Abstract
Several studies have shown that combination treatment with natural products and chemotherapy agents can improve the sensitivity and cytotoxicity of chemotherapy agents. Resveratrol, a natural product, has many biological effects including antitumor and antiviral activities, as well as vascular protective effect. The aim of this study is to investigate the synergistic anticancer effect of resveratrol in combination with cisplatin and the potential anticancer mechanisms involved in A549 cells. The results obtained from Cell Counting Kit-8 and isobolographic analysis demonstrated that combination of resveratrol and cisplatin resulted in synergistic cytotoxic effects in A549 cells. Results from Hoechst staining, flow cytometry and western blot analysis suggested that resveratrol enhanced cisplatin-mediated apoptosis. Meanwhile, the changes of LC3-II and P62 levels and formation of autophagosome suggested that resveratrol in combination with cisplatin triggered autophagy. More importantly, inhibiting autophagy by 3-methyladenine markedly attenuated the apoptosis caused by combination of resveratrol and cisplatin in A549 cells. Taken together, our study provides the first evidence that resveratrol combined with cisplatin synergistically induce apoptosis via modulating autophagic cell death in A549 cells. These findings also help us to understand the role of natural products in combination with chemotherapy agents in lung cancer.
Keywords: resveratrol, apoptosis, autophagy, cisplatin, non-small cell lung cancer
Introduction
Lung cancer is one of the most common malignancies with the highest incidence and mortality rate worldwide. According to reports, the incidence of lung cancer is still increasing and approximately 85% of lung cancer is non-small cell lung cancer (NSCLC) [1]. Although many efforts have been made in the therapy of lung cancer, the 5-year overall survival is still below 20% [2]. Currently, surgery, chemotherapy, radiation therapy, molecular-targeted therapy, and intervention therapy are the main therapies for lung cancer. Most NSCLC patients were in end-stage when the diagnosis was ensured. For these patients, systemic therapy including chemotherapy becomes the main therapy [3].
As one of platinum-based chemotherapy, cisplatin (cis-diammineplatinum dichloride) is the standard therapy for lung cancer. The prominent anticancer effects of cisplatin are induction of oxidative stress, DNA damage, and apoptosis [4–6]. However, the emergence of drug resistance results in the failure of chemotherapy. Various studies have revealed that the phosphoinositide 3-kinase (PI3K), mitogen-activated protein kinases (MAPKs) pathways, Bax-like proteins, and Bcl2-like proteins are involved in cisplatin resistance [7–10]. Therefore, new strategies, such as the combination of natural products and chemotherapy agents, are needed to overcome cisplatin resistance or sensitize tumor cells to cisplatin.
As the ultimate goal of translational medicine is to develop new treatment strategies and to improve health across populations, more and more researchers are focusing on the therapy of natural products combined with chemotherapy. Resveratrol (3,4,5-trihydroxystilbene) is one of natural phytoalexin products widely existed in many spermatophyte, such as berries, peanuts, and Chinese medicine Polygonum cuspidatum, etc., especially rich in fresh grape skins [11]. The famous ‘French Paradox’ demonstrates that resveratrol can elicit beneficial effects in cardiovascular diseases [12]. It has also been reported that resveratrol has antitumor effects on different cancer types including breast cancer, bladder cancer, and lung cancer. Studies on the antitumor effects showed that resveratrol can inhibit proliferation and induce apoptosis via modulating the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) and MAPKs pathways [13–15]. In addition, its antiangiogenic effects and suppression of invasion have also been reported [16]. More recently, some studies suggested that resveratrol exerts its antitumor effects through the induction of autophagy, which plays an important role in the therapy of tumors [17, 18].
Although the effects of resveratrol and cisplatin in A549 cells have been studied, the relationship between apoptosis and autophagy in A549 cells treated with combination of resveratrol and cisplatin has not been resolved. Herein, based on the crosstalk between autophagy and apoptosis, we hypothesize that combination treatment may have synergistically effects on A549 cells through increasing apoptosis induced by autophagy.
Materials and Methods
Cell culture
Human lung adenocarcinoma cell line (A549) was purchased from the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China). Cells were cultured in RPMI-1640 medium (Gibico-BRL, Grand Island, USA) supplemented with 10% heat-inactivated fetal bovine serum, 100 U/ml penicillin G, and 100 μg/ml streptomycin at 37°C in a humidified atmosphere containing 5% CO2. All experiments were performed during the exponential phase of cell growth.
Regents and antibodies
Cisplatin was obtained from The First Affiliated Hospital of Nanjing Medical University (Nanjing, China). Resveratrol, 3-methyladenine (3-MA), and solvent dimethyl sulfoxide (DMSO) were purchased from Sigma (St Louis, USA). Resveratrol was dissolved in DMSO (0.1%, v/v, final concentration) and sterilized by passing through a 0.22 μm pore size filter (Merck Millipore, Bedford, USA). Cell Counting Kit-8 (CCK-8) was purchased from Obio Technology (Shanghai, China). RPMI-1640 medium, fetal bovine serum, and penicillin/streptomycin were purchased from Gibico-BRL (Gaithersburg, USA). Antibodies to Bax, phosphor-AKT, AKT, p62, and LC3I/II were purchased from Cell Signaling Technology (Danvers, USA). Antibody to Bcl2 was purchased from Abcam (Cambridge, UK). Antibody to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and horseradish peroxidase-conjugated anti-rabbit IgG secondary antibody were purchased from Bioworld (Nanjing, China). Alexa Fluor® 555-conjugated anti-rabbit IgG (H+L) secondary antibody was purchased from Invitrogen (Carlsbad, USA). Hoechst 33342 was purchased from Guangzhou Ribobio (Guangzhou, China). The annexin V-fluorescein isothiocyanate (FITC) kit was purchased from Bender MedSystems (Vienna, Austria).
