Abstract
Background
Cigarette smoking has been shown to be a risk factor for adult glioma by some but not all studies. We conducted a meta-analysis to systematically assess the potential association.
Methods
PubMed and EMBASE were searched from the date of their inception to October 1, 2015, to identify relevant articles. Reference lists from these articles were reviewed to identify additional studies. Both cohort and case–control studies were included. Fixed-effects models were used to calculate the overall relative risk (RR) with corresponding 95% confidence intervals (CIs).
Results
The final analysis included 24 studies (seven cohort and 17 case–control studies), involving more than 2.3 million individuals. The combined RR was 1.04 (95% CI: 1.00, 1.09; P=0.073) for ever-smokers, 0.97 (95% CI: 0.88, 1.07; P=0.574) for current-smokers, and 1.07 (95% CI: 0.98, 1.16; P=0.130) for past smokers, with little evidence of heterogeneity. Omission of any single study from the analysis had little effect on the result. No evidence of publication bias was found. A small but statistically significant increase was found in past smokers in females (RR: 1.13, 95% CI: 1.00, 1.28; P=0.046) but not in males.
Conclusion
In general, there was no association between cigarette smoking and adult glioma. The small but statistically significant association in females requires further investigation.
Keywords: cigarette smoking, glioma, meta-analysis, risk
Introduction
Glioma accounts for >70% of all brain tumors in adults, with an estimated incidence of 6.0/100,000 per year.1,2 The development of glioma is clearly associated with ionizing radiation.3,4 Putative association with other factors, including pesticide exposure,5 alcohol consumption,6 smoking,7 obesity,8 and sex hormones,9 has also been noted. Cigarette smoking is the most important modifiable cause of many types of human cancers, including the respiratory, digestive, hematologic, and urinary systems.10
Association between adult glioma and cigarette smoking has been reported both in females11,12 and in males.13 However, increased risk of glioma in smokers has not been rep licated in many other studies, including case–control studies11,14–16 and cohort studies.17–21 A meta-analysis of 17 studies in 2009 failed to show significant association, but a small and significant increased risk was noted in a cohort study.7 Recently, a series of cohort studies and case–control studies have been published,21–25 again, with conflicting results. In particular, a recent large population-based case–control study involving >13,000 individuals in the People’s Republic of China supported a positive association between cigarette smoking and gliomas.25 As a result, a reanalysis is appropriate.
Materials and methods
Literature search
Two investigators (HXL and XXP) independently conducted a systematic search of the PubMed and EMBASE databases to identify relevant publications from the inception of the databases to October 1, 2015. The search terms were (glioma OR brain neoplasm OR brain cancer OR brain tumor) AND (smoke OR smoking OR cigarette OR tobacco OR smoker). The references of the retrieved articles were checked to identify additional studies.
Inclusion and exclusion criteria
The criteria for data inclusion were: 1) studies investigating the association between cigarette smoking and risk of adult glioma; 2) the study design being cohort or case–control study; 3) odds ratio (OR), relative risk (RR), or hazard ratio (HR), with corresponding 95% confidence intervals (CIs), being provided or calculated from raw data; and 4) at least one of the following smoking exposure variables being provided: ever- versus never-smokers, current versus never-smokers, past versus never-smokers, duration, intensity, or cumulative smoking (pack-year). In the cases of multiple publications, only the one with the most complete information was used. Case report, meta-analysis, reviews, comments, and editorials were not included.
Data extraction
Two investigators (HXL and XXP) independently extracted the following data: first author’s name, year of publication, study design, study country/period, data source (cohort study), follow-up years (cohort study), sample size, age of subjects, diagnostic criteria of glioma, sex strata, research instrument, control source (case–control study), smoking variables and adjustment factors, and risk estimate (RR, OR, HR, and 95% CI). Disagreements were resolved through discussion.
Statistical analysis
The meta-analysis was conducted using STATA 12.0 (StataCorp LP, College Station, TX, USA). Since glioma is a rare disease, OR and HR are practically equivalent to RR.26 RR was used throughout this study. Heterogeneity was evaluated with Cochran’s Q statistic27 and I2 statistic,28 and defined as low (I2≤25%), moderate (I2=25%–50%), or high (I2>50%). RR was calculated using a fixed-effects model when I2<50%, and using a random-effects model otherwise. Sensitivity analysis was carried out to evaluate the potential effects of the individual study to the overall results, as described previously.29 Publication bias was assessed using Begg’s funnel plots and Egger’s regression test.30,31 Sensitivity analysis and publication bias were assessed for ever- versus never-smokers only. All analyses were two sided, with P≤0.05 indicating statistical significance.
Ever-, current-, and past smokers were defined as in the original publications. To probe into potential heterogeneity, several subgroup analyses were performed according to study design (cohort versus case–control study), control source (population-based versus hospital-based), research instrument (questionnaire versus interview for case–control study; mailed versus self-administered questionnaire for cohort studies), sex, geographical area, age at which smoking was started (≤20 versus >20 years), smoking intensity (≤20 versus >20 cigarettes per day), duration (≤20 versus >20 years), cumulative smoking (≤15 versus >15 pack-years), and year of study publication (before 1990 versus 1990–2000 versus 2000–2010 versus 2010–now).
Results
Search results and characteristics of studies
The initial screening identified a total of 873 articles (n=417 from PubMed and n=456 from EMBASE). After reading the titles and abstracts, 32 papers were selected for further processing. Reviewing the full text, eleven articles were eliminated for the following reasons: six for irrelevant articles;32–37 two articles did not provide the data “never-smokers”, “ever-smokers”, or “current-smoker”;38,39 and two articles did not have sufficient data for the variable “ever-, current-, or past smokers”, but were included the subgroup analysis based on smoking “intensity”.40,41 Two articles were from the same study,11,42 and so only the one with most complete information was included.11 One article included three case–control studies.22 The final analysis in this study included a total of 24 studies from 22 articles.11–25,43–49 The selection process is shown in Figure 1.
Figure 1.
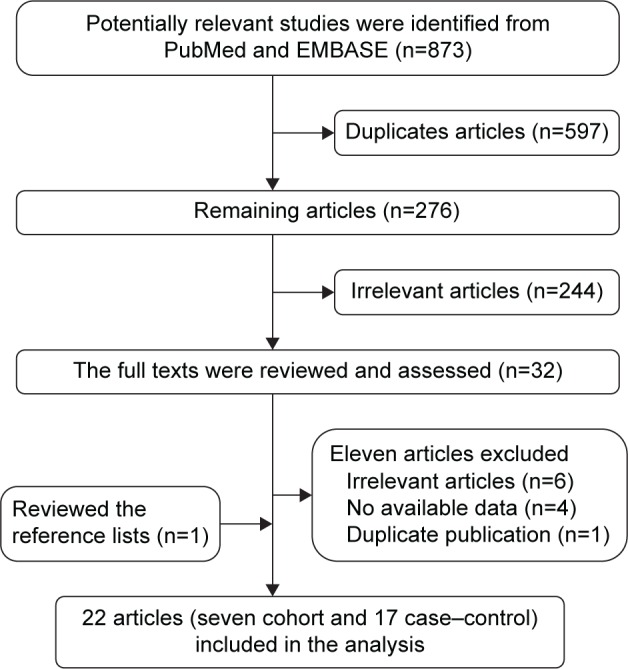
Flowchart of studies included in the meta-analysis.
