Abstract
Background
Serum concentrations of symmetric dimethylarginine (SDMA) detected chronic kidney disease (CKD) in cats an average of 17.0 months before serum creatinine (Cr) concentrations increased above the reference interval.
Objectives
To report on the utility of measuring serum SDMA concentrations in dogs for detection of CKD before diagnosis by measurement of serum Cr.
Animals
CKD dogs (n = 19) included those persistently azotemic for ≥3 months (n = 5), dogs that were azotemic at the time of death (n = 4), and nonazotemic dogs (n = 10). CKD dogs were compared with healthy control dogs (n = 20).
Methods
Retrospective study, whereby serum Cr concentrations were determined by enzymatic colorimetry and serum SDMA concentrations were determined by liquid chromatography‐mass spectrometry in dogs with necropsy confirmed CKD.
Results
Serum SDMA increased before serum Cr in 17 of 19 dogs (mean, 9.8 months; range, 2.2–27.0 months). Duration of elevations in serum SDMA concentrations before the dog developed azotemia (N = 1) or before the dog died (N = 1) was not determined. Serum SDMA and Cr concentrations were linearly related (r = 0.84; P < .001). Serum SDMA (r = −0.80) and serum Cr (r = −0.89) concentrations were significantly related to glomerular filtration rate (both P < .001).
Conclusion and Clinical Importance
Using serum SDMA as a biomarker for CKD allows earlier detection of kidney dysfunction in dogs than does measurement of serum Cr. Earlier detection might be desirable for initiating renoprotective interventions that slow progression of kidney disease.
Keywords: Endogenous, Canine, Pet foods, Predictor
Abbreviations
- ADMA
asymmetric dimethylarginine
- BUN
blood urea nitrogen
- CKD
chronic kidney disease
- GFR
glomerular filtration rate
- IRIS
International Renal Interest Society
- Cr
creatinine
- SDMA
symmetric dimethylarginine
- UPC
urine protein: creatinine
- USG
urine specific gravity
- IBD
inflammatory bowel disease
Chronic kidney disease (CKD) is a common cause of morbidity and mortality in dogs. Antemortem prevalence of CKD varies from 0.9% in dogs of all ages examined to 2.4% in dogs between 10 and 15 years of age.1 The prevalence of CKD in dogs in UK veterinary practices is 0.37%.2
Regardless of the initiating cause, CKD tends to be progressive.3 Progression occurs because of persistence of primary disease or addition of other renal insults or complications. Once glomerular filtration rate (GFR) has decreased to 30–50% of normal, progression to end‐stage renal failure tends to be inevitable. Glomerular hyperperfusion leads to glomerulosclerosis and proteinuria. Compensatory hyperfunctioning renal tubules progress to tubulointerstitial lesions, destructive fibrosis, and loss of nephrons. Even in laboratory dogs maintained under optimal environmental conditions, the development of renal lesions is progressive over their lifetime.4 Progressive renal disease is likely present when renal disease is first diagnosed.5 Thus, early recognition of CKD is desirable in order to test for renoprotective interventions, such as dietary modifications that might slow its progression.6, 7, 8
Measurement of GFR is the gold standard method for estimating renal function and staging kidney disease.9 In a euhydrated animal with normal ability to pass urine, GFR is directly related to functional renal mass. Detecting a decrease in GFR is cumbersome if done by assessing iohexol clearance because of the expense and requirement for multiple and accurately timed blood draws. Therefore, serum creatinine (Cr) concentration remains the standard surrogate for estimating GFR because it is easily measured and less expensive.10 However, limitations of using serum Cr as a biomarker to monitor renal function are that serum Cr does not increase above the reference range until approximately 75% of nephrons are nonfunctioning,11 falsely increased concentrations can occur with some types of assays,12 and there is secretion of serum Cr into renal tubules in male dogs.13 Most importantly, daily production of creatinine is determined largely by muscle mass, such that lean body mass influences serum Cr concentration. Overall, it is less than ideal as an early biomarker of CKD.9
Symmetric dimethylarginine (SDMA) is produced by post‐translational methylation of arginine residues in proteins. There are 3 main species of methylated arginines: monomethylarginine, asymmetric dimethylarginine (ADMA), and SDMA.14 Free methylarginines are released into the cytosol after proteolysis and then enter the blood circulation. Both SDMA and ADMA are excreted by glomerular filtration and accumulate in patients with renal failure. Symmetric dimethylarginine is excreted primarily (≥90%) by renal clearance.15, 16 Most of the ADMA is converted to l‐citrulline and dimethylamine by dimethylarginine dimethylaminohydrolases. Because serum SDMA is not metabolized by this route, it correlates better with GFR than ADMA.17 A meta‐analysis of 18 studies in humans showed that serum SDMA concentration is highly correlated with GFR.17 Serum SDMA also correlates with GFR in cats.18, 19 Furthermore, SDMA concentrations in dogs are not affected by lean body mass.20
The purpose of this retrospective study was to report on the utility of measuring serum SDMA concentrations to detect CKD in dogs before diagnosis by single‐point serum Cr measurements. Our goal was to measure serum SDMA concentrations in dogs before they became azotemic, provided banked serum samples were available for measurement, in order to compare serum SDMA and Cr as biomarkers for early detection of CKD. The diagnosis of CKD was based on histopathology confirmed findings at necropsy.
Materials and Methods
Animals and Study Design
All study protocols were reviewed and approved by the Institutional Animal Care and Use Committee, Hill's Pet Nutrition, Inc, Topeka, KS (Permit Number: CP354). Each dog had an annual physical examination, CBC, serum biochemical analyses, urinalysis, and urine culture if indicated by the urinalysis results. In addition, after 2010, serum was frozen at −70°C and banked for retrospective analyses. Dogs were housed in pairs in indoor runs or in groups in spacious rooms with natural light that varied with seasonal changes. All dogs were exercised daily and were provided with regular opportunities for socialization and environmental enrichment. All dogs were owned by the commercial funders of this research or their affiliates, who gave permission for them to be included in this study.
All dogs had been fed many types of commercial and noncommercial foods of varying nutrient compositions, including dry and canned dog foods, in palatability studies. All foods met the requirements established by the Association of American Feed Control Officials for complete and balanced pet foods for adult dogs. All dogs had food withheld for 20 hours prior to blood collection.