Cell viability assay
Cell viability was evaluated by CCK-8 assay. Cells in logarithmic growth phase were seeded in 96-well plates at a density of 1 × 103 cells per well and incubated for 24 h. Then, the cells were incubated with different concentrations of resveratrol (2.5, 5, 10, 20, and 40 µM), cisplatin (3.125, 6.25, 12.5, 25, and 50 µM), and the combination of resveratrol (2.5 µM) and cisplatin with or without 3-MA for 48 h, respectively. After that, 10 µl of CCK-8 dissolved in 100 µl RPMI-1640 was added to each well and incubated for another 1–4 h. The absorbance was measured at 450 nm wavelength with a microplate reader (Thermo Scientific, Waltham, USA). The 50% inhibitory concentration (IC50) was calculated according to the dose–response curve.
Analysis of combined drug effects
To evaluate the synergistic effect of resveratrol in combination with cisplatin on A549 cells, isobolographic analysis was performed. The IC50 values of resveratrol alone and cisplatin alone were plotted in the X and Y axes, respectively. The straight line connecting the two intercept points indicated an additive effect of two drugs. The points below or above the line indicated a synergistic or antagonistic effect, respectively.
Flow cytometry analysis
Cells in logarithmic growth phase were seeded in six-well plates and treated with resveratrol (2.5 µM), cisplatin (20 µM), and the combination for 24 h. Then, the cells were collected and washed twice with cold phosphate-buffered saline (PBS). Cells were resuspended in 500 µl 1 × binding buffer and stained with annexin V and propidium iodide (PI) (Bender MedSystems, Vienna, Austria), according to the manufacturer’s protocol. After 15 min of incubation at room temperature in the dark, cells were analyzed by flow cytometry (FACScan, Becton Dickinson, Mountain View, USA). At least 10,000 cells were collected for each sample.
Hoechst 33342 staining
A549 cells were seeded in 24-well plates and treated with different drugs with or without 3-MA for 24 h. Then cells were fixed with 4% paraformaldehyde for 30 min and washed twice with PBS for 10 min to remove any residual solvent. Then, cells were subject to Hoechst 33342 staining for 15 min, followed by two times wash with PBS for 5 min, and finally observed under a fluorescence microscope (Becton Dickinson).
Western blot analysis
A549 cells were seeded in 60-mm dishes and treated with different drugs with or without 3-MA for 24 h. Then, the cells were harvested and lysed by RIPA buffer (Thermo Scientific). Protein lysates were collected and separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electrotransferred onto polyvinylidene difluoride (PVDF) membrane (Millipore) in Tris-glycine buffer (PH 8.4) containing 20% methanol. Then, the PVDF membranes were blocked in 5% fat-free dry milk in Tris-buffered-saline containing 0.1% Tween-20 (TBST) for 1 h at room temperature. After extensive wash, membranes were incubated with different specific antibodies overnight at 4°C. After three times wash with TBST, membranes were incubated with appropriate secondary antibodies for 1 h at room temperature. After three times wash with TBST, protein bands were visualized with enhanced chemiluminescence detection reagents (Thermo Scientific) and the Bio-Rad Gel Doc/Chemi Doc Imaging System (Bio-Rad, Hercules, USA). Data were analyzed using the Quantity One software (Bio-Rad).
Transmission electron microscope
The cellular ultrastructure and autophagosome were detected by transmission electron microscope (TEM; JEOL, Tokyo, Japan). A549 cells were seeded in six-well plates and treated with different drugs for 24 h. Then, cells were harvested and washed three times with PBS and fixed with 4% paraformaldehyde at 4°C overnight. The cell pellet was then placed in 1% osmium tetroxide, fixed at room temperature for 1 h. After a series of graded ethanol dehydration, samples embedded in resin were cut into ultrathin sections and fixed to the copper line. Samples were observed under a JEOL 1200EX TEM. Autophagosomes were defined as double-membrane vacuoles measuring 0.5 or 2 µm.
Immunofluorescence analysis
A549 cells were seeded in 24-well plates and treated with different drugs for 24 h. Afterwards, cells were fixed with 4% paraformaldehyde for 30 min at room temperature, and subsequently incubated with 0.5% Triton X-100 for permeabilization. After being washed with PBS, the cells were blocked in 5% BSA for 60 min, followed by overnight incubation with a previously described anti-LC3 antibody (1:100) at 4°C. Then, the cells were washed with PBS, incubated with FITC-conjugated IgG (1:500) for 2 h. The nuclei were counterstained with Hoechst and cells were viewed with a fluorescence microscope (Becton Dickinson).
