Abstract
Background
Feline pancreas‐specific lipase (Spec fPL) is considered a useful test for the antemortem diagnosis of pancreatitis in cats. A recent study found good agreement between the results of the Spec fPL and catalytic 1,2‐o‐dilauryl‐rac‐glycero‐3‐glutaric acid‐(6′‐methylresorufin) ester (DGGR) lipase assay. Prospective studies evaluating their sensitivity and specificity are lacking.
Objectives
To compare the results of the Spec fPL and the DGGR assays with a standardized histologic assessment of the pancreas.
Animals
Sixty client‐owned cats presented for necropsy.
Methods
Prospective study: Spec fPL concentrations and serum DGGR lipase activity were measured from the same blood sample. The pancreas was removed within 3 hours after euthanasia; serial transverse sections were made every 0.5 cm throughout the entire pancreas and reviewed using a histologic grading scheme. Sensitivity and specificity for the Spec fPL and DGGR assay results were determined.
Results
The sensitivity and specificity for the Spec fPL assay (cutoff value ≥5.4 μg/L) was 42.1 [95% confidence interval (95% CI), 29.4–55.9%] and 100% (95% CI, 31.0–100.0%). The sensitivity and specificity for the DGGR assay (cutoff value >26 U/L) was 36.8 (95% CI, 24.7–50.7%) and 100% (95% CI, 31.0–100.0%). When lymphocytic inflammation up to 10% of a section was considered normal, the sensitivity and specificity for Spec fPL assay (cutoff value ≥5.4 μg/L) was 61.1 (95% CI, 36.1–81.7%) and 69.0% (95% CI, 52.8–81.9%) and the sensitivity and specificity for the DGGR assay (cutoff value >26 U/L) was 66.7 (95% CI, 41.2–85.6%) and 78.6% (95% CI, 62.8–89.2%).
Conclusions and Clinical Importance
Both lipase assays performed similarly well, but their agreement with histologic pancreatic inflammation was limited.
Keywords: Feline, Feline pancreas‐specific lipase, Histopathology, Lipase, Pancreas
Abbreviations
- 95% CI
95% confidence interval
- AI
disease activity index
- AUC
area under the curve
- CV
coefficient of variation
- DGGR
1,2‐o‐dilauryl‐rac‐glycero‐3‐glutaric acid‐(6′‐methylresorufin) ester
- fPLI
feline pancreatic lipase immunoreactivity
- MCS
mean cumulative score
- ROC
receiver operating characteristic
- SD
standard deviation
- SE
standard error
- Spec fPL
feline pancreas‐specific lipase
- κ
Cohen's kappa coefficient
Histologic pancreatic inflammation appears to be a common finding in cats1 with the consequence that pancreatitis is also surmised to be a common clinical disorder in cats. However, reports on clinically relevant pancreatitis in cats are scarce2, 3, 4 and the actual prevalence of clinically relevant pancreatitis remains currently unknown. Nonetheless, antemortem diagnosis continues to be difficult because of vague clinical signs and nonspecific clinicopathologic findings.4, 5 Although ultrasonographic examination of the pancreas is an option in many clinics, its sensitivity and specificity for the diagnosis of feline pancreatitis are operator dependent and therefore highly variable.3, 6, 7 Moreover, there is poor agreement between serum lipase results and ultrasonographic findings that until recently were considered to represent pancreatitis in cats.8 The commercially available Spec fPL test, an enzyme‐linked immunosorbent assay, is widely thought to be a useful test for diagnosing pancreatitis in cats.9 However, details of its development and validation have not been published in a peer‐reviewed article. More recently, a catalytic assay for the determination of serum lipase activity using the substrate 1,2‐o‐dilauryl‐rac‐glycero‐3‐glutaric acid‐(6′‐methylresorufin)‐ester (DGGR)10 was validated for use in feline serum and has good agreement with the Spec fPL assay.11 The short turnaround time and low cost of the DGGR assay are of particular benefit to clinicians and clients. Nevertheless, the results of the Spec fPL and DGGR assay have not been compared to a gold standard. Although the selection of a gold standard for diagnosing pancreatitis in cats is controversial,9 histologic examination of the pancreas currently constitutes the only modality that allows a definitive diagnosis. Therefore, the goal of this study was to compare the results of the Spec fPL and DGGR assays with standardized histologic examination of the pancreas. We hypothesized that the performance of both tests is similar for the diagnosis of pancreatitis in cats.
