Abstract
Physarum myosin is a Ca2+-binding protein and its activity is inhibited by Ca2+. In the present study, to clarify the light chains (LCs) from the different species (Physarum and scallop) and to determine the specific Ca2+-regulated effects, we constructed hybrid myosins with a Physarum myosin heavy chain (Ph·HC) and Physarum and/or scallop myosin LCs, and examined Ca2+-mediated regulation of ATPases and motor activities. In these experiments, it was found that Ca2+ inhibited motilities and ATPase activities of Physarum hybrid myosin with scallop regulatory light chain (ScRLC) and Physarum essential light chain (PhELC) but could not inhibit those of the Physarum hybrid myosin mutant Ph·HC/ScRLC/PhELC-3A which lacks Ca2+-binding ability, indicating that PhELC plays a critical role in Ca2+-mediated regulation of Physarum myosin. Furthermore, the effects of Ca2+ on ATPase activities of Physarum myosin constructs are in the following order: Ph·HC/PhRLC/PhELC > Ph·HC/ScRLC/PhELC > Ph·HC/PhRLC/ScELC > Ph·HC/ScRLC/ScELC, suggesting that the presence of PhRLC and PhELC leads to the greatest Ca2+ sensitivity of Physarum myosin. Although we did not observe the motilities of Physarum hybrid myosin Ph·HC/PhRLC/ScELC and Ph·HC/ScRLC/ScELC, our results suggest that Ca2+-binding to the PhELC may alter the flexibility of the regulatory domain and induce a ’closed’ state, which may consequently prevent full activity and force generation.
Keywords: actin, ATPase activity, calcium, in vitro motility assay, Physarum hybrid myosin
Introduction
Myosins constitute a superfamily of motor proteins that play important roles in several cellular processes that produce force, translocation, and muscle contraction [1–4]. Regulation via myosin follows either Ca2+-dependent phosphorylation of myosin regulatory light chains (RLCs) or direct binding of Ca2+ to essential light chains (ELCs). The former mode of regulation occurs in vertebrate smooth muscle, and it is widely accepted that contraction of smooth muscle is initiated by increases in intracellular Ca2+ concentrations. Specifically, Ca2+–calmodulin binding results in a complex that binds and activates myosin light chain kinase (MLCK). Subsequently, activated MLCK phosphorylates myosin RLCs to induce the contraction of smooth muscle [5,6]. The latter mechanism involving direct Ca2+-binding has been shown in some molluscan muscles [7,8] and in lower eukaryotes [9,10]. Mollusk scallop myosin belongs to the myosin II family, and its activity is regulated by Ca2+-binding to ELC [11]. Moreover, a similar myosin II family member from Physarum polycephalum is also regulated by Ca2+-binding to ELC [12]. However, in contrast to scallop myosin, the activity of Physarum myosin is inhibited by Ca2+ [13]. Despite these diametrically opposing effects, both of these myosins comprise heavy chain (HC) and pairs of RLC class and ELC class molecules that bind Ca2+ [14]. Several previous studies have demonstrated the Ca2+-activating regulation of scallop myosin [15,16]. However, the mechanism by which Ca2+ inhibits the activity of Physarum myosin remains poorly characterized.
Previously, we constructed hybrid heavy meromyosin (HMM) of smooth muscle HMM-HC associated with ELCs and RLCs from Physarum/scallop myosins, and purified recombinant hybrid smooth muscle HMMs following expression in Sf-9 cells. The ATPase assay and in vitro motility assay demonstrated that the inhibiting and activating effects were related to Physarum ELC (PhELC) and scallop ELC (ScELC), respectively [14]. Subsequently, recombinant full-length Physarum myosin was obtained and the Ca2+-mediated regulation was identified [12]. However, whether light chains (LCs) from different species (Physarum and scallop) show specific Ca2+-regulated effects or not still remains unclear. Here, we further examine the roles of LCs from Physarum and scallop to assess the role of LCs and examine the interactions between RLC and ELC.
We expressed hybrid myosins of Physarum, including myosin with full-length Physarum HC (Ph·HC) associated with Physarum light chains (PhRLC and PhELC), and with scallop light chains (ScRLC and ScELC). We also generated a hybrid mutant Physarum myosin with ScRLC and PhELC-3A and showed loss of Ca2+-mediated regulation. Finally, we measured the effect of Ca2+ on motilities and the ATPase activities of hybrid Physarum myosins and mutants.
Materials and Methods
Materials
ATP was purchased from Sigma-Aldrich (St Louis, USA). Dithiothreitol (DTT) and 4-(2-aminoethyl)-benzenesulfonyl fluoride hydrochloride (ABSF) were purchased from Wako Pure Chemical Industries (Osaka, Japan). Glucose oxidase and catalase were purchased from Sigma-Aldrich. Restriction and other modifying enzymes were purchased from Takara Shuzo Co. (Kyoto, Japan). All other chemicals were commercial products of reagent grade. Solutions were prepared using MilliQ water (Millipore, Billerica, USA).
Actin was purified from the acetone powder of chicken skeletal muscle according to Spudich and Watt [17] with slight modifications and used as actin filaments after polymerization.
