Abstract
Background
Blood pressure is relevant to the diagnosis and management of many medical, cardiovascular and critical diseases. The accuracy of many commonly used noninvasive blood pressure (NIBP) monitors and the accuracy of NIBP measurements in hypo‐ and hypertensive standing horses has not been determined.
Hypothesis/Objectives
The objective of this study was to investigate the accuracy of an oscillometric BP monitor in standing horses before and during pharmacologically induced hyper‐ and hypotension and to compare results in standing and anesthetized horses.
Animals
Eight standing mares from a research herd (SG) and eight anesthetized horses from a hospital population (AG).
Methods
Prospective experimental and observational studies. Invasive blood pressure (IBP) and NIBP, corrected to heart level, were measured simultaneously. In the SG hyper‐ and hypotension were induced by administration of phenylephrine (3 μg/kg/min IV for 15 minutes) and acepromazine (0.05 mg/kg IV), respectively. In the AG NIBP and IBP were recorded during regular hospital procedures.
Results
There was a significant correlation between mean NIBP and IBP in standing (R = 0.88, P < .001) and anesthetized horses (R = 0.81, P < .001). The mean bias (lower, upper limit of agreement) was 16.4(−16.1, 48.9) mmHg for mean BP in the SG and 0.5(−22.3, 23.2) mmHg in the AG. The NIBP device was capable of identifying the increase and decrease in BP in all horses, but in the SG significant correlation between NIBP and IBP was only detected for the normotensive phase.
Conclusion and Clinical Importance
While the evaluated oscillometric BP device allowed estimation of BP and adequately differentiated marked trends, the accuracy and precision were low in standing horses.
Keywords: Acepromazine, Equine, Hypertension, Hypotension, Oscillometric, Phenylephrine
Abbreviations
- AG
anesthetized group
- BP
blood pressure
- DAP
diastolic arterial pressure
- HR
heart rate
- IBP
invasive blood pressure
- MAP
mean arterial pressure
- NIBP
noninvasive blood pressure
- R
Pearson correlation coefficient
- SAP
systolic arterial pressure
- SG
standing group
Arterial blood pressure (BP) is defined as the product of cardiac output and systemic vascular resistance. While cardiac output is a better marker of flow and perfusion, its measurement is cumbersome in many clinical situations. Arterial BP measurements are a good alternative to learn about potential flow and tissue perfusion.1, 2 Direct techniques require cannulation of an artery, which can be impractical, labor intensive, time‐consuming and even impossible in certain settings. Noninvasive oscillometric blood pressure readings (NIBP) are easily obtained and measure changes in oscillations in the sphygmomanometer cuff pressure induced by alterations in blood flow due to deflation of a cuff placed around an artery. The maximal oscillations are used to determine mean arterial pressure (MAP) and different algorithms estimate systolic (SAP) and diastolic (DAP) BP.2
The potential applications of BP measurements in standing horses encompass the evaluation and monitoring of renal disease, cardiac disease, severe or chronic pain, fluid resuscitation monitoring and is well established in small animals and human medicine. BP monitoring in horses is more commonly used to recognize hypotension and is useful in guiding and monitoring treatment in events such as: hypovolemia, acute hemorrhage, systemic inflammatory response syndrome, sepsis, anaphylaxis, heart failure, or aortic valve disease.2, 3 Hypertension is uncommonly recognized in horses but can occur in any cases of acute of chronic pain, laminitis, Equine Metabolic Syndrome and renal failure.2, 4, 5, 6 Four studies have demonstrated good agreement between oscillometric NIBP and invasive blood pressure (IBP) in foals and anesthetized adult horses.7, 8, 9, 10 However, ambiguous findings have been reported in studies assessing the accuracy of different oscillometric BP devices in the standing adult horse.1 , 2 The importance for validation of devices in the species of interest and under circumstances in which the patient is being tested has been emphasized.11 To the authors' knowledge, the accuracy of NIBP in adult standing horses in hypo‐ and hypertensive ranges has not been evaluated. The aims of this study were to investigate the agreement of oscillometric NIBP measurements and IBP measurements in different BP ranges in the standing horse and compare it to horses under general anesthesia.
Methods
The study was performed with the approval and supervision of the appropriate committee for animal care and experimentation. The study population consisted of a standing research group (SG) and a group of client‐owned horses presented to the University Teaching Hospital for surgical procedures under general anesthesia (AG). The SG was composed of 8 Warmblood mares 11.6 ± 1.5 years old and weighing 568 ± 62 kg. The AG consisted of 6 geldings and 2 mares (1 Icelandic horse, 1 Pony and 6 Warmbloods) 10.8 ± 7.6 years old and weighing 481.8 ± 89.1 kg.
The mares from the SG were placed in the stalls at least 15 minutes before being instrumented and a general physical examination was performed. A 13G indwelling catheter was placed in a jugular vein using standard aseptic technique and all drugs were administered through this catheter. Horses were equipped with a digital telemetry unit3 following manufacturer's instructions.
