Abstract
Background
Doberman Pinschers with dilated cardiomyopathy (DCM) are at high risk of sudden cardiac death (SCD). Risk factors for SCD are poorly defined.
Aim
To assess cardiac biomarkers, Holter‐ECG, echocardiographic variables and canine characteristics in a group of Doberman Pinschers with DCM dying of SCD and in a DCM control group to identify factors predicting SCD.
Methods/Animals
A longitudinal prospective study was performed in 95 Doberman Pinschers with DCM. Forty‐one dogs died within 3 months after the last cardiac examination (SCD‐group) and were compared to 54 Doberman Pinschers with DCM surviving 1 year after inclusion. Holter‐ECG, echocardiography, measurement of N‐terminal prohormone of brain‐natriuretic peptide (NT‐proBNP), and cardiac Troponin I (cTnI) concentrations were recorded for all dogs.
Results
Volume overload of the left ventricle (left ventricular end‐diastolic volume (LVEDV/BSA) > 91.3 mL/m²) was the single best variable to predict SCD. The probability of SCD increases 8.5‐fold (CI 0.95 = 0.8–35.3) for every 50 mL/m²‐unit increment in LVEDV/BSA. Ejection fraction (EF), left ventricular end‐systolic volume (LVESV/BSA) and NT‐proBNP were highly correlated with LVEDV/BSA (r = −0.63, 0.96, 0.86, respectively). Generated conditional inference trees (CTREEs) revealed that the presence of ventricular tachycardia (VT), increased concentration of cTnI, and the fastest rate (FR) of ventricular premature complexes (VPC) ≥260 beats per minute (bpm) are additional important variables to predict SCD.
Conclusion
Conditional inference trees provided in this study might be useful for risk assessment of SCD in Doberman Pinschers with DCM.
Keywords: 24‐hour ECG, Biomarker, cTnI, DCM, Doberman Cardiomyopathy, Echocardiography, Electrocardiography, NT‐proBNP, Survival
Abbreviations
- bpm
beats per minute
- BSA
body surface area
- CI
confidence interval
- cTnI
cardiac Troponin I
- CTREE
conditional inference tree
- CHF
congestive heart failure
- DCM
dilated cardiomyopathy
- EF
ejection fraction
- Holter‐ECG
24‐hour ECG
- LVEDV
left ventricular end‐diastolic volume
- LVESV
left ventricular end‐systolic volume
- NT‐proBNP
N‐terminal prohormone of brain‐natriuretic peptide
- VIS
variable importance score
- SCD
sudden cardiac death
- SD
standard deviation
- FR
fastest rate
- VPC
ventricular premature complexes
- VT
ventricular tachycardia
Dilated cardiomyopathy (DCM) is the most common cardiac disease of Doberman Pinschers.1, 2, 3 Prognosis of DCM in Doberman Pinschers is consistently poor.4, 5 The progression of this disease can be divided into three stages.4 Dogs in stage one exhibit no morphological changes and to date no clinical relevant instrument for diagnosis exists at this early stage. Stage two is the occult stage in which, from an owner's perspective, no clinical signs are apparent. This stage can be diagnosed using echocardiography, 24‐hour ECG (Holter‐ECG) and cardiac biomarkers.4, 6, 7, 8, 9 Doberman Pinschers in this stage have either ventricular arrhythmias, systolic dysfunction with or without a secondary left ventricular volume overload, or both, and about one‐third of the affected Doberman Pinschers die of sudden cardiac death (SCD).2, 3, 5, 7, 10 Dogs reaching the third stage have congestive heart failure (CHF)4. About one‐third of these dogs also die of SCD.4, 11 SCD is presumed to be caused by ventricular tachycardia (VT) which leads to ventricular fibrillation and death.5, 12, 13
In human medicine, left ventricular dysfunction measured by ejection fraction (EF) ≤30% is the most important measurement used for risk stratification to identify patients at high risk of dying of SCD.14, 15, 16 However, EF alone is insufficient to predict SCD, because many patients who die of SCD show no systolic dysfunction in the first place.16, 17 Hence, additional variables for risk evaluation are currently investigated, such as electrophysiology testing,18 signal‐averaged electrocardiography,19 cardiac magnetic resonance imaging,18 and measurement of cardiac biomarker concentrations.20, 21 Currently, there is no consensus or general recommendation which additional variables should be used, but a combination of cardiac magnetic resonance imaging and electrophysiology testing appears to be promising in human medicine field. However, any of the above methods are still critically discussed, so that further studies are warranted.18
In Doberman Pinschers suffering from DCM, only a very limited amount of research has been devoted to evaluate prognostic factors that can be helpful to predict SCD. In Doberman Pinschers with DCM, VT is suspected to be a risk for SCD.5, 6 Ventricular late potentials, detected by signal‐averaged electrocardiography, are also presumed to have prognostic value.22 Furthermore, anecdotally SCD is often associated with enlarged heart dimensions.10
Despite these findings, no clear framework of prognostic factors has been presented that could help to predict the probability of SCD in Doberman Pinschers. Identifying prognostic factors for SCD could help defining which patient might benefit most from antiarrhythmic or other treatment options. This might help to prevent unnecessary treatment, as many antiarrhythmic drugs are known to have either adverse effects or being pro‐arrhythmogenic.23, 24
Therefore, the main goal of this study was to evaluate several echocardiographic and Holter‐ECG variables, canine characteristics as well as cardiac Troponin I (cTnI) and N‐terminal prohormone of brain‐natriuretic peptide (NT‐proBNP) concentrations as possible predictors of SCD in Doberman Pinschers. Secondary objectives were to provide conditional inference trees (CTREEs) including several variables for clinicians to estimate the risk of SCD. The authors of this study hypothesized that the evaluation of Holter‐ECG, echocardiographic variables as well as the measurement of NT‐proBNP and cTnI concentrations would yield single variables, or pairs of variables, with significant prognostic value for assessing the probability of SCD of Doberman Pinschers suffering from DCM.
Materials and Methods
Animals
Ninety‐five privately owned purebred Doberman Pinschers suffering from occult or clinically overt DCM that were examined at the Cardiology Department of the Clinic of Small Animal Medicine, Ludwig‐Maximilians University, Munich between March 3, 2005 and February 28, 2014 for screening or treatment of DCM, were included. These dogs were selected from a larger cohort of Doberman Pinschers participating in a continuing prospective longitudinal study. Dogs were selected in accordance with inclusion and exclusion criteria as described below. This study complies with the regulations of the German Animal Welfare Act and owners provided written consent to participate in that study.