Construction of recombinant baculovirus transfer vectors
Recombinant baculovirus vectors were constructed using the Bac-to-Bac system (Invitrogen, Carlsbad, USA) with genes for Ph·HC (GenBank™ accession number AF335500), PhRLC (GenBank™ accession number AB076705), ScRLC (GenBank™ accession number M17208), PhELC (GenBank™ accession number J03499), and ScELC (GenBank™ accession number M17201) according to the manufacturer’s protocol. PhELC with no Ca2+-binding activity was generated by substituting D15, D17, and E26 positions with alanines and was designated PhELC-3A as described in our previous report [12]. To facilitate purification of Physarum myosin, a His-tag sequence was attached to the C-terminus of Ph·HC cDNA based on our previous report [14]. Recombinant baculovirus constructs were generated as previously reported [12,14].
Expression and purification of recombinant hybrid myosins
Recombinant hybrid myosins were expressed and purified as described previously [12,14]. Briefly, Sf-9 cells were co-infected with three separate viruses (HC, RLC, and ELC) at a multiplicity of infection of 1. Infected cells were grown for 72 h at 27°C and those expressing Physarum hybrid myosins were pelleted by centrifugation and resuspended in buffer containing 20 mM Tris–HCl (pH 7.5), 1 mM MgCl2, 1 mM ethylene glycol tetraacetic acid (EGTA), 5 mM 2-mercaptethanol, 1 mM ABSF, and His-tag protein protease inhibitor cocktail (Roche, Indianapolis, USA). To release recombinant myosin from endogenous actin, the lysis buffer was adjusted to 0.2 M NaCl and 1 mM ATP after sonication, and lysates were then centrifuged for 1 h at 15,000 g to remove cell debris and unbroken cells. Subsequently, supernatants were mixed with Ni-nitrilotriacetic acid agarose affinity column chromatography solution (Qiagen, Hilden, Germany) in a 50-ml beaker with a rotating wheel for 3 h at 4°C and were then loaded onto a column. The column was washed with binding buffer containing 0.6 M NaCl, 20 mM Tris–HCl (pH 7.5), 5 mM MgCl2, 1 mM EGTA, 20 mM imidazole (pH 7.5), 0.1 mM ABSF, and 5 mM 2-mercaptethanol, and Physarum hybrid myosin was eluted with buffer containing 0.6 M NaCl, 20 mM Tris–HCl (pH 7.5), 5 mM MgCl2, 1 mM EGTA, 200 mM imidazole (pH 7.5), 0.1 mM ABSF, and 5 mM 2-mercaptethanol. Fractions were subject to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis, and those containing myosin were pooled and concentrated into 500 μl using an Amicon Ultra centrifugal Filter Unit (Millipore). Concentrates were then subject to SuperoseTM6 column chromatography (GE Healthcare, Buckingham, UK), and eluents containing myosin were concentrated into 0.05–0.1 ml using the filter unit. Approximately 100 μg of myosin was obtained from 1 × 108 cells using this procedure. All protein samples were used within 1 week and were kept on ice throughout the procedures.
ATPase assay
ATPase activities (specific activity of Physarum myosin, s−1 per myosin head) were determined by continuous measurements of inorganic phosphate (Pi) release using an EnzChek Phosphate Assay Kit (Invitrogen) according to the method of Webb as described in our previous report [18]. Briefly, ATPase activity was measured in reaction mixtures containing 20 mM Tris–HCl (pH 7.5), 40 mM KCl, 1.5 mM MgCl2, 0.1 mM DTT, 0.5 mM ATP, 0.05 μM Physarum myosin, 5 μM actin filaments, and 0.1 mM EGTA or various concentrations of Ca2+ (pCa: 8, 7, 6, 5, and 4) obtained by using Calcon 3.6 software to calculate the amount of Ca2+ (Supplementary Table S1). After 10 min of pre-incubation at 25°C, reactions were initiated by adding ATP, and Pi release was monitored for 5 min. It must be noted that our results may not reflect the changes in maximal ATPase activity since we did not do ATPase measurements at saturating actin concentrations.
In vitro motility assays
In vitro motility assays were performed as described previously [19] with slight modifications. Briefly, 0.2% nitrocellulose-coated coverslips were coated with Physarum myosin by standing on ice for 10 min in a buffer consisting of 60 mM KCl, 25 mM imidazole (pH 7.5), 4 mM MgCl2, and 1 mM DTT; actin filaments (3 nM) labeled with rhodamine–phalloidin (Molecular Probes, Eugene, USA) were introduced into flow cells that were constructed between a glass slide and a coverslip coated with Physarum myosin in motility buffer containing 10 mM KCl, 2 mM ATP, 1 mM MgCl2, 10 mM imidazole (pH 7.5), 25 mM DTT, and 0.1 mM EGTA or various concentrations of Ca2+ (pCa: 8, 7, 6, 5, and 4), along with anti-oxidization reagents consisting of 0.2 mg/ml glucose oxidase, 0.04 mg/ml catalase, and 4.5 mg/ml glucose. Movements of actin filaments were recorded using a fluorescence microscope equipped with a silicone-intensifier target camera. Average velocities were determined using WCIF ImageJ and were presented as the means ± standard deviations (SD) of at least 60 actin filaments for each experiment.
Other procedures
Protein concentrations were determined using Bio-Rad protein assays [20] with bovine serum albumin as a standard. SDS-PAGE was performed on 12.5% polyacrylamide gels using the Laemmli buffer system [21] with slight modifications. Statistical significance was determined by Student’s t-test or one-way ANOVA using Sigma Stat version 3.1. A P-value <0.05 was considered statistically significant.
