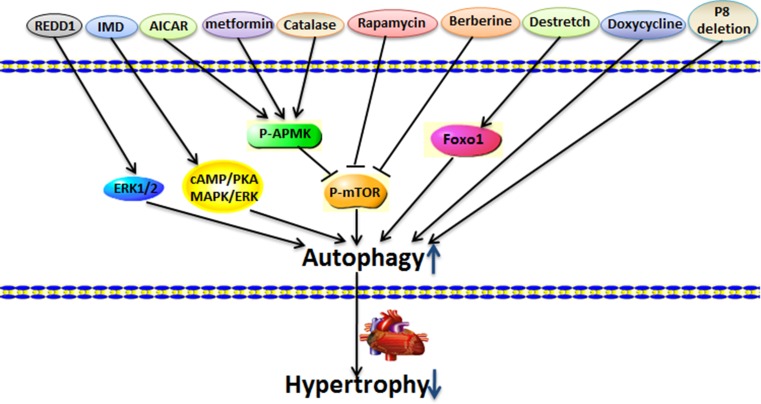
Autophagy also participates in the degradation of cytoplasmic components and contributes to the removal of damaged cellular components and the preservation of cell physiological function [79]. Several studies have suggested that high levels of autophagy may ameliorate cardiac hypertrophy (Fig. 5).
Figure 5.
High level of autophagy ameliorates cardiac hypertrophy Regulated in development and DNA damage 1 (REDD1), intermedin (IMD), 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR), metformin, catalase, rapamycin, berberine, destretch, doxycycline, and transcriptional regulator p8 deletion significantly increase autophagy level, improve cardiac function, and attenuate cardiac hypertrophy.
Regulated in development and DNA damage 1 (REDD1) is a stress-responsive protein found to be essential for inhibiting cardiac hypertrophy through autophagy [80,81]. REDD1 knockdown impairs autophagy and aggravates PE-induced cardiac hypertrophy via the mTOR signaling pathway. Rapamycin reverses the pro-hypertrophic effect of REDD1 knockdown [82].
Intermedin (IMD) exhibits important protective effects on heart hypertrophy. Several studies have reported that IMD induces remarkable autophagy in hypertrophic cardiomyocyte models both in vivo and in vitro. Transverse aortic contraction induction, Ang II, or isoprenaline treatment induces prominent cardiac hypertrophy, which is inhibited by IMD treatment. Furthermore, 3-MA reduces IMD-induced autophagy and abrogates the protection of IMD on cardiac hypertrophy [83].
AMPK is a serine/threonine protein kinase that regulates the process of cardiac hypertrophy. It has been shown that AMPK is an important positive regulator of autophagy. Both 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) and metformin, which are AMPK activators, significantly enhance autophagosome formation, lysosomal function and autophagy, and block pressure-overload-induced hypertrophy [84].
The antioxidant catalase plays a beneficial role in the restoration of autophagy. A high-fat diet intake induces ROS production and inhibits autophagy by promoting the phosphorylation of mTOR in the heart. Catalase reduces the ROS level and promotes autophagy by inhibiting the phosphorylation of mTOR. Catalase also significantly alleviates high-fat diet–induced cardiac hypertrophy and inhibits autophagy [85].
Autophagy inducer rapamycin plays a significant role in cardiac hypertrophy. Rapamycin has been found to ameliorate cardiac hypertrophy induced by mechanical stress or cardiac injury [86–88]. Low doses of rapamycin could be used to treat human heart failure [89]. It has been shown that berberine has cardioprotective effects on cardiac hypertrophy. The administration of berberine enhances autophagy in TAC-induced hypertrophic hearts and is accompanied by reduced heart size and cardiac apoptosis, as well as suppression of cardiac hypertrophy. Furthermore, 3-MA, an autophagy inhibitor, reverses the berberine-induced autophagy level and abrogates the protection of berberine against heart hypertrophy. These data indicated that berberine could attenuate cardiac hypertrophy through an autophagy-dependent mechanism and was associated with enhanced autophagy through the inhibition of mTOR [90].
Mechanical overload promotes the development of cardiac hypertrophy. However, autophagy signaling reduces cardiac hypertrophy. Cardiac hypertrophy in TAC-treated mice models showed that TAC reduces the level of constriction. This process is called DeTAC. LC3-II expression and p62 degradation are significantly increased during DeTAC. Furthermore, the expression of FoxO1 is increased during DeTAC. The cardiac-specific over-expression of FoxO1 ameliorates cardiac hypertrophy by activating autophagy. In vitro, the overexpression of FoxO1 reduces cardiomyocyte size by autophagy. The inhibition of autophagy reverses the protective effect of FoxO1. These results suggested that autophagy prevents cardiac hypertrophy [91]. Another study also supported that autophagy is involved in preventing cardiac hypertrophy [92]. When Ang II was continuously infused into Atg5-deficient mice, the mice developed similar levels of cardiac hypertrophy compared with the WT control mice. When Ang II infusion was stopped after 7 days, the cardiac hypertrophy was regressed in control mice, but the Atg5-deficient mice showed significantly less reduction in cardiac hypertrophy. Therefore, autophagy appeared to be necessary for the regression of cardiac hypertrophy [92].