The characteristics of the included studies are listed in Table 1. Of the 24 studies included, seven were cohort studies12,17–21,43 and the remaining 17 were case–control studies.11,13–16,22–25,44–49 All together, these studies involved >2.3 million individuals. Among these studies, 13 were conducted in the USA,12–15,17,19–22,44,46 four in Europe,17,24,47,48 three in Canada,23,43,45 two in Australia,11,16 and two in the People’s Republic of China.25,49 Seven studies did not present the results using “ever- versus never-smokers” results; we synthesized the data by pooling the variables, from current- and past smokers,13,15 or from males and females.17,18,21,44,48 Of the 17 case–control studies, eight studies were hospital based,22–24,44,45,47 and the remaining nine were population based.11,13–16,23,25,46,48 In the 17 case–control studies, smoking was evaluated using interview in eleven studies,13,14,16,22–25,45,48,49 questionnaire in three studies,11,15,22 medical records in two studies,44,46 and a mixture of questionnaire and interview in one study.47 Among the seven cohort studies, smoking was assessed using questionnaire via mail in four studies,17–20 and self-administered questionnaire in three studies.12,21,43 Among these studies, two reported increased risk in females but not in males,11,12 and one reported an increase in males only.13 No association was found in the two studies exclusively on females.17,43 The two case–control studies conducted in males only did not find increased risk.14,44 The latest case–control study involving 13,000 individuals found increased risk regardless of sex.46 However, several studies that included both sexes failed to find an association between smoking and glioma risk in either males or females.15,16,18,21
Table 1.
Study characteristics of published cohort and case–control studies on cigarette smoking and the risk of adult glioma
| References | Country/period | Data source | Follow-up (years) | No of case/participants (overall) | No of case/participants (N) | No of case/participants (E) | No of case/participants (P) | No of case/participants (C) | Sex strata/age (years) | Diagnostic criteria | Research instrument | Smoking variables assessed and adjustment | Risk estimate: ever-, current-, or past- vs never-smokers, RR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort studies | |||||||||||||
| Braganza et al21 | USA/1995–1996 | NIH-AARP | 10.5 (mean) | 704/477,095 | 265/174,244 | 439/837,848 | 374/243,407 | 65/594,441 | MF/50–71 | First primary malignant glioma and histopathologically | Self-administered questionnaire | Past; current; intensity; cigar/pipe smoking; time since quitting; adjustment (sex, education, marital status, and race/ethnicity) | C: 0.83 (0.63, 1.09); P: 0.95 (0.81, 1.12) |
| Benson et al17 | UK/May 1996–April 2001 | National Health Service (UK) | 6.2 (mean) | 681/1,177,087 | 322/599,949 | 296/577,138 | 189/332,775 | 107/244,363 | F/50–65 | Registers of National Health Service (UK) | Mailed questionnaire | Never, past, current smoking; adjustment (height, BMI, socioeconomic status, alcohol intake, strenuous exercise, age at first birth, parity, and oral contraceptive use) | C: 0.91 (0.73, 1.15); P: 1.09 (0.91, 1.31) |
| Holick et al18 | USA/1976–NR | HPFS; NHS; NHS II | 14–27 | 365/257,918 | 165/72,838 | 200/429,326 | 145/46,051 | 55/383,275 | MF/25–75 | Medical records | Mailed questionnaire | Current, past, intensity, duration, pack-years, age at start; adjustment (age, total meat intake, alcohol, and coffee consumption) | C: 1.06 (0.76, 1.47); P: 1.21 (0.98, 1.49) |
| Silvera et al43 | Canada/1980–1985 | CNBSS | 16.5 (mean) | 117/89,709 | 59/NR | 58/NR | 38/NR | 20/NR | F/40–59 | Canadian cancer database | Self-administered questionnaire | Ever, current, past, intensity, duration, pack-years, age at start; adjustment (age, education, BMI, parity, age at first live birth, age at menarche, menopausal status, center) | E: 1.30 (0.88, 1.93); C: 1.05 (0.62, 1.78); P: 1.51 (0.97, 2.34) |
| Efird et al12 | USA/1977–1985 | KPMCP-NC | 13.3 (mean) | 130/133,811 | 51/65,544 | 79/NR | 45/NR | 34/NR | MF/≥25 | Tumor registry; biopsied and histologically; radiographic and clinical history | Self-administered questionnaire | Ever, current, past, intensity; adjustment (cigars, pipes, sex, race, education, alcohol, and coffee consumption) | E: 1.40 (1.00, 2.10); C: 1.60 (1.00, 2.50); P: 1.30 (0.90, 2.00) |
| McLaughlin et al19 | USA/1953–1980 | US veterans | 26 | 468/177,903 | NR | NR | NR | NR | MF/31–84 | Death certificates | Mailed questionnaire | Ever, current, past; adjustment (attained age and calendar year time period) | E: 1.10 (0.90, 1.30); C: 1.10 (0.90, 1.30); P: 1.10 (0.90, 1.40) |
| Mills et al20 | USA/1976–1982 | CSDA | 6 | 18/34,000 | 13/NR | 18/NR | NR | NR | MF/≥25 | Histopathologically confirmed | Mailed questionnaire | Ever; adjustment (age and sex) | E: 0.82 (0.28, 2.39) |
| References | Country/period | No of case/control (overall) | No of case/control (N) | No of case/control (E) | Sex strata/age (years) | Diagnostic criteria | Control source | Research instrument | Smoking variables assessed and adjustment | Risk estimate: ever-, current-, or past vs never-smokers, RR (95% CI) | |||
| Case–control studies | |||||||||||||
| Hou et al25 | People’s Republic of China/1989–1991 | 4,556/9,112 | 2,676/5,542 | 1,880/3,570 | MF/≥30 | Autopsy, histological test, surgical operation, imaging or laboratory tests, clinical assessment, deduction after death | PCC | Interviews | Current, sex, urban or rural residence, years of smoking, cigarettes smoked daily; adjustment (age, urban, or rural residence) | E: 1.11 (1.03, 1.21) | |||