An oscillometric monitor,4 with a cuff bladder width to tail girth ratio of 0.4–0.6 (manufacturer's recommendations), was centered over the coccygeal artery around the base of the unclipped tail and used for NIBP measurements. To correct readings to the level of the heart base, the vertical distance between the base of the heart, as estimated by the point of the shoulder, and the base of the tail was measured and a correction factor of 0.77 mmHg/cm was applied.12
Noninvasive blood pressure was measured eight times. When pulse rate obtained by the oscillometric device differed by more than twenty percent of the heart rate (HR) obtained electrocardiographically,13 the value was excluded. As recommended by ACVIM guidelines for device validation in small animals, the first measurement was discarded and the average of three to seven consecutive readings was calculated.11
For IBP monitoring, a 20 or 22G over the needle catheter was placed in the facial or transversal facial artery, after aseptic preparation, and secured with cyanoacrylate glue and tape. The catheter was connected via nondistensible heparin‐saline filled tubing to a disposable BP transducer, which was positioned and zeroed to the level of the heart base and connected to a monitor.5 Catheter placement was attempted in the unsedated horse and if necessary, xylazine6 0.5 mg/kg IV was administered. BP recordings were performed a minimum of 30 minutes after xylazine administration.
Paired readings of invasive and noninvasive SAP, DAP and MAP were obtained simultaneously, with IBP readings taken at the end of the deflation cycle of the noninvasive blood pressure monitor. Hypertension was induced with an IV phenylephrine7 infusion (3 μg/kg/min diluted in 1 L 0.9% NaCl solution over 15 minutes) and hypotension with IV acepromazine8 (0.05 mg/kg).
Blood pressure was obtained at four different time points: before pharmacological intervention, immediately after phenylephrine infusion, 35 minutes after phenylephrine infusion, and 30 minutes after acepromazine. Time points were chosen based on previously described temporal effects of acepromazine and phenylephrine to detect maximal changes in BP.14, 15, 16
Noninvasive blood pressure was obtained and corrected in the AG using the same device and protocol described above. Horses received anesthetic protocols adjusted individually to the needs of the horse and surgical procedure (Supporting Information). Only recordings from surgeries performed in dorsal recumbency were used. Three BP ranges were defined for the AG as: hypotension (MAP < 65 mmHg), normotension (MAP = 65–90 mmHg), and hypertension (MAP > 90 mmHg). Anesthetic procedures were not modified for the study. If necessary, dobutamine9 was administered intraoperatively targeting a MAP of 70 mmHg (Table S1).
Statistical Analysis
A Shapiro–Wilk Test was used to evaluate normality. Pearson correlation coefficient (R) was calculated to assess the correlation between NIBP and IBP for each phase (normo‐, hypo‐ and hypertensive) separately and for all phases together. Agreement between NIBP and IBP was quantified using the Bland and Altman method for repeated measurements.17 The bias was reported as the mean difference between NIBP and IBP. A positive bias reflected underestimation and a negative bias reflected overestimation of IBP by NIBP. The limits of agreement were calculated as bias ± (1.96 × standard deviation of the bias). A paired sample t‐test was used to determine significant differences between measurements at different time points in the SG and AG. All analyses were done with a commercial statistical software10 and significance was set at P < .05.
Results
The SG had normal general physical examinations and no complications during or after completing the protocol. All horses in the SG showed frequent second degree atrioventricular and/or sinus block/arrest after phenylephrine. Three mares in the SG developed reflex tachycardia after acepromazine.
A total of 148 and 124 sets of paired NIBP and IBP measurements were obtained in the SG and AG, respectively. The cuff width to tail circumference ratio was 0.47 ± 0.03 and 0.49 ± 0.05, and the vertical distance was 26.0 ± 3.0 and 24.1 ± 6.0 in the SG and the AG, respectively. One SG mare was sedated for catheter placement. One mare in the SG had no pulse rates within 20% of the HR obtained electrocardiographically after phenylephrine and this data point was excluded from analysis. One intra‐arterial catheter was lost before IBP after acepromazine could be obtained. The AG data were collected for the interval below 65 mmHg in 6/8 horses, between 65–90 mmHg in 8/8 horses and above 90 mmHg in 6/8 horses. A summary of IBP and NIBP in standing and anesthetized horses is shown in Table 1.
Table 1.