Inclusion criteria
Doberman Pinschers diagnosed with occult or clinically overt DCM and without clinical signs of another cardiac or systemic diseases were selected from the continuing prospective longitudinal study. Diagnosis of DCM was based on Holter‐ECG, echocardiographic criteria, or both. Each dog was brought in for regular follow‐up examinations every 3–6 months. Dogs that had their last examination no longer than 3 months before SCD were included in the SCD‐group and data from this last cardiac examination was used for statistical analysis. SCD was defined as cause of death when (1) death was sudden and instantaneous as the result of an unexpected collapse without evidence of dyspnea; or when (2) death occurred during sleep and no evidence of prior dyspnea was seen.5 Doberman Pinschers diagnosed with occult or clinically overt DCM surviving longer than 1 year post diagnosis were included in the control group. To identify suitable dogs, we queried our database for Doberman Pinschers that had survived more than 1 year after being diagnosed with DCM and for which a series of examinations was available. Hence, for statistical analysis, the data record from approximately 1 year before the most recent examination was selected to assure the stated time frame of greater than 1 year could be abided to. If Doberman Pinschers of the control group subsequently died after a period greater than 1 year, dogs remained in the control group nonetheless.
Exclusion criteria
Doberman Pinschers dying of SCD more than 3 months after their last examination were excluded from this study. Doberman Pinschers that died of other causes than SCD within 1 year after being diagnosed with DCM or were diagnosed with significant systemic diseases, congenital or acquired heart diseases other than DCM, were excluded too.
Examinations
Baseline clinical history was gathered with special attention to observed syncope or collapse episodes as well as signs of CHF. Information about sex, age, body weight, medication, and known systemic diseases were obtained. A complete general clinical examination, echocardiography, Holter‐ECG, and measurement of plasma NT‐proBNP‐, serum cTnI‐, urea‐, and creatinine concentrations were performed. Complete blood count analyses and chemistry screens were not routinely undertaken. However, further blood examination was conducted when the result of the general clinical examination was abnormal. In case of clinical signs of CHF X‐ray examinations were undertaken.
Echocardiography
Echocardiography was performed and assessed by cardiology residents or diplomates (GW, JS, PH, GT, PS) in right and left lateral recumbency on unsedated dogs using a 2.0/3.5 MHz transducer1 according to official recommendations.25 Applying Simpson`s method of disc, left ventricular end‐systolic volume (LVESV) and left ventricular end‐diastolic volume (LVEDV) were measured and values were normalized to body surface area (BSA). LVESV/BSA > 55 mL/m², LVEDV/BSA > 95 mL/m², or both were considered to be indicative of DCM.26 M‐mode measurements were performed, too. As Simpson`s method of disc is superior to M‐mode, those measurements were used for statistical analyses.26
Holter‐ECG
Holter‐ECG devices were attached to Doberman Pinschers for 24 hours. Over the course of the study, three Holter‐ECG analysis systems,2 , 3, 4 were used. Manual adjustments and accuracy verification of the arrhythmias recognized by the software were performed by veterinarians of the Cardiology Department with comprehensive experience in Holter analysis and supervision of a cardiology diplomate (GW). Table S1 illustrates the 26 Holter‐ECG variables that were used for statistical analysis. Diagnosis of DCM with Holter‐ECG was considered when (1) >100 ventricular premature complexes (VPC) within 24 hours; or (2) >50 VPC/24 hours diagnosed in two examinations within 12 months; or (3) ventricular couplet, triplet or VT were detected.5, 6, 27 Presence of >3 consecutive VPC and instantaneous ventricular rate during occurrence of these VPC >160 beats per minute (bpm) were criteria for VT.
Blood sampling and analysis
Frozen plasma (−70°C) was used to determine NT‐proBNP concentrations5 in batches as described previously9 and serum to determine cTnI concentrations6 (immediately—not in batched analyses). Both analyses were conducted by an external laboratory.7 Urea and creatinine concentrations were measured in‐house.8 Concentrations of NT‐proBNP and cTnI were only used for further statistical analysis, if urea and creatinine concentrations were within normal limits to avoid falsely increased values of NT‐proBNP and cTnI.
Cardiac treatment
Dogs with systolic dysfunction were treated with pimobendan9 and dogs with CHF received furosemide10 in addition. Sotalol hydrochloride,11 amiodarone hydrochloride,12 mexiletine,13 metildigoxin,14 carvedilol,15 and ramipril16 were administered to dogs with several malignancy criteria in the Holter‐ECG, such as having couplets, triplets, or VT.
Statistical Analysis
Statistical analyses were performed using commercially available statistics software programs.17, 18 For all statistical evaluations, a probability of P < .05 was regarded as statistically significant. Continuous variables were depicted descriptively as median (interquartile range) for non‐normally distributed variables or mean (±standard deviation (SD)) for normally distributed variables. With categorical data the frequency of occurrence was determined. A CTREE was built to illustrate the association of prognostic factors and SCD in a non‐parametric manner.28 All displayed p‐values are Bonferroni‐corrected. Random Forest method was used to create variable importance scores (VIS) for all analyzed variables. The VIS were established to determine the importance of variables more precisely.28 This method of evaluation randomly generated 10,000 CTREEs from the original data, each constructed on a certain randomization of variables and data points. A Bravais‐Pearson correlation coefficient was then calculated to detect correlations between the identified metric variables with a high VIS. To estimate the influence of measured variables on SCD a multiple logistic regression model was used. To ensure stability of logistic regression estimates, the number of covariates had to be limited with respect to the number of observations. Therefore, the model size was restricted to six covariates, which were selected on the basis of VIS, correlation between the variables, and expert opinion (GW). Thereafter, a coefficient plot was generated that contained variables with (1) a high VIS but without statistical significance in the regression analysis or with (2) statistical significance in the regression analysis. The result is depicted as hazard ratio with 95% confidence interval (CI). Although confidence intervals (CIs) provided by the logistic regression do not account for uncertainty of the proceeding model selection procedure and are thus not based on correct theoretical limits, the intervals can be used to access a measure of variability for calculated regression estimates. In line with the definition of statistical significance based on CIs, a particular importance is given by the variables, for which the corresponding CI does not cover the value zero. The whole statistical evaluation was then repeated after excluding all Doberman Pinschers with CHF.