Dogs with CKD came from a colony of over 340 Beagles. Dogs in the colony ranged in age from <1 to 16.7 years, with approximately 25% of dogs >10 years. Inclusion criteria included histopathologically confirmed evidence of CKD at necropsy (fibrosis, tubular proteinosis, chronic interstitial nephritis, mononuclear inflammatory infiltrates, and glomerulosclerosis). Dogs with concurrent disease were included as long as CKD was present at necropsy. Under veterinary supervision, all dogs had been euthanized when their quality of life deteriorated indicating the need for euthanasia for humane purposes.
Dogs with CKD (n = 19) included 5 dogs that were persistently azotemic for ≥3 months (mean, 12 months; range, 4–26 months). In these persistently azotemic dogs, acute kidney disease might have been present in some dogs with CKD and gone undiagnosed. Four additional dogs were azotemic at the time of death. Ten dogs with elevated serum SDMA were nonazotemic at death. Mean age of dogs with CKD at the time serum SDMA was first detected as elevated was 12.8 years (range, 6.5–15.8 years). There were 9 ovariohysterectomized females and 10 neutered males. Mean body weight was 11.7 kg (range, 7.7–14.3 kg).
A similar number of healthy control dogs (N = 20) was selected from the same colony. These dogs were a cohort of adult dogs that were fed maintenance food.1 All dogs had been immunized against canine distemper, adenovirus, parvovirus, bordetella, and rabies, and none had chronic systemic disease on the basis of results of annual physical examination, complete blood count determination, serum biochemical analyses, urinalysis, and fecal examination for parasites. Criteria for inclusion were age >8 years and requirement of 4 normal GFR tests and 4 normal serum Cr concentrations over a 6‐month period. Urine specific gravity (USG) was also assessed at the time of GFR testing. In addition, these dogs lacked historical, physical, or biochemical evidence of concurrent disease at the time of inclusion (including no abnormal findings on annual urinalysis) and had banked serum samples available for determination of serum SDMA concentrations. Mean age of these adult dogs was 10.5 years (range, 8.2–13.3 years). There were 10 ovariohysterectomized females and 10 neutered males. Mean body weight was 14.7 kg (range, 8.5–20.8 kg).
Retrospective data were used to document serum Cr concentrations in dogs with CKD. Because serum samples had been banked as part of annual examinations or as part of protocols for other studies, exact interval data were not available. Serum SDMA concentrations were determined from serum stored in serum banks. Serum creatinine and SDMA concentrations also were measured from blood collected prospectively in CKD dogs prior to death.
Analyses
Serum Biomarkers
Serum Cr and blood urea nitrogen (BUN) concentrations were determined by enzymatic colorimetric methods2 by the in‐house laboratory.3
Urinalysis
Urine specific gravity was determined using a refractometer. Urine creatinine concentration was used as an internal reference and measured with the same assay as serum Cr. Urine protein concentrations were determined using urine supernatant (benzethonium chloride turbidimetric method).2 Urine protein to creatinine (UPC) ratio calculations are reported as mg/dL protein: mg/dL creatinine.
Glomerular Filtration Rate
GFR was determined by iohexol clearance, as previously reported,21 in healthy control dogs at baseline and at 1.5, 3, and 6 months. In addition, 7 CKD dogs had GFR determinations performed as part of their medical work up around the time that serum SDMA increased ≥14 μg/dL. In brief, a single intravenous injection of iohexol at a dose of 300 mg I/kg BW was administered and 3 serum samples were collected at 2, 3, and 4 h after iohexol injection. Serum concentrations of iohexol were measured by a commercial laboratory4 using an inductively coupled plasma‐atomic emission spectroscopy method. GFR was estimated from calculations made using a one‐compartment model for serum iohexol clearance and normalized according to body weight in kg.
An empirical correction formula for GFR (Brochner‐Mortensen formula22: Cl = 0.990778 Cl1 ‐ 0.001218 Cl1 2), normalized to body weight in kg, was applied to GFR values for healthy dogs and dogs with CKD. It has been shown that in most dogs (excluding small dogs < 6 kg), GFR estimates predicted by use of the Brochner‐Mortensen formula22, 23 are closely related to the estimates predicted by use of a dog‐specific correction formula.24 However, corrected GFR estimates using the Brochner‐Mortensen‐formula were 60 to 100% higher in the smallest dogs with the highest GFR, i.e., one dog was approximately 8 mL/min/kg, whereas corrected GFR using the dog‐specific formula were ≤ 4 mL/min/kg in all dogs.23 Therefore, we also used an upper threshold of ≤ 4 mL/min/kg in the healthy mature‐adult dogs in our study for GFR values.
Symmetric Dimethylarginine
Serum SDMA concentrations were determined from banked serum frozen at −70 °C, using liquid chromatography‐mass spectrometry as previously described,20 with an assay validated in dogs.25 The oldest serum sample used in this study was dated October 2007. Most SDMA concentrations were determined prior to or during October 2014. Thus, sample storage time ranged from 1 week to 7 years. The reference interval for serum SDMA concentrations in healthy dogs was < 14 μg/dL.5
Necropsy
All CKD dogs had a systematic gross necropsy and histopathologic evaluation (light microscopy; H&E staining; 4‐μm sections). The underlying cause of death was determined at necropsy.
Statistical Analysis
Statistical analyses were performed using Statistical Analysis Software version 9.2.6 Data were assessed for normality by the Shapiro‐Wilk test. To investigate the relationships between serum SDMA and GFR, serum Cr and GFR, and serum SDMA and serum Cr, correlation coefficients were measured between these response variables for control and CKD dogs. To accomplish this, serum SDMA and serum Cr were plotted against GFR data from both control dogs and CKD dogs (only 7 of 19 CKD dogs had GFR measurements), and best‐fit equations were derived from data plots. These analyses were performed using repeated‐measures‐in‐time and the PROC MIXED general linear model in SAS. Significance was accepted as P < .05. Using derived equations, the GFR that corresponded to the upper limit of the reference interval for serum SDMA (14 μg/dL)5 and serum Cr (1.4 mg/dL; based on International Renal Interest Society [IRIS] guidelines for differentiating Stage 1 from Stage 2 CKD) concentrations were calculated.
All healthy dogs had 4 evaluations for serum Cr, BUN, and SDMA concentrations, USG, and GFR over a 6‐month period. These measurements were used to calculate standard deviation and coefficient of variation for these variables. The data for serum Cr, BUN, and SDMA concentrations were not normally distributed. To achieve normal distribution, we first replaced less than 5% of the highest values with a ceiling value and then log‐transformed the data. The means of the transformed data were then used in comparisons described below. Raw means, median, and range are reported. To describe the variability of GFR in healthy control dogs, mean, range, and percentiles were determined. The lower 2.5 percentile was used to establish the lower limit of normal. Any dog with GFR consistently below the lower 2.5 percentile was considered to have abnormal renal function, whereas dogs above this threshold were considered to have normal renal function.