Materials and Methods
Animals and Study Design
A total of 60 cats that were euthanized for a variety of reasons at the Clinic for Small Animal Internal Medicine, Vetsuisse Faculty, University of Zurich and subsequently submitted for necropsy were used in the study. Collection of a serum sample within 12 hours before euthanasia, and removal of the entire pancreas from each cat within 3 hours of euthanasia were criteria for inclusion in the study. Pancreata were placed in 10% buffered formalin, and the Spec fPL concentration and serum lipase activity using the DGGR assay were measured in the same blood sample.
Serum Lipase Determination
Serum lipase activity was measured within one hour using the DGGR assay.1 Spec fPL concentration was measured by IDEXX Laboratories.2 The reference interval for the DGGR assay (8–26 U/L) was previously established using 80 clinically healthy, male and female cats of various breeds.11
Histologic Evaluation
Each pancreas was cut transversely at the midpoint of the body yielding a left and right side, which were cut transversely into smaller pieces (Fig 1). Serial transverse sections of the entire pancreas were made every 0.5 cm and stained with hematoxylin and eosin. Light microscopy was used for examination of all sections by a board‐certified pathologist (MH) in a blinded fashion.6 A histologic scoring scheme was designed and modified based on previously reported scoring schemes.1, 12 All tissue sections of the pancreas were evaluated for the presence of neutrophilic inflammation, lymphocytic inflammation, pancreatic edema, pancreatic necrosis, peripancreatic fat necrosis, fibrosis, cystic degeneration, atrophy, nodular hyperplasia, islet cell amyloidosis, and neoplasia. The severity of lesions (with the exception of neoplasia) in each section was scored as follows: 0 = 0% of the section affected, grade 1 = <25% of the section affected, grade 2 = 25–50% of the section affected, and grade 3 = >50% of the section affected. Because mild lymphocytic inflammation (Fig 2) has been shown to be a common finding in feline pancreata,1 additional statistical analyses were carried out with 0–10% lymphocytic inflammation defined as absence of lymphocytic inflammation, and grade 1 defined as 10–25% of the section affected. For each variable, a mean cumulative score (MCS) was calculated as MCS = ∑ score of single sections/number of sections. A disease activity index (AI) was calculated as AI = (MCSneutrophilic inflammation + MCSlymphocytic inflammation + MCSpancreatic edema + MCSpancreatic necrosis + MCSfat necrosis)/5. The right and left side of the pancreas were compared.
Figure 1.
A specimen of feline pancreas that has been fixated in formalin and cut transversely every 0.5 cm.
Figure 2.
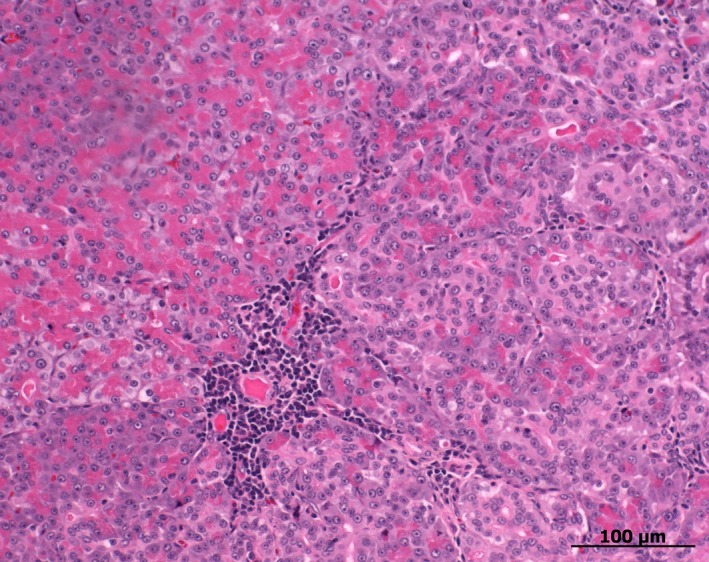
Small nests of lymphocytes located predominantly perivascularly in the pancreas of a cat. The overall grade of lymphocytic inflammation in the corresponding slide was graded as 0–10%.