Results
Expression and purification of Physarum hybrid myosin constructs
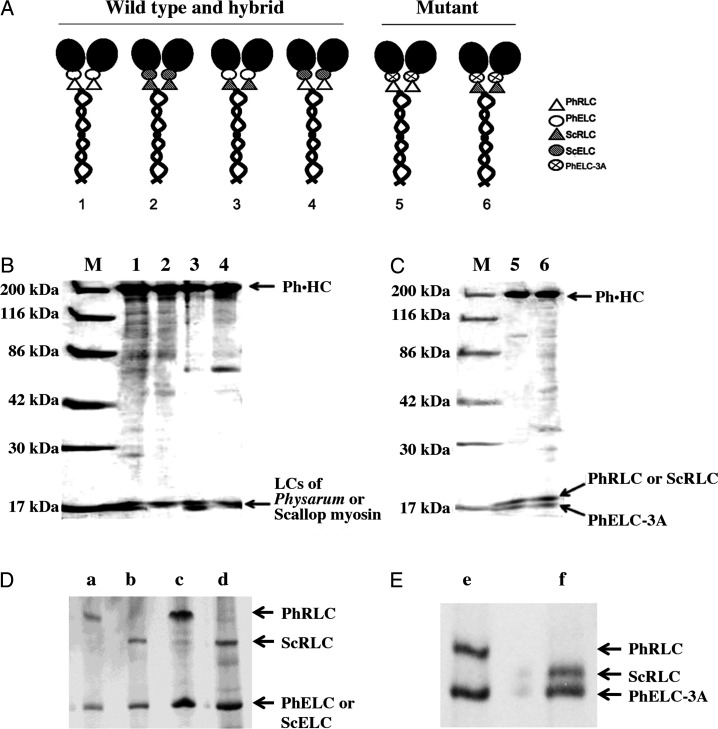
Figure 1A shows the illustration of six constructs with a Ph·HC and Physarum and/or scallop LCs: wild-type (Ph·HC/PhRLC/PhELC; Fig. 1A, model 1); three hybrids (Ph·HC/ScRLC/ScELC, Ph·HC/ScRLC/PhELC, and Ph·HC/PhRLC/ScELC; Fig. 1A, models 2, 3, and 4); and two mutants (Ph·HC/PhRLC/PhELC-3A and Ph·HC/ScRLC/PhELC-3A; Fig. 1A, models 5 and 6). To produce hybrid myosins, Sf-9 cells were co-infected with genes for HCs of Physarum myosin and LCs of Physarum and/or scallop myosin as previously described [14]. After 72 h of culture, co-infected cells were harvested and proteins were purified according to our previous reports [12,14]. SDS-PAGE of purified Physarum hybrid myosins (Fig. 1B) showed three types of hybrid myosin: (i) Ph·HC/ScRLC/ScELC (lane 2), (ii) Ph·HC/ScRLC/PhELC (lane 3), and (iii) Ph·HC/PhRLC/ScELC (lane 4). Because the molecular weights of ScRLC, ScELC, and PhRLC are close (≈18 kDa), they are indistinguishable by SDS-PAGE; the association of them with the hybrid Physarum myosin was confirmed by separating the LCs with glycerol PAGE (Fig. 1D). In addition, two Physarum myosin mutants were isolated as shown in Fig. 1, Ph·HC/PhRLC/PhELC-3A (Fig. 1C, lane 5) and Ph·HC/ScRLC/PhELC-3A (Fig. 1C, lane 6). As shown in glycerol PAGE (Fig. 1E), two LCs of them were clearly visible. All Physarum myosin constructs contained HC, ELC, and RLC with a stoichiometry of 1.0:0.9–1.1:0.9–0.97 as determined by using Image J software to analyze the density of protein bands based on the previous reports [22,23].
Figure 1.
Schematic diagram and purification of Physarum myosin hybrid constructs (A) Schematic diagram of Physarum myosin hybrid constructs. (B) SDS-PAGE (12.5%) of purified Physarum myosin hybrid constructs associated with LCs of Physarum and/or scallop myosins. Lane M, molecular weight marker; lane 1, Ph·HC/PhRLC/PhELC; lane 2, Ph·HC/ScRLC/ScELC; lane 3, Ph·HC/ScRLC/PhELC; lane 4, Ph·HC/PhRLC/ScELC. (C) SDS-PAGE (12.5%) of purified myosin mutant Ph·HC/PhRLC/PhELC-3A and hybrid myosin mutant Ph·HC/ScRLC/PhELC-3A. Lane M, molecular weight marker; lane 5, Ph·HC/PhRLC/PhELC-3A; lane 6, Ph·HC/ScRLC/PhELC-3A. (D) Glycerol PAGE showing LCs of expressed Physarum hybrid myosin Ph·HC/PhRLC/ScELC (a), expressed Physarum hybrid myosin Ph·HC/ScRLC/ScELC (b), Physarum myosin (c), and purified scallop myosin (d). (E) Glycerol PAGE showing LCs of expressed Physarum myosin mutants of Ph·HC/PhRLC/PhELC-3A (e) and hybrid myosin mutant Ph·HC/ScRLC/PhELC-3A (f).