Summary of invasive blood pressure (IBP) and noninvasive blood pressure (NIBP) and heart rate (HR) in standing and anesthetized horses
| SYS (mmHg) | DIA (mmHg) | MEAN (mmHg) | HR (bpm) | ||||
|---|---|---|---|---|---|---|---|
| IBP | NIBP | IBP | NIBP | IBP | NIBP | ||
| Standing group | |||||||
| Hypotensive Phase (n = 7) | 119.5 ± 9.3a | 103.6 ± 8.7a (83.3 ± 7.2a) | 73.9 ± 7.9a | 65.5 ± 10.0a (45.2 ± 7.8a) | 89.1 ± 6.8a | 78.6 ± 9.7a (58.2 ± 7.7a) | 45.5 ± 9.5a |
| Normotensive Phase (n = 8) | 169.0 ± 24.6 | 142.3 ± 20.5 (122.2 ± 20.4) | 110.4 ± 16.3 | 100.2 ± 17.9 (80.2 ± 17.4) | 131.5 ± 18 | 116.2 ± 19.4 (96.2 ± 19.1) | 34.0 ± 4.2 |
| Hypertensive Phase (n = 7) | 236.7 ± 17.9a | 179.6 ± 35.6a (159.5 ± 33.4a) | 148.2 ± 15.6a | 141.4 ± 28.5a (121.3 ± 26.6a) | 180.7 ± 11.9a | 155.7 ± 30.0a (135.6 ± 28.0a) | 35.5 ± 4.6 |
| Anesthetized group | |||||||
| Hypotensive Phase (n = 6) | 82.4 ± 7.8a | 79.3 ± 8.6a (97.7 ± 8.0a) | 43.0 ± 3.1a | 40.8 ± 10.0a (59.2 ± 9.1a) | 57.1 ± 4.7a | 54.9 ± 10.6a (73.2 ± 10.0) | 35.9 ± 3.1 |
| Normotensive Phase (n = 8) | 106.6 ± 8.5 | 103.3 ± 10.8 (121.9 ± 10.6) | 59.7 ± 5.3 | 59.6 ± 10.1 (78.2 ± 9.7) | 76.1 ± 5.0 | 75.7 ± 8.6 (94.3 ± 8.6) | 30.3 ± 4.1 |
| Hypertensive Phase(n = 6) | 125.5 ± 14.1a | 122.0 ± 20.8 (139.5 ± 18.8) | 75.8 ± 5.2a | 76.0 ± 17.0a (93.5 ± 15.1a) | 93.2 ± 3.1a | 94.5 ± 17.4a (111.9 ± 15.4a) | 32.6 ± 3.4 |
SYS, systolic; DIA, diastolic. NIBP values in brackets = noncorrected measurements.
Significant difference compared to normotensive phase.
The mean bias, lower and upper limit of agreement, and R are listed in Table 2. Scatter and Bland and Altman plots for mean BP are shown in Figures 1 and 2. When phases were studied separately, significant correlations were detected for the normotensive phase in the SG and the SAP of the normo‐ and hypertensive phase in the AG (Table 2). Validation criteria recommended by the ACVIM for small animals was only met for SAP in anesthetized horses. The MAP in anesthetized horses met all criteria aside from a lower than recommended R (Table 3).
Table 2.
Results of Bland and Altman analysis and correlation between invasive and noninvasive blood pressure for the standing and the anesthetized group
| Bias (mmHg) | LLA (mmHg) | ULA (mmHg) | R | P | |
|---|---|---|---|---|---|
| Standing group: all phases combined (n = 8) | |||||
| Systolic BP | 30.0 | −18.8 | 78.7 | 0.84 | <0.001 |
| Diastolic BP | 8.9 | −25.5 | 43.3 | 0.84 | <0.001 |
| Mean BP | 16.4 | −16.1 | 48.9 | 0.88 | <0.001 |
| Standing group: hypotensive phase (n = 7) | |||||
| Systolic BP | 15.9 | −0.4 | 32.2 | 0.58 | 0.18 |
| Diastolic BP | 8.4 | −9.8 | 26.6 | 0.45 | 0.31 |
| Mean BP | 10.5 | −5.1 | 26.1 | 0.58 | 0.17 |
| Standing group: normotensive phase (n = 8) | |||||
| Systolic BP | 26.8 | −2.1 | 51.4 | 0.85 | 0.01 |
| Diastolic BP | 10.2 | −15.3 | 35.6 | 0.72 | 0.05 |
| Mean BP | 15.3 | −9.6 | 40.1 | 0.78 | 0.02 |
| Standing group: hypertensive phase (n = 7) | |||||
| Systolic BP | 57.2 | −10.9 | 125.3 | 0.30 | 0.51 |
| Diastolic BP | 6.8 | −54.1 | 67.6 | 0.10 | 0.83 |
| Mean BP | 25.0 | −29.8 | 79.7 | 0.37 | 0.42 |
| Anesthetized group: all phases combined (n = 8) | |||||
| Systolic BP | 3.3 | −13.3 | 19.8 | 0.92 | <0.001 |
| Diastolic BP | 0.6 | −22.8 | 24.0 | 0.76 | <0.001 |
| Mean BP | 0.5 | −22.3 | 23.2 | 0.81 | <0.001 |
| Anesthetized group: <65 mmHg phase (n = 6) | |||||
| Systolic BP | 3.0 | −11.6 | 17.6 | 0.59 | 0.22 |
| Diastolic BP | 2.1 | −16.7 | 21.0 | 0.26 | 0.61 |
| Mean BP | 2.3 | −17.8 | 22.4 | 0.28 | 0.59 |
| Anesthetized group: 65–90 mmHg phase (n = 8) | |||||
| Systolic BP | 3.3 | −9.8 | 16.4 | 0.79 | 0.02 |
| Diastolic BP | 0.0 | −24.1 | 24.2 | −0.20 | 0.64 |
| Mean BP | 0.4 | −20.6 | 21.3 | −0.18 | 0.67 |
| Anesthetized group: >90 mmHg phase (n = 6) | |||||
| Systolic BP | 3.5 | −20.8 | 27.8 | 0.81 | 0.05 |
| Diastolic BP | −0.2 | −30.2 | 29.8 | 0.46 | 0.35 |
| Mean BP | −1.2 | −31.5 | 29.0 | 0.68 | 0.14 |
LLA, Lower limit of agreement; ULA, upper limit of agreement; P, probability level; R, Pearson correlation coefficient.