| Vida et al23 | Canada/2002–2004 | 166/648 | 78/311 | 88/337 | MF/30–59 | Histologically confirmed or based on unequivocal diagnostic imaging | PCC | Interviews | Ever, pack-years, duration, sex, by education level; adjustment (age, sex, education level, region) | E: 0.96 (0.67, 1.38) | |||
| Cabaniols et al24 | France/January 2005–December 2005 | 116/116 | 54/50 | 62/66 | MF/≥18 | All new cases of malignant primitive brain tumors | HCC | Interviews | Ever; adjustment (age, sex) | E: 0.86 (0.50, 1.48) | |||
| Lachance et al22 | USA/1997–2008 | 855/1,160 | 429/539 | 426/621 | MF/≥20 | Histologically confirmed | HCC | Interviews and questionnaire | Ever | E: 1.02 (0.67, 1.57)a; 1.05 (0.79, 1.38)b; 0.74 (0.55, 1.00)c | |||
| Zheng et al15,d | USA/NR | 375/2,434 | 190/1,107 | 185/1,327 | MF/40–85 | Histologically confirmed | PCC | Questionnaire | Ever, past, current, duration, intensity, pack-years, by sex; adjustment (age, body mass index, education, exercise, duration living in area served by chlorinated surface water, first-degree relative with brain cancer) | E: 0.84 (0.66, 1.08); C: 0.87 (0.63, 1.19); P: 0.87 (0.66, 1.19) | |||
| Hu et al49 | People’s Republic of China/September 1989–May 1995 | 218/436 | 113/235 | 105/201 | M, 39.2/F, 40.3 | Histologically confirmed | HCC | Interviews | Ever | E: 1.13 (0.80, 1.58) | |||
| Lee et al13 | USA/August 1991–March 1994 | 434/430 | 192/189 | 242/241 | MF/≥20 | Histopathologically confirmed | PCC | Interviews | Ever, filtered, unfiltered, both; pack-years, by sex; adjustment (age, education, income) | E: 1.03 (0.66, 1.59) | |||
| Hurley et al11 | Australia/July 1987–December 1991 | 416/422 | 174/190 | 242/232 | MF/20–70 | Histopathologically confirmed | PCC | Questionnaire | ever, pack-years, duration, age at start, by sex; adjustment (age, sex, reference date) | E: 1.29 (0.95,1.75) | |||
| Ryan et al16 | Australia/February 1997–April 1990 | 110/417 | NR | NR | MF/25–74 | Newly diagnosed primary gliomas | PCC | Interviews | Ever, current, past, pack-years; adjustment (age, sex) | E: 1.19 (0.73, 1.95); C: 1.11 (0.62, 1.99); P: 1.39 (0.74, 2.63) | |||
| Brownson et al44 | USA/January 1984–December 1988 | 312/1,248 | NR | NR | M/54.6 (mean) | Histopathologically confirmed | Other cancers | Cancer registry | Current, past | C: 0.90 (0.70, 1.30); P: 1.00 (0.70, 1.50) | |||
| Schlehofer et al48 | Germany/1987–1988 | 115/418 | NR | NR | MF/NR | Histopathologically confirmed | PCC | Interviews | Current, past; adjustment (age, sex) | C: 0.70 (0.50,1.10); P: 0.90 (0.60, 1.50) | |||
| Preston-Martin et al14 | USA/1980–1984 | 202/202 | NR | NR | M/25–69 | Diagnosis of primary glioma | Neighborhood | Interviews | Ever | E: 0.70 (0.40, 1.00) | |||
| Burch et al45 | Canada/1979–1982 | 215/215 | NR | NR | MF/25–80 | Histopathologically confirmed | HCC | Interview | Ever, plain, filter, dose–response | E: 1.44 (0.89, 2.34) | |||
| Carpenter et al46 | USA/1943–1979 | 41/04 | 16/47 | 25/57 | MF/NR | Death certificates | Employees from nuclear facilities | Medical records | Ever | E: 1.10 (0.50, 2.70) | |||
| Musicco et al47 | Italy/January 1979–March 1980 | 42/201 | 24/127 | 18/74 | MF/≥20 | Histologically; radiologic and arteriography | HCC | Questionnaire and Interview | Ever, heavy-smokers; adjustment (sex, age, residence) | E: 1.80 (0.55, 5.90) |
Notes:
Data were collected from Mayo Clinic, Rochester, MN, USA;
Data were collected from University of California, San Francisco (UCSF), CA, USA;
Data were collected from Duke University Medical Center, Raleigh, NC, USA; and University of Illinois, Chicago (Duke-UIC), IL, USA;
Only this study provides the number of cases in group current-smokers and past-smokers.
Abbreviations: N, never-smokers; E, ever-smokers; C, current-smokers; P, past-smokers; M, males; F, Females; MF, males and females; BMI, body mass index; NIH-AARP, American Association of Retired Persons; HPFS, The Health Professionals Follow-up Study; NHS, The Nurses’ Health Study I; NHS II, The Nurses’ Health Study II; CNBSS, Canadian National Breast Screening Study; KPMCP-NC, Kaiser Permanente Medical Care Program of Northern California; CSDA, California Seventh-Day Adventists; PCC, population-based case–control; HCC, hospital-based case–control; NR, not reported, if information is part of the scope of the study, but not reported, CI, confidence interval, RR, risk ratio.
Main analysis
Ever-smokers
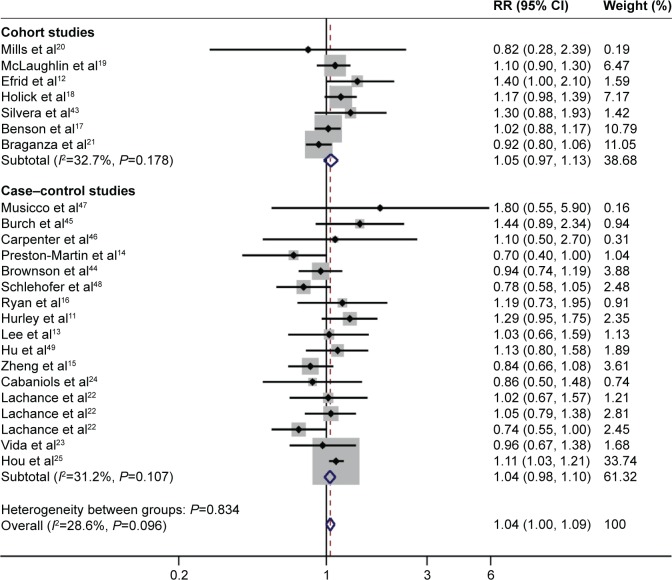
All 24 studies estimated the risk in ever-smokers. The analysis of never-smokers included >920,912 participants, with >4,812 glioma cases, while the analysis of ever-smokers included >1,851,038 participants, with >4,121 glioma cases. The pooled RR for ever-smokers (n>1,855,159) versus never-smokers (n>925,724) was 1.04 (95% CI: 1.00, 1.09; P=0.072). No significant heterogeneity was found (I2=28.6%, P=0.096; Figure 2).
Figure 2.
Forest plot of cigarette smoking and the risk of glioma (ever-smokers versus never-smokers).
Notes: The pooled RR for current-smokers versus never-smokers was 0.97 (95% CI: 0.88, 1.07), with low heterogeneity (P=0.225, I2=23.7%).
Abbreviations: RR, relative risk; CI, confidence interval.
Current-smokers
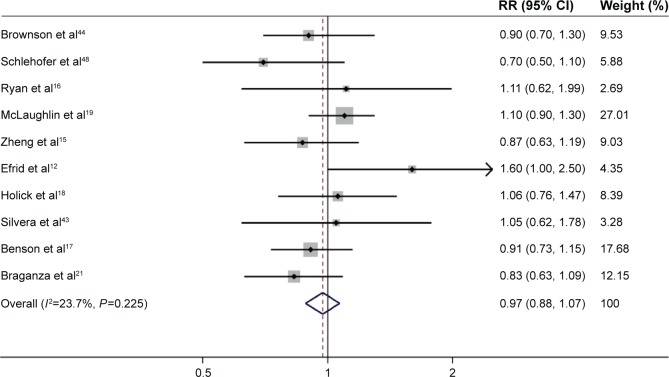
Risk estimates for current-smokers were reported in ten studies.12,15–19,21,43,44,48 The analysis of current-smokers included >1,222,544 participants, with >368 glioma cases. The pooled RR for current-smokers (n>1,222,912) versus never-smokers (n>925,724) was 0.97 (95% CI: 0.88, 1.07; P=0.574), with low heterogeneity (P=0.225, I2=23.7%; Figure 3).