Statistical Analyses
A commercial software3 was used for statistical analysis. Cohen's kappa coefficient (κ) was calculated to measure agreement between Spec fPL and DGGR assays and between both lipase assays and histologic results. Differences in MCS, AI, and CI of the right and left side of the pancreas were evaluated using the Wilcoxon signed‐rank test. Bonferroni correction was applied to multiple comparisons. The performance of both lipase assays was evaluated using Receiver operating characteristic (ROC) curves and the corresponding area under the curve (AUC). For the calculation of Cohen's Kappa, ROC‐Curve, and sensitivity and specificity, the AI was dichotomized into AI = 0 (no evidence of histologic pancreatic inflammation) and AI > 0 (evidence of histologic pancreatic inflammation). In addition, logistic regressions with either SpecfPL or DGGR assay as predictors were performed to assess which one showed a better model fit based on AIC (Akaike's information criterion). Logistic regression analysis was performed for the original AI [AI = 0 (no evidence of histologic pancreatic inflammation) and AI > 0 (evidence of histologic pancreatic inflammation)] as well as for the modified AI when the presence of up to 10% lymphocytes was considered normal. We utilized AIC as a goodness‐of‐model fit with lower values (<2) indicating a better model fit.
Results
Study Population
The study population consisted of 60 cats that included 30 male (30 neutered) and 30 female (28 spayed) cats, ranging in age from 10 months to 19 years (median 11.5). Breeds included domestic shorthair (n = 46), domestic longhair (n = 3), Persian (n = 3), Siberian (n = 2), Angora (n = 1), British longhair (n = 1), British shorthair (n = 1), Ragdoll (n = 1), Siamese (n = 1), and mixed breed cats (n = 1).
Lipase Assay Results
The Spec fPL concentration was ≤3.5 μg/L in 30/60 (50%) cats, 3.6–5.3 μg/L in 6/60 (10%) cats, and ≥5.4 μg/L in 24/60 (40%) cats. Serum lipase activity was ≤26 U/L in 39/60 (65%) cats and ≥27 U/L in 21/60 (35%) cats (Table 1). Agreement between the Spec fPL (cutoff value >3.5 μg/L) and DGGR assays (cutoff value >26 U/L) was κ = 0.63 (standard error [SE], 0.10), and agreement between the Spec fPL (cutoff value ≥5.4 μg/L) and DGGR assays (cutoff value >26 U/L) was κ = 0.82 (SE, 0.08).
Table 1.
Contingency table showing the frequency distribution of the results of the Spec fPL and DGGR‐lipase assays using different cut‐off values
| Spec fPL | ||||
|---|---|---|---|---|
| ≤3.5 μg/L | 3.6–5.3 μg/L | ≥5.4 μg/L | Total | |
| DGGR‐lipase | ||||
| ≤26 U/L | 29 | 6 | 4 | 39 |
| ≥27 U/L | 1 | 0 | 20 | 21 |
| Total | 30 | 6 | 24 | 60 |
Pancreatic Histology
The mean number of sections was 15.43 (range, 9–22) per formalin‐fixated pancreas with 6.78 (range, 3–10) for the right side and 8.65 (range, 3–13) for the left side of the pancreas. The mean length of the right side of the formalin‐fixated pancreas was 6.87 cm (range, 1.50–8.50 cm) and the mean length of the left formalin‐fixated side was 8.51 cm (range, 2.0–9.50 cm). Nodular hyperplasia was the most common histopathologic finding and was seen in 57/60 (95%) cats, followed by lymphocytic inflammation in 56/60 (93%), cystic degeneration in 43/60 (72%), fibrosis in 37/60 (62%), islet cell amyloidosis in 26/60 (43%), atrophy in 16/60 (27%), neutrophilic inflammation in 11/60 (18%), edema in 9/60 (15%), peripancreatic fat necrosis in 9/60 (15%), neoplasia in 9/60 (16%) (5 lymphoma, 3 adenocarcinoma, and 1 mastocytoma), and pancreatic necrosis in 8/60 (13%). The numbers of cats with pancreatic lesions and the type of lesions are shown in Tables 2 and 3. Detailed results of the cats with pancreatitis and neoplasia (AI, MCS for neutrophilic and lymphocytic inflammation, edema, necrosis, as well as results of Spec fPL and DGGR assay) are available in Table S1. There were no significant differences in the MCS between the right and left sides of the pancreas with regard to neutrophilic inflammation (P = .053), lymphocytic inflammation (P = .142), edema (P = .612), pancreatic necrosis (P = .161), peripancreatic fat necrosis (P = .594), fibrosis (P = .202), cystic degeneration (P = .139), atrophy (P = .414), nodular hyperplasia (P = .28), and islet cell amyloidosis (P = .269).
Table 2.
Frequency distribution of cats with neutrophilic and lymphocytic inflammation of the pancreas of different grades
| Histologic variable | 0% | <10% | 10–25% | 25–50% | >50% |
|---|---|---|---|---|---|
| Neutrophilic inflammation | 49 | 7 | 2 | 2 | 0 |
| Lymphocytic inflammation | 4 | 51 | 3 | 1 | 1 |
Table 3.