Effects of Ca2+ on ATPase activities of Physarum hybrid myosin constructs
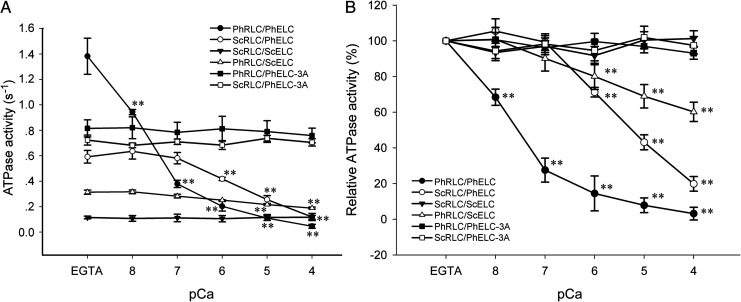
To elucidate the role of PhRLC on Ca2+-mediated regulation of myosins, a hybrid myosin Ph·HC/ScRLC/PhELC was constructed by substituting ScRLC for PhRLC and the actin-activated ATPase activities were measured in the presence or absence of Ca2+ (Fig. 2A,B). Subsequent ATPase activity assays showed reduced activity of the Physarum myosin hybrid Ph·HC/ScRLC/PhELC, from 0.592 ± 0.050 s−1 in 0.1 mM EGTA to 0.119 ± 0.029 s−1 in 0.1 mM Ca2+ (pCa: 4), suggesting that PhRLC is not necessary for Ca2+-mediated inhibitory regulation of Physarum myosin. Furthermore, a hybrid mutant myosin Ph·HC/ScRLC/PhELC-3A was constructed by substituting PhELC-3A for PhELC, which showed actin-activated ATPase activities of 0.723 ± 0.039 s−1 in 0.1 mM EGTA and 0.705 ± 0.029 s−1 in 0.1 mM Ca2+ (pCa: 4). These data showed no Ca2+ inhibition of ATPase activity of this Physarum hybrid mutant myosin. To compare the effects of Ca2+, the measurement of ATPase activity was repeated on both Physarum myosin wild-type Ph·HC/PhRLC/PhELC and mutant Ph·HC/PhRLC/PhELC-3A. The ATPase activity of Ph·HC/PhRLC/PhELC was clearly inhibited by Ca2+ from 1.382 ± 0.142 s−1 in 0.1 mM EGTA to 0.046 ± 0.016 s-1 in 0.1 mM Ca2+ (pCa: 4). However, Ca2+ did not inhibit ATPase activity of the Physarum myosin mutant Ph·HC/PhRLC/PhELC-3A, as indicated in our previous report [12].
Figure 2.
Specific actin-activated ATPase activities of Physarum myosin hybrid constructs in EGTA and in the presence of various concentrations of Ca 2+ (A) ATPase activity was measured in the presence of Physarum myosin hybrid constructs in EGTA and at different concentrations of Ca2+ as described in ‘Materials and Methods’ section. (B) Relative effects of Ca2+ on Physarum myosin hybrid constructs. Data are presented as means ± SEM, n = 5. **P < 0.01.
In further experiments, we substituted ScELC for PhELC to obtain another hybrid myosin construct Ph·HC/PhRLC/ScELC. The ATPase activity of this Physarum hybrid myosin was reduced from 0.314 ± 0.016 s−1 in 0.1 mM EGTA to 0.189 ± 0.008 s−1 in 0.1 mM Ca2+ (pCa, 4). In addition, after substituting two LCs from scallop myosin (ScRLC and ScELC) for PhRLC and PhELC, the ATPase activity of the consequent hybrid myosin construct was 0.115 ± 0.011 s−1 in 0.1 mM EGTA and 0.117 ± 0.016 s−1 in 0.1 mM Ca2+, indicating no inhibition by Ca2+ (pCa: 4) (Fig. 2A,B).
Effects of Ca2+ on motor activity of Physarum hybrid myosin constructs
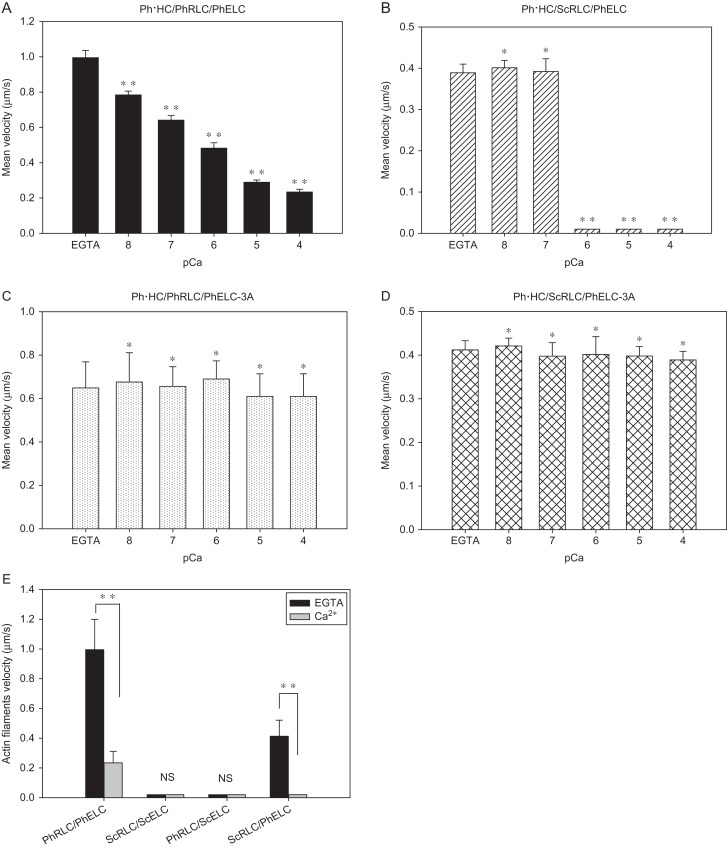
Ca2+-dependent regulation of motor activities of expressed Physarum myosin constructs was determined using in vitro motility assays. In these experiments, glass coverslip surfaces were coated with six different Physarum myosin constructs, and movements of actin filaments were monitored (Supplementary Fig. S1). Initially, the velocity of actin filaments in the presence of Physarum myosin hybrid Ph·HC/ScRLC/PhELC in the presence of 0.1 mM EGTA was 0.389 ± 0.087 μm/s (Fig. 3B and Supplementary Video S1). However, in the presence of 0.1 mM Ca2+ (pCa: 4), no movement was observed (Fig. 3B and Supplementary