Doxycycline has used to treat heart failure in animals. Doxycycline treatment significantly attenuated cardiac hypertrophy by activating autophagy and increasing autophagosome formation and LC3-II expression [93]. Pressure overload resulted in the development of cardiac hypertrophy. However, transcriptional regulator p8 deletion (p8−/−) mice showed lower cardiomyocyte hypertrophy. TAC induced significant autophagy in the left ventricle but much higher autophagy in the p8−/− mice. P8 deletion regulates autophagy signaling pathways and plays a beneficial role in cardiac hypertrophy [94].
Controversies
As previously mentioned, autophagy appears to play dual roles in cardiac hypertrophy [95]. The exact mechanism of autophagy in cardiac hypertrophy requires further investigation. For example, different opinions exist concerning the role of autophagy in the pressure stress-induced cardiac hypertrophy. A few studies have indicated that autophagy is an adaptive response in pressure stress-induced cardiac hypertrophy [78,96], but other reports have suggested that excessive autophagy aggravates pressure stress-induced cardiac hypertrophy [46]. Therefore, depending on the conditions and the extent of stimulation, autophagy may play adaptive or maladaptive roles [3].
The mechanism of autophagy in cardiac hypertrophy is complex. A consensus has been reached that the basal levels of autophagy are essential for homeostasis, while imbalanced levels of autophagy may lead to cell death [3]. However, the protective or destructive properties of autophagy vary in different situations [76]. Furthermore, differences in surgical models, animal strains, and genetic manipulations may contribute to these variations. TAC, PAH, static pressure, mechanical stress, cold stress, and chronic alcohol intake have been shown to induce autophagy and promote cardiac hypertrophy, whereas metformin, catalase, berberine, and doxycycline have been shown to prevent cardiac hypertrophy by increasing autophagy.
The role of autophagy in cardiac hypertrophy may also depend on disease stage and severity [73]. Typically, during the early stages of cardiac hypertrophy, autophagy occurs at low, beneficial, basal levels. In contrast, as cardiac hypertrophy develops, sustained excessive autophagy in hypertrophied hearts may be responsible for heart failure [46,97]. Furthermore, recent studies have revealed two new roles for cardiac hypertrophy: frustrated autophagy and autophagy interrupts. Frustrated autophagy and autophagy interrupts typically occur when autophagosomes are unable to fuse with lysosomes. These activated lysosomes may promote uncontrolled autodigestion and lead to cell death [98]. Autophagy has an essential homeostatic function in normal, physiological states; however, frustrated autophagy or autophagy interrupts can have deleterious consequences.
Autophagy is a highly dynamic, multistep process. Therefore, the mere detection of the number of autophagosomes and the presence of LC3 processing is insufficient for an overall evaluation of the entire autophagic system. Autophagic flux, which includes autophagosome formation, maturation, fusion with lysosomes, and subsequent breakdown and release [99], is used to represent the dynamic process of autophagy. Low-level autophagic flux is necessary for an adaptive response during cardiac hypertrophy [73]. However, autophagy flux in cardiomyocytes ranges from low-level activation required for cellular homeostasis to high-level activation, which is detrimental and disease promoting [100]. In a mouse model of pressure overload induced by TAC surgery, the increase in autophagic flux correlates with the degree of left ventricular hypertrophy; the flux response increases initially and then ultimately reverts to a higher steady-state level [46]. Genetic models, in which autophagic flux is increased in cardiomyocytes by over-expression of beclin-1, showed amplified pressure overload-induced heart failure. Autophagy flux may be a more effective and important way than traditional autophagy protein markers. To provide more evidence concerning the role of autophagy in cardiac hypertrophy, additional detailed studies on autophagic flux are required. In selective autophagy, maladaptive autophagy or frustrated autophagy may target at specific cellular substrates. Aggrephagy, mitophagy, xenophagy, ribophagy, and pexophagy are different types of selective autophagy. Degradation or removal of entire aggregates by selective autophagy is named aggrephagy. In particular, aggrephagy participates in Alzheimer’s disease and age-related macular degeneration [101]. Mitophagy targets at the degradation of damaged mitochondria. Recent studies indicated that the serine/threonine kinase PTEN-induced putative kinases 1 and the E3 ubiquitin ligase Parkin regulated the mammalian mitophagy [102]. 3-MA reverses cardiac remodeling and attenuates mitophagy in heart failure. BNIP3 over-expression promotes mitophagy, leading to the gradual decline of cardiac function and LV remodeling [103]. In a Parkin knockout Drosophila model, the disruption of mitophagy triggers the dilated cardiomyopathy [104]. Xenophagy, which is a form of selective autophagy that targets at intracellular pathogens for degradation, mainly contributes to the immune response [105]. Ribophagy is a selective degradation of ribosomes. 60S and 40S ribosomal subunits appear to be independently targeted for degradation [106]. The level of ribophagy increases obviously in neonatal hypoxia/ischemia [107]. Pexophagy is a selective autophagy process where damaged and/or superfluous peroxisomes undergo vacuolar degradation [108]. Pexophagy has been studied extensively in yeasts and plants [109]. But there are no related reports about the role of pexophagy in cardiac hypertrophy. So far, research about selective autophagy in cardiac hypertrophy is still very limit. Future studies will outline the role of selective autophagy and its function in pathophysiological cardiac hypertrophy [110]. Selective autophagy may have potential importance in cardiac hypertrophy [111,112]. Studies that focus on selective autophagy rather than general autophagy may be more effective in determining the role of autophagy in cardiac hypertrophy.