Figure 1.
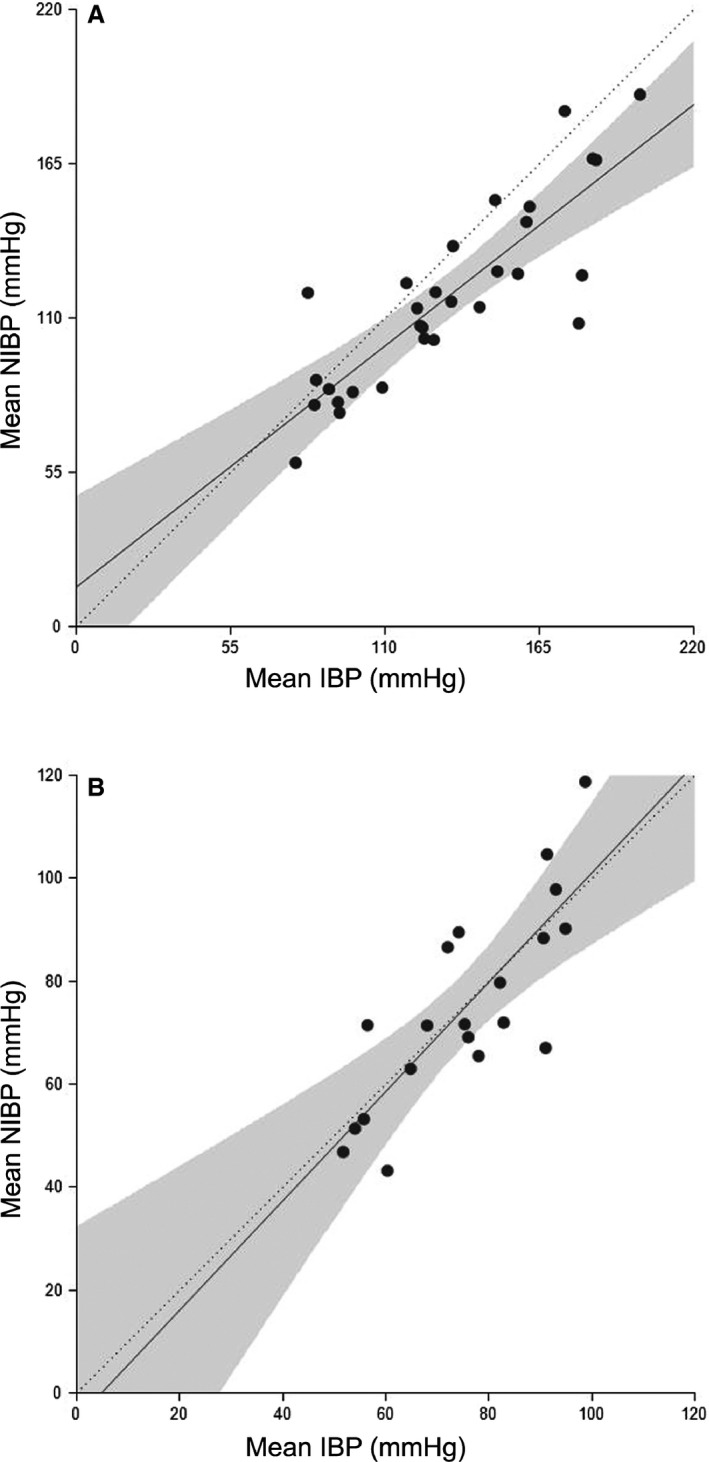
Mean noninvasive blood pressure (NIBP) values plotted against the invasive blood pressure (IBP) (n = 8) in the standing horse (A) and under general anesthesia (GA) (B). The solid line represents the regression line, the dotted line the 45° line and the filled area the confidence band.
Figure 2.