Frequency distribution of cats with pancreatic lesions of different grades
| Histologic variable | 0% | 1–25% | 25–50% | >50% |
|---|---|---|---|---|
| Edema | 51 | 5 | 4 | 0 |
| Pancreatic necrosis | 52 | 6 | 2 | 0 |
| Fat necrosis | 51 | 5 | 4 | 0 |
| Fibrosis | 23 | 30 | 5 | 2 |
| Cystic degeneration | 17 | 39 | 4 | 0 |
| Atrophy | 44 | 10 | 5 | 1 |
| Nodular hyperplasia | 3 | 42 | 10 | 5 |
| Islet cell amyloidosis | 34 | 18 | 2 | 6 |
The mean AI was 0.19 (SD, 0.25; range, 0.00–1.12). The AI was 0 in 3/60 (5%) cats, >0 but <1 in 55/60 (92%) cats, and >1 in 2/60 (3%) cats. The AI did not differ significantly between the right and left sides of the pancreas (P = .7).
When normal pancreas was considered to include up to 10% lymphocytic inflammation, the AI was 0 in 42/60 (70%) cats, >0 but <1 in 17/60 (28%) cats, and >1 in 1/60 (2%) cats, and the mean AI was 0.10 (SD, 0.26; range, 0.00–1.06). The AI did not differ significantly between the right and left sides of the pancreas (P = .124).
Agreement Between Lipase Assay Results and Pancreatic Histology
Agreement between AI and the results of the Spec fPL assay (cutoff value >3.5 μg/L) was slight (κ = 0.10; SE, 0.06). Agreement between AI and the results of the Spec fPL assay (cutoff value ≥5.4 μg/L) was slight (κ = 0.07; SE, 0.04), and agreement between AI and the results of the DGGR assay (cutoff value >26 U/L) was also slight (κ = 0.06; SE, 0.03).
When normal pancreas was considered to include up to 10% lymphocytic inflammation, agreement between AI and the results of the Spec fPL assay (cutoff value >3.5 μg/L) was slight (κ = 0.13; SE, 0.12), and agreement between AI and the results of the Spec fPL assay (cutoff value ≥5.4 μg/L) was fair (κ = 0.28; SE, 0.13). Agreement between AI and the results of the DGGR assay (cutoff value >26 U/L) was moderate (κ = 0.43; SE, 0.12).
Logistic Regression Analysis
Based on AIC, as a goodness‐of‐fit‐criterion, the models with the DGGR assay indicated a better model fit compared to SpecfPL for both, the original AI (DGGR assay: 67.4 versus SpecfPL: 88) as well as the modified AI when up to 10% lymphocytic inflammation was considered normal (DGGR assay: 75.7 versus SpecfPL: 91.17).
ROC Curve of Lipase Assays Versus AI as Gold Standard
Receiver operating characteristic curves of both lipase assays are shown in Figure 3; the gold standard used was an AI for which up to 10% lymphocytic inflammation was considered normal. The AUC for the Spec fPL assay was 0.60 [95% confidence interval (95% CI), 0.40–0.80] and the AUC for the DGGR assay was 0.71 (95% CI, 0.55–0.88).
Figure 3.
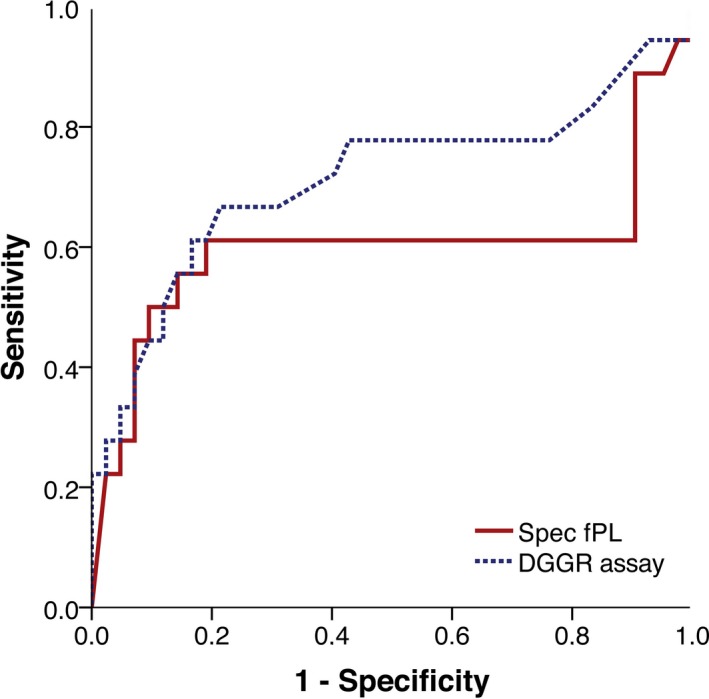
ROC curve for the histopathologic diagnosis of pancreatitis by use of the Spec fPL assay (solid line) and the DGGR assay (dashed line). The gold standard was an AI with up to 10% lymphocytic inflammation defined as normal.