Video S2). In subsequent experiments with the Physarum myosin hybrid mutant Ph·HC/ScRLC/PhELC-3A, actin filaments moved at a velocity of 0.412 ± 0.087 μm/s in the presence of 0.1 mM EGTA and 0.389 ± 0.020 μm/s in the presence of 0.1 mM Ca2+ (pCa: 4) (Fig. 3D). In addition, to confirm the accuracy of these results, we repeated the previous published experiments with the constructs of Ph·HC/PhRLC/PhELC and Ph·HC/PhRLC/PhELC-3A [12]. Ca2+ remarkably inhibited the movements of actin filaments in the presence of the Physarum myosin Ph·HC/PhRLC/PhELC (Fig. 3A) but did not affect that of the Physarum myosin mutant Ph·HC/PhRLC/PhELC-3A (Fig. 3C). These observations were consistent with our previous report that PhELC played an important role on Ca2+-mediated regulation [12]. However, no movements of actin filaments were observed on glass coated with Physarum hybrid myosins Ph·HC/PhRLC/ScELC or Ph·HC/ScRLC/ScELC (Fig. 3E).
Figure 3.
Effects of Ca 2+ on the velocity of actin filaments on Physarum myosin hybrid constructs (A) Velocity of actin filaments on Physarum myosin Ph·HC/PhRLC/PhELC in EGTA or various concentrations of Ca2+. (B) Velocity of actin filaments on Physarum hybrid myosin Ph·HC/ScRLC/PhELC in the presence of EGTA or various concentrations of Ca2+. (C) Velocity of actin filaments on the Physarum myosin mutant Ph·HC/PhRLC/PhELC-3A in the presence of EGTA or various concentrations of Ca2+. (D) Velocity of actin filaments on the Physarum hybrid myosin mutant Ph·HC/ScRLC/PhELC-3A in the presence of EGTA or various concentrations of Ca2+. (E) Effects of Ca2+ on the velocity of actin filaments on Physarum myosin constructs with LCs from Physarum (PhRLC and PhELC) and scallop myosin (ScRLC and ScELC) in the presence of EGTA or Ca2+. Data are presented as means ± SD, n ≥ 60. **P < 0.01; *P > 0.05; NS, no significant.
Discussion
In the present study, to elucidate the roles of LCs from different species (Physarum and scallop) in Ca2+-mediated regulation, we generated four recombinant, full-length Physarum myosin constructs (Ph·HC/PhRLC/PhELC, Ph·HC/ScRLC/ScELC, Ph·HC/ScRLC/PhELC, and Ph·HC/PhRLC/ScELC) comprising Ph·HC associated with Physarum and/or scallop LCs in Sf-9 cells. In addition, we produced two Physarum mutant myosins (Ph·HC/PhRLC/PhELC-3A and Ph·HC/ScRLC/PhELC-3A) comprising Ph·HC associated with ScRLC and PhELC-3A. Subsequent examinations of Ca2+-dependent regulation of motor and ATPase activities indicate that PhELC plays a critical role in Ca2+-mediated regulation of Physarum myosin. Although PhRLC is not necessary for Ca2+-mediated regulation of Physarum myosin, the presence of PhRLC and PhELC leads to the highest degree of Ca2+ regulation of Physarum myosins.
In addition, we used Sf-9 cells to express recombinant Physarum hybrid myosins. Our preparations were pure, except for a potential contaminant from the native Sf-9 myosin, which may possibly associate with the recombinant myosin during purification. We were unable to estimate the presence and the exact amount of this contaminant, but it is believed to be equal in all preparations, and thus should not significantly affect the comparison between the myosin species.