Results
Animals
Overall, 120 Doberman Pinschers in the occult (n = 103) or overt (n = 17) stage of DCM were examined. Nineteen Doberman Pinschers experiencing SCD more than 3 months after the last examination and six dogs that died within 12 months not due to SCD after the cardiac examination were excluded (Fig 1). Ninety‐five Doberman Pinschers (39 female and 56 male, mean age 7.4 ± 2.2 years, mean body weight 36.7 ± 5.3 kg) were included. Forty‐one Doberman Pinschers (9 female, 32 male, mean age at the last examination 7.6 ± 2.1 (minimum 3.4, maximum 12.5) years, mean body weight 36.8 ± 4.7 kg) died of SCD within 3 months after the last examination (SCD‐group). Fifty‐four Doberman Pinschers (30 female, 24 male, 7.3 ± 2.2 years, 36.7 ± 5.7 kg) survived the end of the follow‐up period (control group). Group allocation is depicted in Fig 1.
Figure 1.
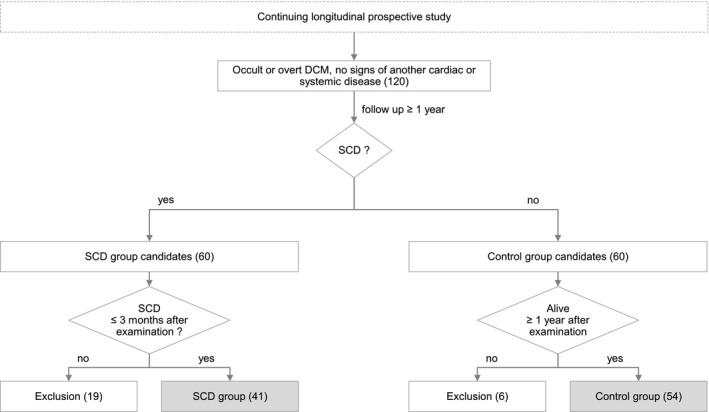
Flowchart to demonstrate the group allocation. Forty‐one Doberman Pinschers with DCM that died within 3 months after their last cardiac examination of SCD (SCD‐group) were compared to 54 Doberman Pinschers with DCM surviving 1 year after their cardiac examination (control group). DCM, dilated cardiomyopathy; SCD, sudden cardiac death.
CHF and syncope events
At the time of the last cardiac examination 14/41 (34%) Doberman Pinschers that experienced SCD were diagnosed with CHF. All dogs with CHF were treated with furosemide and were showing no signs of CHF. None of the dogs in the control group was diagnosed with CHF at the time of the cardiac examination. According to the history, 7/95 (7%) dogs (4 cases in SCD‐group, 3 cases in control group) showed a syncope before the cardiac examination.
Medical treatment
By the time of the cardiac examination 66/95 (70%) Doberman Pinschers had already received medical treatment. After the cardiac examination additional 19/95 (20%) Doberman Pinschers were started on medical treatment. Accordingly, 85/95 (90%) Doberman Pinschers were treated with cardiac medication after the cardiac examination (Table 1). Antiarrhythmic drugs were only prescribed if several malignancy criteria were fulfilled, such as having >50 couplets, triplets, VT, or a fastest instantaneous rate (FR) of VPC >280 bpm.
Table 1.
Cardiac medication that Doberman Pinschers received by the time of SCD (SCD‐group) or after their last cardiac examination (control group), respectively. SCD, sudden cardiac death
| n (%) | ||
|---|---|---|
| Control group | SCD‐group | |
| Cardiac medication | 47 (87) | 38 (93) |
| Antiarrhythmics | 25 (46) | 27 (66) |
| Sotalol Hydrochloride | 15 (28) | 15 (37) |
| Amiodarone Hydrochloride | 8 (15) | 8 (20) |
| Carvedilol | 1 (2) | 1 (2) |
| Metildigoxin | 1 (2) | 2 (5) |
| Mexiletine | 0 (0) | 1 (2) |
| Pimobendan | 25 (46) | 34 (83) |
| Ramipril | 29 (54) | 29 (71) |
| Furosemide | 0 (0) | 14 (34) |
Examinations and conditional inference trees
Results of echocardiographic measurements and 26 elected Holter‐ECG variables for the SCD‐ and control group are shown, respectively, in Table 2 and in Table S2. The following mean (±SD) and median [interquartile range] concentrations of NT‐proBNP and cTnI were detected: NT‐proBNP (pmol/L): SCD‐group (n = 35): 4,165 (±3,070) and 3,030 [2,196–5,550]; control group (n = 29): 1,149 (±906) and 901 [430–1,712]. Cardiac Troponin I (ng/mL): SCD‐group (n = 20): 0.722 (±0.487) and 0.521 [0.397–0.864]; control group (n = 41): 0.286 (±0.227) and 0.268 [0.010–0.472]. Because of a mild increase in urea and creatinine concentrations in four dogs, NT‐proBNP and cTnI concentrations from these dogs were not considered for statistical analysis. Over the course of the study, these dogs did not develop any clinical signs or worsening of kidney function. Furthermore, 11 dogs (SCD‐group: n = 7; control group: n = 4) of this study had stabilized hypothyroidism and received optimal medical treatment for this reason.
Table 2.
Echocardiographic values of SCD‐ and control group. Data of the SCD‐group are from the examination after that the dog died of SCD (within 3 month). Data of the control group presented are from the examination after which the dog was still alive at least 1 year
| Control group | SCD‐group | |||
|---|---|---|---|---|
| Mean (±SD) | Range | Mean (±SD) | Range | |
| LVEDV/BSA (mL/m²) | 91.4 (22.5) | 56.9–143.3 | 137.3 (44.3) | 70.1–264.5 |
| LVESV/BSA (mL/m²) | 53.0 (20.3) | 25.6–136.4 | 94.1 (44.8) | 30.5–202.9 |
| LA/Ao | 1.40 (0.17) | 1.04–1.70 | 1.66 (0.49) | 1.11–2.75 |
| EF (%) | 43 (10) | 5–61 | 34 (12) | 6–57 |
EF, ejection fraction; LVEDV/BSA and LVESV/BSA, left ventricular end‐diastolic and end‐systolic volume normalized to body surface area; LA/Ao, short‐axis ratio of diastolic left atrial diameter to aortic root diameter; SCD, sudden cardiac death; SD, standard deviation.