Statistical analysis
Data are presented as the mean ± standard error of the mean. All statistical analyses were performed using one-way ANOVA, followed by a Dunnett post hoc test, employing Prism 6.00 software (GraphPad Software, San Diego, USA) and SPSS version 20 (SPSS, Inc., Chicago, USA). P < 0.05 was considered as statistically significant difference.
Results
Combination of resveratrol and cisplatin results in synergistic cytotoxic effects in A549 cells
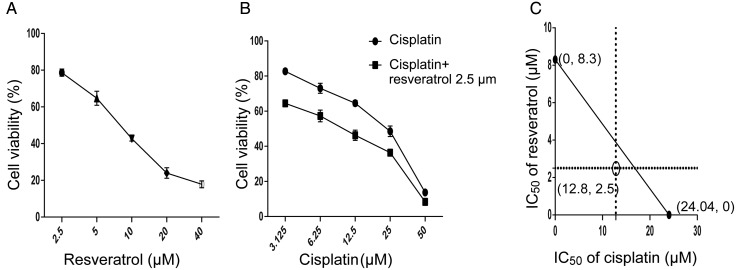
To investigate the effect of resveratrol in combination with cisplatin on cell viability, A549 cells were treated with different concentrations of resveratrol (2.5, 5, 10, 20, and 40 µM) or cisplatin (3.125, 6.25, 12.5, 25, and 50 µM) for 48 h, respectively. As shown in Fig. 1A,B both resveratrol and cisplatin showed a dose-dependent cytotoxic effect to A549 cells. The IC50 values of resveratrol and cisplatin on A549 cells are 8.3 and 24.04 µM, respectively. Afterwards, we chose 2.5 µM of resveratrol to combine with different concentrations of cisplatin. As shown in Fig. 1B, combination treatment enhanced cytotoxicty of cisplatin, compared with cisplatin alone in A549 cells. The IC50 value of combination treatment is 12.8 µM, which was reduced to ~50% of that of cisplatin alone. Meanwhile, the isobologram was plotted according to the IC50 values of resveratrol, cisplatin, and combination. As shown in Fig. 1C, the result indicated that combination of resveratrol and cisplatin resulted in synergistic cytotoxic effect in A549 cells.
Figure 1.
Resveratrol and cisplatin alone or in combination decreases the viability of A549 cells A549 cells were treated with various concentrations of resveratrol and cisplatin alone or combination for 48 h. (A,B) Cell viability was determined using the CCK-8 assay. (C) After treated with combination of resveratrol (2.5 µM) and cisplatin (20 µM), isobolographic analysis was performed using the pre-described method. The hollow dot below the line indicated a synergistic effect.
Resveratrol enhances the effect of cisplatin on apoptosis in A549 cells
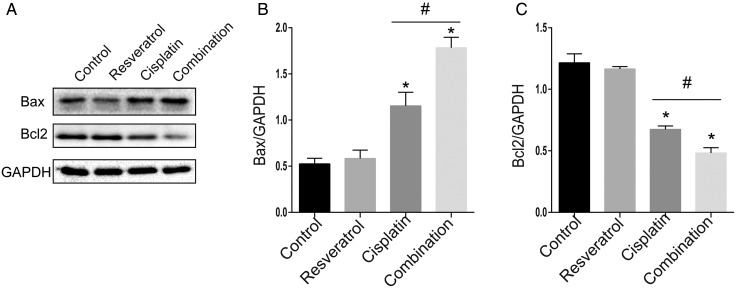
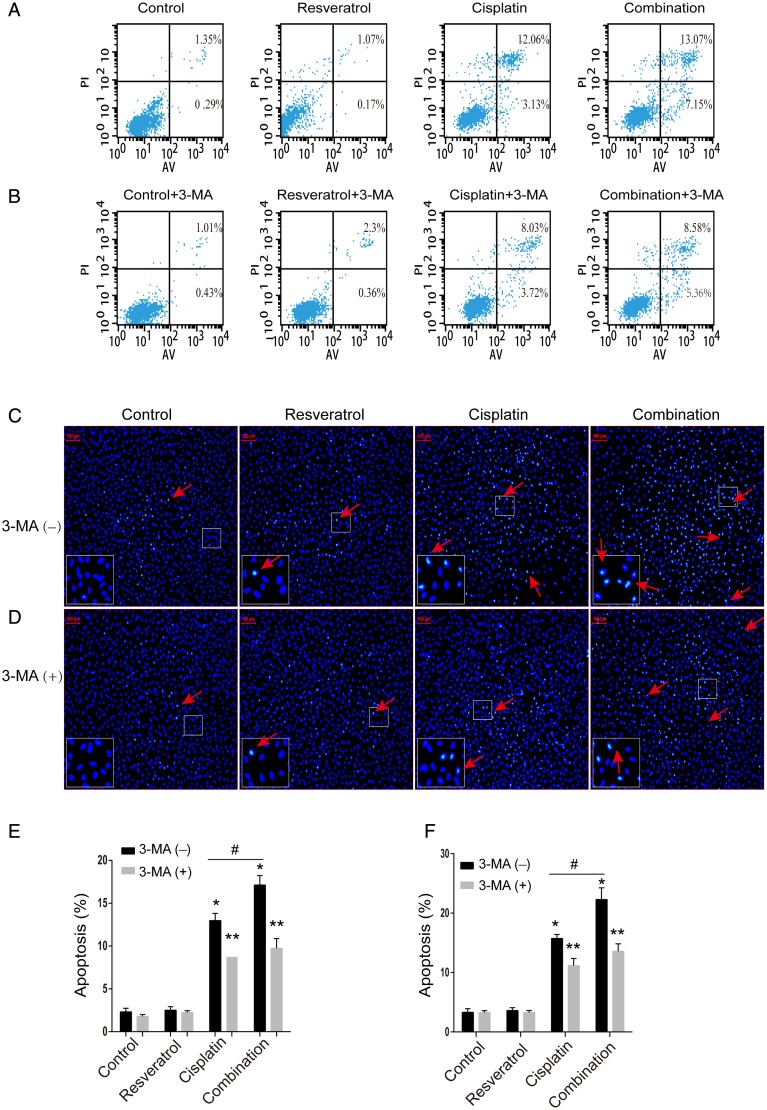
To determine whether combination treatment-induced cell death was related to apoptosis, A549 cells were treated with resveratrol (2.5 µM), cisplatin (20 µM), or combination for 24 h. Bax and Bcl2 are important classical molecules involved in apoptosis signaling pathway, therefore we detected the protein levels of Bax and Bcl2 under the treatment of the drugs by western blot analysis. As shown in Fig. 2, Bax level was increased in the combination treatment group compared with that in cisplatin alone treatment group, but Bcl2 level was decreased in the combination treatment group. To further ensure the association between combination treatment and apoptosis, apoptosis