Figure 3.
Forest plot of the risk of developing adult glioma in current-smokers.
Note: The pooled RR for current-smokers versus never-smokers was 0.97 (95% CI: 0.88, 1.07), with low heterogeneity (P=0.225, I2=23.7%).
Abbreviations: RR, relative risk; CI, confidence interval.
Past-smokers
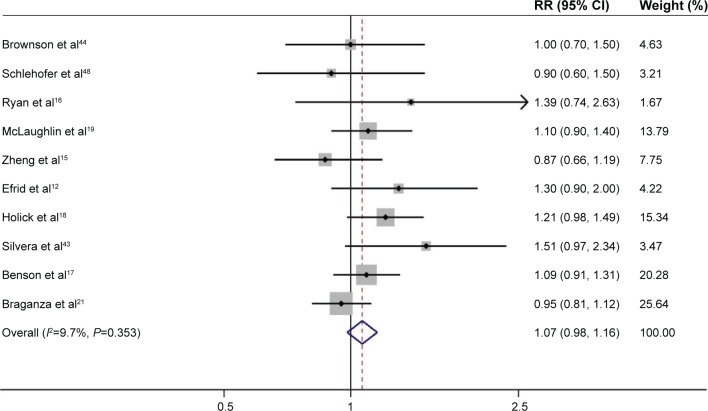
Ten studies evaluated the risk in past-smokers.12,15–19,21,40,43,44 The analysis of current-smokers included >623,095 participants, with >839 glioma cases. The pooled RR for past-smokers (n>623,934) versus never-smokers (n>925,724) was 1.07 (95% CI: 0.98, 1.16; P=0.130), with low heterogeneity (P=0.353, I2=9.7%; Figure 4).
Figure 4.
Forest plot of the risk of developing adult glioma in past-smokers.
Note: The pooled RR for past-smokers versus never-smokers was 1.07 (95% CI: 0.98, 1.16), with low heterogeneity (P=0.353, I2=9.7%).
Abbreviations: RR, relative risk; CI, confidence interval.
Stratified analysis
To examine the potential heterogeneity, several subgroup analyses were performed. The results of “ever smoker versus never smoker” are listed in Table 2.
Table 2.
Results of meta-analysis for cigarette smoking and risk of glioma
| Group | No of studies | RR (95% CI) | P-value | I2 (%) | P-heterogeneity | Analysis model |
|---|---|---|---|---|---|---|
| Total | 24 | 1.04 (1.00, 1.09) | 0.072 | 28.6 | 0.096 | Fixed-effects model |
| Study design | ||||||
| Cohort | 7 | 1.05 (0.97, 1.13) | 0.200 | 32.7 | 0.178 | Fixed-effects model |
| Case–control | 17 | 1.04 (0.98, 1.10) | 0.201 | 31.2 | 0.107 | Fixed-effects model |
| Hospital-based | 8 | 0.98 (0.87, 1.11) | 0.773 | 0.0 | 0.423 | Fixed-effects model |
| Population-based | 9 | 1.06 (0.99, 1.13) | 0.106 | 43.1 | 0.080 | Fixed-effects model |
| Research instrument (cohort) | ||||||
| Mailed questionnaire | 4 | 1.08 (0.98, 1.19) | 0.105 | 0.0 | 0.633 | Fixed-effects model |
| Self-admin questionnaire | 3 | 1.14 (0.84, 1.54) | 0.403 | 67.9 | 0.045 | Random-effects model |
| Research instrument (case–control) | ||||||
| Interview | 11 | 1.05 (0.98, 1.12) | 0.142 | 36.3 | 0.118 | Fixed-effects model |
| Questionnaires | 3 | 1.00 (0.84, 1.19) | 0.972 | 35.1 | 0.201 | Fixed-effects model |
| Sex | ||||||
| Male | 10 | 1.03 (0.96, 1.10) | 0.408 | 49.2 | 0.038 | Fixed-effects model |
| Female | 10 | 1.12 (1.03, 1.22) | 0.007 | 24.7 | 0.216 | Fixed-effects model |
| Geographic area | ||||||
| USA | 13 | 0.99 (0.92, 1.06) | 0.782 | 29.2 | 0.168 | Fixed-effects model |
| Europe | 4 | 0.97 (0.86, 1.10) | 0.637 | 20.7 | 0.286 | Fixed-effects model |
| Canada | 3 | 1.17 (0.93, 1.48) | 0.178 | 6.4 | 0.344 | Fixed-effects model |
| Australia | 2 | 1.26 (0.97, 1.64) | 0.079 | 0.0 | 0.785 | Fixed-effects model |
| People’s Republic of China | 2 | 1.11 (1.03, 1.20) | 0.008 | 0.0 | 0.920 | Fixed-effects model |
| Age at start smoking (years) | ||||||
| Younger (≤20) | 3 | 1.15 (0.95, 1.39) | 0.145 | 42.1 | 0.178 | Fixed-effects model |
| Older (>20) | 3 | 1.25 (1.02, 1.52) | 0.029 | 0.0 | 0.553 | Fixed-effects model |
| Duration (years) | ||||||
| Short-term (≤20) | 5 | 0.97 (0.76, 1.24) | 0.799 | 50.3 | 0.090 | Random-effects model |
| Long-term (>20) | 6 | 1.06 (0.90, 1.26) | 0.473 | 58.1 | 0.036 | Random-effects model |
| Intensity (years) | ||||||
| Light (≤20) | 7 | 1.02 (0.96, 1.09) | 0.547 | 41.9 | 0.112 | Fixed-effects model |
| Heavy (>20) | 7 | 1.11(0.91, 1.35) | 0.309 | 65.4 | 0.008 | Random-effects model |
| Pack-years | ||||||
| Light (≤15) | 4 | 1.16 (0.96, 1.42) | 0.132 | 0.0 | 0.821 | Fixed-effects model |
| Heavy (>15) | 7 | 0.91 (0.80, 1.06) | 0.218 | 28.4 | 0.211 | Fixed-effects model |
| Year of study publication | ||||||
| Before 1990 | 7 | 0.91 (0.78, 1.07) | 0.261 | 18.1 | 0.292 | Fixed-effects model |
| 1990–2000 | 5 | 1.14 (1.00, 1.30) | 0.055 | 0.0 | 0.908 | Fixed-effects model |
| 2000–2010 | 5 | 1.09 (0.93, 1.26) | 0.284 | 50.7 | 0.088 | Random-effects model |
| 2010–now | 5 | 1.04 (0.97, 1.10) | 0.293 | 49.2 | 0.096 | Fixed-effects model |
Abbreviations: CI, confidence interval; RR, relative risk.
In the stratified analysis by study design, more than 2,756,887 participants and 1,956 glioma cases were included in cohort studies, and more than 6,977 participants and 15,063 glioma cases were included in case–control studies. The pooled RR for ever-smokers (n>1,329,577) versus never-smokers (n>913,450) was 1.05 (95% CI: 0.97, 1.13; P=0.200) in cohort studies, and the RR for ever-smokers (n>9,999) versus never-smokers (n>12,283) was 1.04 (95% CI: 0.98, 1.10; P=0.201) in case–control studies. Within the case–control studies, the RR for ever-smokers was 0.98 (95% CI: 0.87, 1.11) in hospital-based studies and 1.06 (95% CI: 0.99, 1.13) in population-based studies.