Healthy dogs were compared with CKD dogs at the time serum SDMA concentrations were first increased (≥14 μg/dL) and at the time serum Cr concentrations were first increased (≥1.4 mg/dL) as repeated‐measures‐in‐time using the PROC MIXED general linear model. Comparisons included age; body weight; serum Cr, BUN, and SDMA concentrations; USG; and GFR measurements. In addition, the estimated time (in months) that serum SDMA concentration was increased before serum Cr concentration was increased or the dog died was calculated for each dog. Data are reported as mean (range) unless otherwise indicated. A P < .05 was considered statistically significant, and P values are indicated for all P < .10.
Results
Concurrent serum SDMA and serum Cr concentrations were plotted for CKD dogs and healthy control dogs (1 time point only) using a scatter plot (Fig 1; r = 0.84; P < .001). In dogs with CKD, repeated measures (3 sets of data) within the same animal were included (ie, at a time when serum SDMA and serum Cr were both normal, when serum SDMA first increased above normal [≥14 μg/dL], and at the time when serum Cr was first increased above normal [≥1.4 mg/dL] or at death; Fig 1; Table 1). There were no situations in which serum SDMA concentration was within the reference interval and serum Cr concentration was increased above the reference interval. All healthy control dogs had serum SDMA and Cr concentrations within the reference interval. As CKD dogs became azotemic, serum SDMA and Cr data points moved to the upper right quadrant (Fig 1).
Figure 1.
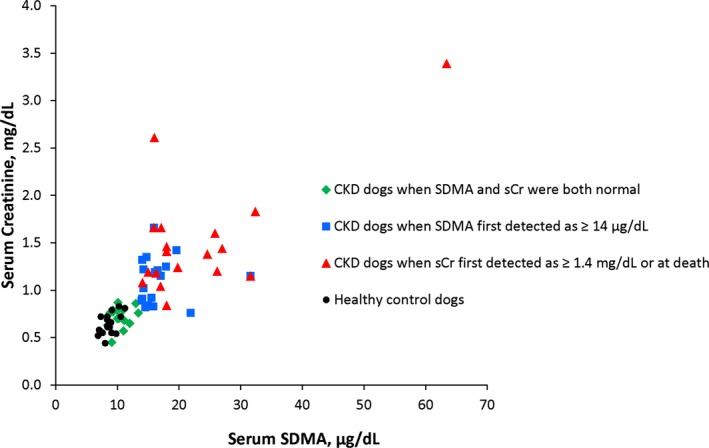
Relationship between serum symmetric dimethylarginine (SDMA; μg/dL) and serum creatinine (Cr; mg/dL) concentrations in 20 healthy dogs (mean age, 10.5 years; range, 8.2–13.3 years; circles) and 19 dogs with chronic renal disease (CKD). CKD dogs are shown at 3 time points: before serum SDMA concentrations were elevated (≥14 μg/dL; mean age, 11.7 years; range, 5.9–15.3 years; diamonds), at the time serum SDMA concentrations were first detected as elevated (mean age, 12.8 years; range, 6.5–15.8 years; squares), and when serum Cr concentrations were first detected as elevated (≥1.4 mg/dL) or at death (mean age, 13.6 years; range, 8.6–16.1 years; triangles). There is a positive linear relationship between serum SDMA and serum Cr concentrations (r = 0.84; P < .001). No dogs with serum Cr concentrations above the reference interval (≥1.4 mg/dL) had normal serum SDMA concentrations (<14 μg/dL).
Table 1.
Demographic data, mean (median; range), are summarized for healthy dogs (N = 20) and dogs with CKD (N = 19 unless otherwise indicated) at 3 time points: before serum SDMA concentrations were elevated, at the time serum SDMA concentrations were first detected as elevated, and when serum Cr concentrations were first detected as elevated or at death
| Healthy Dogs | Dogs with CKD | ||||||
|---|---|---|---|---|---|---|---|
| Data before Serum SDMA Was Detected as ≥14 μg/dL | P Valuec | Data when Serum SDMA First Detected as ≥14 μg/dLa | P Valuec | Data when Serum Cr First Detected as ≥1.4 mg/dL or at Deathb | P Valuec | ||
| Age (years) | 10.5 (9.9; 8.2–13.3) | 11.7 (12.0; 5.9–15.3) | .06 | 12.8 (12.8; 6.5–15.8) | <.001 | 13.6 (13.8; 8.6–16.1) | <.001 |
| Body weight (kg) | 14.7 (14.1; 8.5–20.8) | 11.9 (11.4; 8.6–15.6) | .004 | 11.7 (11.8; 7.7–14.3) | <.001 | 11.2 (11.1; 8.1–13.1) | <.001 |
| Serum Cr (mg/dL) | 0.6 (0.6; 0.4–1.1) | 0.7 (0.7; 0.4–0.9) | >.10 | 1.1 (1.2; 0.8–1.7) | <.001 | 1.5 (1.4; 0.8–3.4) | <.001 |
| Blood urea nitrogen (mg/dL) | 8.7 (8.4; 4.1–14.2) | 15.5 (14.3; 7.2–36.1) | <.001 | 27.2 (25.8; 14.4–50.5) | <.001 | 42.2 (37.8; 16.4–102.1) | <.001 |
| Urine specific gravity | 1.022 (1.021; 1.005–1.049) | 1.019 (N = 9) (1.020; 1.009–1.023) | >.10 | 1.016 (N = 15) (1.016; 1.009–1.023) | .01 | 1.017 (N = 12) (1.015; 1.008–1.027) | .05 |
| Urine protein to creatinine ratio | NA | 2.3 (N = 6) (0.6; 0.2–7.2) | 2.6 (N = 13) (0.8; 0.1–11.7) | 2.1 (N = 6) (0.7; 0.1–7.5) | |||
| Glomerular filtration rate (mL/min/kg)d | 4.38 (3.98; 2.33–7.08) | 1.38 (N = 7) (1.44; 0.98–1.64) | <.001 | ||||
| Serum SDMA (μg/dL) | 8.7 (8.5; 6.4–13.5) | 10.4 (N = 16) (10.2; 8.4–13.4) | <.001 | 16.8 (15.8; 14.0–31.6) | <.001 | 22.5 (18.0; 14.0–63.4) | <.001 |
| Approximate time SDMA increased before serum Cr (months)e | 0.0 (0.0–0.0) | 9.8 (7.0; 2.2–27.0) | <.001 | ||||
CKD, chronic kidney disease; SDMA, symmetric dimethylarginine; Cr, creatinine.