cells were detected by Annexin V-FITC/PI staining and flow cytometry analysis. As shown in Fig. 3A,E, when compared with cisplatin alone, combination treatment increased apoptosis rate in A549 cells (12.98% ± 0.84% vs. 17.12% ± 1.10%, P < 0.05). Meanwhile, the nuclear morphology change results (Fig. 3C,F) were also consistent with above results. Taken together, these results indicated that resveratrol enhanced the effect of cisplatin on apoptosis in A549 cells.
Figure 2.
Combination treatment of resveratrol and cisplatin modulates apoptosis-related protein levels in A549 cells A549 cells were treated with resveratrol (2.5 µM), cisplatin (20 µM) alone, or in combination for 24 h. (A) The protein level of Bax and Bcl2 was evaluated by western blot analysis. GAPDH was used as the loading control. (B,C) Quantitative analysis of Bax and Bcl2 levels. *P < 0.05 vs. control group, #P < 0.05 vs. cisplatin group.
Figure 3.
Effect of 3-MA on the resveratrol and cisplatin-induced apoptosis in A549 cells A549 cells were treated with resveratrol (2.5 µM), cisplatin (20 µM) alone, or in combination with or without 3-MA (500 µM) for 24 h. (A,B) Cell apoptosis was evaluated by flow cytometry. The apoptosis rate = (annexin V+PI+ + annexin V+PI−)/total cells × 100%. (C,D) Cell apoptosis was evaluated by Hoechst staining assay. The cells pointed by red arrows represent apoptotic cells. The lower left corner rectangles represent the enlarged parts. (E,F) Quantitative analysis of cell apoptosis. *P < 0.05 vs. control group, **P < 0.05 vs. the group with 3-MA(-), and #P < 0.05 vs. cisplatin group.
Combination of resveratrol and cisplatin induces autophagy in A549 cells
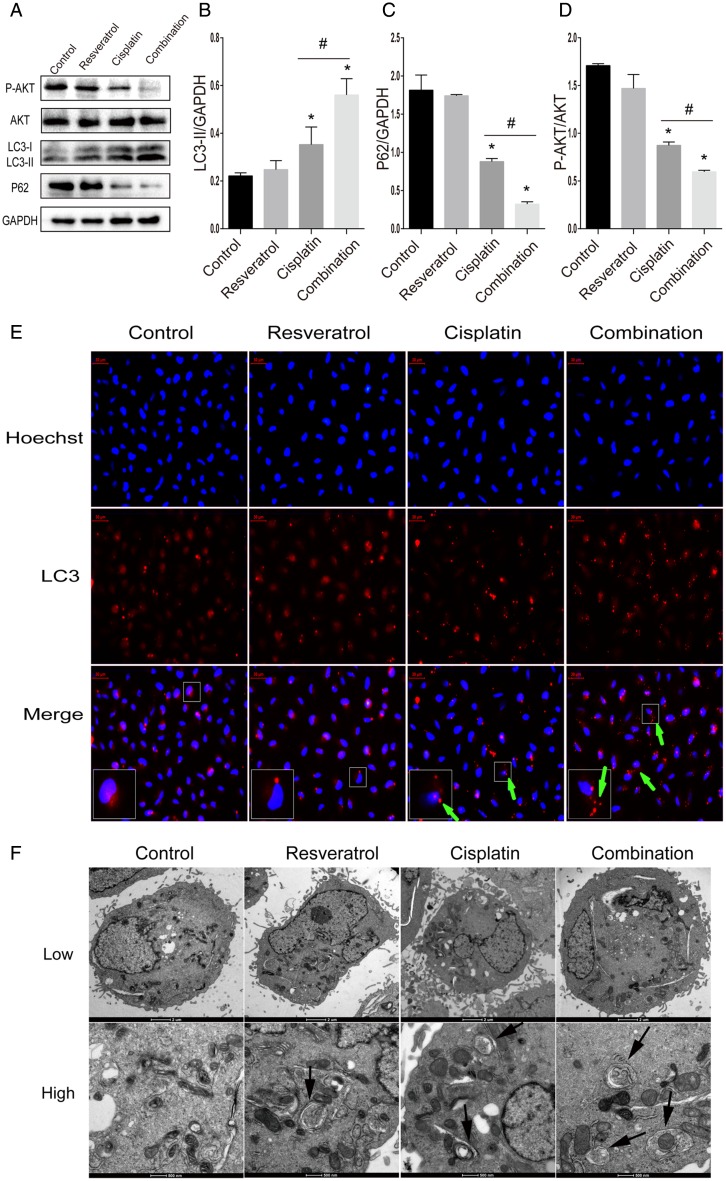
To determine whether the combination of resveratrol and cisplatin led to the activation of autophagy in A549 cells, protein levels of LC3-II and P62 were detected by western blot analysis. As shown in Fig. 4A,B, the level of LC3-II, an important component of autophagosome, was significantly increased in combination treatment than in the cisplatin alone treatment (P < 0.05). Meanwhile, the level of P62, a substrate of autophagy, was dramatically decreased in combination treatment than in the cisplatin alone (Fig. 4A,C).
Figure 4.
Combination treatment of resveratrol and cisplatin leads to the activation of autophagy in A549 cells A549 cells were treated with resveratrol (2.5 µM), cisplatin (20 µM) alone, or in combination for 24 h. (A) The protein levels of LC3-II, P62, and P-AKT were evaluated by western blot analysis. (B–D) Quantitative analysis of LC3-II, P62, and P-AKT levels. (E) A549 cells were fixed and double-labeled with an anti-LC3 antibody with higher selectivity for LC3-II (red) and Hoechst (blue). Increase of red punctuate aggregation pointed by green arrows indicated the activation of autophagy. The lower left corner rectangles represent the enlarged parts. (F) Autophagosomes observed by TEM. Arrows indicate autophagosomes. The low magnification with scale bar: 2 μm. The high magnification with scale bar: 500 nm. *P < 0.05 vs. control group, #P < 0.05 vs. cisplatin group.