In the cohort studies, the RR for ever-smokers was 1.08 (95% CI: 0.98, 1.19) in studies using mailed questionnaire and 1.14 (95% CI: 0.84, 1.54) in studies using self-administered questionnaire. In the case–control studies, the RR for ever-smokers was 1.05 (95% CI: 0.98, 1.12) among studies using interview and 1.00 (95% CI: 0.84, 1.19) among studies using questionnaire. Homogeneity was detected (I2=67.9%) among studies using questionnaire.
In the stratified analysis by sex, a total of 12 studies were included. Among these studies, ten studies11–13,15,17,18,21,23,25,43 were included to evaluate the association between ever-smokers and the risk of glioma in females, and the pooled RR for ever-smokers (n>1,458,747) versus never-smokers (n>1,457,322) was 1.12 (95% CI: 1.03, 1.22; P=0.007). Ten studies11–15,18,21,23,25,44 were included to evaluate the association between ever-smokers and the risk of glioma in males, and the pooled RR for ever-smokers (n>229,901) versus never-smokers (n>111,759) was 1.03 (95% CI: 0.96, 1.10) (P=0.408). In addition, after stratified analysis by current and past in females smokers, the summary RR for current-smokers (n>629,003) versus never-smokers (n>1,457,322) was 1.06 (95% CI: 0.95, 1.18; P=0.275), including six studies,15,17,18,21,25,43 and for past smokers (n>827,870) versus never-smokers (n>1,457,322) was 1.13 (95% CI: 1.00, 1.28; P=0.046), involving five studies.15,17,18,21,25 These results suggested that females who reported being past smokers were at increased risk of glioma compared with never-smokers, while current-smokers did not appear to be at an increased risk.
Upon a stratified analysis based on geographical area, no significant association was observed, and the combined RR was 0.99 (95% CI: 0.92, 1.06) for the USA, 1.17 (95% CI: 0.93, 1.48) for Canada, 0.97 (95% CI: 0.86, 1.10) for Europe, 1.26 (95% CI: 0.97, 1.64) for Australia, and 1.11 (95% CI: 1.03, 1.20) for the People’s Republic of China.
There are six studies providing the sufficient data for the smoking duration subgroup.11,15,18,23,25,43 The summary RR was 0.97 (95% CI: 0.76, 1.24) for short-term (≤20 years), and 1.06 (95% CI: 0.90, 1.26) for long-term (>20 years).
Eight studies provided the information for smoking intensity.12,15,18,20,25,40,41,43 The pooled RR was 1.02 (95% CI: 0.96, 1.09) for light (≤20 cigarettes per day), and 1.11 (95% CI: 0.91, 1.35) for heavy (>20 cigarettes per day) smokers.
There were no significant differences between publication times. The summary RR for ever-smokers were 0.91 (95% CI: 0.78, 01.07), 1.14 (95% CI: 1.00, 1.30), 1.09 (95% CI: 0.93, 1.26), and 1.04 (95% CI: 0.97, 1.10) according to studies published in before 1990, 1991–2000, 2001–2010, and 2011–now, respectively.
Little evidence of heterogeneity was observed in most analyses of our study except in subgroups of self-administered questionnaire (heterogeneity: I2=67.9%, P=0.045), short-term smoking duration (heterogeneity: I2=50.3%, P=0.09), long-term smoking duration (heterogeneity: I2=58.1%, P=0.036), heavy intensity smoking (heterogeneity: I2=65.4%, P=0.008), and year of study publication (2000–2010; heterogeneity: I2=50.7%, P=0.088).
Sensitivity analysis and publication bias
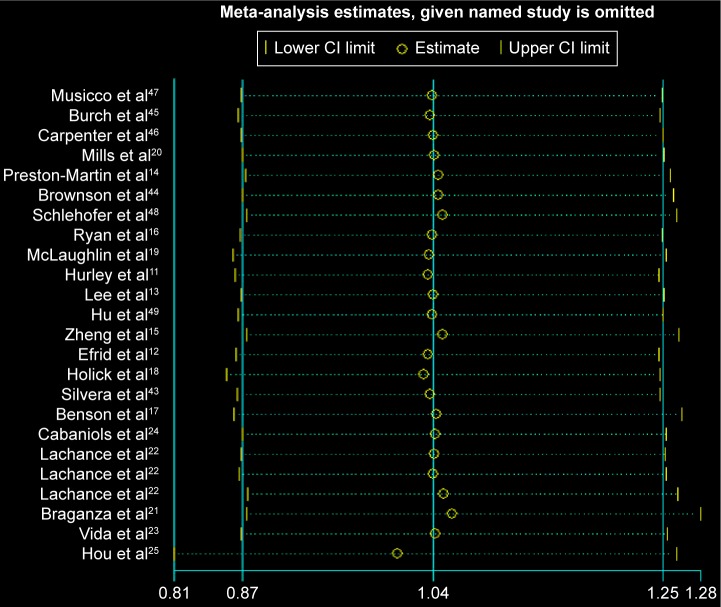
Removing one study at a time did not significantly alter the results, resulting in the pooled RR having a narrow variation range from 1.01 (95% CI: 0.81, 1.26) to 1.06 (95% CI: 0.88, 1.28; Figure 5). No evidence of publication bias was detected for Begg’s (P=0.862) and Egger’s test (P=0.630; Figure 6).
Figure 5.
Sensitivity analysis for the association between ever-smoking and glioma.
Notes: The two ends of the lines represent the 95% CI.
Abbreviation: CI, confidence interval.
Figure 6.
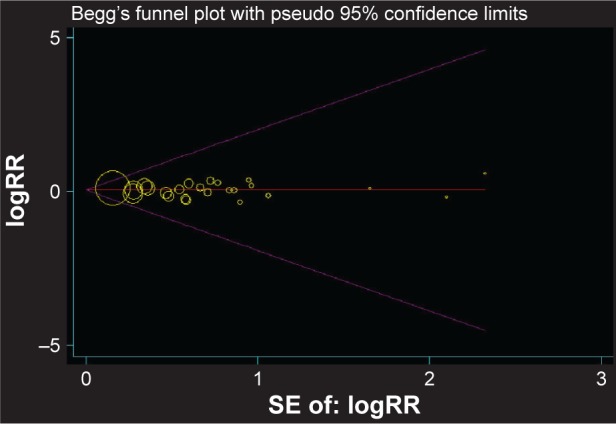
Begg’s funnel plot of all 24 studies for the associations between ever-smoking and glioma.
Note: Each point represents separate study for the indicated association.
Abbreviations: RR, relative risk; SE, standard error.
Discussion
To the best of our knowledge, this meta-analysis is the most comprehensive analysis of a putative association between cigarette smoking and adult glioma risk up to date. The analysis included seven cohort studies and 17 case–control studies involving >2.3 million subjects. The overall analysis did not find significant association between cigarette smoking and glioma, regardless of smoking definition (ever-, current-, or past smokers). Subgroup analysis also failed to find an association regardless of the stratification factors, with an exception of a small but statistically significant increase in females for past smokers (RR: 1.13, 95% CI: 1.00, 1.28; P=0.046).