Two dogs had serum Cr ≥1.4 mg/dL at the time serum SDMA concentrations were first detected as ≥14 μg/dL. Banked serum samples were not available to measure SDMA concentrations before the dogs developed azotemia.
Data for the 2 dogs that had serum Cr ≥1.4 mg/dL at the time serum SDMA concentrations were first detected as ≥14 μg/dL are also included. Ten dogs with elevated serum SDMA concentrations died with serum Cr <1.4 mg/dL. All had necropsy confirmed evidence of CKD.
Dogs with CKD were compared with healthy dogs at each of the 3 time points.
GFR for healthy dogs in this study determined from 4 iohexol clearance tests per dog over a 6‐month period.
Serum SDMA concentrations increased before serum Cr in 17 of 19 dogs. In 2 of 19 dogs, although both dogs had increased SDMA concentrations at the time of euthanasia, we did not have banked serum samples to determine how long SDMA had been increased before the dog developed azotemia (N = 1) or before the dog died (N = 1).
Mean (range) of GFR measurements in healthy mature adult dogs in this study determined from 4 iohexol clearance tests per dog was 4.38 mL/min/kg (2.33 to 7.08 mL/min/kg). The lower 2.5 percentile, which was used to establish the lower limit of normal, was determined to be 2.25 mL/min/kg. All dogs with GFR 49% decrease below the mean GFR) were considered to have CKD whereas dogs above this threshold were considered to have normal renal function. Using the Brochner‐Mortensen formula, mean corrected GFR (median; range) was 4.32 mL/min/kg (3.93; 2.30‐6.95 mL/min/kg). For CKD dogs, mean corrected GFR (median; range) was 1.36 mL/min/kg (1.43; 0.97‐1.62 mL/min/kg). Corrected mean GFR estimates in both healthy and CKD dogs were within 1% of uncorrected values. Using an upper threshold of ≤ 4 mL/min/kg in the healthy mature‐adult dogs, mean corrected GFR (median; range) was 3.68 mL/min/kg (3.93; 2.30 to 4.00 mL/min/kg).
Using serum from data banks to measure SDMA concentrations, serum SDMA concentrations increased above the reference interval before serum Cr concentrations increased above the reference interval in 17 of 19 dogs by a mean of 9.8 months (range, 2.2–27.0 months). A representative example of the relationship between serum SDMA and Cr concentrations across time is shown for 1 dog in an age versus analytes concentration bar graph (Fig 2). In 2 of 19 dogs, although both dogs had increased SDMA concentrations at the time of euthanasia (one dog had SDMA of 32 μg/dL, sCr was 1.15 mg/dL; the other dog had SDMA of 16 μg/dL, sCr was 1.66 mg/dL, 5 days prior sCr was 1.38 mg/dL), we did not have banked serum samples to determine how long SDMA had been elevated. In 10 of 19 dogs with CKD, serum Cr concentration did not increase above the reference interval antemortem (mean serum Cr, 1.11; median, 1.17; range, 0.80–1.38 mg/dL) and the diagnosis of CKD was based on necropsy findings of renal disease. In these 10 dogs, the estimated time that serum SDMA concentration was increased before serum Cr increased was recorded as months until death.
Figure 2.
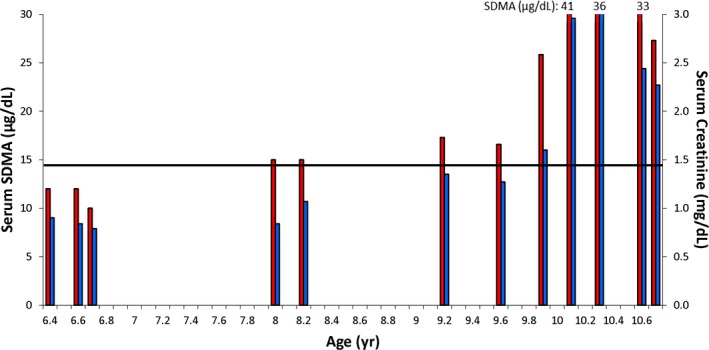
Representative dog (neutered male) with serum symmetric dimethylarginine (SDMA; red bars) and serum creatinine (Cr; blue bars) concentrations indicated across time. Serum SDMA was increased at 8.0 years (15 μg/dL). Glomerular filtration rate was measured at 8.7 years and found to be 1.45 mL/min/kg, which was 67% below the mean of 4.38 mL/min/kg for the healthy control dogs. The dog became azotemic at 9.9 years (serum Cr, 1.60 mg/dL), approximately 22 months after serum SDMA was increased. The dog died at 10.7 years. Renal histopathology revealed lymphocytic/plasmacytic interstitial nephritis with interstitial and periglomerular fibrosis, glomerulosclerosis, and tubular proteinosis. The horizontal line represents the upper limit of the reference intervals for serum Cr and SDMA concentrations.
The best‐fit equation for the relationship between serum SDMA concentration and GFR was a quadratic relationship: [GFR in mL/min/kg] = 0.0167 [serum SDMA concentration in μg/dL]2 – 0.7837 [serum SDMA concentration in μg/dL] + 9.93 (r = −0.80; P < .0001). Setting the upper limit of the reference interval for serum SDMA at 14 μg/dL corresponded to a GFR of 2.23 mL/min/kg, which represents an approximately 49% decrease from mean GFR of healthy dogs.
The best‐fit equation for the relationship between serum Cr concentration and GFR was a quadratic relationship: [GFR in mL/min/kg] = 5.32 [serum Cr concentration in μg/dL]2 – 15.12 [serum Cr concentration in μg/dL] + 11.84 (r = −0.89; P < .0001). Setting the upper limit of the reference interval for serum Cr at 1.4 mg/dL corresponded to a GFR of 1.10 mL/min/kg, which represents approximately 75% decrease from the mean GFR of healthy dogs.