To further examine the autophagy induced by combination, the LC3 immunoreactivity was examined by immunofluorescence in A549 cells. As shown in Fig. 4E, combination treatment altered the distribution of LC3 to form many coarse dots and punctuate staining when compared with the cisplatin alone. TEM, as a classical and direct method to detect autophagy, was also used to observe the formation of autophagosome. A lot of double membranes structure in cytoplasm and many autophagosomes were observed in A549 cells treated with different drugs (Fig. 4F).
For PI3K/Akt pathway is a key regulator of authophagy, we also detected the levels of P-AKT and AKT by western blot analysis. As shown in Fig. 4A,D, combination treatment significantly decreased the expression of P-AKT, compared with cisplatin alone. All the above results suggested that combination of resveratrol and cisplatin induced autophagy in A549 cells.
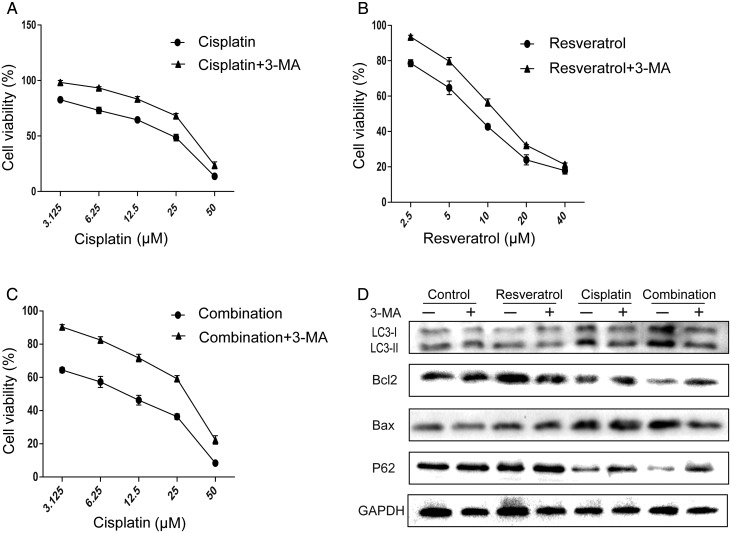
Combination of resveratrol and cisplatin induces apoptosis by modulating autophagy in A549 cells
According to the above results, it was found that combination treatment can increase apoptosis and induce autophagy in A549 cells. For the relationship between apoptosis and autophagy in A549 cells treated with drugs was unclear, we hypothesized that combination treatment increased apoptosis by inducing autophagy in A549 cells. To confirm the hypothesis, we first detected the cytotoxicity of combination, cisplatin, and resveratrol in the presence of 3-MA, respectively. Interestingly, cell viability of groups in the presence of 3-MA was significantly increased, compared with that in the absence of 3-MA (Fig. 5A–C). The IC50 values in A549 cells treated with combination, cisplatin, and resveratrol were significantly increased when co-treated with 3-MA, compared with those in the absence of 3-MA (30.4 µM vs. 12.8 µM, 34.8 µM vs. 24.04 µM, 12.7 µM vs. 8.3 µM). Then, the protein levels of Bax, Bcl2, LC3, and P62 were also detected in the presence of 3-MA. As shown in Fig. 5D, the increase in LC-II level and reduction of P62 level in A549 cells treated with combination were reversed by 3-MA. At the same time, the increase in Bax level and reduction of Bcl2 level were attenuated by 3-MA, indicating that inhibition of autophagy abolished combination-induced apoptosis in A549 cells. Furthermore, as shown in Fig. 3, Hoechst 33342 staining and flow cytometry results also confirmed the above results. Taken together, combination of resveratrol and cisplatin increased apoptosis by triggering autophagy in A549 cells.