Subgroup analysis in this study identified a small but statistically significant association between smoking and glioma among females but not males. The association was in accordance with results from two studies,20,43 but not others.11,13,15,17,18,21,23,25 Efird et al12 reported on a cohort of 133,811 subjects to the Kaiser Permanente Medical Care Program of Northern California, with follow-up of up to 21 years; 130 cases were diagnosed with glioma, and compared with never-smokers, an increased risk of gliomas was seen among female smokers smoking <1 pack (RR=1.7, 95% CI=0.9, 3.1), 1–2 packs (RR=1.8, 95% CI=0.8, 4.1), and >2 packs (RR=3.0, 95% CI=0.9, 10.6) per day, respectively; but no association was observed in males.
Our result stratified analysis by past and current smokers in females showed that past females smokers had increased risk of glioma, but not current smokers. The result was consistent with Silvera et al’s study,43 but our result could have been due to chance. Silvera et al43 reported that pastsmokers were at increased risk of glioma compared with never-smokers in females, but upon stratifying past smokers by years since having quit smoking, they found an inverse association between past-smokers who stopped smoking >10 years prior to baseline compared with those who stopped smoking within the 10 years prior to baseline (HR =0.39, 95% CI: 0.19, 0.82), indicating that the association between past smokers and glioma may have been driven by females who recently stopped smoking.43
The mechanism underlying the association between cigarette smoking and adult glioma in females but not in males is unknown. However, some causal relationships are conceivable. First, several animal studies showed that smoking increases the levels of certain sex hormones and sex hormones promote tumor progression,50,51 including glioma.9 Second, the risk of smoking-related cancers (such as in the esophagus, lungs, and oral cavity) has been observed to be higher in females compared to males in some studies,52–54 and these phenomena may also appear in brain cancer. Third, females may be more susceptible to carcinogens in cigarette smoke than males in terms of a greater frequency for specific mutations in the p5355 and K-RAS56 genes, which increased the concentration of carcinogen adducts in smoking-affected tissue,57 elevated the expression of certain enzymes in the cytochrome p450 family,58 reduced capacity for DNA repair,59 and may also induce glioma. Furthermore, cigarette smoking affects both the innate and adoptive immune arms, and leads to a series of immunological disorders (such as atopic diseases and asthma),60 and females are more susceptible to the immunological disorders.61 It may be considered that smoking affects immunological function, and immunological factors induce brain cancer.62–64
Little evidence of heterogeneity was observed in the current analysis. High between-study heterogeneity was only observed from subgroup results: the group self-administered questionnaire, duration (short-term, long-term), intensity (heavy), and year of study publication (2000–2010). It is not surprising given the differences in study designs, characteristics of populations, definition of cigarette smoking, geographic area, and adjustment for confounding factors. As a result, a random-effects model, a conservative method to estimate the pooled effect, was used in these subgroup analyses.
As a crucial barrier that maintains brain homeostasis, the blood–brain barrier (BBB) selectively excludes many endogenous and xenobiotic substances, including some carcinogens, from entering the brain.65,66 Whether N-nitroso compounds, the major carcinogens in cigarette smoke from cigarette smoking, could cross the BBB in adults remains uncertain. A previous study in rats showed that, upon intravenous administration, N-nitroso compounds could induce glioma formation.67 Nicotine could also stimulate the malignant behavior of glioma cells.68 There are some in vivo evidences that showed that nicotine could increase the permeability of the BBB, by allowing carcinogens (eg, nitrosamines) to reach the brain.69 However, another study failed to show that N-nitrosamines could cause cancer in the brain.70 Therefore, future studies should explore the biological mechanisms between cigarette smoking and glioma risk.
Several important strengths should be mentioned in our analysis. First, the major strength of this meta-analysis is the large number of participants and diversity of studied populations. Compared with the former meta-analysis in 2009,7 our study included 17 case–control and seven cohort studies involving more than 2.3 million individuals. Second, a number of subgroup analyses were conducted in our study. No publication bias was found in this study, which further supported the robustness of the study results.
There are some potential limitations in this study. First, only English language publications were included, which may omit other languages studies. Second, both cohort and case–control studies were included, and so methodological differences and confounding factors were unavoidable. However, we conducted a separate analysis for the two types of studies, with consistent results. Third, moderate-to-high heterogeneity was observed in some subgroups, which could not be avoided because of the confounding factors from original studies.
Conclusion
Cigarette smoking is not significantly associated with adult glioma in the overall population. However, there is a small but statistically increased association in females smokers.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ricard D, Idbaih A, Ducray F, et al. Primary brain tumours in adults. Lancet. 2012;379(9830):1984–1996. doi: 10.1016/S0140-6736(11)61346-9. [DOI] [PubMed] [Google Scholar]
- 2.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 3.Bondy ML, Scheurer ME, Malmer B, et al. Brain tumor epidemiology: consensus from the Brain Tumor Epidemiology Consortium. Cancer. 2008;113(7 Suppl):1953–1968. doi: 10.1002/cncr.23741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson DE, Mabuchi K, Ron E, et al. Cancer incidence in atomic bomb survivors. Part II: solid tumors, 1958–1987. Radiat Res. 1994;137(2 Suppl):S17–S67. [PubMed] [Google Scholar]
- 5.Li HX, Meng HY, Peng XX, et al. A meta-analysis of association between pesticides exposure and glioma risk in adults. J Craniofac Surg. 2015;26(7):e672–e673. doi: 10.1097/SCS.0000000000001707. [DOI] [PubMed] [Google Scholar]
- 6.Qi ZY, Shao C, Yang C, Wang Z, Hui GZ. Alcohol consumption and risk of glioma: a meta-analysis of 19 observational studies. Nutrients. 2014;6(2):504–516. doi: 10.3390/nu6020504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mandelzweig L, Novikov I, Sadetzki S. Smoking and risk of glioma: a meta-analysis. Cancer Causes Control. 2009;20(10):1927–1938. doi: 10.1007/s10552-009-9386-z. [DOI] [PubMed] [Google Scholar]
- 8.Niedermaier T, Behrens G, Schmid D, et al. Body mass index, physical activity, and risk of adult meningioma and glioma: a meta-analysis. Neurology. 2015;85(15):1342–1350. doi: 10.1212/WNL.0000000000002020. [DOI] [PubMed] [Google Scholar]
- 9.Qi ZY, Shao C, Zhang X, Hui GZ, Wang Z. Exogenous and endogenous hormones in relation to glioma in women: a meta-analysis of 11 case-control studies. PLoS One. 2013;8(7):e68695. doi: 10.1371/journal.pone.0068695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canadian Cancer Society [homepage on the Internet] Smoking and cancer. [Accessed October 25, 2015]. Available from: http://www.cancer.ca/en/prevention-and-screening/live-well/smoking-and-tobacco/smoking-and-cancer/?region=qc.