In healthy control dogs, the standard deviation and coefficient of variation for other variables measured over a 6‐month period were serum Cr (0.11 mg/dL; 16.93%), BUN (1.78 mg/dL; 20.42%), serum SDMA (1.40 μg/dL; 16.06%), and USG (0.01; 1.01%), respectively. Compared with healthy control dogs, dogs with CKD at the time when serum SDMA was first detected as being ≥14 μg/dL (Table 1) were older (P < .001), with lower body weight (P < .001), and had higher concentrations of serum Cr, BUN, and SDMA (all P < .001) and lower GFR (P < .001) and USG (P = .01). The UPC ratios were not measured (no abnormal findings on urinalysis) in control dogs for comparison. Dogs with CKD at the time when serum Cr was first detected as being ≥1.4 mg/dL, or when dogs died, were older (P < .001), with lower body weight (P < .001), and continued to have higher serum Cr, BUN, and SDMA concentrations (all P < .001) compared with healthy control dogs.
Retrospective IRIS staging of CKD dogs at the time that serum SDMA concentrations were first detected as elevated (≥14 μg/dL) showed that 17 of 19 dogs were nonazotemic IRIS stage 1 (serum Cr <1.4 mg/dL) and 2 of 19 dogs were IRIS stage 2 (serum Cr 1.42 and 1.66 mg/dL). Five dogs were nonproteinuric (UPC ratio ≤0.2), 3 were borderline proteinuric (UPC ratio >0.2–0.5), 7 were proteinuric (UPC ratio >0.5), and 4 were not assessed. (One of the dogs whose proteinuria was not assessed when SDMA concentration was first detected as elevated was proteinuric with 0.6 UPC ratio 14 months later, at least 8 months before it became azotemic.) Blood pressure data were not available. In the 7 proteinuric dogs in this study, serum SDMA concentrations increased above the reference interval either before serum Cr concentrations increased above the reference interval or dogs died, by a mean of 6.6 months (range, 0–13 months). In the 5 nonproteinuric dogs in this study, serum SDMA concentrations increased above the reference interval either before serum Cr concentrations increased above the reference interval or dogs died, by a mean of 10.8 months (range, 4–27 months; P = .34).
After necropsy and evaluation of gross and microscopic lesions, the reason for euthanasia in 14 dogs was determined to be CKD. The remaining 5 dogs were euthanized because of other primary conditions (liver disease/failure, clinical neurological disease, mammary carcinoma, and 2 with severe arthritis), but had concurrent CKD. Interstitial fibrosis was the most consistent CKD finding (n = 17). Lymphoplasmacytic interstitial nephritis, glomerulosclerosis, and tubular proteinosis were each reported in 15 dogs. One dog also had microscopic metastatic renal neoplasia (bilateral; carcinoma of unknown origin). One dog had renal amyloidosis. Concurrent diseases (of minor clinical significance or clinically undetected) were reported in 9 of the 14 dogs with CKD as main cause of death: 7 of these dogs had inflammatory bowel disease (IBD), 4 of which also had hepatic vacuolar degeneration and 2 of which also had nonrenal neoplasia; and 2 of these 14 dogs had nonrenal neoplasia in addition to CKD.
Ten of 19 dogs with CKD diagnosed by necropsy examination were nonazotemic at the time of death. Four had concurrent disease listed as underlying cause of death. In 6 nonazotemic dogs, after evaluation of gross and microscopic lesions, the reason for euthanasia was determined by the pathologist to be CKD. Three dogs had GFR measurements of 1.64, 0.98, and 1.38 mL/min/kg and serum Cr of 1.08, 1.38, and 1.18 mg/dL, respectively. These dogs had interstitial fibrosis, tubular proteinosis, and mineralization; interstitial fibrosis, interstitial nephritis, and amyloid; and periglomerular fibrosis, interstitial fibrosis, interstitial nephritis, glomerulosclerosis, and tubular proteinosis, respectively. Three more nonazotemic dogs at necropsy (no GFR available) all had interstitial fibrosis, glomerulosclerosis, and tubular proteinosis.
Discussion
Using retrospective serum samples, we were able to show that serum SDMA increases before serum Cr in dogs with CKD by a mean of 9.8 months (range, 2.2–27.0 months). A similar observation has been made in cats with CKD.18 Because serum samples had been banked as part of annual examinations or as part of protocols for other studies, exact interval data were not available. Conversely, all healthy control dogs had serum SDMA and Cr concentrations within the reference interval. Thus, serum SDMA represents a promising biomarker for early detection of CKD in dogs with reduced renal function. Early detection is desirable because it allows testing to determine if dietary or other renoprotective interventions can slow progression of CKD, as was shown for cats with IRIS stages 2 and 3 CKD.26
The diagnosis of CKD was made based on histopathology confirmed chronic renal lesions at necropsy. The necropsy findings were not diagnostic for a specific kidney disease, but rather a morphologic diagnosis of structural changes in the kidney, and equivalent to what has been referred to as chronic interstitial nephritis.27 Thus, regardless of inciting cause, all dogs had chronic kidney disease and elevated serum SDMA, but slightly less than 50% were azotemic at the time of death.
A potential limitation of this study is that control dogs did not have renal biopsies. Each had 4 normal GFR assessments, and lacked historical, physical, or biochemical evidence of concurrent renal disease over the 6‐month study period. When assessing renal disease in a mature or aged animal, normal aging changes can occur in the absence of any specific renal insult; these background changes could lead to decreased renal reserve but usually not to renal failure.3 In aged dogs, for example renal weight is reduced by 20–30% because of decreased size and number of nephrons.3 In humans, GFR decreases with age because the renal fraction of cardiac output decreases and because of intrarenal vascular changes such as coalescence of glomerular capillaries in juxtamedullary glomeruli and atrophy of arterioles of cortical glomeruli.3 Glomerular mesangial volume increases, proximal tubule volume and length decrease, and interstitial connective tissue increases.3 Based on normal serum SDMA in all control dogs, we can assume that any purely senescent renal changes quantitatively represented less than 49% decrease from mean GFR of healthy dogs.