Figure 5.
Activation of autophagy contributes to the apoptosis triggered by combination treatment in A549 cells A549 cells were treated with various concentrations of resveratrol, cisplatin alone, or in combination with or without 3-MA (500 µM). (A–C) Cell viability were determined by CCK-8 assay. (D) The protein levels of LC3-II, P62, Bax, and Bcl2 were evaluated by western blot analysis.
Discussion
In this study, we investigated the synergistic effects of resveratrol in combination with cisplatin on A549 cells and explored the possible mechanisms. Our results demonstrated that resveratrol dose-dependently inhibited the viability of A549 cells. Moreover, resveratrol exerted a synergistic effect with cisplatin on A549 cells. The anticancer effect of resveratrol depends on its promotion of apoptosis by triggering autophagy.
Apoptosis, which occurs after sufficient cellular damage, is a normal component of the growth and health of multicellular organisms. Moreover, apoptosis is involved in a variety of pathological processes, especially in cancer. The blockage of cell apoptosis plays an important role in the development of chemo-resistance. Therefore, promotion of apoptosis is used as a potential anticancer strategy. Bax and Bcl2 are the regulators of apoptosis. While Bax acts as apoptosis inducer, Bcl2 evokes a survival signal for the cells [19]. Results from this study showed that cisplatin increased the protein level of Bax and decreased the protein level of Bcl2. Furthermore, resveratrol can increase the effects of cisplatin via modulating Bax/Bcl2 ratio in A549 cells. This was further confirmed by flow cytometry and Hoechst staining, which suggested the combination treatment could synergistically induce apoptosis in A549 cells.
Autophagy is a lysosomal degradation pathway, which is important for keeping cellular homeostasis, mammalian development, and immunity [20–22]. Autophagy also plays a crucial regulatory role in many pathological processes, most notably in cancer. More and more researches suggested that autophagy might be a target pathway of anticancer therapeutic agents. It is worth to note that autophagy is a double-edged sword in the initiation, development, and metastasis of cancer [23]. On one hand, autophagy plays an antitumor role, on the other hand, autophagy, as an adaptive response, protects tumor cells against stress. Autophagy initiation which is completed with the accumulation of the ULK1/2-ATG13-FIP200 complex results in the development of isolation membrane, also known as a phagosome. On receiving autophagic stimulus, the phagosome converts into autophagosome depending on autophagy-related genes (Atg) proteins, then, its maturation is completed upon fusion with lysosome to form autophagolysosome [24]. Therefore, the formation of autophagosome observed by TEM was considered as the gold standard for detecting autophagy. LC3, an important component of autophagy, was studied first. LC3 appears as LC3-I and LC3-II in the cytoplasm. LC3-I is converted to LC3-II when autophagy occurs. Therefore, LC3-II was considered as the molecular marker of autophagosome and the level of LC3-II was employed to reflect the degree of cell autophagy [25]. In this study, we found that the combination treatment could induce autophagy via increasing the accumulation of autophagosome and the level of LC3-II. Meanwhile, the protein level of P62, a substrate of autophagy [26], was markedly reduced, further indicating an enhancement of autophagy. Because many reports have suggested that Class I PI3Ks can active AKT/PKB through phosphorylation and the latter inhibit autophagy in turn [27, 28], we detected the protein level of P-AKT. Our results revealed that combination treatment could decrease the phosphorylation of AKT. Therefore, we believe that the combination treatment might induce autophagy via decreasing the phosphorylation of AKT. In addition, various studies demonstrated that some molecules, such as Atgs, mTOR, P53, and Beclin-1, also played important roles in modulating autophagic activity [29–31].