Conclusions and Perspectives
Recent findings have suggested an important role of autophagy in cardiac hypertrophy [113]. Basal autophagy is essential for the maintenance of cellular homeostasis, whereas excess or frustrated autophagic activity can aggravate cardiac hypertrophy and may contribute to the pathogenesis of heart failure [114]. The suppression of excessive or frustrated autophagy may be an effective therapy for cardiac hypertrophy. Future studies should emphasize the relationship between autophagy and the different stages of cardiac hypertrophy, as well as autophagic flux and selective autophagy [115]. While the successful treatment of pathophysiological autophagy will require further investigation, autophagy should be considered as a potential therapeutic target for cardiac hypertrophy.
Funding
This work was supported by the grants from the National Natural Science Foundation of China (Nos. 81470434, 81270420, 81470435, and 30971169), the Hunan Provincial Natural Science Foundation (No. 14JJ3102), the Hunan Provincial Science and Technology Project (No. 2015RS4040), the China Postdoctoral Science Foundation (Nos. 2014M560647 and 2015T80875), the Administration of Traditional Chinese Medicine of Hunan Province (No. 201578), the Health and Family Planning Commission of Hunan Province (No. B2015-48), and the Aid Program for Science and Technology Innovative Research Team in Higher Educational Institutions of Hunan Province (No. 2008-244).
References
- 1.Mathew J, Sleight P, Lonn E, Johnstone D, Pogue J, Yi Q, Bosch J, et al. . Reduction of cardiovascular risk by regression of electrocardiographic markers of left ventricular hypertrophy by the angiotensin-converting enzyme inhibitor ramipril. Circulation 2001, 104: 1615–1621. [DOI] [PubMed] [Google Scholar]
- 2.Wang ZV, Ferdous A, Hill JA.. Cardiomyocyte autophagy: metabolic profit and loss. Heart Fail Rev 2013, 18: 585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nemchenko A, Chiong M, Turer A, Lavandero S, Hill JA.. Autophagy as a therapeutic target in cardiovascular disease. J Mol Cell Cardiol 2011, 51: 584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Z, Klionsky DJ.. Eaten alive: a history of macroautophagy. Nat Cell Biol 2010, 12: 814–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rothermel BA, Hill JA.. Myocyte autophagy in heart disease: friend or foe? Autophagy 2007, 3: 632–634. [DOI] [PubMed] [Google Scholar]
- 6.Lavandero S, Troncoso R, Rothermel BA, Martinet W, Sadoshima J, Hill JA.. Cardiovascular autophagy: concepts, controversies, and perspectives. Autophagy 2013, 9: 1455–1466. [DOI] [PubMed] [Google Scholar]
- 7.Singh R, Cuervo AM.. Autophagy in the cellular energetic balance. Cell Metab 2011, 13: 495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gurusamy N, Das DK.. Autophagy, redox signaling, and ventricular remodeling. Antioxid Redox Signal 2009, 11: 1975–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uchiyama Y, Shibata M, Koike M, Yoshimura K, Sasaki M.. Autophagy-physiology and pathophysiology. Histochem Cell Biol 2008, 129: 407–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pedrozo Z, Torrealba N, Fernandez C, Gatica D, Toro B, Quiroga C, Rodriguez AE, et al. . Cardiomyocyte ryanodine receptor degradation by chaperone-mediated autophagy. Cardiovasc Res 2013, 98: 277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gottlieb RA, Mentzer RM.. Autophagy during cardiac stress: joys and frustrations of autophagy. Annu Rev Physiol 2010, 72: 45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mijaljica D, Prescott M, Devenish RJ.. Microautophagy in mammalian cells: revisiting a 40-year-old conundrum. Autophagy 2011, 7: 673–682. [DOI] [PubMed] [Google Scholar]