Two reasons exist to explain nonazotemic dogs with CKD lesions at necropsy. First, as in cats, we have shown that serum SDMA concentration is an earlier indicator of reduction in GFR than serum Cr.18 In our study, the upper reference interval for serum SDMA of <14 μg/dL corresponded to a GFR of 2.23 mL/min/kg (approximately 49% decrease from mean), whereas the upper reference interval for serum Cr of <1.4 mg/dL corresponded to a GFR of 1.10 mL/min/kg (approximately 75% decrease from mean). Thus, serum SDMA detects a reduction in GFR before serum Cr in dogs with CKD on average 9.8 months earlier. In dogs, we have previously shown that reduction in lean body mass lowers serum Cr concentration, but not SDMA concentration.21 In this study, 6 nonazotemic dogs were euthanized for poor quality of life that had clinically unrecognized CKD. Serum SDMA is also a more sensitive biomarker than serum Cr for assessing renal dysfunction in humans.7, 8
Second, not all dogs that had underlying CKD died from renal failure. Five dogs were euthanized because of other primary conditions, 4 of which were nonazotemic. Concurrent diseases were also reported in 9 dogs where CKD was determined to be the main cause of death. These included IBD, hepatic vacuolar degeneration, and nonrenal neoplasia (neoplasia of the lymph nodes, urinary bladder, lung, and mammary glands). Not surprisingly, elevated serum SDMA concentrations are also found in human patients with multiple disorders compounded by reduced renal function. For example, investigators have looked at the association of serum SDMA concentrations in patients with cardiovascular risk,28, 29, 30, 31, 32 with stroke,33 during the acute inflammatory response,34 with microvascular dysfunction caused by critical illness and sepsis,35 with alcoholic liver disease,36 with hepatorenal syndrome,37 with essential hypertension,38 with type 2 diabetes with albuminuria,39 and with IBD.40 Serum SDMA was increased in most patient groups compared with appropriate control groups. Yet, as previously reviewed,21 researchers concluded that serum SDMA accumulates because of reduced renal clearance and reflects concurrent renal dysfunction. There is no evidence that elevated serum SDMA concentrations contribute to lesions or death.41
A significant positive correlation (r = 0.84) was present between serum SDMA and serum Cr concentrations, similar to what has been reported previously in cats with CKD.18, 42 These results support that serum SDMA is excreted primarily by the kidneys in both species.
GFR is directly related to functional renal mass. Early detection of a decrease in GFR might be important for initiating dietary or medical interventions in dogs with CKD when serum Cr concentrations are still within the reference range (<1.4 mg/dL). In this study, serum SDMA concentrations were inversely related to GFR (r = −0.80) and similar to what we have published for cats (r = −0.79).18 These results show that serum SDMA has advantages over serum Cr because it increases above the reference interval before serum Cr in dogs with CKD and because serum SDMA concentrations are not affected by lean body mass in dogs20 or cats.19 In support, in Fig 2, the decrease in serum Cr from 3.1 mg/dL at 10.3 months to 2.44 and 2.27 mg/dL at 10.6 and 10.7 months is likely the result of decreased lean body mass, which was not measured in this study, although total body weight decreased from 15.7 kg to 14.3 and 13.3 kg, respectively. Symmetric dimethylarginine was also recently reported in human volunteers to provide an accurate and precise estimate of GFR and to serve as a more sensitive biomarker of renal dysfunction than serum Cr.7
Mean GFR was higher in healthy control dogs compared with healthy control cats18 (4.38 versus 1.94 mL/min/kg, respectively) similar to what has been previously reported,9 and the 2.5 percentile for the reference interval was higher in dogs compared with cats (2.25 versus 1.36 mL/min/kg, respectively). There was also more variability in GFR values from dogs (range, 2.33–7.08 mL/min/kg) compared with cats (1.34–3.79 mL/min/kg).18 This variability was reduced using correction formulas for GFR, including the Brochner‐Mortensen formula22 and an approximation of the dog‐specific formula23 by setting an upper threshold for GFR. Whether we used GFR values normalized according to body weight in kg, or GFR values corrected with the Brochner‐Mortensen formula, or with an upper threshold to approximate the dog‐specific formula, our conclusions remained the same. No dog with CKD had higher GFR than any healthy control dog, and serum SDMA (r = −0.80) and serum Cr (r = −0.89) concentrations were significantly correlated to GFR (both P < .001).
The upper limit for the serum SDMA reference interval (<14 μg/dL) corresponded to a 49% decrease in GFR in dogs compared with the upper limit for the serum SDMA reference interval (<14 μg/dL) corresponding to a 24% decrease in GFR in cats.18 We speculate that more cats with greater reductions in renal function were included in the normal populations used to establish the feline serum SDMA reference interval (GFR was not measured).9 Also, it is not surprising that the number of months that serum SDMA was increased before azotemia was detected was greater in cats compared with dogs, knowing that a smaller decrease in GFR from normal is detected in cats with serum SDMA ≥14 μg/dL.
The use of a biomarker to help identify dogs with CKD earlier in the disease course might provide additional options for slowing progressive loss of kidney function in order to ameliorate clinical and biochemical consequences of CKD, while maintaining adequate nutrition.10 Feeding a renal diet to dogs with IRIS stages 3 and 4 CKD is considered the current standard of care with strong evidence supporting this recommendation.10 Dietary modifications include decreased protein, phosphorus, and sodium content; increased water soluble vitamins and fiber content; increased caloric density; and additional n‐3 fatty acids, antioxidants, and potassium.10 Previous studies in dogs with a remnant kidney model of CKD have shown that feeding foods enriched in (n‐3) PUFA (15%) reduces glomerular hypertension, proteinuria, tubulointerstitial fibrosis, glomerulosclerosis and limits the production of proinflammatory eicosanoid mediators such as PGE2 and TxA1.6, 7 Studies in 6‐ to 8‐yr‐old Beagles fed dietary (n‐3) FA supplements (2.5% dry matter basis) in combination with antioxidants (vitamin E, carotenoids, and lutein at concentrations comparable to those found in commercial canine renal disease foods) showed independent and additive protective effects, best explained as causing a decrease in renal oxidant injury.43 Specifically, the rate of decline of GFR was slowed by the use of (n‐3) PUFA and by the addition of dietary antioxidants in this dog remnant model of CKD.43 In another study, obese dogs fed a high‐fat food for 7–9 weeks, or 24 weeks, exhibited increased GFR and renal plasma flow.44
In summary, this study demonstrated the utility of using serum SDMA as an early indicator of compromised renal function in dogs with CKD. Future studies are needed to determine if early interventions can improve the outcome of CKD in dogs.
Supporting information
Discussion S1. Glomerular filtration rate (GFR) correction formulas.
Discussion S2. Symmetric dimethylarginine (SDMA) concentrations from banked serum samples.
Acknowledgments
None.