- 11.Hurley SF, McNeil JJ, Donnan GA, et al. Tobacco smoking and alcohol consumption as risk factors for glioma: a case-control study in Melbourne, Australia. J Epidemiol Community Health. 1996;50(4):442–446. doi: 10.1136/jech.50.4.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Efird JT, Friedman GD, Sidney S, et al. The risk for malignant primary adult-onset glioma in a large, multiethnic, managed-care cohort: cigarette smoking and other lifestyle behaviors. J Neurooncol. 2004;68(1):57–69. doi: 10.1023/b:neon.0000024746.87666.ed. [DOI] [PubMed] [Google Scholar]
- 13.Lee M, Wrensch M, Miike R. Dietary and tobacco risk factors for adult onset glioma in the San Francisco Bay Area (California, USA) Cancer Causes Control. 1997;8(1):13–24. doi: 10.1023/a:1018470802969. [DOI] [PubMed] [Google Scholar]
- 14.Preston-Martin S, Mack W, Henderson BE. Risk factors for gliomas and meningiomas in males in Los Angeles County. Cancer Res. 1989;49(21):6137–6143. [PubMed] [Google Scholar]
- 15.Zheng T, Cantor KP, Zhang Y, Chiu BC, Lynch CF. Risk of brain glioma not associated with cigarette smoking or use of other tobacco products in Iowa. Cancer Epidemiol Biomarkers Prev. 2001;10(4):413–414. [PubMed] [Google Scholar]
- 16.Ryan P, Lee MW, North B, McMichael AJ. Risk factors for tumors of the brain and meninges: results from the Adelaide Adult Brain Tumor Study. Int J Cancer. 1992;51(1):20–27. doi: 10.1002/ijc.2910510105. [DOI] [PubMed] [Google Scholar]
- 17.Benson VS, Pirie K, Green J, Casabonne D, Beral V. Lifestyle factors and primary glioma and meningioma tumours in the Million Women Study cohort. Br J Cancer. 2008;99(1):185–190. doi: 10.1038/sj.bjc.6604445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holick CN, Giovannucci EL, Rosner B, Stampfer MJ, Michaud DS. Prospective study of cigarette smoking and adult glioma: dosage, duration, and latency. Neuro Oncol. 2007;9(3):326–334. doi: 10.1215/15228517-2007-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLaughlin JK, Hrubec Z, Blot WJ, Fraumeni JF., Jr Smoking and cancer mortality among U.S. veterans: a 26-year follow-up. International journal of cancer. Int J Cancer. 1995;60(2):190–193. [PubMed] [Google Scholar]
- 20.Mills PK, Preston-Martin S, Annegers JF, et al. Risk factors for tumors of the brain and cranial meninges in Seventh-Day Adventists. Neuroepidemiology. 1989;8(5):266–275. doi: 10.1159/000110193. [DOI] [PubMed] [Google Scholar]
- 21.Braganza MZ, Rajaraman P, Park Y, et al. Cigarette smoking, alcohol intake, and risk of glioma in the NIH-AARP Diet and Health Study. Br J Cancer. 2014;110(1):242–248. doi: 10.1038/bjc.2013.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lachance DH, Yang P, Johnson DR, et al. Associations of high-grade glioma with glioma risk alleles and histories of allergy and smoking. Am J Epidemiol. 2011;174(5):574–581. doi: 10.1093/aje/kwr124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vida S, Richardson L, Cardis E, et al. Brain tumours and cigarette smoking: analysis of the INTERPHONE Canada case-control study. Environ Health. 2014;13:55. doi: 10.1186/1476-069X-13-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cabaniols C, Giorgi R, Chinot O, et al. Links between private habits, psychological stress and brain cancer: a case-control pilot study in France. J Neurooncol. 2011;103(2):307–316. doi: 10.1007/s11060-010-0388-1. [DOI] [PubMed] [Google Scholar]
- 25.Hou L, Jiang J, Liu B, et al. Smoking and adult glioma: a population-based case-control study in China. Neuro Oncol. 2015;18(1):105–113. doi: 10.1093/neuonc/nov146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Copas J, Shi JQ. Meta-analysis, funnel plots and sensitivity analysis. Biostatistics. 2000;1(3):247–262. doi: 10.1093/biostatistics/1.3.247. [DOI] [PubMed] [Google Scholar]
- 30.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 31.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang S, Zhao Y, Ruan Z, et al. Association between EGF +61 G/A and glioma risk in a Chinese population. BMC Cancer. 2010;10:221. doi: 10.1186/1471-2407-10-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J, Li D, Qu F, et al. Association of leukocyte mitochondrial DNA content with glioma risk: evidence from a Chinese case-control study. BMC Cancer. 2014;14:680. doi: 10.1186/1471-2407-14-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benson VS, Green J, Pirie K, Beral V. Cigarette smoking and risk of acoustic neuromas and pituitary tumours in the Million Women Study. Br J Cancer. 2010;102(11):1654–1656. doi: 10.1038/sj.bjc.6605695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walker AJ, Card T, Bates TE, Muir K. Tricyclic antidepressants and the incidence of certain cancers: a study using the GPRD. Br J Cancer. 2011;104(1):193–197. doi: 10.1038/sj.bjc.6605996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greenop KR, Blair EM, Bower C, Armstrong BK, Milne E. Factors relating to pregnancy and birth and the risk of childhood brain tumors: results from an Australian case-control study. Pediatr Blood Cancer. 2014;61(3):493–498. doi: 10.1002/pbc.24751. [DOI] [PubMed] [Google Scholar]
- 37.Seliger C, Ricci C, Meier CR, et al. Diabetes, use of antidiabetic drugs, and the risk of glioma. Neuro Oncol. 2015;18(3):340–349. doi: 10.1093/neuonc/nov100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blowers L, Preston-Martin S, Mack WJ. Dietary and other lifestyle factors of women with brain gliomas in Los Angeles County (California, USA) Cancer Causes Control. 1997;8(1):5–12. doi: 10.1023/a:1018437031987. [DOI] [PubMed] [Google Scholar]
- 39.Ahlbom A, Navier IL, Norell S, Olin R, Spannare B. Nonoccupational risk indicators for astrocytomas in adults. Am J Epidemiol. 1986;124(2):334–337. doi: 10.1093/oxfordjournals.aje.a114393. [DOI] [PubMed] [Google Scholar]
- 40.Hochberg F, Toniolo P, Cole P, Salcman M. Nonoccupational risk indicators of glioblastoma in adults. J Neurooncol. 1990;8(1):55–60. doi: 10.1007/BF00182087. [DOI] [PubMed] [Google Scholar]
- 41.Zampieri P, Meneghini F, Grigoletto F, et al. Risk factors for cerebral glioma in adults: a case-control study in an Italian population. J Neurooncol. 1994;19(1):61–67. doi: 10.1007/BF01051049. [DOI] [PubMed] [Google Scholar]
- 42.Giles GG, McNeil JJ, Donnan G, et al. Dietary factors and the risk of glioma in adults: results of a case-control study in Melbourne, Australia. Int J Cancer. 1994;59(3):357–362. doi: 10.1002/ijc.2910590311. [DOI] [PubMed] [Google Scholar]