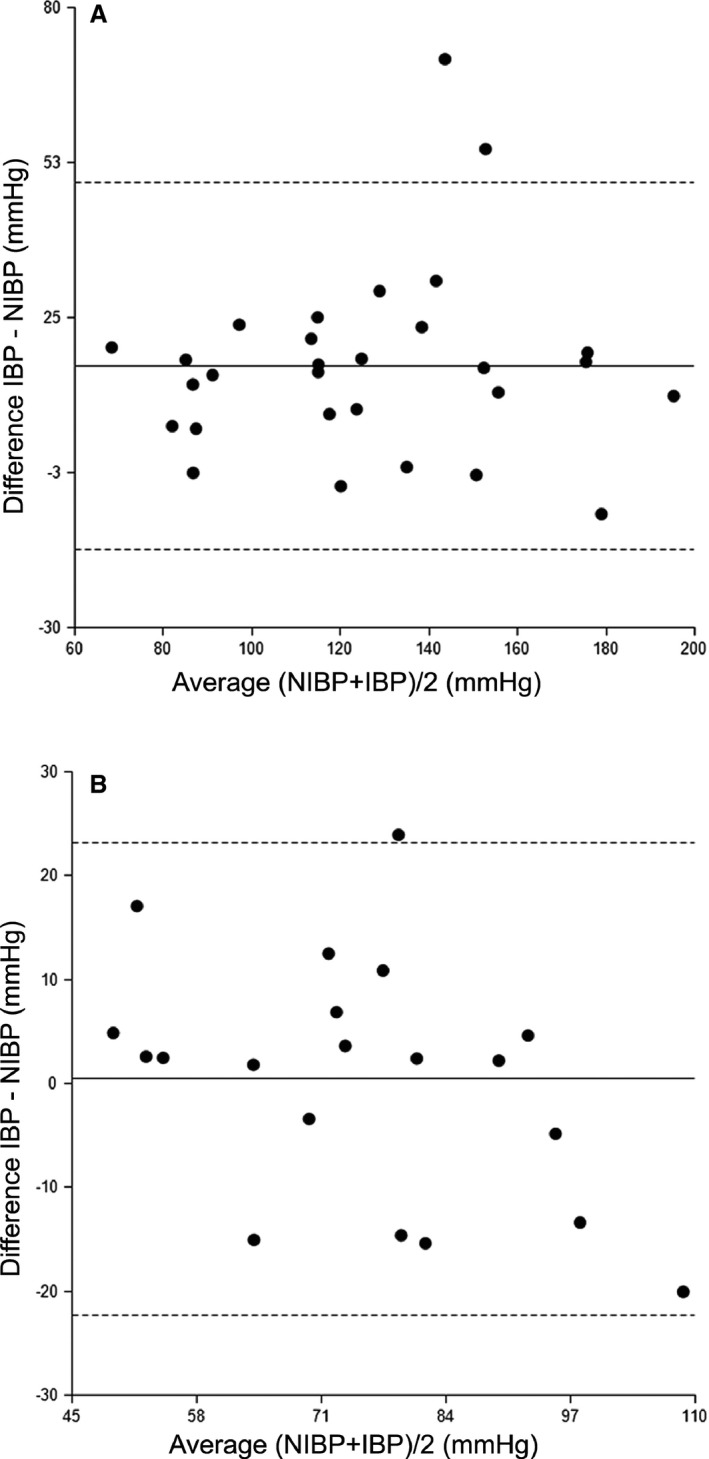
Bland and Altman plot of mean arterial blood pressure (BP) (n = 8) in the standing horse (A) and under general anesthesia (GA) (B). Noninvasive blood pressure= NIBP, Invasive blood pressure= IBP. Mean bias (solid line) and Lower and upper limits of agreement (dotted line) are indicated.
Table 3.
Adherence to the validation criteria for blood pressure devices recommended by ACVIM consensus statement establishing the “Guidelines for the Identification, Evaluation, and Management of Systemic Hypertension in Dogs and Cats”
| Standing Group (n = 8) | Anesthetized Group (n = 8) | ACVIM Criteria | |||||
|---|---|---|---|---|---|---|---|
| SYS | DIA | MEAN | SYS | DIA | MEAN | ||
| Mean Difference of paired readings (mmHg) | 30.0 | 8.9 | 16.4 | 3.3 | 0.6 | 0.5 | ≤ ±10 |
| Standard Deviation of the difference (mmHg) | 24.9 | 17.5 | 16.6 | 8.5 | 12.0 | 11.6 | ≤ 15 |
| Correlations (R) between paired pressures | 0.84 | 0.84 | 0.88 | 0.92 | 0.76 | 0.81 | ≥0.9 |
| Within 10 mmHg of IBP (%) | 17 | 52 | 31 | 80 | 70 | 55 | ≥50 |
| Within 20 mmHg of IBP (%) | 34 | 79 | 72 | 95 | 90 | 95 | ≥80 |
IBP, invasive blood pressure; SYS, Systolic blood pressure; DIA, diastolic blood pressure; MEAN, mean blood pressure.
Underlined values meet validation criteria.
Discussion
Noninvasive blood pressure was strongly correlated with IBP and reliably differentiated increases and decreases in BP in the SG and AG. However, in standing horses the NIBP underestimated IBP and the accuracy and precision were low, indicated by the large bias and wide limits of agreement, respectively.
Several studies have investigated the agreement of NIBP and IBP monitors in horses (Table S2). Different study designs, including different age, size, position, consciousness, correction factors, devices, and arteries used, make direct comparison difficult. Similar to previous findings,9 the overall agreement in our study was higher in anesthetized animals. This could be because of factors such as motion, muscle tone and anxiety causing erroneous detection of pressure waveforms. Previous studies suggested that more accurate NIBP measurements could be obtained in neonates,7, 9 however, in the study presented here performance of the oscillometric device in the AG was similar to previous studies in foals. Compared to other studies in adult horses under general anesthesia, the device used in this study showed better accuracy and similar or better precision.8, 18 Results in standing horses1 , 2 vary more between studies. Explanations for discrepancies include one or more of: the use of different devices, horses' compliance and different BP ranges.