- 13.Arias E, Cuervo AM.. Chaperone-mediated autophagy in protein quality control. Curr Opin Cell Biol 2011, 23: 184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mizushima N, Levine B.. Autophagy in mammalian development and differentiation. Nat Cell Biol 2010, 12: 823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y, Klionsky DJ.. The regulation of autophagy — unanswered questions. J Cell Sci 2011, 124: 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kroemer G, Marino G, Levine B.. Autophagy and the integrated stress response. Mol Cell 2010, 40: 280–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nair U, Jotwani A, Geng J, Gammoh N, Richerson D, Yen WL, Griffith J, et al. . SNARE proteins are required for macroautophagy. Cell 2011, 146: 290–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Z, Klionsky DJ.. Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol 2010, 22: 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galluzzi L, Pietrocola F, Levine B, Kroemer G.. Metabolic control of autophagy. Cell 2014, 159: 1263–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li TY, Lin SY, Lin SC.. Mechanism and physiological significance of growth factor-related autophagy. Physiology (Bethesda) 2013, 28: 423–431. [DOI] [PubMed] [Google Scholar]
- 21.Qi D, Young LH.. AMPK: energy sensor and survival mechanism in the ischemic heart. Trends Endocrinol Metab 2015, 26: 422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szatmari Z, Sass M.. The autophagic roles of Rab small GTPases and their upstream regulators: a review. Autophagy 2014, 10: 1154–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Webb AE, Brunet A.. FOXO transcription factors: key regulators of cellular quality control. Trends Biochem Sci 2014, 39: 159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu J, Wang Y, Tan X, Jing H.. MicroRNAs in autophagy and their emerging roles in crosstalk with apoptosis. Autophagy 2012, 8: 873–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su Z, Yang Z, Xu Y, Chen Y, Yu Q.. Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol Cancer 2015, 14: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tong XP, Chen Y, Zhang SY, Xie T, Tian M, Guo MR, Kasimu R, et al. . Key autophagic targets and relevant small-molecule compounds in cancer therapy. Cell Prolif 2015, 48: 7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gros F, Muller S.. Pharmacological regulators of autophagy and their link with modulators of lupus disease. Br J Pharmacol 2014, 171: 4337–4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim YC, Guan KL.. mTOR: a pharmacologic target for autophagy regulation. J Clin Invest 2015, 125: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Xu X, Ren J.. MTOR overactivation and interrupted autophagy flux in obese hearts: a dicey assembly? Autophagy 2013, 9: 939–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Decuypere JP, Monaco G, Bultynck G, Missiaen L, De Smedt H, Parys JB.. The IP(3) receptor-mitochondria connection in apoptosis and autophagy. Biochim Biophys Acta 2011, 1813: 1003–1013. [DOI] [PubMed] [Google Scholar]
- 31.O’Farrell F, Rusten TE, Stenmark H.. Phosphoinositide 3-kinases as accelerators and brakes of autophagy. FEBS J 2013, 280: 6322–6337. [DOI] [PubMed] [Google Scholar]
- 32.Weng LQ, Zhang WB, Ye Y, Yin PP, Yuan J, Wang XX, Kang L, et al. . Aliskiren ameliorates pressure overload-induced heart hypertrophy and fibrosis in mice. Acta Pharmacol Sin 2014, 35: 1005–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oka T, Hikoso S, Yamaguchi O, Taneike M, Takeda T, Tamai T, Oyabu J, et al. . Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature 2012, 485: 251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yin X, Peng C, Ning W, Li C, Ren Z, Zhang J, Gao H, et al. . miR-30a downregulation aggravates pressure overload-induced cardiomyocyte hypertrophy. Mol Cell Biochem 2013, 379: 1–6. [DOI] [PubMed] [Google Scholar]
- 35.Pan W, Zhong Y, Cheng C, Liu B, Wang L, Li A, Xiong L, et al. . MiR-30-regulated autophagy mediates angiotensin II-induced myocardial hypertrophy. PLoS One 2013, 8: e53950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.An L, Zhao X, Wu J, Jia J, Zou Y, Guo X, He L, et al. . Involvement of autophagy in cardiac remodeling in transgenic mice with cardiac specific over-expression of human programmed cell death 5. PLoS One 2012, 7: e30097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeffery TK, Morrell NW.. Molecular and cellular basis of pulmonary vascular remodeling in pulmonary hypertension. Prog Cardiovasc Dis 2002, 45: 173–202. [DOI] [PubMed] [Google Scholar]
- 38.Long L, Yang X, Southwood M, Lu J, Marciniak SJ, Dunmore BJ, Morrell NW.. Chloroquine prevents progression of experimental pulmonary hypertension via inhibition of autophagy and lysosomal bone morphogenetic protein type II receptor degradation. Circ Res 2013, 112: 1159–1170. [DOI] [PubMed] [Google Scholar]