For CTREE computations, the following variables were considered: all Holter‐ECG variables, echocardiographic values (LVEDV/BSA, LVESV/BSA, EF, LA/Ao), concentrations of NT‐proBNP and cTnI, sex, age, body weight, and information about CHF, syncopes, and administered medication. CTREEs detected the following variables having prognostic value for predicting SCD: LVEDV/BSA, cTnI concentration, incidence of VT, antiarrhythmic treatment yes/no, and FR of all VPC. The term “FR of all VPC” refers to the highest FR from all detected forms of VPC (regardless of single VPC, couplet, triplet, or VT).
Selected variables found to be significant in predicting SCD are depicted in Fig 2a and b (CTREE A and B). In dogs with cardiac enlargement (LVEDV/BSA > 91.3 mL/m2; P < .001) analysis determined that FR of all VPC >260 bpm (P = .011) was the best predictor of SCD, as 82% (28/34) of the dogs with FR of all VPC >260 bpm died of SCD within 3 months (Fig 2a). According to our antiarrhythmic treatment regimen, dogs with incidence of VT and dogs with FR of all VPC >280 bpm received antiarrhythmic treatment, if necessary. Accordingly, Doberman Pinschers with a higher presumed SCD risk received antiarrhythmic treatment and precisely those (88% (7/8)) dogs died of SCD more frequently than Doberman Pinschers where a lower SCD risk was presumed and which had not received antiarrhythmic treatment (18% (3/17)). However, CTREE analysis did not identify more prognostic variables for those dogs.
Figure 2.

Conditional inference trees to demonstrate the relationship of prognostic factors and SCD in a nonparametric manner. (A) shows variables and cut‐off values based on which Doberman Pinschers that died of SCD within 3 months after cardiac examination could be determined. (B) illustrates the CTREE for Doberman Pinschers after exclusion of decompensated dogs. CTnI, cardiac Troponin I; CTREE, conditional inference tree; FR, fastest rate; LVEDV/BSA, left ventricular end‐diastolic volume/body surface area; SCD, sudden cardiac death; VPC, ventricular premature complexes; VT, ventricular tachycardia.
Figure 2b shows variables and cut‐off values predicting SCD when dogs with CHF (n = 14) are excluded from statistical analysis. Dogs with cardiac enlargement (LVEDV/BSA > 91.3 mL/m²) and cTnI (P = .019) >0.34 ng/mL died of SCD with a probability of 72% (23/32). Dogs presented with LVEDV/BSA > 91.3 mL/m² and cTnI ≤ 0.34 ng/mL died of SCD with a probability of 8% (1/13). Dogs with DCM but normal cardiac size (LVEDV/BSA ≤ 91.3 mL/m²), and with incidence of VT (P = .035) in the Holter‐ECG, died of SCD with a probability of 33% (3/9). In all dogs with VT, VT did occur intermittently.
Random forest variable importance score
After randomly generating 10,000 CTREEs from the original data, the following variables with the highest VIS could be determined by the Random Forest method: LVEDV/BSA (VIS = 0.0276), LVESV/BSA (VIS = 0.0257), and NT‐proBNP (VIS = 0.0209) (Fig 3a). Figure 3b depicts the VIS for the SCD‐group after excluding Doberman Pinschers with CHF.
Figure 3.
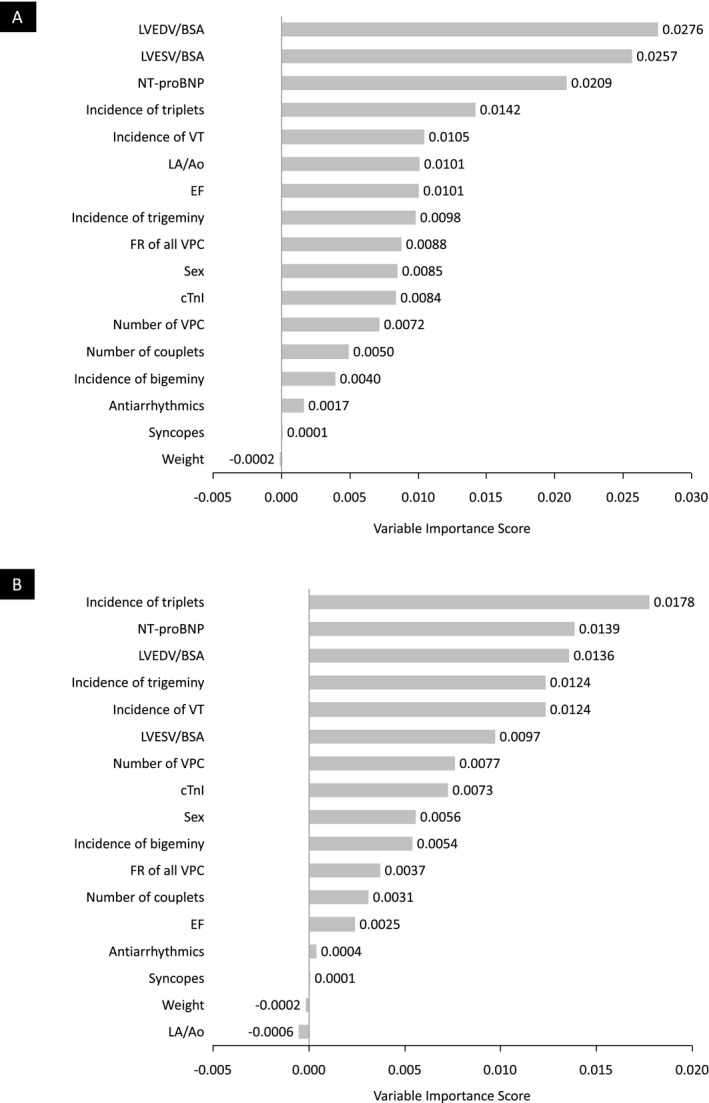
Random Forest Variable Importance Score for all analyzed variables. (A) shows the VIS for Doberman Pinschers with DCM that died within 3 months after their last cardiac examination of SCD (SCD‐group). (B) shows the VIS for the SCD‐group after excluding Doberman Pinschers with CHF. CHF, congestive heart failure; CTnI, cardiac Troponin I; EF, ejection fraction; FR, fastest rate; LA/Ao, short‐axis ratio of diastolic left atrial diameter to aortic root diameter; LVEDV/BSA, left ventricular end‐diastolic volume/body surface area; LVESV/BSA, left ventricular end‐systolic volume/body surface area; NT‐proBNP, N‐terminal prohormone of brain‐natriuretic peptide; SCD, sudden cardiac death; VIS, variable importance score; VPC, ventricular premature complexes; VT, ventricular tachycardia.