According to the ATPase assays, the ATPase activities of Physarum myosins associated with Physarum and/or scallop LCs showed considerable changes in 0.1 mM EGTA (Fig. 2A). Their ATPase activities showed the following order: Ph·HC/PhRLC/PhELC > Ph·HC/PhRLC/PhELC-3A > Ph·HC/ScRLC/PhELC-3A > Ph·HC/ScRLC/PhELC > Ph·HC/PhRLC/ScELC > Ph·HC/ScRLC/ScELC, suggesting that the presence of the PhELC is important for maintaining ATPase activity (even at low calcium). In addition, wild-type myosin showed higher activity than any other hybrid myosins. This could be due to that the hybrid myosins are not functioning as optimal as the wild-type due to the structural differences noted in the next discussion. On the other hand, Ph·HC/PhRLC/PhELC exhibited remarkable activity changes in 0.1 mM Ca2+ relative to those in 0.1 mM EGTA (96.67% inhibitory effect; Table 1). However, Ph·HC/ScRLC/ScELC was almost impervious to Ca2+ concentrations (Fig. 2 and Table 1), suggesting that PhRLC and PhELC, but not Ph·HC, mediate Ca2+ inhibitory regulation of Physarum myosin. However, whereas Ph·HC/PhRLC/ScELC showed a 39.81% inhibitory effect of 0.1 mM Ca2+, greater inhibitory effects were observed with Ph·HC/ScRLC/PhELC in 0.1 mM Ca2+ (79.90% inhibitory effect; Table 1), indicating that PhELC plays a more important role in Ca2+ inhibition than PhRLC. These observations were also confirmed in assays of actin filament motility and ATPase activities of Physarum myosin mutant Ph·HC/PhRLC/PhELC-3A [12] and Ph·HC/ScRLC/PhELC-3A, which showed no inhibitory effect of Ca2+ (Figs. 2 and 3C,D, and Table 1). Moreover, the effects of Ca2+ on the ATPase activity of the Physarum myosin hybrid Ph·HC/ScRLC/PhELC and Ph·HC/PhRLC/ScELC (79.90% and 39.81% inhibitory effects; Table 1) were less obvious than that observed with the Physarum myosin Ph·HC/PhRLC/PhELC (96.67% inhibitory effect). These results suggest that the presence of PhRLC and PhELC facilitates the full inhibitory effect of Ca2+.
Table 1.
The relative actin-activated ATPase activities of Physarum myosin constructs in EGTA and in Ca2+ and the effects of Ca2+ on them
| Physarum myosin constructs | The relative actin-activated ATPase activities | ||
|---|---|---|---|
| EGTA (%)a | Ca2+ (%)b | Effect of Ca2+ (%)c | |
| Ph·HC/PhRLC/PhELC | 100 | 3.33 | 96.6↓↓↓ |
| Ph·HC/ScRLC/PhELC | 100 | 20.10 | 79.90↓↓ |
| Ph·HC/PhRLC/ScELC | 100 | 60.19 | 39.81↓ |
| Ph·HC/ScRLC/ScELC | 100 | 101.74 | 1.74
|
| Ph·HC/PhRLC/PhELC-3A | 100 | 93.13 | 6.87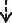
|
| Ph·HC/ScRLC/PhELC-3A | 100 | 97.51 | 2.49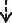
|
aThe actin-activated ATPase activities of Physarum myosin constructs were considered as 100% in EGTA.
bThe actin-activated ATPase activities in Ca2+ / in EGTA × 100.
cEffect of Ca2+ (%)c = ∣Ca2+ (%)b − EGTA (%)a∣/EGTA (%)a × 100; ↓means inhibition;  means little activation and no significant difference;
means little activation and no significant difference; 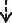 means little inhibition and no significant difference.
means little inhibition and no significant difference.
Although we were not able to determine the velocity of movements of actin filaments with Physarum myosin hybrid Ph·HC/PhRLC/ScELC or Ph·HC/ScRLC/ScELC, the waggling movements of actin filaments in EGTA were observed using the fluorescence microscope (Supplementary Videos S3 and S4). These observations suggest that interactions between actin and myosin were intact and were consistent with a previous report [16]. Moreover, lower ATPase activities of Physarum myosin hybrids Ph·HC/PhRLC/ScELC and Ph·HC/ScRLC/ScELC than that of Physarum myosin wild-type were observed in EGTA (Fig. 2), indicating that they may not be able to slide the actin filament. VanBuren et al. [24] showed that ELC is required for full force production by skeletal muscle myosin, and ELC removal reduced both force and velocity more than RLC removal in skeletal muscle myosin. In addition, the ELC is closer to the motor domain and thus the replacement of the ELC is more disruptive than replacing or removing the RLC [25,26]. It is therefore not too surprising that no movements of actin filaments on the Physarum myosin hybrids Ph·HC/PhRLC/ScELC and Ph·HC/ScRLC/ScELC were observed.
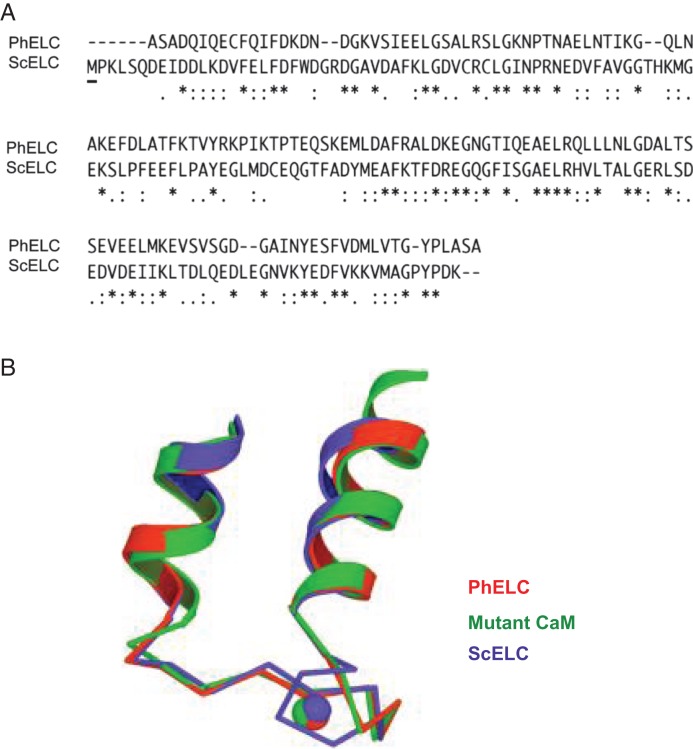