- 39.Rawat DK, Alzoubi A, Gupte R, Chettimada S, Watanabe M, Kahn AG, Okada T, et al. . Increased reactive oxygen species, metabolic maladaptation, and autophagy contribute to pulmonary arterial hypertension-induced ventricular hypertrophy and diastolic heart failure. Hypertension 2014, 64: 1266–1274. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, Li ZL, Crane JA, Jordan KL, Pawar AS, Textor SC, Lerman A, et al. . Valsartan regulates myocardial autophagy and mitochondrial turnover in experimental hypertension. Hypertension 2014, 64: 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li L, Zeng H, Chen JX.. Apelin-13 increases myocardial progenitor cells and improves repair postmyocardial infarction. Am J Physiol Heart Circ Physiol 2012, 303: H605–H618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li F, Li L, Qin X, Pan W, Feng F, Chen F, Zhu B, et al. . Apelin-induced vascular smooth muscle cell proliferation: the regulation of cyclin D1. Front Biosci 2008, 13: 3786–3792. [DOI] [PubMed] [Google Scholar]
- 43.Li L, Zeng H, Hou X, He X, Chen JX.. Myocardial injection of apelin-overexpressing bone marrow cells improves cardiac repair via upregulation of Sirt3 after myocardial infarction. PLoS One 2013, 8: e71041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xie F, Liu W, Feng F, Li X, Yang L, Lv D, Qin X, et al. . A static pressure sensitive receptor APJ promote H9c2 cardiomyocyte hypertrophy via PI3K-autophagy pathway. Acta Biochim Biophys Sin 2014, 46: 699–708. [DOI] [PubMed] [Google Scholar]
- 45.Lin L, Tang C, Xu J, Ye Y, Weng L, Wei W, Ge J, et al. . Mechanical stress triggers cardiomyocyte autophagy through angiotensin II type 1 receptor-mediated p38MAP kinase independently of angiotensin II. PLoS One 2014, 9: e89629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu H, Tannous P, Johnstone JL, Kong Y, Shelton JM, Richardson JA, Le V, et al. . Cardiac autophagy is a maladaptive response to hemodynamic stress. J Clin Invest 2007, 117: 1782–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu S, Xu D.. Cold stress accentuates pressure overload-induced cardiac hypertrophy and contractile dysfunction: role of TRPV1/AMPK-mediated autophagy. Biochem Biophys Res Commun 2013, 442: 8–15. [DOI] [PubMed] [Google Scholar]
- 48.Chou IP, Chiu YP, Ding ST, Liu BH, Lin YY, Chen CY.. Adiponectin receptor 1 overexpression reduces lipid accumulation and hypertrophy in the heart of diet-induced obese mice--possible involvement of oxidative stress and autophagy. Endocr Res 2014, 39: 173–179. [DOI] [PubMed] [Google Scholar]
- 49.Guo R, Hu N, Kandadi MR, Ren J.. Facilitated ethanol metabolism promotes cardiomyocyte contractile dysfunction through autophagy in murine hearts. Autophagy 2012, 8: 593–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Willis MS, Min JN, Wang S, McDonough H, Lockyer P, Wadosky KM, Patterson C.. Carboxyl terminus of Hsp70-interacting protein (CHIP) is required to modulate cardiac hypertrophy and attenuate autophagy during exercise. Cell Biochem Funct 2013, 31: 724–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun M, Ouzounian M, de Couto G, Chen M, Yan R, Fukuoka M, Li G, et al. . Cathepsin-L ameliorates cardiac hypertrophy through activation of the autophagy-lysosomal dependent protein processing pathways. J Am Heart Assoc 2013, 2: e000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dennemarker J, Lohmuller T, Muller S, Aguilar SV, Tobin DJ, Peters C, Reinheckel T.. Impaired turnover of autophagolysosomes in cathepsin L deficiency. Biol Chem 2010, 391: 913–922. [DOI] [PubMed] [Google Scholar]
- 53.Rothermel BA, Hill JA.. Autophagy in load-induced heart disease. Circ Res 2008, 103: 1363–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fidzianska A, Bilinska ZT, Walczak E, Witkowski A, Chojnowska L.. Autophagy in transition from hypertrophic cardiomyopathy to heart failure. J Electron Microsc (Tokyo) 2010, 59: 181–183. [DOI] [PubMed] [Google Scholar]
- 55.Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF.. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci USA 2004, 101: 10308–10313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Redout EM, Wagner MJ, Zuidwijk MJ, Boer C, Musters RJ, van Hardeveld C, Paulus WJ, et al. . Right-ventricular failure is associated with increased mitochondrial complex II activity and production of reactive oxygen species. Cardiovasc Res 2007, 75: 770–781. [DOI] [PubMed] [Google Scholar]