Multiple logistic regression analysis
The Bravais‐Pearson correlation test revealed strong correlations between the following variables: LVEDV/BSA, LVESV/BSA, EF, LA/Ao, and NT‐proBNP concentration. LVEDV/BSA was representatively used for the regression analysis as this variable has the highest predictive value judging by the VIS. VIS and expert opinion (GW) constituted further criteria for selecting variables with expert opinion (GW) being based on relevant veterinary literature5, 10, 23 and on personal experience. The following variables were selected for regression analysis: LVEDV/BSA, incidence of triplets, incidence of VT, FR of all VPC, sex, and total number of VPC. The statistical evaluation revealed LVEDV/BSA as the sole statistically significant variable for identifying Doberman Pinschers suffering from DCM that are at high risk of SCD. LVEDV/BSA showed statistical significance in both evaluated groups (SCD‐group: P = .00079, SCD‐group without CHF: P = .0024) (Table S3). In addition, the multiple logistic regression analysis was conducted using NT‐proBNP concentration as the representative variable. In this context, the statistical analysis calculated NT‐proBNP concentration as the sole statistically significant variable (SCD‐group: P = .00439, SCD‐group without CHF: P = .00472).
A coefficient plot depicting the multiplier effect of different variables on SCD risk and the effect of incremental modifications of continuous variables is seen in Fig 4 and the exact values of the variables are shown in Table S4. Altering LVEDV/BSA by 50 mL/m² increased SCD risk 8.5‐fold (CI0.95 = 2.8–35.3). Interestingly, several variables showed a clear tendency toward an increased SCD risk but were not found to be statistically significant (eg, accelerating “FR of all VPC” by 80 bpm increased SCD risk 2.0‐fold (CI0.95 = 0.7–6.7)). A coefficient plot that was calculated after exclusion of decompensated Doberman Pinschers shows similar results.
Figure 4.
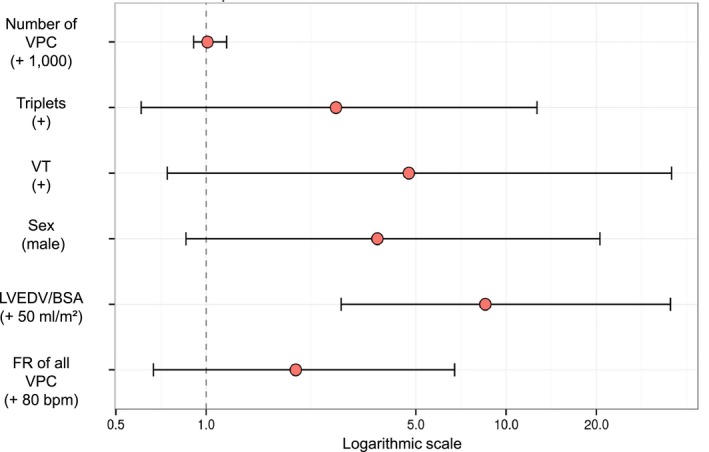
Coefficient plot to illustrate the multiplier effect of different variables on SCD risk (hazard ratio with 95% confidence interval). Displayed variables are 50 mL/m²—unit increment in LVEDV/BSA, and variables that showed a high VIS but had no statistical significance in regression analysis: an alteration of 1,000 VPC in the total number of VPC, an increase of 80 bpm in FR of all VPC; incidence of triplets; incidence of VT; and male sex. Bpm, beats per minute; FR, fastest rate; LVEDV/BSA, left ventricular end‐diastolic volume/body surface area; SCD, sudden cardiac death; VIS, variable importance score; VPC, ventricular premature complexes; VT, ventricular tachycardia.
Discussion
This prospective study examined several variables with the potential to predict SCD in Doberman Pinschers suffering from DCM. A single variable, LVEDV/BSA, was found to be statistically significant in predicting SCD in this population of dogs. Distinguishing from other studies this current study draws on a greater population of Doberman Pinschers with DCM (both in the SCD‐ and control group) and comprises a high number of analyzed variables. A special statistical software18 calculated CTREEs using all available variables, combined them in various ways, and provided cut‐off values for the chosen variables. These cut‐off values were calculated autonomously by the software and were not predetermined. These CTREEs might be very useful for clinicians to assess the risk of SCD. Several other authors also investigated the predictive value of different variables for assessing the SCD risk of Doberman Pinschers suffering from DCM. However, most of the previously existing studies determined a combination of CHF and SCD as terminal point, drew on a rather small population, did not compile cut‐off values, or the control group was not based on Doberman Pinschers with DCM.5, 7, 11, 29, 30
LVEDV/BSA was identified as the best statistically significant variable for assessing SCD risk where every 50 mL/m² increment increases SCD risk 8.5‐fold (CI0.95 = 2.8–35.3). In accordance with this finding, other studies similarly reported that Doberman Pinschers with DCM that died of SCD or CHF had enlarged hearts.5, 29 A recent study showed that treatment of Doberman Pinschers in the occult stage of DCM with pimobendan delayed the occurrence of CHF or SCD by 9 months.30 The effect on SCD may be explained by the fact that pimobendan reduces cardiac size, although this assumption warrants further examination.30 LVEDV/BSA > 91.3 mL/m² was statistically suggested as a cut‐off value for identifying Doberman Pinschers at high risk of dying of SCD. This cut‐off value is very similar to the previously published upper cut‐off value of Simpson's method of disc for Doberman Pinschers to diagnose DCM.26
Due to the strong correlation between the variables LVEDV/BSA, LVESV/BSA, LA/Ao, EF, and NT‐proBNP, only one of these variables was considered for the multiple logistic regression analysis. LVEDV/BSA was selected for this purpose, but can be regarded representative of the other strongly correlated variables in the context of this study. For this reason, the term “enlarged heart” is used below referring to all five variables. In human medicine, severe left ventricular systolic dysfunction—recognized by measuring EF—represents the core variable for assessing SCD risk.14, 15, 16 This further confirms the findings of our study that an enlarged heart can be used as prognostic variable for assessing SCD risk. EF was also included in the CTREE‐ and Random Forest VIS method, but LVEDV/BSA was actually superior to EF in predicting SCD and was therefore selected for regression analysis. NT‐proBNP concentration has a high predictive value but was not considered for further analysis because of its strong correlation with LVEDV/BSA as well. However, measurement of NT‐proBNP concentration could be a suitable alternative when echocardiography is not available, as it can predict cardiac enlargement.9, 31