Mean arterial pressure is a better marker of potential flow than SAP or DAP and is minimally affected by amplifications of pressure waveform in peripheral vessels.2, 19 The maximum oscillation amplitude, representing MAP, is more accurately detected by oscillometric monitors than the algorithm‐dependent SAP and DAP,20, 21 which is why some previous studies only report MAP.7, 22 We chose to report all three variables, as one of our aims was device evaluation.11 Interestingly in the AG, while the bias was slightly larger than for MAP (3 versus 0.5 mmHg), the best accuracy, precision and fulfillment ACVIM criteria were seen in SAP. Validation criteria for small animals were based on challenges when acquiring standardized readings, current literature on indirect devices and intervention points for treatment in these species.11 The fact that guidelines were only met for the systolic NIBP in the AG in this study should be interpreted with caution as requirements for horses have not been formally established.
Although direct techniques measure the pressure within a vessel, indirect methods use cuff pressure to estimate arterial pressure.23 Therefore, changes in the physical properties of the arterial wall mainly affect indirect methods. Both phenylephrine and acepromazine have antagonistic effects on postsynaptic alpha‐adrenergic receptors, acting on smooth muscle of vasculature, inducing vasoconstriction and vasodilation, respectively.24, 25 This alters the viscoelastic properties of the artery, possibly causing larger discrepancies seen during hypo‐ and hypertension.26
Controversies exist about the influence of different pressure states on the accuracy of the oscillometric BP in horses and other species. Some reports show negative effects of hypotension on the accuracy of NIBP in horses,27 cats28 and dogs,29 whereas other reports in adult horses30 and foals found no effect.9 Previous studies reported no effects when pressure was modulated by anesthetic depth and administration of dobutamine and phenylephrine in anesthetized foals.7 In contrast, a recent report on anesthetized adult horses inducing hypertension with dobutamine and norepinephrine and hypotension with isoflurane and nitroglycerine, found poor agreement between IBP and NIBP in hypo‐ and hypertensive ranges.18 In anesthetized small animals, measurements during phenylephrine‐induced hypertension were accurate,31 although an increase in bias was shown with higher pressure ranges in another study.32 In clinical practice, inotropes and vasopressors are used frequently in hemodynamically compromised animals. The differences between measurements in naturally occurring and pharmacologically induced hypo‐ or hypertension deserve attention.
The effect of gravity and the large vertical distance between the coccygeal artery and the base of the heart in horses results in underestimation of BP when this is measured in the coccygeal artery with the equine standing and overestimation of BP using the same location with a horse in dorsal recumbency. Measurement results should therefore be corrected to heart level or described as “uncorrected” values.33 Although a correction factor of 0.77 mmHg/cm of vertical distance between the base of the tail and the point of the shoulder12 was added or subtracted in standing or dorsally recumbent horses, respectively, most corrected NIBP readings underestimated IBP readings in the standing group in this study. This correction factor is physiologically derived and has been validated in humans.34 The factor has been described and traditionally used in horses.12 The evaluation of different correction factors would be interesting but is beyond the scope of this experiment.
The use of cuff width to tail circumference ratios from 0.2 to 0.92, 7, 9, 13 have been described, Cuffs that are too wide will cause underestimation and cuffs that are too small cause overestimation of NIBP.2, 13, 33 Arguably, smaller ratios could have reduced the bias in this study similarly to the report in anesthetized horses.22 In a recent study, a reduction in the mean difference between IBP and NIBP measurements from 9.91 to 0.39 mmHg was seen with a change in cuff width to appendage circumference ratio from 0.42 to 0.25 in horses placed in lateral recumbency using a similar oscillometric monitor. Interestingly, the mean bias in the AG we report, where a cuff width to tail circumference ratio of 0.49 ± 0.05 was used, was 0.5 mmHg, similar to the lowest mean difference reported in the mentioned study,22 making it uncertain if a different cuff size would improve the accuracy of NIBP measurements.
Incompatibilities between changes in pulse wave forms and the applied algorithm induced by changes in HR and rhythm might be another explanation for the low accuracy and precision. It has been reported that the rate of the cuff deflation to the HR can be a source of error, if the deflation rate is too rapid or the HR is too slow.35 It has been shown that changes in HR cause a significant alteration in the shape of the plateau of the oscillometric curve, which particularly affects the MAP.26 In our study, all of the horses developed frequent second degree atrioventricular, sinus block/arrest, or both after phenylephrine and reflex tachycardia was seen in 3 mares in the SG after acepromazine administration, which represents a plausible source of inaccuracy. Moreover, phenylephrine modulates the pulse wave contour and impedance in distally located vessels, which might interfere with the device's accuracy.31
Limitations of this study include the use of a different peripheral artery for IBP and NIBP. Pressures vary between arteries because of reflections of the forward propagating pressure pulse wave, leading to amplification of the SAP27 and slight dampening of the DAP in distally located vessels, whereas the MAP stays fairly unaffected.19 The aortic root might be considered the gold standard for IBP measurements. Central BP can be assessed in the common carotid artery, as a surrogate for the aortic pressures19 and a recent report in anesthetized horses showed good agreement in blood pressure measurements between the facial and the carotid artery,10 making the facial artery appropriate for our aims. Another limitation of the study is the limited sample size and the homogeneity of size and breed within the study population. It is therefore true that making extrapolations for the entire equine population could be difficult, yet the sample is representative of horses of average size and weight. The choice of sample size was based on the ACVIM guidelines that recommended a minimum of 8 animals when intra‐arterial methods are used for comparison.11
While the evaluated device allows an estimation of BP and adequate differentiation of trends in pressure, the device's accuracy and precision was limited in standing horses compared to those under general anesthesia. Observations in larger populations, in nonpharmacologically induced, naturally occurring hyper‐ and hypotensive horses, and studies with different pharmacologic interventions could help to further evaluate the source of the inaccuracy and define factors to be taken into account when interpreting NIBP measurement in compromised, standing horses.