Conflict of Interest Declaration: One of the authors (DEJ) has an affiliation to the commercial funders of this research, as an employee of Hill's Pet Nutrition, Inc. The work presented in this study was funded by and performed at the Pet Nutrition Center, Hill's Pet Nutrition, Inc, Topeka, KS (http://www.hillspet.com/our-company.html). The funding decision makers had no role in study design, data collection and analysis, or preparation of the article. Three of the authors (MY, EO, and MY) have an affiliation to a commercial company, as employees of IDEXX Laboratories, Inc, which holds a patent on the ELISA methodology for measuring SDMA concentration. (http://www.idexx.com/view/xhtml/en_us/corporate/home.jsf). IDEXX Laboratories, Inc performed the SDMA analyses. The patent no. is US Patent No. US 481,690 B2; Date: July 9, 2013 Murthy et al., Methods for Detecting Symmetrical Dimethylarginine. This does not alter our adherence to JVIM policies on sharing data and materials. Data are freely available upon request. JAH has received consulting fees from Hill's Pet Nutrition, Inc and has been paid speaking honoraria by IDEXX Laboratories, Inc in the past 12 months.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Work was done at Pet Nutrition Center, Hill's Pet Nutrition, Inc., 1035 NE 43rd Street, Topeka, KS 66617‐1587, United States.
Work was supported by Pet Nutrition Center, Hill's Pet Nutrition, Inc., 1035 NE 43rd Street, Topeka, KS 66617‐1587, United States.
Previously presented at 2014 ACVIM Forum in Nashville, TN, June 3–6, 2015.
Footnotes
Hill's Science Diet Mature Adult, Hill's Pet Nutrition, Inc, Topeka, KS
Roche Diagnostics, Cobas 6000 series, c501 module, Indianapolis, IN
Pet Nutrition Center, Hill's Pet Nutrition, Inc, Topeka, KS
Diagnostic Center for Population and Animal Health, Michigan State University, E. Lansing, MI
Rentko V, Nabity M, Yerramilli M, et al. Determination of serum symmetric dimethylarginine reference limit in clinically healthy dogs. J Vet Intern Med 2013;27:750
SAS Institute, Cary, NC
Dixon JJ, Lane K, Dalton RN, et al. Symmetrical dimethylarginine is a more sensitive biomarker of renal dysfunction than creatinine. Crit Care 2013;17:P423
Payto D, El‐Khoury JM, Bunch D, et al. SDMA outperforms serum creatinine‐based equations in estimating kidney function compared with measured GFR. Clin Chem 2014;60:S26
IDEXX Laboratories, Inc, Westbrook, ME
References
- 1. Polzin DJ, Osborne CA, Bartges JW, et al. Chronic renal failure In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine, 4th ed Philadelphia, PA: WB Saunders Co; 1995:1734–1760. [Google Scholar]
- 2. O'Neill DG, Elliott J, Church DB, et al. Chronic kidney disease in dogs in UK veterinary practices: Prevalence, risk factors, and survival. J Vet Intern Med 2013;27:814–821. [DOI] [PubMed] [Google Scholar]
- 3. Maxie MG, Newman SJ. Urinary system In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals, 5th ed Philadelphia, PA: Elsevier Saunders; 2007:425–521. [Google Scholar]
- 4. Pomeroy MJ, Robertson JL. The relationship of age, sex, and glomerular location to the development of spontaneous lesions in the canine kidney: Analysis of a life‐span study. Toxicol Pathol 2004;32:237–242. [DOI] [PubMed] [Google Scholar]
- 5. Finco DR, Brown SA, Brown CA, et al. Progression of chronic renal disease in the dog. J Vet Intern Med 1999;13:516–528. [DOI] [PubMed] [Google Scholar]
- 6. Brown SA, Brown CA, Crowell WA, et al. Beneficial effects of chronic administration of dietary omega‐3 polyunsaturated fatty acids in dogs with renal insufficiency. J Lab Clin Med 1998;131:447–455. [DOI] [PubMed] [Google Scholar]
- 7. Brown SA, Brown CA, Crowell WA, et al. Effects of dietary polyunsaturated fatty acid supplementation in early renal insufficiency in dogs. J Lab Clin Med 2000;135:275–286. [DOI] [PubMed] [Google Scholar]
- 8. Jacob F, Polzin DJ, Osborne CA, et al. Clinical evaluation of dietary modification for treatment of spontaneous chronic renal failure in dogs. J Am Vet Med Assoc 2002;220:1163–1170. [DOI] [PubMed] [Google Scholar]
- 9. Von Hendy‐Willson VE, Pressler BM. An overview of glomerular filtration rate testing in dogs and cats. Vet J 2011;188:156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Polzin DJ. Evidence‐based step‐wise approach to managing chronic kidney disease in dogs and cats. J Vet Emerg Crit Care (San Antonio) 2013;23:205–215. [DOI] [PubMed] [Google Scholar]
- 11. Finco DR, Duncan JR. Evaluation of blood urea nitrogen and serum creatinine concentrations as indicators of renal dysfunction: A study of 111 cases and a review of related literature. J Am Vet Med Assoc 1976;168:593–601. [PubMed] [Google Scholar]
- 12. Balint P, Visy M. True creatinine and pseudocreatinine in blood plasma of dog. Acta Physiol Hung 1965;28:265–272. [Google Scholar]
- 13. O'Connell JMB, Romeo JA, Mudge GH. Renal tubular secretion of creatinine in the dog. Am J Phyiol 1962;203:985–990. [Google Scholar]
- 14. Bedford MT, Richard S. Arginine methylation an emerging regulator of protein function. Mol Cell 2005;18:263–272. [DOI] [PubMed] [Google Scholar]
- 15. Schwedhelm E, Boger RH. The role of asymmetric and symmetric dimethylarginines in renal disease. Nat Rev Nephrol 2011;7:275–285. [DOI] [PubMed] [Google Scholar]
- 16. Kielstein JT, Boger RH, Bode‐Boger SM, et al. Marked increase of asymmetric dimethylarginine in patients with incipient primary chronic renal disease. J Am Soc Nephrol 2002;13:170–176. [DOI] [PubMed] [Google Scholar]
- 17. Kielstein JT, Salpeter SR, Bode‐Boeger SM, et al. Symmetric dimethylarginine (SDMA) as endogenous marker of renal function–A meta‐analysis. Nephrol Dial Transplant 2006;21:2446–2451. [DOI] [PubMed] [Google Scholar]
- 18. Hall JA, Yerramilli M, Obare E, et al. Comparison of serum concentrations of symmetric dimethylarginine and creatinine as kidney function biomarkers in cats with chronic kidney disease. J Vet Intern Med 2014;28:1676–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hall JA, Yerramilli M, Obare E, et al. Comparison of serum concentrations of symmetric dimethylarginine and creatinine as kidney function biomarkers in healthy geriatric cats fed reduced protein foods enriched with fish oil, L‐carnitine, and medium‐chain triglycerides. Vet J 2014;202:588–596. [DOI] [PubMed] [Google Scholar]