Sudden cardiac death risk for Doberman Pinschers with normal cardiac size (LVEDV/BSA ≤ 91.3 mL/m²), but with ventricular arrhythmias, can be assessed using Holter‐ECG evaluation. In our study population Doberman Pinschers with DCM, but normal cardiac size and with VT in the Holter‐ECG carry a higher risk of dying of SCD. For those dogs, evaluating FR of all VPC could further improve SCD risk assessment (Fig 4). Existing presumptions of other authors, that sustained VT can be used to identify Doberman Pinschers at high risk of dying of SCD, could thus be confirmed.5, 12, 29 It must be pointed out though, that no distinction between sustained and nonsustained VT was made in the scope of this study. In human medicine, several authors also act on the assumption that SCD risk can be assessed by means of evaluating nonsustained VT in combination with EF, which corroborates our findings.15, 32 Furthermore, a randomized trial showed that human patients with non‐sustained VT, low EF, and inducible VT, benefited from an implantable cardioverter defibrillator.33
Doberman Pinschers with DCM and cardiac enlargement can also have ventricular arrhythmias at the same time. Additional measurement of FR of all VPC in the Holter‐ECG could improve SCD risk assessment for such dogs. Presumptions exist that certain arrhythmias could be electrically instable and that rapid arrhythmias increase the risk of developing ventricular fibrillation.34 For this reason, some authors recommend antiarrhythmic treatment for Doberman Pinschers when diagnosing arrhythmias characterized by a high ventricular rate.10, 23
The total number of VPC was found statistically not significant in risk assessment for SCD and furthermore showed no tendency in either direction when being altered by 1,000 VPC (Fig 4). This could lead to the assumption that the total number of VPC does not influence SCD risk. Our findings thus contradict the presumption of various authors that a high number of VPC represents a malignancy criterion and that antiarrhythmic treatment should be commenced upon occurrence of a critical number of VPC.10, 23
According to CTREE A (Fig 2a), Doberman Pinschers of this study population that received antiarrhythmic treatment were at higher risk of dying of SCD than Doberman Pinschers not receiving antiarrhythmic treatment. This result could be interpreted as antiarrhythmic treatment being actually harmful to the patient. However, according to our antiarrhythmic treatment regimen described above, these observations could suggest that Doberman Pinschers that had received antiarrhythmic treatment died of SCD due to malignant arrhythmias and not because they had received antiarrhythmic treatment. But further studies are required to evaluate these presumptions. Other studies draw similar conclusions; namely that dogs suffering from arrhythmias are at higher risk of dying of SCD.5, 12, 29 Furthermore, some authors report about retarded SCD29 or reductions in severity of arrhythmia35 for dogs that received antiarrhythmic treatment. It could also be argued that antiarrhythmic drugs are potentially pro‐arrhythmogenic and therefore increased the SCD risk, as in human medicine. For example, the Cardiac Arrhythmia Suppression Trial (CAST) was a groundbreaking, double‐blind, randomized study which demonstrated that suppression of ectopy and nonsustained VT after myocardial infarction with antiarrhythmic drug treatment (Vaughan Williams classification type 1c) increased mortality in this population.36 However, Doberman Pinschers with malignant arrhythmias not receiving antiarrhythmic treatment had shorter median survival times compared to Doberman Pinschers receiving treatment.29 Therefore, our interpretation of the fact that Doberman Pinschers receiving antiarrhythmic drugs were at a higher risk of dying of SCD was more likely to be influenced by the preselection of dogs with more malignant arrhythmias to receive treatment.
Another interesting result of this study is that cTnI concentrations are useful for risk assessment of SCD in Doberman Pinschers with an enlarged heart. This result corresponds with previous studies reporting cTnI concentration measurement as a useful additional test to Holter‐ECG and echocardiography.8 Some authors recommend measuring cardiac biomarkers in humans for SCD risk assessment, because high cardiac biomarker concentrations were found to predict SCD in their studies populations.20, 21
Conditional inference trees are constructed in a greedy manner, which means that the statistical software picks the most important variable for each step. Variables that have a high correlation and thus provide similar information are therefore not showing up in the CTREEs, as adding another nodal point would not result in additional information. The Random Forest method for calculation of the VIS overcomes this limitation being based on 10,000 CTREEs, each constructed on a certain randomization of variables and data points. As an enlarged heart is the most important predictor of SCD in our study, the echocardiographic variables LVEDV/BSA, LVESV/BSA, EF, and NT‐proBNP also have a high VIS. Although the CTREEs selected VT and FR of all VPC as the most important arrhythmia variables, triplets, and trigeminy also have a considerably high VIS. For couplets and triplets, malignancy criteria are reported in the literature6, whereas in the author`s best knowledge presence of trigeminy was not reported as a potential predictor in the veterinary literature. In human patients with frequent VT also VPC, particularly numerous bigeminy and trigeminy, have been reported37 and thus incidence of trigeminy is considered as an indicator for occurrence of malignant VT.
Evaluation of VIS found male sex as higher risk for SCD. Reports of increased SCD occurrence in male versus female Doberman Pinschers are also found in veterinary literature.5 The VIS for “male sex” could be attributed to the fact that male dogs develop earlier and more often the classical DCM type with echocardiographic detectable changes of cardiac enlargement1 and therefore higher risk for SCD.