Supporting information
Table S1. Summary of anesthetic protocol and procedures used in the 8 horses in the anesthetized group.
Table S2. Comparison of recent studies evaluating oscillometric blood pressure monitors.
Acknowledgments
The authors gratefully acknowledge Vinzenz Gerber, Dominik Burger and Murielle Lauper for assistance with organization of the experiment and evaluation of results. The authors thank the Swiss National Stud for their support.
Grant support: The study was supported by a grant of the Department for Clinical Veterinary Medicine of the University of Bern. The data were partially presented at the 2015 ACVIM Forum in Indianapolis, Indiana.
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Footnotes
Olsen E, Kronborg C, Buhl R, Andersen PH. How to obtain indirect blood pressure in the standing horse? ACVIM Forum 2011 (abstract)
Zacche E, Gravena K, Gering AP, Hernandez‐Tovar MC, Gomide, Lacerda‐Neto JC, Camacho AA. Validation of three different noninvasive devices to assess arterial blood pressure in standing and nonsedated horses. Journal Of Veterinary Internal Medicine 2013, 655–655. (abstract)
Televet, Engel Engineering Service GmbH, Offenbach am Main, Germany
Cardell Veterinary Monitor 9402, CAS Medical Systems, Brandford, CT, USA
Datex Ohmeda, S3, GE, Datex‐ Ohmeda Division, Helsinki, Finland
Xylasol, Graeub AG, Bern, Switzerland
Phenylephrine HCL, Dr. G. Bichsel AG, Interlaken, Switzerland
Prequillan, Fatro S.p.A., Ozzano Emilia, Italy
Dobutrex 250 mg/5 mL, Teva Pharma AG, Basel, Switzerland
NCSS 9. NCSS, LLC. Kaysville, UT
References
- 1. Shih A. Cardiac output monitoring in horses. Vet Clin North Am Equine Pract 2013;29:155–167. [DOI] [PubMed] [Google Scholar]
- 2. Marsh PS. Approach to equine critical care: Monitoring Arterial Blood Pressure In: Reed SM, Bayly WM, Sellon DC, eds. Equine Imternal Medicine, 3rd ed St. Louis, MO: Saunder; 2010:254–256. [Google Scholar]
- 3. Shoemaker WC. Invasive and noninvasive monitoring In: Shoemaker WC, Ayres SM, Grenvik A, eds. Textbook of Critical Care, 4th ed Philadelphia, PA: Saunders; 2000:74–92. [Google Scholar]
- 4. Rugh KS, Garner HE, Sprouse RF, Hatfield DG. Left ventricular hypertrophy in chronically hypertensive ponies. Lab Anim Sci 1987;37:335–338. [PubMed] [Google Scholar]
- 5. Bailey SR, Habershon‐Butcher JL, Ransom KJ, et al. Hypertension and insulin resistance in a mixed‐breed population of ponies predisposed to laminitis. Am J Vet Res 2008;69:122–129. [DOI] [PubMed] [Google Scholar]
- 6. Navas de Solis C, Slack J, Boston RC, Reef VB. Hypertensive cardiomyopathy in horses: 5 cases (1995–2011). J Am Vet Med Assoc 2013;243:126–130. [DOI] [PubMed] [Google Scholar]
- 7. Giguere S, Knowles HA, Valverde A, et al. Accuracy of indirect measurement of blood pressure in neonatal foals. J Vet Intern Med 2005;19:571–576. [PubMed] [Google Scholar]
- 8. Branson KR. A clinical evaluation of an oscillometric blood pressure monitor on anesthetized horses. J Equine Vet Sci 1997;17:537–540. [Google Scholar]
- 9. Nout YS, Corley KT, Donaldson LL, Furr MO. Indirect oscillometric and direct blood pressure measurements in anesthetized and conscious neonatal foals. J Vet Emerg Crit Care 2002;12:75–80. [Google Scholar]
- 10. Gent TC, Schwarz A, Hatz LA, et al. Evaluation of accuracy of invasive and non‐invasive blood pressure monitoring in relation to carotid artery pressure in anaesthetised ponies. Pferdeheilkunde 2015;31:33–38. [Google Scholar]
- 11. Brown S, Atkins C, Bagley R, et al. Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med 2007;21:542–558. [DOI] [PubMed] [Google Scholar]
- 12. Parry BW, McCarthy MA, Anderson GA. Survey of resting blood pressure values in clinically normal horses. Equine Vet J 1984;16:53–58. [DOI] [PubMed] [Google Scholar]
- 13. Magdesian KG. Monitoring the critically ill equine patient. Vet Clin North Am Equine Pract 2004;20:11–39. [DOI] [PubMed] [Google Scholar]
- 14. Pequito M, Amory H, Serteyn D, et al. Comparison of the sedative and hemodynamic effects of acepromazine and promethazine in the standing horse. J Equine Vet Sci 2012;32:799–804. [Google Scholar]