Combination treatment can induce apoptosis and autophagy in A549 cells. However, what is the relationship between autophagy and apoptosis? In some instances blockage of autophagy will sensitize cells to apoptosis [32], while in other cases autophagy can act as an initiating factor for apoptosis-induction, which means that excessive autophagic activity can directly induce apoptotic cell death and interruption of autophagy will delay apoptosis [33]. Moreover, there are some crosstalk between apoptosis and autophagy. For example, Beclin-1, an essential molecular for autophagy, interacts with Bcl2 [34, 35]. Increased Beclin-1 level may release Bak/Bax from Bcl2 to promote apoptosis, while decreased Bcl2 level may result in excessive beclin-1-dependent autophagy. In this study, we found that combination treatment-induced apoptosis could be abolished by 3-MA, which means that activation of autophagy increased apoptosis in A549 cells. However, the exact mechanisms of the crosstalk between autophagy and apoptosis are still unresolved in this study.
In conclusion, in the current study, we revealed that resveratrol increased the sensitivity of A549 cells to cisplatin via inducing apoptosis, followed by activation of autophagy. These findings support the possibility of resveratrol as a potential adjuvant drug or chemosensitizer for the treatment of NSCLC. Although we demonstrated that autophagy can induce apoptosis, the potential mechanisms of autophagy and apoptosis remain to be further elucidated. Meanwhile, our findings should be further confirmed in models of lung cancer in future.
Funding
This work was supported by the grants from the National Natural Science Foundation of China (No. 81273571), the National Major Scientific and Technological Project for ‘Significant New Drugs Development’ (No. 2011ZX09302-003-02), Jiangsu Province Major Scientific and Technological Special Project (No. BM2011017), and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
References
- 1.Sacco PC, Casaluce F, Sgambato A, Rossi A, Maione P, Palazzolo G, Napolitano A. Current challenges of lung cancer care in an aging population. Expert Rev Anticancer Ther 2015, 15: 1419–1429. [DOI] [PubMed] [Google Scholar]
- 2.Ettinger D, Wood DE, Akerley W, Bazhenova LA, Borghaei H, Camidge DR, Cheney RT, et al.. Non-small cell lung cancer, version 6.2015. J Natl Compr Canc Netw 2015, 13: 515–524. [DOI] [PubMed] [Google Scholar]
- 3.NSCLC Meta-Analyses Collaborative Group Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J Clin Oncol 2008, 26: 4617–4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van den Berg JH, Beijnen JH, Balm AJ, Schellens JH. Future opportunities in preventing cisplatin induced ototoxicity. Cancer Treat Rev 2006, 32: 390–397. [DOI] [PubMed] [Google Scholar]
- 5.Mukhopadhyay P, Choi YM, Kim HK, Shim W, Anwar MA, Kwon JW, Kwon HK, et al.. Mechanism of cisplatin-induced cytotoxicity is correlated to impaired metabolism due to mitochondrial ROS generation. PLoS One 2015, 10: e0135083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kohno K, Wang KY, Takahashi M, Kurita T, Yoshida Y, Hirakawa M, Harada Y, et al.. Mitochondrial transcription factor A and mitochondrial genome as molecular targets for cisplatin-based cancer chemotherapy. Int J Mol Sci 2015, 16: 19836–19850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu M, Qi Z, Liu B, Ren Y, Li H, Yang G, Zhang Q. RY-2f, an isoflavone analog, overcomes cisplatin resistance to inhibit ovarian tumorigenesis via targeting the PI3K/AKT/mTOR signaling pathway. Oncotarget 2015, 6: 25281–25294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li W, Melton DW. Cisplatin regulates the MAPK kinase pathway to induce increased expression of DNA repair gene ERCC1 and increase melanoma chemoresistance. Oncogene 2012, 31: 2412–2422. [DOI] [PubMed] [Google Scholar]
- 9.Lo SJ, Fan LC, Tsai YF, Lin KY, Huang HL, Wang TH, Liu H, et al.. A novel interaction of nucleophosmin with BCL2-associated X protein regulating death evasion and drug sensitivity in human hepatoma cells. Hepatology 2013, 57: 1893–1905. [DOI] [PubMed] [Google Scholar]
- 10.Tajeddine N, Galluzzi L, Kepp O, Hangen E, Morselli E, Senovilla L, Araujo N, et al.. Hierarchical involvement of Bak, VDAC1 and Bax in cisplatin-induced cell death. Oncogene 2008, 27: 4221–4232. [DOI] [PubMed] [Google Scholar]