Sensitivity and Specificity
When the AI was used as the gold standard, the sensitivity and specificity of the Spec fPL assay (cutoff value >3.5 μg/L) were 52.6% (95% CI, 39.1–65.8%) and 100.0% (95% CI, 31.0–100.0%). The sensitivity and specificity of the Spec fPL assay (cutoff value ≥5.4 μg/L) were 42.1% (95% CI, 29.4–55.9%) and 100.0% (95% CI, 31.0–100.0%). The sensitivity and specificity of the DGGR assay were 36.8% (95% CI, 24.7–50.7%) and 100.0% (95% CI, 31.0–100.0%).
When the AI was used as the gold standard and up to 10% lymphocytic inflammation was considered normal, the sensitivity and specificity of the Spec fPL assay (cutoff value >3.5 μg/L) were 61.1% (95% CI, 36.1–81.7%) and 54.8% (95% CI, 38.8–69.8%). The sensitivity and specificity of the Spec fPL assay (cutoff value ≥5.4 μg/L) were 61.1% (95% CI, 36.1–81.7%) and 69.0% (95% CI, 52.8–81.9%). The sensitivity and specificity of the DGGR assay were 66.7% (95% CI, 41.2–85.6%) and 78.6% (95% CI, 62.8–89.2%).
Discussion
This study compares the results of an immunoassay and a catalytic lipase assay with those of standardized histologic examination of the pancreas in cats. There was very good agreement between the lipase assays, which was similar to the results of our previous studies using different populations of cats.8, 11 In this study, the sensitivity of the Spec fPL assay with a cutoff value of ≥5.4 μg/L for the diagnosis of pancreatic inflammation ranged from 42.1% to 61.1%, while the DGGR‐lipase assay had a sensitivity of 36.8–66.8%. The specificity of the Spec fPL assay with a cutoff value of ≥5.4 μg/L ranged from 69.0 to 100%, whereas that of the DGGR assay was 78.6 to 100%; the value depended on whether up to 10% lymphocytic inflammation was considered normal or abnormal. A recent retrospective study reported similar sensitivities and specificities for the Spec fPL (57% sensitivity, 63% specificity) and DGGR assays (48% sensitivity, 63% specificity) in 31 cats. However, histopathologic evaluation was based on pancreatic tissue obtained during necropsy (28) or biopsy (3),11 and the time interval between histopathologic evaluation and lipase measurements in that study ranged from a couple of hours to 5 days.
Based on our scoring system, 57 of 60 cats had pancreatic inflammation, which was equivalent to a prevalence of 95%. This is even higher than the results of the largest histopathologic study to date, in which the prevalence of pancreatitis in a comparable cat population was 67%.1 However, that study did not specify how many sections per pancreas were examined. It is conceivable that the overall prevalence of pancreatic inflammation increases when the organ is sectioned at closer intervals because fewer lesions would remain undetected. Similarly, a recent study in dogs reported histopathologic evidence of pancreatitis in 63 of 70 dogs.13
The relevance of mild lymphocytic pancreatic inflammation in cats is currently unknown. In a study that evaluated the feline pancreatic lipase immunoreactivity (fPLI) test, small nests of lymphocytes were considered normal in feline pancreata.6 We therefore decided to establish an alternative AI that defined lymphocytic inflammation affecting <10% of a section as normal. Subsequently, the sensitivity of the Spec fPL assay (cutoff value ≥5.4 μg/L) increased from 42.1 to 61.1% because more cats with normal lipase results were classified as healthy. This was slightly lower than the sensitivity of 67% reported for the fPLI assay in the study mentioned above.6 Likewise, the sensitivity of the DGGR assay increased from 36.5 to 78.6%, making it the test with the highest sensitivity in this study. It has been argued that a diagnostic test with a maximal sensitivity might not be an absolute priority because clinical signs compatible with pancreatitis may be already detected in an initial clinical assessment and thus could be viewed as some sort of screening test.14 Rather, a true diagnostic test would be needed to confirm the suspicion of pancreatitis, and the clinician would thus be interested in a test with maximal specificity rather than maximal sensitivity.14 While these considerations might relate to the diagnosis of pancreatitis in dogs, we feel that a test with a high sensitivity is more useful in cats because signs of pancreatitis in this species are vague and nonspecific.