- 15. Leise BS, Fugler LA, Stokes AM, et al. Effects of intramuscular administration of acepromazine on palmar digital blood flow, palmar digital arterial pressure, transverse facial arterial pressure, and packed cell volume in clinically healthy, conscious horses. Vet Surg 2007;36:717–723. [DOI] [PubMed] [Google Scholar]
- 16. Hardy J, Bednarski RM, Biller DS. Effect of phenylephrine on hemodynamics and splenic dimensions in horses. Am J Vet Res 1994;55:1570–1578. [PubMed] [Google Scholar]
- 17. Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res 1999;8:135–160. [DOI] [PubMed] [Google Scholar]
- 18. Tünsmeyer J, Hopster K, Feige K, Kästner SB. Agreement of high definition oscillometry with direct arterial blood pressure measurement at different blood pressure ranges in horses under general anaesthesia. Vet Anaesth Analg 2015;42:286–291. [DOI] [PubMed] [Google Scholar]
- 19. Avolio AP, Van Bortel LM, Boutouyrie P, et al. Role of pulse pressure amplification in arterial hypertension experts' opinion and review of the data. Hypertension 2009;54:375–383. [DOI] [PubMed] [Google Scholar]
- 20. Kittleson MD. Measurement of systemic arterial blood pressure (Dogs, cats, disease diagnosis). Vet Clin North Am Small Anim Pract 1983;13:321–336. [DOI] [PubMed] [Google Scholar]
- 21. Geddes LA, Voelz M, Combs C, et al. Characterization of the oscillometric method for measuring indirect blood pressure. Ann Biomed Eng 1982;10:271–280. [DOI] [PubMed] [Google Scholar]
- 22. Tearney CC, Guedes AGP, Brosnan RJ. Equivalence between invasive and oscillometric blood pressures at different anatomic locations in healthy normotensive anaesthetised horses. Equine Vet J 2015. doi:10.1111/evj.12443. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23. Podell M. Use of blood pressure monitors In: Kirk R, Bonagura JD, eds. Kirk's Current Veterinary Therapy XI: Small Animal Practice. Philadelphia, PA: WB Saunders Co; 1992:834–837. [Google Scholar]
- 24. Plumb DC. Phenylephrine In: Plumb DC, ed. Plumb's Veterinary Drug Handbook. Ames: Blackwell Publishing; 2008:724. [Google Scholar]
- 25. Plumb DC. Acepromazine maleate In: Plumb DC, ed. Plumb's Veterinary Drug Handbook. Ames: Blackwell Publishing; 2008:3. [Google Scholar]
- 26. Ursino M, Cristalli C. A mathematical study of some biomechanical factors affecting the oscillometric blood pressure measurement. IEEE Trans Biomed Eng 1996;43:761–778. [DOI] [PubMed] [Google Scholar]
- 27. Muir WW, Wade A, Grospitch B. Automatic noninvasive sphygmomanometry in horses. J Am Vet Med Assoc 1983;182:1230–1233. [PubMed] [Google Scholar]
- 28. Caulkett NA, Cantwell SL, Houston DM. A comparison of indirect blood pressure monitoring techniques in the anesthetized cat. Vet Surg 1998;27:370–377. [DOI] [PubMed] [Google Scholar]
- 29. Bosiack AP, Mann FA, Dodam JR, et al. Comparison of ultrasonic Doppler flow monitor, oscillometric, and direct arterial blood pressure measurements in ill dogs. J Vet Emerg Crit Care 2010;20:207–215. [DOI] [PubMed] [Google Scholar]
- 30. Latshaw H, Fessler JF, Whistler SJ, Geddes LA. Indirect measurement of mean blood pressure in the normotensive and hypotensive horse. Equine Vet J 1979;11:191–194. [DOI] [PubMed] [Google Scholar]
- 31. McMurphy RM, Stoll MR, McCubrey R. Accuracy of an oscillometric blood pressure monitor during phenylephrine‐ induced hypertension in dogs. Am J Vet Res 2006;67:1541–1545. [DOI] [PubMed] [Google Scholar]
- 32. Binns SH, Sisson DD, Buoscio DA, Schaeffer DJ. Doppler ultrasonographic, oscillometric sphygmomanometric, and photoplethysmographic techniques for noninvasive blood pressure measurement in anesthetized cats. J Vet Intern Med 1995;9:405–414. [DOI] [PubMed] [Google Scholar]
- 33. Parry BW, McCarthy MA, Anderson GA, Gay CC. Correct occlusive bladder width for indirect blood pressure measurement in horses. Am J Vet Res 1982;43:50–54. [PubMed] [Google Scholar]
- 34. Gornik HL, Garcia B, Wolski K, et al. Validation of a method for determination of the ankle‐brachial index in the seated position. J Vasc Surg 2008;48:1204–1210. [DOI] [PubMed] [Google Scholar]
- 35. Ramsey M III. Blood pressure monitoring: Automated oscillometric devices. J Clin Monit 1991;7:56–67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Summary of anesthetic protocol and procedures used in the 8 horses in the anesthetized group.
Table S2. Comparison of recent studies evaluating oscillometric blood pressure monitors.