Physarum polycephalum, a lower eukaryote, shows vigorous shuttling of cytoplasmic streaming [27]. This cytoplasmic streaming is regulated by Ca2+ and driven by a conventional myosin II; the increase in [Ca2+]i is a signal for myosin activation and the decrease in [Ca2+]i for myosin inhibition [10]. This regulatory mode was confirmed in the living plasmodium in our recent study and the Ca2+ regulation was observed in recombinant myosin purified from Sf-9 cells [12,28]. However, it is unclear how this regulation plays a role in its physiological function. Similar to Physarum myosin, regulation of scallop myosin is also controlled by direct binding of Ca2+ to the ELC [7]. However, the effect of Ca2+ is totally opposite to that of Physarum myosin; Ca2+ activates its activity [7,11]. Further biochemical studies demonstrated that Ca2+ binding to the ELC to inhibit/activate the ATPase activity and in vitro motility [13]. Structural studies showed that the Ca2+ inhibition/activation difference between the two conventional myosins may be due to difference in Ca2+-induced change in the conformational flexibility of the regulatory domain (RD) comprising ELC, RLC, and a portion of the HC [25]. By comparing the amino acid sequence of PhELC with that of ScELC, we found relatively low sequence similarity between these two Ca2+-binding ELCs (Fig. 4A). In addition, methionine is absent in the N-lobe of PhELC and only one methionine exists in ScELC (Fig. 4A). Interestingly, the larger numbers of methionines in CaM (more than 5%) are thought to adopt the open conformation in Ca2+-binding state [29]. Accordingly, the absence of methionine in the PhELC may prevent the large conformational transition. By comparison of the structure of Ca2+-binding EF-hands of PhELC with that of ScELC, an extra turn in the ScELC was observed (Fig. 4B). These differences between PhELC and ScELC may result in conformational flexibility or intramolecular interactions between Ph·HC and ScELC that are not appropriate.
Figure 4.
Comparisons of amino acid sequences and structure of the Ca 2+-binding EF-hands between PhELC and ScELC (A) Comparisons of amino acid sequences between PhELC and ScELC using the program of CLUSTAL W (1.83). The first row shows amino acid sequences of PhELC and the second row shows that of ScELC. ’*’ indicates positions that have a single, fully conserved residue; ’:’ indicates that ’strong’ group is fully conserved; ’.’ indicates that ’weaker’ group is fully conserved. The underlined letter ’M’ in the beginning of second row indicates the only one methionine in N-lobe of ScELC. (B) Structure and comparison of the Ca2+-binding EF-hand I of PhELC (red), ScELC (blue), and mutant CaM (green) [25]. Note that the extra turn in the first helix of ScELC. Adapted from Debreczeni et al. [25].
Based on these results, we have proposed a simple model to explain the regulatory effect of Ca2+. In EGTA condition (in Ca2+-free state), Physarum myosin is the active ‘open’ state in which the myosin shows high actin-activated ATPase activity and induces the sliding movement of actin. This ‘open’ state of Physarum myosin may require that the PhRLC and the PhELC maintain specific orientations relative to each other so that active conformation can be formed. Substitution of the PhRLC or the PhELC could not, however, produce a normal ‘open’ state. Ca2+-binding to the PhELC may alter the flexibility of the RD and induce a ‘closed’ state, which may consequently prevent full activity and force generation. Although the opposite effects of Ca2+ are seen on Physarum and scallop myosin, the effects of Ca2+-binding to the ELC on the changes of RD flexibility seem to be similar [25,30]. To fully understand the opposite regulatory effect of Ca2+-binding on Physarum and scallop myosin clearly, further studies should be carried out to express scallop myosin HC with the Physarum LCs and determine if the LCs can reverse the regulation of Ca2+.
Supplementary Data
Supplementary data is available at ABBS online.
Acknowledgment
We would like to dedicate to the late Andrew Szent-Gorgyi, Professor Emeritus of Brendeis University.
Funding
This work was supported by the grants from the Smoking Research Foundation and by Grants-in-Aid for Scientific Research of the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Nos. 23590295 and 15K00809 to A.N.; No. 26670128 to K.K.).