- 57.Qipshidze N, Tyagi N, Metreveli N, Lominadze D, Tyagi SC.. Autophagy mechanism of right ventricular remodeling in murine model of pulmonary artery constriction. Am J Physiol Heart Circ Physiol 2012, 302: H688–H696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kong Y, Tannous P, Lu G, Berenji K, Rothermel BA, Olson EN, Hill JA.. Suppression of class I and II histone deacetylases blunts pressure-overload cardiac hypertrophy. Circulation 2006, 113: 2579–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Granger A, Abdullah I, Huebner F, Stout A, Wang T, Huebner T, Epstein JA, et al. . Histone deacetylase inhibition reduces myocardial ischemia-reperfusion injury in mice. FASEB J 2008, 22: 3549–3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cao DJ, Wang ZV, Battiprolu PK, Jiang N, Morales CR, Kong Y, Rothermel BA, et al. . Histone deacetylase (HDAC) inhibitors attenuate cardiac hypertrophy by suppressing autophagy. Proc Natl Acad Sci USA 2011, 108: 4123–4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yitzhaki S, Huang C, Liu W, Lee Y, Gustafsson AB, Mentzer RM Jr, Gottlieb RA.. Autophagy is required for preconditioning by the adenosine A1 receptor-selective agonist CCPA. Basic Res Cardiol 2009, 104: 157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boengler K, Buechert A, Heinen Y, Roeskes C, Hilfiker-Kleiner D, Heusch G, Schulz R.. Cardioprotection by ischemic postconditioning is lost in aged and STAT3-deficient mice. Circ Res 2008, 102: 131–135. [DOI] [PubMed] [Google Scholar]
- 63.Boengler K, Schulz R, Heusch G.. Loss of cardioprotection with ageing. Cardiovasc Res 2009, 83: 247–261. [DOI] [PubMed] [Google Scholar]
- 64.Hua Y, Zhang Y, Ceylan-Isik AF, Wold LE, Nunn JM, Ren J.. Chronic Akt activation accentuates aging-induced cardiac hypertrophy and myocardial contractile dysfunction: role of autophagy. Basic Res Cardiol 2011, 106: 1173–1191. [DOI] [PubMed] [Google Scholar]
- 65.Kawano J, Arora R.. The role of adiponectin in obesity, diabetes, and cardiovascular disease. J Cardiometab Syndr 2009, 4: 44–49. [DOI] [PubMed] [Google Scholar]
- 66.Fujita K, Maeda N, Sonoda M, Ohashi K, Hibuse T, Nishizawa H, Nishida M, et al. . Adiponectin protects against angiotensin II-induced cardiac fibrosis through activation of PPAR-alpha. Arterioscler Thromb Vasc Biol 2008, 28: 863–870. [DOI] [PubMed] [Google Scholar]
- 67.Lopaschuk GD, Folmes CD, Stanley WC.. Cardiac energy metabolism in obesity. Circ Res 2007, 101: 335–347. [DOI] [PubMed] [Google Scholar]
- 68.Guo R, Zhang Y, Turdi S, Ren J.. Adiponectin knockout accentuates high fat diet-induced obesity and cardiac dysfunction: role of autophagy. Biochim Biophys Acta 2013, 1832: 1136–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R.. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev 2006, 20: 515–524. [DOI] [PubMed] [Google Scholar]
- 70.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009, 136: 215–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Su M, Wang J, Wang C, Wang X, Dong W, Qiu W, Wang Y, et al. . MicroRNA-221 inhibits autophagy and promotes heart failure by modulating the p27/CDK2/mTOR axis. Cell Death Differ 2015, 22: 986–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ucar A, Gupta SK, Fiedler J, Erikci E, Kardasinski M, Batkai S, Dangwal S, et al. . The miRNA-212/132 family regulates both cardiac hypertrophy and cardiomyocyte autophagy. Nat Commun 2012, 3: 1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, et al. . The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med 2007, 13: 619–624. [DOI] [PubMed] [Google Scholar]
- 74.Bhuiyan MS, Pattison JS, Osinska H, James J, Gulick J, McLendon PM, Hill JA, et al. . Enhanced autophagy ameliorates cardiac proteinopathy. J Clin Invest 2013, 123: 5284–5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mughal W, Dhingra R, Kirshenbaum LA.. Striking a balance: autophagy, apoptosis, and necrosis in a normal and failing heart. Curr Hypertens Rep 2012, 14: 540–547. [DOI] [PubMed] [Google Scholar]
- 76.Xu X, Ren J.. Unmasking the janus faces of autophagy in obesity-associated insulin resistance and cardiac dysfunction. Clin Exp Pharmacol Physiol 2012, 39: 200–208. [DOI] [PubMed] [Google Scholar]