- 11.Novelle MG, Wahl D, Dieguez C, Bernier M, de Cabo R. Resveratrol supplementation: where are we now and where should we go? Ageing Res Rev 2015, 21: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Catalgol B, Batirel S, Taga Y, Ozer NK. Resveratrol: French paradox revisited. Front Pharmacol 2012, 3: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai H, Scott E, Kholghi A, Andreadi C, Rufini A, Karmokar A, Britton RG, et al.. Cancer chemoprevention: evidence of a nonlinear dose response for the protective effects of resveratrol in humans and mice. Sci Transl Med 2015, 7: 298ra117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oi N, Yuan J, Malakhova M, Luo K, Li Y, Ryu J, Zhang L, et al.. Resveratrol induces apoptosis by directly targeting Ras-GTPase-activating protein SH3 domain-binding protein 1. Oncogene 2015, 34: 2660–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alkhalaf M, Jaffal S.. Potent antiproliferative effects of resveratrol on human osteosarcoma SJSA1 cells: novel cellular mechanisms involving the ERKs/p53 cascade. Free Radic Biol Med 2006, 41: 318–325. [DOI] [PubMed] [Google Scholar]
- 16.Tsai JH, Hsu LS, Lin CL, Hong HM, Pan MH, Way TD, Chen WJ. 3,5,4’-Trimethoxystilbene, a natural methoxylated analog of resveratrol, inhibits breast cancer cell invasiveness by downregulation of PI3K/Akt and Wnt/beta-catenin signaling cascades and reversal of epithelial-mesenchymal transition. Toxicol Appl Pharmacol 2013, 272: 746–756. [DOI] [PubMed] [Google Scholar]
- 17.Filomeni G, Graziani I, Rotilio G, Ciriolo MR. trans-Resveratrol induces apoptosis in human breast cancer cells MCF-7 by the activation of MAP kinases pathways. Genes Nutr 2007, 2: 295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miki H, Uehara N, Kimura A, Sasaki T, Yuri T, Yoshizawa K, Tsubura A. Resveratrol induces apoptosis via ROS-triggered autophagy in human colon cancer cells. Int J Oncol 2012, 40: 1020–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karch J, Molkentin JD. Regulated necrotic cell death: the passive aggressive side of Bax and Bak. Circ Res 2015, 116: 1800–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radogna F, Dicato M, Diederich M. Cancer-type-specific crosstalk between autophagy, necroptosis and apoptosis as a pharmacological target. Biochem Pharmacol 2015, 94: 1–11. [DOI] [PubMed] [Google Scholar]
- 21.Schneider JL, Cuervo AM. Autophagy and human disease: emerging themes. Curr Opin Genet Dev 2014, 26: 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hale AN, Ledbetter DJ, Gawriluk TR, Rucker EB III. Autophagy: regulation and role in development. Autophagy 2013, 9: 951–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Apel A, Zentgraf H, Buchler MW, Herr I. Autophagy-A double-edged sword in oncology. Int J Cancer 2009, 125: 991–995. [DOI] [PubMed] [Google Scholar]
- 24.Jin M, Klionsky DJ.. Regulation of autophagy: modulation of the size and number of autophagosomes. FEBS Lett 2014, 588: 2457–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanida I, Ueno T, Kominami E. LC3 conjugation system in mammalian autophagy. Int J Biochem Cell Biol 2004, 36: 2503–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katsuragi Y, Ichimura Y, Komatsu M. p62/SQSTM1 functions as a signaling hub and an autophagy adaptor. FEBS J 2015, 282: 4672–4678. [DOI] [PubMed] [Google Scholar]
- 27.Xing C, Zhu B, Liu H, Yao H, Zhang L.. Class I phosphatidylinositol 3-kinase inhibitor LY294002 activates autophagy and induces apoptosis through p53 pathway in gastric cancer cell line SGC7901. Acta Biochim Biophys Sin 2008, 40: 194–201. [DOI] [PubMed] [Google Scholar]
- 28.Zhang DM, Liu JS, Deng LJ, Chen MF, Yiu A, Cao HH, Tian HY, et al.. Arenobufagin, a natural bufadienolide from toad venom, induces apoptosis and autophagy in human hepatocellular carcinoma cells through inhibition of PI3K/Akt/mTOR pathway. Carcinogenesis 2013, 34: 1331–1342. [DOI] [PubMed] [Google Scholar]
- 29.Fu LL, Cheng Y, Liu B. Beclin-1: autophagic regulator and therapeutic target in cancer. Int J Biochem Cell Biol 2013, 45: 921–924. [DOI] [PubMed] [Google Scholar]
- 30.Naves T, Jawhari S, Jauberteau MO, Ratinaud MH, Verdier M. Autophagy takes place in mutated p53 neuroblastoma cells in response to hypoxia mimetic CoCl(2). Biochem Pharmacol 2013, 85: 1153–1161. [DOI] [PubMed] [Google Scholar]
- 31.Hsu CM, Tsai Y, Wan L, Tsai FJ. Bufalin induces G2/M phase arrest and triggers autophagy via the TNF, JNK, BECN-1 and ATG8 pathway in human hepatoma cells. Int J Oncol 2013, 43: 338–348. [DOI] [PubMed] [Google Scholar]
- 32.Pan WR, Chen PW, Chen YL, Hsu HC, Lin CC, Chen WJ. Bovine lactoferricin B induces apoptosis of human gastric cancer cell line AGS by inhibition of autophagy at a late stage. J Dairy Sci 2013, 96: 7511–7520. [DOI] [PubMed] [Google Scholar]
- 33.Zou W, Wang X, Vale RD, Ou G. Autophagy genes promote apoptotic cell corpse clearance. Autophagy 2012, 8: 1267–1268. [DOI] [PubMed] [Google Scholar]
- 34.Pedro JM, Wei Y, Sica V, Maiuri MC, Zou Z, Kroemer G, Levine B. BAX and BAK1 are dispensable for ABT-737-induced dissociation of the BCL2-BECN1 complex and autophagy. Autophagy 2015, 11: 452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mukhopadhyay S, Panda PK, Sinha N, Das DN, Bhutia SK. Autophagy and apoptosis: where do they meet? Apoptosis 2014, 19: 555–566. [DOI] [PubMed] [Google Scholar]