- 43.Silvera SA, Miller AB, Rohan TE. Cigarette smoking and risk of glioma: a prospective cohort study. Int J Cancer. 2006;118(7):1848–1851. doi: 10.1002/ijc.21569. [DOI] [PubMed] [Google Scholar]
- 44.Brownson RC, Reif JS, Chang JC, Davis JR. An analysis of occupational risks for brain cancer. Am J Public Health. 1990;80(2):169–172. doi: 10.2105/ajph.80.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burch JD, Craib KJ, Choi BC, et al. An exploratory case-control study of brain tumors in adults. J Natl Cancer Inst. 1987;78(4):601–609. [PubMed] [Google Scholar]
- 46.Carpenter AV, Flanders WD, Frome EL, Cole P, Fry SA. Brain cancer and nonoccupational risk factors: a case-control study among workers at two nuclear facilities. Am J Public Health. 1987;77(9):1180–1182. doi: 10.2105/ajph.77.9.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Musicco M, Filippini G, Bordo BM, et al. Gliomas and occupational exposure to carcinogens: case-control study. Am J Epidemiol. 1982;116(5):782–790. doi: 10.1093/oxfordjournals.aje.a113468. [DOI] [PubMed] [Google Scholar]
- 48.Schlehofer B, Kunze S, Sachsenheimer W, et al. Occupational risk factors for brain tumors: results from a population-based case-control study in Germany. Cancer Causes Control. 1990;1(3):209–215. doi: 10.1007/BF00117472. [DOI] [PubMed] [Google Scholar]
- 49.Hu J, Johnson KC, Mao Y, et al. Risk factors for glioma in adults: a case-control study in northeast China. Cancer Detect Prev. 1998;22(2):100–108. doi: 10.1046/j.1525-1500.1998.cdoa22.x. [DOI] [PubMed] [Google Scholar]
- 50.Avtsyn AP, Yablonovskaya LY. Effects of disturbances in the hormonal status on experimental brain tumors. Acta Unio Int Contra Cancrum. 1964;20:1519–1522. [PubMed] [Google Scholar]
- 51.Law MR, Cheng R, Hackshaw AK, Allaway S, Hale AK. Cigarette smoking, sex hormones and bone density in women. Eur J Epidemiol. 1997;13(5):553–558. doi: 10.1023/a:1007389712487. [DOI] [PubMed] [Google Scholar]
- 52.Ahsan H, Neugut AI, Gammon MD. Association of adenocarcinoma and squamous cell carcinoma of the esophagus with tobacco-related and other malignancies. Cancer Epidemiol Biomarkers Prev. 1997;6(10):779–782. [PubMed] [Google Scholar]
- 53.Zang EA, Wynder EL. Differences in lung cancer risk between men and women: examination of the evidence. J Natl Cancer Inst. 1996;88(3–4):183–192. doi: 10.1093/jnci/88.3-4.183. [DOI] [PubMed] [Google Scholar]
- 54.Muscat JE, Richie JP, Jr, Thompson S, Wynder EL. Gender differences in smoking and risk for oral cancer. Cancer Res. 1996;56(22):5192–5197. [PubMed] [Google Scholar]
- 55.Kure EH, Ryberg D, Hewer A, et al. p53 mutations in lung tumours: relationship to gender and lung DNA adduct levels. Carcinogenesis. 1996;17(10):2201–2205. doi: 10.1093/carcin/17.10.2201. [DOI] [PubMed] [Google Scholar]
- 56.Nelson HH, Christiani DC, Mark EJ, et al. Implications and prognostic value of K-ras mutation for early-stage lung cancer in women. J Natl Cancer Inst. 1999;91(23):2032–2038. doi: 10.1093/jnci/91.23.2032. [DOI] [PubMed] [Google Scholar]
- 57.Ryberg D, Hewer A, Phillips DH, Haugen A. Different susceptibility to smoking-induced DNA damage among male and female lung cancer patients. Cancer Res. 1994;54(22):5801–5803. [PubMed] [Google Scholar]
- 58.Guengerich FP, Shimada T. Activation of procarcinogens by human cytochrome P450 enzymes. Mutat Res. 1998;400(1–2):201–213. doi: 10.1016/s0027-5107(98)00037-2. [DOI] [PubMed] [Google Scholar]
- 59.Wei Q, Cheng L, Amos CI, et al. Repair of tobacco carcinogen-induced DNA adducts and lung cancer risk: a molecular epidemiologic study. J Natl Cancer Inst. 2000;92(21):1764–1772. doi: 10.1093/jnci/92.21.1764. [DOI] [PubMed] [Google Scholar]
- 60.Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun. 2010;34(3):J258–J265. doi: 10.1016/j.jaut.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 61.Zein JG, Erzurum SC. Asthma is different in women. Curr Allergy Asthma Rep. 2015;15(6):28. doi: 10.1007/s11882-015-0528-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Madany M, Thomas TM, Edwards L, Yu JS. Immunobiology and immunotherapeutic targeting of glioma stem cells. Adv Exp Med Biol. 2015;853:139–166. doi: 10.1007/978-3-319-16537-0_8. [DOI] [PubMed] [Google Scholar]
- 63.George J, Levy Y, Shoenfeld Y. Smoking and immunity: an additional player in the mosaic of autoimmunity. Scand J Immunol. 1997;45(1):1–6. doi: 10.1046/j.1365-3083.1997.d01-366.x. [DOI] [PubMed] [Google Scholar]
- 64.Johnson JD, Houchens DP, Kluwe WM, Craig DK, Fisher GL. Effects of mainstream and environmental tobacco smoke on the immune system in animals and humans: a review. Crit Rev Toxicol. 1990;20(5):369–395. doi: 10.3109/10408449009089870. [DOI] [PubMed] [Google Scholar]
- 65.Bernacki J, Dobrowolska A, Nierwinska K, Malecki A. Physiology and pharmacological role of the blood–brain barrier. Pharmacol Rep. 2008;60(5):600–622. [PubMed] [Google Scholar]
- 66.Mazzone P, Tierney W, Hossain M, et al. Pathophysiological impact of cigarette smoke exposure on the cerebrovascular system with a focus on the blood-brain barrier: expanding the awareness of smoking toxicity in an underappreciated area. Int J Environ Res Public Health. 2010;7(12):4111–4126. doi: 10.3390/ijerph7124111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lantos PL. Development of nitrosourea-induced brain tumours – with a special note on changes occurring during latency. Food Chem Toxicol. 1986;24(2):121–127. doi: 10.1016/0278-6915(86)90346-7. [DOI] [PubMed] [Google Scholar]
- 68.Khalil AA, Jameson MJ, Broaddus WC, Lin PS, Chung TD. Nicotine enhances proliferation, migration, and radioresistance of human malignant glioma cells through EGFR activation. Brain Tumor Pathol. 2013;30(2):73–83. doi: 10.1007/s10014-012-0101-5. [DOI] [PubMed] [Google Scholar]
- 69.Hawkins BT, Egleton RD, Davis TP. Modulation of cerebral micro-vascular permeability by endothelial nicotinic acetylcholine receptors. Am J Physiol Heart Circ Physiol. 2005;289(1):H212–H219. doi: 10.1152/ajpheart.01210.2004. [DOI] [PubMed] [Google Scholar]
- 70.Dietrich M, Block G, Pogoda JM, et al. A review: dietary and endogenously formed N-nitroso compounds and risk of childhood brain tumors. Cancer Causes Control. 2005;16(6):619–635. doi: 10.1007/s10552-005-0168-y. [DOI] [PubMed] [Google Scholar]