- 77.Roe ND, Xu X, Kandadi MR, Hu N, Pang J, Weiser-Evans MC, Ren J.. Targeted deletion of PTEN in cardiomyocytes renders cardiac contractile dysfunction through interruption of Pink1-AMPK signaling and autophagy. Biochim Biophys Acta 2015, 1852: 290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu X, Hua Y, Nair S, Bucala R, Ren J.. Macrophage migration inhibitory factor deletion exacerbates pressure overload-induced cardiac hypertrophy through mitigating autophagy. Hypertension 2014, 63: 490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kaushik S, Cuervo AM.. Autophagy as a cell-repair mechanism: activation of chaperone-mediated autophagy during oxidative stress. Mol Aspects Med 2006, 27: 444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bauml MA, Underwood DA.. Left ventricular hypertrophy: an overlooked cardiovascular risk factor. Cleve Clin J Med 2010, 77: 381–387. [DOI] [PubMed] [Google Scholar]
- 81.Lang CH, Korzick DH.. Chronic alcohol consumption disrupts myocardial protein balance and function in aged, but not adult, female F344 rats. Am J Physiol Regul Integr Comp Physiol 2014, 306: R23–R33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu C, Xue R, Wu D, Wu L, Chen C, Tan W, Chen Y, et al. . REDD1 attenuates cardiac hypertrophy via enhancing autophagy. Biochem Biophys Res Commun 2014, 454: 215–220. [DOI] [PubMed] [Google Scholar]
- 83.Chen H, Wang X, Tong M, Wu D, Wu S, Chen J, Wang X, et al. . Intermedin suppresses pressure overload cardiac hypertrophy through activation of autophagy. PLoS One 2013, 8: e64757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li Y, Chen C, Yao F, Su Q, Liu D, Xue R, Dai G, et al. . AMPK inhibits cardiac hypertrophy by promoting autophagy via mTORC1. Arch Biochem Biophys 2014, 558: 79–86. [DOI] [PubMed] [Google Scholar]
- 85.Liang L, Shou XL, Zhao HK, Ren GQ, Wang JB, Wang XH, Ai WT, et al. . Antioxidant catalase rescues against high fat diet-induced cardiac dysfunction via an IKKbeta-AMPK-dependent regulation of autophagy. Biochim Biophys Acta 2015, 1852: 343–352. [DOI] [PubMed] [Google Scholar]
- 86.Korolchuk VI, Saiki S, Lichtenberg M, Siddiqi FH, Roberts EA, Imarisio S, Jahreiss L, et al. . Lysosomal positioning coordinates cellular nutrient responses. Nat Cell Biol 2011, 13: 453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Buss SJ, Muenz S, Riffel JH, Malekar P, Hagenmueller M, Weiss CS, Bea F, et al. . Beneficial effects of Mammalian target of rapamycin inhibition on left ventricular remodeling after myocardial infarction. J Am Coll Cardiol 2009, 54: 2435–2446. [DOI] [PubMed] [Google Scholar]
- 88.Soesanto W, Lin HY, Hu E, Lefler S, Litwin SE, Sena S, Abel ED, et al. . Mammalian target of rapamycin is a critical regulator of cardiac hypertrophy in spontaneously hypertensive rats. Hypertension 2009, 54: 1321–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bishu K, Ogut O, Kushwaha S, Mohammed SF, Ohtani T, Xu X, Brozovich FV, et al. . Anti-remodeling effects of rapamycin in experimental heart failure: dose response and interaction with angiotensin receptor blockade. PLoS One 2013, 8: e81325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li MH, Zhang YJ, Yu YH, Yang SH, Iqbal J, Mi QY, Li B, et al. . Berberine improves pressure overload-induced cardiac hypertrophy and dysfunction through enhanced autophagy. Eur J Pharmacol 2014, 728: 67–76. [DOI] [PubMed] [Google Scholar]
- 91.Hariharan N, Ikeda Y, Hong C, Alcendor RR, Usui S, Gao S, Maejima Y, et al. . Autophagy plays an essential role in mediating regression of hypertrophy during unloading of the heart. PLoS One 2013, 8: e51632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oyabu J, Yamaguchi O, Hikoso S, Takeda T, Oka T, Murakawa T, Yasui H, et al. . Autophagy-mediated degradation is necessary for regression of cardiac hypertrophy during ventricular unloading. Biochem Biophys Res Commun 2013, 441: 787–792. [DOI] [PubMed] [Google Scholar]
- 93.Zhu H, Sun X, Wang D, Hu N, Zhang Y.. Doxycycline ameliorates aggregation of collagen and atrial natriuretic peptide in murine post-infarction heart. Eur J Pharmacol 2015, 754: 66–72. [DOI] [PubMed] [Google Scholar]
- 94.Georgescu SP, Aronovitz MJ, Iovanna JL, Patten RD, Kyriakis JM, Goruppi S.. Decreased metalloprotease 9 induction, cardiac fibrosis, and higher autophagy after pressure overload in mice lacking the transcriptional regulator p8. Am J Physiol Cell Physiol 2011, 301: C1046–C1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang EY, Biala AK, Gordon JW, Kirshenbaum LA.. Autophagy in the heart: too much of a good thing? J Cardiovasc Pharmacol 2012, 60: 110–117. [DOI] [PubMed] [Google Scholar]