The specificity of both lipase assays was 100% when mild lymphocytic inflammation (<10% of section affected) was considered to be indicative of pancreatitis. Because only 3 cats were classified as healthy in this study, calculation of specificity was based on a small number of cases, reflected in the wide 95% CI of 31.0–100.0%. A specificity of 100% was also reported for the fPLI assay in a study that used 8 healthy shelter cats.6 When we considered lymphocytic inflammation in <10% of the section as normal, the number of healthy cats increased to 42 and the specificity decreased to 61.1% for the Spec fPL assay (cutoff value ≥5.4 μg/L) and to 66.7% for the DGGR assay. Because the significance of minimal to mild lymphocytic inflammation is not known, we are unable to conclude which calculation of specificity better reflects clinical pancreatitis.
The performance of the Spec fPL and DGGR assays was similar based on the agreement of results, areas under the curve, and sensitivity. The DGGR assay had a slightly higher specificity, which must be interpreted cautiously because of overlapping confidence intervals. It is often stated that serum lipase activity is not pancreas‐specific, implying that sensitivity is higher because of extrapancreatic sources of lipase, and specificity lower compared with the pancreas‐specific Spec fPL assay. In human medicine, the DGGR assay is generally considered to be specific for pancreatic lipase; several cofactors such as colipase, bile salts, and calcium ions play an important role in the activation process of pancreatic lipase, thus increasing the assay's specificity.10, 15 The high specificity of the DGGR assay in this study suggests that this also applies to cats. Another possible explanation is that extrapancreatic serum lipase activities were coincidentally normal in this study population, but because the cats were included irrespective of the cause of euthanasia, this seems unlikely.
The reference interval for the Spec fPL assay (0–3.5 μg/L) was established using 41 healthy cats several years ago.4 The cutoff value of ≥5.4 μg/L was based on 141 cats with “clinical signs consistent with pancreatitis.”4 Two internists blinded to the Spec fPL results divided the 141 cats into 6 groups with different probabilities of having pancreatitis based on history, the results of clinical examination, CBC, biochemistry panel, urinalysis, and abdominal ultrasonography, and clinical outcome.4 This was an innovative approach because, normally, establishment of the reference interval is based only on healthy individuals and does not allow a definitive statement about the condition of individuals with increased values. However, the data are only available as an abstract and thus we do not know whether the cats had acute or chronic pancreatitis or, perhaps more importantly when considering the often unspecific clinical picture of feline pancreatitis, how much importance was placed on the results of ultrasonography, which has since been shown to have poor agreement with Spec fPL results.8 On the other hand, there is only one reference interval available for the DGGR assay (8–26 U/L).11 In a study of 251 cats with suspicion of pancreatitis, the best agreement between the Spec fPL and DGGR assay was seen with cutoff values of ≥5.4 μg/L for Spec fPL and >34 U/L for the DGGR assay.11 However, in the course of the statistical evaluation, we checked this possible cutoff value and could not find a better agreement with the Spec fPL cutoff of ≥5.4 μg/L nor with the AI compared with the cutoff value of >26 U/L for the DGGR assay. Based on the results of this study, we were unable to determine whether there is a DGGR assay cutoff value that is more consistent with pancreatitis than the reference interval used in this study.
We chose to include cats with concurrent pancreatic neoplasia because those cases also had pancreatitis. To the authors' knowledge, there are no previous reports on the results of serum lipase measurements in cats with pancreatic neoplasia. A previous study in dogs using an assay that contained 1,2‐diglyceride as a substrate suggested that marked hyperlipasemia may be a noninvasive marker for pancreatic neoplasia.16 However, this does not seem to be the case based on these results; the values for the lipase assays, which were similar to the AI, did not discriminate between spontaneous pancreatitis and inflammation secondary to neoplastic growth in the pancreas.