Limitations
There are several limitations, which could have influenced the results of this study. The assignment of patients to the SCD‐group or of having previously a syncope, was based on potentially subjective information provided by the canine owners. Complete blood examination was not performed in every dog and therefore systemic diseases and following potential influences on our study results cannot be entirely excluded. In addition, it has to be stated that the results of CTREEs should not be overestimated as the CTREEs specifically account for our study population due to the high variance of tree‐based algorithms. Other populations could result in different CTREEs. However, since our study included a large number of Doberman Pinschers dying of SCD, the computed CTREEs are most likely valid for risk assessment also in other Doberman Pinschers populations. Another limitation is the lack of a full cardiac examination, including Holter‐ECG, of all dogs at the exact day of SCD. Only dogs that had died within 3 months after the last examination were included in the SCD‐group to retain a comparatively large population and at the same time have a quite short interval between the last examination and SCD. Because this time elapsed between examination and SCD, other causes (also noncardiac) could have affected the results. Furthermore, it cannot be excluded that the disease progressed and that the dogs dying of SCD had even more malignant arrhythmias. Therefore, the results of this study can only provide an approximation of what data might have been obtained immediately before SCD. The variables used in the study to predict SCD are considerably time‐varying. We made the assumption that the best time point for comparison for the control group would be the time point, where at least a 12‐month follow‐up is available. If this time point or a time point 3 month before the last examination would be more appropriate is a matter of statistical debate. Repeated measurements per dog could be used to conduct survival analysis including time‐varying covariates and thereby possibly leading to more accurate predictions. As not enough repeated measurements per dog are available, our analysis is limited in its prognostic statements. Another limitation is related to the inhomogeneous medical treatment regime and to the not actually proven malignancy criteria, which were used to decide which dog was treated with antiarrhythmics and which not.
Conclusion
An enlarged heart, represented by a LVEDV/BSA > 91.3 mL/m2, was found to be the most important and single statistically significant variable to identify Doberman Pinschers with DCM carrying a high risk to die of SCD. The probability of SCD occurrence increases 8.5‐fold for each 50 mL/m²‐unit increment of LVEDV/BSA. Other variables were not statistically significant in the multiple logistic regression analysis, but incidence of VT, an increased cTnI concentration, and a FR of all VPC >260 bpm might be additional prognostic markers for SCD risk assessment according to CTREE analysis.
Conflict of Interest Declaration
Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
Supporting information
Table S1. List of 26 Holter‐ECG variables that were used for analysis.
Table S2. Results of 26 Holter‐ECG variables in the SCD‐ and control group expressed as mean ± standard deviation, median [interquartile range], or frequencies (%).
Table S3. Results of the multiple logistic regression analysis.
Table S4. Values of the coefficient plot (hazard ratio with 95% CI).
Acknowledgment
The study was not supported by a grant.
Footnotes
Vivid 7 dimensions; General Electric Medical Systems, Waukesha, WI
Custo tera; Arcon Systems GmbH, Starnberg, Germany
Amedtech ECGpro Holter software, EP 810 digital Recorder; Medizintechnik Aue GmbH, Aue, Germany
Trillium Platinum Holter Analysis Software, Trillium 5000 Holter Recorder; Forest Medical, East Syracuse, NY
Cardiopet proBNP test; IDEXX Laboratories, Ludwigsburg, Germany
Immulite 2000 troponin I test; Siemens Healthcare Diagnostics, Eschborn, Germany
IDEXX Laboratories, Ludwigsburg, Germany
Cobas Integra 400 plus; Roche Diagnostics, Rotkreuz, Switzerland
Vetmedin®; Boehringer Ingelheim, Ingelheim, Germany
Dimazon®; MSD Animal Health GmbH, Unterschleißheim, Germany
Sotalol‐ratiopharm®; Ratiopharm GmbH, Ulm, Germany
Amiodaron 200®; 1 A Pharma GmbH, Oberhaching, Germany
Ritalmex 200®; Valeant Pharmaceuticals International Inc., Laval, QC
Lanitop®; Riemser Pharma GmbH, Berlin, Germany
Dilatrend®; Roche Deutschland Holding GmbH, Grenzach, Germany
Vasotop®; MSD Animal Health GmbH, Unterschleißheim, Germany
PASW Statistics, Version 18.0; IBM Corporation, Armonk, NY
RStudio, Version 3.1.2, Boston, MA
References
- 1. Wess G, Schulze A, Butz V, et al. Prevalence of dilated cardiomyopathy in Doberman Pinschers in various age groups. J Vet Intern Med 2010;24:533–538. [DOI] [PubMed] [Google Scholar]
- 2. O'Grady MR, Horne R. The prevalence of dilated cardiomyopathy in doberman pinschers: a 4.5 year follow‐up (abstract). J Vet Intern Med 1998;12:199. [Google Scholar]
- 3. Calvert CA. Dilated congestive cardiomyopathy in doberman pinschers. Comp Cont Educ Pract 1986;8:417–430. [Google Scholar]
- 4. O'Grady MR, O'Sullivan ML. Dilated cardiomyopathy: an update. Vet Clin Small Anim Pract 2004;34:1187–1207. [DOI] [PubMed] [Google Scholar]
- 5. Calvert CA, Hall G, Jacobs G, et al. Clinical and pathologic findings in Doberman pinschers with occult cardiomyopathy that died suddenly or developed congestive heart failure: 54 cases (1984–1991). J Am Vet Med Assoc 1997;210:505–511. [PubMed] [Google Scholar]
- 6. Calvert CA, Jacobs G, Pickus CW, et al. Results of ambulatory electrocardiography in overtly healthy Doberman Pinschers with echocardiographic abnormalities. J Am Vet Med Assoc 2000;217:1328–1332. [DOI] [PubMed] [Google Scholar]
- 7. Singletary GE, Morris NA, Lynne O'Sullivan M, et al. Prospective evaluation of NT‐proBNP assay to detect occult dilated cardiomyopathy and predict survival in Doberman Pinschers. J Vet Intern Med 2012;26:1330–1336. [DOI] [PubMed] [Google Scholar]
- 8. Wess G, Simak J, Mahling M, et al. Cardiac troponin I in Doberman Pinschers with cardiomyopathy. J Vet Intern Med 2010;24:843–849. [DOI] [PubMed] [Google Scholar]
- 9. Wess G, Butz V, Mahling M, et al. Evaluation of N‐terminal pro‐B‐type natriuretic peptide as a diagnostic marker of various stages of cardiomyopathy in Doberman Pinschers. Am J Vet Res 2011;72:642–649. [DOI] [PubMed] [Google Scholar]