- 20. Hall JA, Yerramilli M, Obare E, et al. Relationship between lean body mass and serum renal biomarkers in healthy dogs. J Vet Intern Med 2015;29:808–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Braselton WE, Stuart KJ, Kruger JM. Measurement of serum iohexol by determination of iodine with inductively coupled plasma‐atomic emission spectroscopy. Clin Chem 1997;43:1429–1435. [PubMed] [Google Scholar]
- 22. Brochner‐Mortensen J. A simple method for the determination of glomerular filtration rate. Scand J Clin Lab Invest 1972;30:271–274. [DOI] [PubMed] [Google Scholar]
- 23. Bexfield NH, Heiene R, Gerritsen RJ, et al. Glomerular filtration rate estimated by 3‐sample plasma clearance of iohexol in 118 healthy dogs. J Vet Intern Med 2008;22:66–73. [DOI] [PubMed] [Google Scholar]
- 24. Heiene R, Moe L. The relationship between some plasma clearance methods for estimation of glomerular filtration rate in dogs with pyometra. J Vet Intern Med 1999;13:587–596. [DOI] [PubMed] [Google Scholar]
- 25. Nabity MB, Lees GE, Boggess MM, et al. Symmetric dimethylarginine assay validation, stability, and evaluation as a marker for the early detection of chronic kidney disease in dogs. J Vet Intern Med 2015;29:1036–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ross SJ, Osborne CA, Kirk CA, et al. Clinical evaluation of dietary modification for treatment of spontaneous chronic kidney disease in cats. J Am Vet Med Assoc 2006;229:949–957. [DOI] [PubMed] [Google Scholar]
- 27. Brown SA. Renal pathophysiology: Lessons learned from the canine remnant kidney model. J Vet Emerg Crit Care (San Antonio) 2013;23:115–121. [DOI] [PubMed] [Google Scholar]
- 28. Kiechl S, Lee T, Santer P, et al. Asymmetric and symmetric dimethylarginines are of similar predictive value for cardiovascular risk in the general population. Atherosclerosis 2009;205:261–265. [DOI] [PubMed] [Google Scholar]
- 29. Meinitzer A, Kielstein JT, Pilz S, et al. Symmetrical and asymmetrical dimethylarginine as predictors for mortality in patients referred for coronary angiography: The Ludwigshafen Risk and Cardiovascular Health study. Clin Chem 2011;57:112–121. [DOI] [PubMed] [Google Scholar]
- 30. Pilz S, Edelmann F, Meinitzer A, et al. Associations of methylarginines and homoarginine with diastolic dysfunction and cardiovascular risk factors in patients with preserved left ventricular ejection fraction. J Card Fail 2014;20:923–930. [DOI] [PubMed] [Google Scholar]
- 31. Dimitrow PP, Undas A, Bober M, et al. Plasma biomarkers of endothelial dysfunction in patients with hypertrophic cardiomyopathy. Pharmacol Rep 2007;59:715–720. [PubMed] [Google Scholar]
- 32. Cavalca V, Veglia F, Squellerio I, et al. Circulating levels of dimethylarginines, chronic kidney disease and long‐term clinical outcome in non‐ST‐elevation myocardial infarction. PLoS ONE 2012;7:e48499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Luneburg N, von Holten RA, Topper RF, et al. Symmetric dimethylarginine is a marker of detrimental outcome in the acute phase after ischaemic stroke: Role of renal function. Clin Sci (Lond) 2012;122:105–111. [DOI] [PubMed] [Google Scholar]
- 34. Blackwell S, O'Reilly DS, Reid D, et al. Plasma dimethylarginines during the acute inflammatory response. Eur J Clin Invest 2011;41:635–641. [DOI] [PubMed] [Google Scholar]
- 35. Koch A, Weiskirchen R, Bruensing J, et al. Regulation and prognostic relevance of symmetric dimethylarginine serum concentrations in critical illness and sepsis. Mediators Inflamm 2013;2013:413826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mookerjee RP, Malaki M, Davies NA, et al. Increasing dimethylarginine levels are associated with adverse clinical outcome in severe alcoholic hepatitis. Hepatology 2007;45:62–71. [DOI] [PubMed] [Google Scholar]
- 37. Lluch P, Mauricio MD, Vila JM, et al. Accumulation of symmetric dimethylarginine in hepatorenal syndrome. Exp Biol Med (Maywood) 2006;231:70–75. [DOI] [PubMed] [Google Scholar]
- 38. Wang D, Strandgaard S, Iversen J, et al. Asymmetric dimethylarginine, oxidative stress, and vascular nitric oxide synthase in essential hypertension. Am J Physiol Regul Integr Comp Physiol 2009;296:R195–R200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Krzyzanowska K, Mittermayer F, Shnawa N, et al. Asymmetrical dimethylarginine is related to renal function, chronic inflammation and macroangiopathy in patients with Type 2 diabetes and albuminuria. Diabet Med 2007;24:81–86. [DOI] [PubMed] [Google Scholar]
- 40. Owczarek D, Cibor D, Mach T. Asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA), arginine, and 8‐iso‐prostaglandin F2alpha (8‐iso‐PGF2alpha) level in patients with inflammatory bowel diseases. Inflamm Bowel Dis 2010;16:52–57. [DOI] [PubMed] [Google Scholar]
- 41. Veldink H, Faulhaber‐Walter R, Park JK, et al. Effects of chronic SDMA infusion on glomerular filtration rate, blood pressure, myocardial function and renal histology in C57BL6/J mice. Nephrol Dial Transplant 2013;28:1434–1439. [DOI] [PubMed] [Google Scholar]
- 42. Jepson RE, Syme HM, Vallance C, et al. Plasma asymmetric dimethylarginine, symmetric dimethylarginine, L‐arginine, and nitrite/nitrate concentrations in cats with chronic kidney disease and hypertension. J Vet Intern Med 2008;22:317–324. [DOI] [PubMed] [Google Scholar]
- 43. Brown SA. Oxidative stress and chronic kidney disease. Vet Clin North Am Small Anim Pract 2008;38:157–166. [DOI] [PubMed] [Google Scholar]
- 44. Henegar JR, Bigler SA, Henegar LK, et al. Functional and structural changes in the kidney in the early stages of obesity. J Am Soc Nephrol 2001;12:1211–1217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Discussion S1. Glomerular filtration rate (GFR) correction formulas.
Discussion S2. Symmetric dimethylarginine (SDMA) concentrations from banked serum samples.