The value of histopathology as a gold standard for the diagnosis of feline pancreatitis has been debated.9, 17 The two main limitations of histopathology are the possibility of false negative results because of missed lesions and the unknown clinical significance of pancreatic lesions. Because we evaluated all serially sectioned pancreata in a standardized fashion, the chances of missing lesions were lower than routine pancreatic biopsy. Because the clinical relevance of histopathologic lesions is frequently discussed in relation to feline pancreatitis, it is important to note that cats often present with concurrent inflammation in the liver, pancreas, and intestines (i.e. triaditis).18, 19 Clinical signs of cholangitis, pancreatitis, and enteritis are nearly impossible to distinguish, and it therefore seems almost pointless to try to attribute histopathologic findings of the pancreas to the corresponding clinical signs in the individual patient. Experimentally‐induced pancreatitis might address this problem; however, not only would this be unethical but also it is questionable whether experimentally‐induced pancreatitis reflects the clinical and pathologic findings of spontaneous pancreatitis. Despite its shortcomings, histopathology remains the most definitive diagnostic tool, and it is the authors' opinion that it is the best gold standard currently available for assessing feline pancreatic disease.
Our study had some limitations. Although the number of cats comprised the largest study population to date, 60 cats are a relatively small number for statistical evaluations, which are reflected in the relatively wide 95% CI in the calculation of sensitivity and specificity. All cats were terminally ill, which may have created to a bias toward a more severely diseased population. However, the same limitation would apply to the most frequently cited study characterizing feline pancreatitis by DeCock et al, making the histopathology results of the two studies comparable.1 The time interval between measurement of serum lipase and euthanasia of the cats was a maximum of 12 hours, which may be considered a further limitation. The onset of new pancreatic lesions during this time interval is possible and could theoretically explain some of the discrepancy between lipase results and histologic findings.
For the calculation of Cohen's kappa values and sensitivity and specificity, it was necessary to dichotomize (i.e. normal versus increased) the results of the AI and the serum lipase determinations. It is possible that this type of allocation of continuous scale values might underestimate a relationship in the dataset. Most probably, it is more realistic to look at Spec fPL and DGGR lipase activites as continuous variables that are surrogates for the degree of pancreatic cellular injury, than to dichotomize a test to absence or presence of disease. However, if we had not dichotomized test results we would have used an interpretation different from the cut offs provided by the manufacturer5 and currently used in clinical practice. To address this dilemma, we have added a logistic regression analysis (and AIC as a model selection criterion) in order to assess if AI 0 or 1 is better explained by variations in SpecfPL or the DGGR assay. Thus, giving the reader some idea whether SpecfPL or DGGR assay is closer linked to AI. Results indicate that the DGGR assay performs better than SpecfPL in terms of explaining the variability in the AI.
In summary, both lipase assays had a similar performance when compared to pancreatic histology. We feel that the DGGR assay is at least as useful as the Spec fPL assay and certainly more advantageous when cost is considered. Our results also indicate that it is impossible to use the results of a blood test for determining the presence or absence of pancreatitis without harvesting the whole pancreas in cats. Even when the entire pancreas is harvested, interpretation of diagnostic blood test results remains difficult because of the unknown relevance of minor histopathologic changes in the pancreas. Internists as well as manufacturers of diagnostic tests should acknowledge this shortcoming and be more cautious in their wording of specific test information.
Supporting information
Table S1. Results of cats with pancreatitis and neoplasia.
Acknowledgments
The study was not supported by a grant or other funding.
Conflict of Interest Declaration
Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
Results of this study were presented in parts as an oral research abstract at the 2013 American College of Veterinary Internal Medicine Forum in Seattle, WA.
The study was performed at the Clinic for Small Animal Internal Medicine, Vetsuisse Faculty, University of Zurich.