References
- 1.Sellers JR. Regulation of cytoplasmic and smooth muscle myosin. Curr Opin Cell Biol 1991, 3: 98–104. [DOI] [PubMed] [Google Scholar]
- 2.Matsumura F, Hartshorne DJ.. Myosin phosphatase target subunit: many roles in cell function. Biochem Biophys Res Commun 2008, 369: 149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartman MA, Spudich JA.. The myosin superfamily at a glance. J Cell Sci 2012, 125: 1627–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Zhang H, Tang Z, Kohama K, Lin Y.. Inverse interaction between tropomyosin and phosphorylated myosin in the presence or absence of caldesmon. Acta Biochim Biophys Sin (Shanghai) 2013, 45: 601–606. [DOI] [PubMed] [Google Scholar]
- 5.Yang JX, Feng XH, Zhang Y, Lin Y.. Influence of trace amount of calponin on smooth muscle myosin in different states. Acta Biochim Biophys Sin (Shanghai) 2004, 36: 529–536. [DOI] [PubMed] [Google Scholar]
- 6.Allen BG, Walsh MP.. The biochemical basis of the regulation of smooth-muscle contraction. Trends Biochem Sci 1994, 19: 362–368. [DOI] [PubMed] [Google Scholar]
- 7.Szent-Gyorgyi AG, Kalabokis VN, Perreault-Micale CL.. Regulation by molluscan myosins. Mol Cell Biochem 1999, 190: 55–62. [PubMed] [Google Scholar]
- 8.Chantler PD. Scallop adductor muscles: structure and function In: Parsons GJ, Shumway SE eds. Scallops: Biology, Ecology and Aquaculture. 2nd edn Amsterdam: Elsevier, 2005, 231–289. [Google Scholar]
- 9.Kawamichi H, Zhang Y, Hino M, Nakamura A, Tanaka H, Farkas L, Nyitray L, et al. . Calcium inhibition of Physarum myosin as examined by the recombinant heavy mero-myosin. Adv Exp Med Biol 2007, 592: 265–272. [DOI] [PubMed] [Google Scholar]
- 10.Kohama K. Ca-inhibitory myosins: their structure and function. Adv Biophys 1987, 23: 149–182. [DOI] [PubMed] [Google Scholar]
- 11.Kalabokis VN, Szent-Györgyi AG.. Regulation of scallop myosin by calcium: cooperativity and the “off” state. Adv Exp Med Biol 1998, 453: 235–240. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Kawamichi H, Tanaka H, Yoshiyama S, Kohama K, Nakamura A.. Calcium-dependent regulation of the motor activity of recombinant full-length Physarum myosin. J Biochem 2012, 152: 185–190. [DOI] [PubMed] [Google Scholar]
- 13.Okagaki T, Higashi-Fujime S, Kohama K.. Ca2+ activates actin-filament sliding on scallop myosin but inhibits that on Physarum myosin. J Biochem 1989, 106: 955–957. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Nakamura A, Kawamichi H, Yoshiyama S, Katayama T, Kohama K.. Calcium regulation of the ATPase activity of Physarum and scallop myosins using hybrid smooth muscle myosin: the role of the essential light chain. FEBS Lett 2010, 584: 3486–3491. [DOI] [PubMed] [Google Scholar]
- 15.Himmel DM, Mui S, O’Neall-Hennessey E, Szent-Györgyi AG, Cohen C.. The on-off switch in regulated myosins: different triggers but related mechanisms. J Mol Biol 2009, 394: 496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Khayat HA, Morris EP, Squire JM.. The 7-stranded structure of relaxed scallop muscle myosin filaments: support for a common head configuration in myosin-regulated muscles. J Struct Biol 2009, 166: 183–194. [DOI] [PubMed] [Google Scholar]
- 17.Spudich JA, Watt S.. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem 1971, 246: 4866–4871. [PubMed] [Google Scholar]
- 18.Webb MR. A continuous spectrophotometric assay for inorganic phosphate and for measuring phosphate release kinetics in biological systems. Proc Natl Acad Sci USA 1992, 89: 4884–4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishikawa R, Okagaki T, Higashi-Fujime S, Kohama K.. Stimulation of the interaction between actin and myosin by Physarum caldesmon-like protein and smooth muscle caldesmon. J Biol Chem 1991, 266: 21784–21790. [PubMed] [Google Scholar]
- 20.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976, 72: 248–254. [DOI] [PubMed] [Google Scholar]
- 21.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227: 680–685. [DOI] [PubMed] [Google Scholar]
- 22.Gassmann M, Grenacher B, Rohde B, Vogel J.. Quantifying western blots: pitfalls of densitometry. Electrophoresis 2009, 30: 1845–1855. [DOI] [PubMed] [Google Scholar]
- 23.Tan HY, Ng TW.. Accurate step wedge calibration for densitometry of electrophoresis gels. Opt Commun 2008, 281: 3013–3017. [Google Scholar]
- 24.VanBuren P, Waller GS, Harris DE, Trybus KM, Warshaw DM, Lowey S.. The essential light chain is required for full force production by skeletal muscle myosin. Proc Natl Acad Sci USA 1994, 20: 12403–12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Debreczeni JE, Farkas L, Harmat V, Hetenyi C, Hajdu I, Zavodszky P, Kohama K, et al. . Structural evidence for non-canonical binding of Ca2+ to a canonical EF-hand of a conventional myosin. J Biol Chem 2005, 280: 41458–41464. [DOI] [PubMed] [Google Scholar]
- 26.Rayment I, Rypniewski WR, Schmidt-Bäse K, Smith R, Tomchick DR, Benning MM, Winkelmann DA, et al. . Three-dimensional structure of myosin subfragment-1: a molecular motor. Science 1993, 261: 50–58. [DOI] [PubMed] [Google Scholar]
- 27.Yoshimoto Y, Matsumura F, Kamiya N.. Simultaneous oscillations of Ca2+ efflux and tension generation in the permealized plasmodial strand of Physarum. Cell Motil 1981, 1: 433–443. [DOI] [PubMed] [Google Scholar]
- 28.Yoshiyama S, Ishigami M, Nakamura A, Kohama K.. Calcium wave for cytoplasmic streaming of Physarum polycephalum. Cell Biol Int 2009, 34: 35–40. [DOI] [PubMed] [Google Scholar]
- 29.Nelson MR, Chazin WJ.. An interaction-based analysis of calcium-induced conformational changes in Ca2+ sensor proteins. Protein Sci 1998, 7: 270–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gourinath S, Himmel DM, Brown JH, Reshetnikova L, Szent-Gyorgyi AG, Cohen C.. Crystal structure of scallop myosin S1 in the pre-power stroke state to 2.6 a resolution: flexibility and function in the head. Structure 2003, 11: 1621–1627. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.