- 10. Calvert CA, Meurs KM. Cardiomyopathy in Doberman Pinschers In: Bonagura JD, Twedt DC, eds. Current veterinary therapy. St. Louis: Saunders Elsevier; 2009:800–803. [Google Scholar]
- 11. Calvert CA, Pickus CW, Jacobs GJ, et al. Signalment, survival, and prognostic factors in Doberman pinschers with end‐stage cardiomyopathy. J Vet Intern Med 1997;11:323–326. [DOI] [PubMed] [Google Scholar]
- 12. Rush JE, Keene BW. ECG of the month. The sudden death of a dog with dilatative cardiomyopathy. J Am Vet Med Assoc 1989;194:52–53. [PubMed] [Google Scholar]
- 13. Zipes DP, Camm AJ, Borggrefe M, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. J Am Coll Cardiol 2006;48:e247–e346. [DOI] [PubMed] [Google Scholar]
- 14. Chugh SS. Early identification of risk factors for sudden cardiac death. Nat Rev Cardiol 2010;7:318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grimm W, Christ M, Bach J, et al. Noninvasive arrhythmia risk stratification in idiopathic dilated cardiomyopathy: results of the Marburg Cardiomyopathy Study. Circulation 2003;108:2883–2891. [DOI] [PubMed] [Google Scholar]
- 16. Solomon SD, Zelenkofske S, McMurray JJ, et al. Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N Engl J Med 2005;352:2581–2588. [DOI] [PubMed] [Google Scholar]
- 17. Stecker EC, Vickers C, Waltz J, et al. Population‐based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two‐year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol 2006;47:1161–1166. [DOI] [PubMed] [Google Scholar]
- 18. Zaman S, Kovoor P. Sudden cardiac death early after myocardial infarction: pathogenesis, risk stratification, and primary prevention. Circulation 2014;129:2426–2435. [DOI] [PubMed] [Google Scholar]
- 19. Gomes JA, Cain ME, Buxton AE, et al. Prediction of long‐term outcomes by signal‐averaged electrocardiography in patients with unsustained ventricular tachycardia, coronary artery disease, and left ventricular dysfunction. Circulation 2001;104:436–441. [DOI] [PubMed] [Google Scholar]
- 20. Berger R, Huelsman M, Strecker K, et al. B‐type natriuretic peptide predicts sudden death in patients with chronic heart failure. Circulation 2002;105:2392–2397. [DOI] [PubMed] [Google Scholar]
- 21. Galante O, Amit G, Zahger D, et al. B‐type natruiretic peptide levels stratify the risk for arrhythmia among implantable cardioverter defibrillator patients. Clin Cardiol 2008;31:586–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Calvert CA, Jacobs GJ, Kraus M. Possible ventricular late potentials in Doberman pinschers with occult cardiomyopathy. J Am Vet Med Assoc 1998;213:235–239. [PubMed] [Google Scholar]
- 23. Kraus MS, Thomason JD, Fallaw TL, et al. Toxicity in Doberman Pinchers with ventricular arrhythmias treated with amiodarone (1996–2005). J Vet Intern Med 2009;23:1–6. [DOI] [PubMed] [Google Scholar]
- 24. Waldo AL, Camm AJ, deRuyter H, et al. Effect of d‐sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. The SWORD Investigators. Survival With Oral d‐Sotalol. Lancet 1996;348:7–12. [DOI] [PubMed] [Google Scholar]
- 25. Thomas WP, Gaber CE, Jacobs GJ, et al. Recommendations for standards in transthoracic two‐dimensional echocardiography in the dog and cat. Echocardiography Committee of the Specialty of Cardiology, American College of Veterinary Internal Medicine. J Vet Intern Med 1993;7:247–252. [DOI] [PubMed] [Google Scholar]
- 26. Wess G, Mäurer J, Simak J, et al. Use of Simpson's method of disc to detect early echocardiographic changes in Doberman Pinschers with dilated cardiomyopathy. J Vet Intern Med 2010;24:1069–1076. [DOI] [PubMed] [Google Scholar]
- 27. Geraghty N, Wess G. Vergleich verschiedener Holterkriterien zur Diagnose des arrhythmischen Stadiums der dilatativen Kardiomyopathie beim Dobermann. In: Tierärztliche Fakultät der LMU München; 2011:1–107.
- 28. Strobl C, Malley J, Tutz G. An introduction to recursive partitioning: rationale, application, and characteristics of classification and regression trees, bagging, and random forests. Psychol Methods 2009;14:323–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Calvert CA, Brown J. Influence of antiarrhythmia therapy on survival times of 19 clinically healthy Doberman pinschers with dilated cardiomyopathy that experienced syncope, ventricular tachycardia, and sudden death (1985–1998). J Am Anim Hosp Assoc 2004;40:24–28. [DOI] [PubMed] [Google Scholar]
- 30. Summerfield NJ, Boswood A, O'Grady MR, et al. Efficacy of pimobendan in the prevention of congestive heart failure or sudden death in Doberman Pinschers with preclinical dilated cardiomyopathy (the PROTECT Study). J Vet Intern Med 2012;26:1337–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Oyama MA, Sisson DD, Solter PF. Prospective screening for occult cardiomyopathy in dogs by measurement of plasma atrial natriuretic peptide, B‐type natriuretic peptide, and cardiac troponin‐I concentrations. Am J Vet Res 2007;68:42–47. [DOI] [PubMed] [Google Scholar]
- 32. Meinertz T, Hofmann T, Kasper W, et al. Significance of ventricular arrhythmias in idiopathic dilated cardiomyopathy. Am J Cardiol 1984;53:902–907. [DOI] [PubMed] [Google Scholar]
- 33. Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med 1996;335:1933–1940. [DOI] [PubMed] [Google Scholar]
- 34. Moise NS. Diagnosis and management of canine arrhythmias In: Fox PR, Sisson D, Moise NS, eds. Textbook of canine and feline cardiology: principles and clinical practice, 2nd ed Philadelphia: WB Saunders; 1999:331–385. [Google Scholar]
- 35. Meurs KM, Spier AW, Wright NA, et al. Comparison of the effects of four antiarrhythmic treatments for familial ventricular arrhythmias in Boxers. J Am Vet Med Assoc 2002;221:522–527. [DOI] [PubMed] [Google Scholar]
- 36. Echt DS, Liebson PR, Mitchell LB, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med 1991;324:781–788. [DOI] [PubMed] [Google Scholar]
- 37. Jug M, Batinic Z, Goldner V et al. Ventricular extrasystole in comparison with manifestations of ventricular tachycardia and ventricular fibrillation in acute myocardial infarct. Lijec Vjesn 1995;117:68–72. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. List of 26 Holter‐ECG variables that were used for analysis.
Table S2. Results of 26 Holter‐ECG variables in the SCD‐ and control group expressed as mean ± standard deviation, median [interquartile range], or frequencies (%).
Table S3. Results of the multiple logistic regression analysis.
Table S4. Values of the coefficient plot (hazard ratio with 95% CI).