Footnotes
Lipase colorimetric for Roche Cobas Integra 800; Roche Diagnostics, Rotkreuz, Switzerland)
IDEXX GmbH Ludwigsburg, Germany
IBM SPSS v.21 for Mac OS X; IBM Corporation, New York, NY
Forman MA, Shiroma J, Armstrong PJ, Robertson JE, Buch J, (2009). Evaluation of Feline Pancreas‐Specific Lipase (Spec fPLTM) for the Diagnosis of Feline Pancreatitis. [ACVIM Abstract 165]. J Vet Intern Med 2009; 23: 733–734 (abstract)
References
- 1. De Cock HEV, Forman MA, Farver TB, Marks SL. Prevalence and histopathologic characteristics of pancreatitis in cats. Vet Pathol 2007;44:39–49. [DOI] [PubMed] [Google Scholar]
- 2. Hill RC, Van Winkle TJ. Acute necrotizing pancreatitis and acute suppurative pancreatitis in the cat. J Vet Intern Med 1993;7:25–33. [DOI] [PubMed] [Google Scholar]
- 3. Saunders HM, Van Winkle TJ, Drobatz K, et al. Ultrasonographic findings in cats with clinical, gross pathologic, and histologic evidence of acute pancreatic necrosis: 20 cases (1994–2001). J Am Vet Med Assoc 2002;221:1724–1730. [DOI] [PubMed] [Google Scholar]
- 4. Williams DA. Feline exocrine pancreatic disease In: Bonagura JD, Twedt DC, eds. Kirk's Current Veterinary Therapy XIV. St Louis, MO: Elsevier Saunders; 2009:538–543. [Google Scholar]
- 5. Simpson K, Shiroma J, Biller D, et al. Ante‐mortem diagnosis of pancreatitis in four cats. J Small Anim Pract 1994;35:93–99. [Google Scholar]
- 6. Forman MA, Marks SL, De Cock HEV, et al. Evaluation of serum feline pancreatic lipase immunoreactivity and helical computed tomography versus conventional testing for the diagnosis of feline pancreatitis. J Vet Intern Med 2004;18:807–815. [DOI] [PubMed] [Google Scholar]
- 7. Gerhardt A, Steiner JM, Williams DA, et al. Comparison of the sensitivity of different diagnostic tests for pancreatitis in cats. J Vet Intern Med 2001;15:329–333. [PubMed] [Google Scholar]
- 8. Oppliger S, Hartnack S, Reusch CE, et al. Agreement of serum feline pancreas‐specific lipase and colorimetric lipase assays with pancreatic ultrasonographic findings in cats with suspicion of pancreatitis: 161 cases (2008–2012). J Am Vet Med Assoc 2014;244:1060–1065. [DOI] [PubMed] [Google Scholar]
- 9. Armstrong PJ, Williams DA. Pancreatitis in cats. Topics Companion Anim Med 2012;27:140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Panteghini M, Bonora R, Pagani F. Measurement of pancreatic lipase activity in serum by a kinetic colorimetric assay using a new chromogenic substrate. Ann Clin Biochem 2001;38:365–370. [DOI] [PubMed] [Google Scholar]
- 11. Oppliger S, Hartnack S, Riond B, et al. Agreement of the serum Spec fPLTM and 1,2‐o‐dilauryl‐rac‐glycero‐3‐glutaric acid‐ (60‐methylresorufin) ester lipase assay for the determination of serum lipase in cats with suspicion of pancreatitis. J Vet Intern Med 2013;27:1077–1082. [DOI] [PubMed] [Google Scholar]
- 12. Newman SJ, Steiner JM, Woosley K, et al. Histologic assessment and grading of the exocrine pancreas in the dog. J Vet Diagn Invest 2006;18:115–118. [DOI] [PubMed] [Google Scholar]
- 13. Trivedi S, Marks SL, Kass PH, et al. Sensitivity and specificity of canine pancreas‐specific lipase (cPL) and other markers for pancreatitis in 70 dogs with and without histopathologic evidence of pancreatitis. J Vet Intern Med 2011;25:1241–1247. [DOI] [PubMed] [Google Scholar]
- 14. Steiner JM. Clinical utility of a new lipase activity assay. Vet Clin Pathol 2005;34:176–176. [DOI] [PubMed] [Google Scholar]
- 15. Arzoglou PL, Férard G, Métais P. Differentiating two forms of plasma lipase by use of media with different ionic strengths. Clin Chem 1984;30:360–363. [PubMed] [Google Scholar]
- 16. Quigley KA, Jackson ML, Haines DM. Hyperlipasemia in 6 dogs with pancreatic or hepatic neoplasia: Evidence for tumor lipase production. Vet Clin Pathol 2001;30:114–120. [DOI] [PubMed] [Google Scholar]
- 17. Xenoulis PG, Steiner JM. Canine and feline pancreatic lipase immunoreactivity. Vet Clin Pathol 2012;41:312–324. [DOI] [PubMed] [Google Scholar]
- 18. Simpson KW. Pancreatitis and triaditis in cats: Causes and treatment. J Small Anim Pract 2015;56:40–49. [DOI] [PubMed] [Google Scholar]
- 19. Weiss DJ, Gagne JM. Relationship between inflammatory hepatic disease and inflammatory bowel disease, pancreatitis, and nephritis in cats. J Am Vet Med Assoc 1996;209:1114–1116. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Results of cats with pancreatitis and neoplasia.