- 96.McMullen JR, Sherwood MC, Tarnavski O, Zhang L, Dorfman AL, Shioi T, Izumo S.. Inhibition of mTOR signaling with rapamycin regresses established cardiac hypertrophy induced by pressure overload. Circulation 2004, 109: 3050–3055. [DOI] [PubMed] [Google Scholar]
- 97.Halapas A, Armakolas A, Koutsilieris M.. Autophagy: a target for therapeutic interventions in myocardial pathophysiology. Expert Opin Ther Targets 2008, 12: 1509–1522. [DOI] [PubMed] [Google Scholar]
- 98.Maejima Y, Chen Y, Isobe M, Gustafsson AB, Kitsis RN, Sadoshima J.. Recent progress in research on molecular mechanisms of autophagy in the heart. Am J Physiol Heart Circ Physiol 2015, 308: H259–H268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang XJ, Chen S, Huang KX, Le WD.. Why should autophagic flux be assessed?. Acta Pharmacol Sin 2013, 34: 595–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schiattarella GG, Hill JA.. Therapeutic targeting of autophagy in cardiovascular disease. J Mol Cell Cardiol 2015, pii: S0022-2828(15)30124-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hyttinen JM, Amadio M, Viiri J, Pascale A, Salminen A, Kaarniranta K.. Clearance of misfolded and aggregated proteins by aggrephagy and implications for aggregation diseases. Ageing Res Rev 2014, 18: 16–28. [DOI] [PubMed] [Google Scholar]
- 102.Vasquez-Trincado C, Garcia-Carvajal I, Pennanen C, Parra V, Hill JA, Rothermel BA, Lavandero S.. Mitochondrial dynamics, mitophagy and cardiovascular disease. J Physiol 2016, 594: 509–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chaanine AH, Jeong D, Liang L, Chemaly ER, Fish K, Gordon RE, Hajjar RJ.. JNK modulates FOXO3a for the expression of the mitochondrial death and mitophagy marker BNIP3 in pathological hypertrophy and in heart failure. Cell Death Dis 2012, 3: 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bhandari P, Song M, Chen Y, Burelle Y, Dorn GW II. Mitochondrial contagion induced by Parkin deficiency in Drosophila hearts and its containment by suppressing mitofusin. Circ Res 2014, 114: 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mizumura K, Choi AM, Ryter SW.. Emerging role of selective autophagy in human diseases. Front Pharmacol 2014, 5: 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ossareh-Nazari B, Nino CA, Bengtson MH, Lee JW, Joazeiro CA, Dargemont C.. Ubiquitylation by the Ltn1 E3 ligase protects 60S ribosomes from starvation-induced selective autophagy. J Cell Biol 2014, 204: 909–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Carloni S, Albertini MC, Galluzzi L, Buonocore G, Proietti F, Balduini W.. Increased autophagy reduces endoplasmic reticulum stress after neonatal hypoxia-ischemia: role of protein synthesis and autophagic pathways. Exp Neurol 2014, 255: 103–112. [DOI] [PubMed] [Google Scholar]
- 108.Wang X, Li S, Liu Y, Ma C.. Redox regulated peroxisome homeostasis. Redox Biol 2015, 4: 104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Manjithaya R, Nazarko TY, Farre JC, Subramani S.. Molecular mechanism and physiological role of pexophagy. FEBS Lett 2010, 584: 1367–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shaid S, Brandts CH, Serve H, Dikic I.. Ubiquitination and selective autophagy. Cell Death Differ 2013, 20: 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dorn GW II, Kitsis RN.. The mitochondrial dynamism-mitophagy-cell death interactome: multiple roles performed by members of a mitochondrial molecular ensemble. Circ Res 2015, 116: 167–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kubli DA, Gustafsson AB.. Mitochondria and mitophagy: the yin and yang of cell death control. Circ Res 2012, 111: 1208–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gottlieb RA, Mentzer RM Jr.. Autophagy: an affair of the heart. Heart Fail Rev 2013, 18: 575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rifki OF, Hill JA.. Cardiac autophagy: good with the bad. J Cardiovasc Pharmacol 2012, 60: 248–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xie M, Morales CR, Lavandero S, Hill JA.. Tuning flux: autophagy as a target of heart disease therapy. Curr Opin Cardiol 2011, 26: 216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]