Abstract
Background
Exacerbated postparturient insulin resistance (IR) has been associated with several pathologic conditions in dairy cattle. Oxidative stress (OS) plays a causative role in IR in humans, and an association, but not direct relationship, between OS and IR recently has been reported in transition dairy cattle.
Hypothesis
Supplementation with antioxidants shortly before calving improves glucose tolerance after parturition in dairy cattle.
Animals
Ten late‐pregnant Holstein cows entering their 2nd to 5th lactation.
Methods
Randomized placebo‐controlled trial: 15 ± 2 days before expected calving, the treatment group received an injection of DL‐alpha‐tocopheryl acetate at a dosage of 6 mg/kg body weight (BW) and 0.06 mg/kg BW of sodium selenite, and the control group was injected with isotonic saline. During the first week after calving, both groups underwent glucose tolerance testing (0.25 g glucose/kg BW). Commercial assays were used to quantify the concentrations of glucose, insulin, nonesterified fatty acids (NEFA), beta‐hydroxybutyrate, and markers of redox status in blood. Data were analyzed using the Mann–Whitney U‐test (α = 0.05).
Results
Supplemented cows showed a lower risk for OS, as reflected by a lower OS index (P = .036), different areas under the curve for the concentrations of glucose (P < .01), insulin (P = .043), and NEFA (P = .041), more rapid elimination rates (P = .080, <.01 and .047 respectively), and shorter half‐lives (P = .040, <.01 and .032) of these metabolites.
Conclusions and Clinical Importance
Supplementation with antioxidants before calving resulted in greater insulin sensitivity after calving, thereby suggesting the role of OS in the development of IR in cattle and the potential benefits of antioxidant supplementation in minimizing the consequences of negative energy balance.
Keywords: Inflammation, Insulin resistance, Oxidative stress, Transition period, Vitamin E
Abbreviations
- AUC
area under the curve
- BHBA
beta‐hydroxybutyrate
- BW
body weight
- IQR
interquartile range
- IR
insulin resistance
- IVGTT
intravenous glucose tolerance test
- NEFA
nonesterified fatty acids
- NFκB
nuclear factor kappa B
- OS
oxidative stress
- OSi
oxidative stress index
- ROS
reactive oxygen species
- SAC
serum antioxidant capacity
- SD
standard deviation
- T1/2
time to reach half‐maximal concentration
- Tbasal
time to reach basal concentration
As dairy cows transition from late pregnancy to the onset of lactation, they are faced with marked and sudden metabolic and endocrine changes that negatively impact their performance and health status. Insulin plays a pivotal role in the partitioning processes that take place to support lactation. Cows undergo a period of insulin resistance (IR) before calving to support fetal glucose needs as well as after calving to prioritize the insulin‐independent uptake of glucose by the mammary gland.1 A prolonged IR state has been related to several pathologic processes, including economically important postpartum conditions such as displaced abomasum2 or decreased fertility as a consequence of enhanced lipolysis.3 The mechanisms causing IR are not fully understood in dairy cattle, but this period of IR has physiologic similarities to human type I and type II diabetes,4 with the major difference being that cows have low glucose concentrations.5 In human type II diabetes, strong evidence supports that oxidative stress (OS), the imbalance between pro‐oxidant production and antioxidant capacity, plays a causative role in the development of IR,6, 7 and antioxidant supplementation can be used to decrease the consequences of IR.7, 8, 9, 10 It is now well known that dairy cattle experience OS after calving,11, 12 and antioxidant supplementation can diminish the harmful effects of excessive pro‐oxidant production.13 We recently found a significant association between oxidant status and whole‐body insulin sensitivity, measured by means of surrogate indices, in periparturient dairy cattle.14 We therefore hypothesized that antioxidant supplementation before calving may impact glucose homeostasis (assessed by means of intravenous glucose tolerance testing [IVGTT]) after calving. Hence, our study aimed to establish a causal relationship between oxidant status and insulin sensitivity in dairy cattle during the transition period.
Material and Methods
A randomized placebo‐controlled study was used. The protocols of this study were approved by the Bioethical Committee of the University of Santiago de Compostela (Spain), and the animals were enrolled with owner consent.
Animals, Nutrition and Husbandry
Ten nonlactating, late‐pregnant Holstein cows from the same commercial herd, located in Meira (northwest Spain), were used in this study. Selection criteria included: parity (entering their 2nd or greater lactation), milk production in the preceding lactation (9000 to 9500 kg), body condition score (3 to 3.5, on a 1 [lean] to 5 [obese] scale as previously described15), and proximity in their expected calving date. Cows in both groups were maintained under identical conditions throughout the study. Animals were kept in a free‐stall barn with concrete stalls and fed a total mixed ration (Table 1), delivered once daily at 9:00 am and formulated according to the National Research Council (NRC)16 to meet or exceed their requirements. Lactating animals were milked twice daily and cows were dried‐off 60 days before their expected calving date.
Table 1.
Ingredients and chemical composition of the diets fed to the cows in the different stages of the study
| Diet Composition (kg dry matter/cow/d) | Lactating Diet | Pre‐Fresh Diet |
|---|---|---|
| Total dry matter offered | 21.7 | 13.2 |
| Grass silage | 8.6 | 5.8 |
| Grass hay | — | 7.4 |
| Concentratea | 13.10 | — |
| Nutrient analysis | ||
| Dry matter (%) | 43.7 | 36.7 |
| Crude protein (% DM) | 16.7 | 9.9 |
| Neutral detergent fiber (% DM) | 35.4 | 61.6 |
| Acid detergent fiber (% DM) | 21.9 | 39.3 |
| Starch (% DM) | 21.6 | 13.8 |
| Ether extract content (% DM) | 6.2 | 2.7 |
| Ashes (% DM) | 8.4 | 7.1 |
| ENL (MJ/kg DM) | 6.76 | 5.19 |
DM, dry matter.
Concentrate composition (% as fed): corn (49.7), soybean meal (19.9), rapeseed meal (11.5), barley (7.5), vegetable soapstock (3.6), beet molasses (2.50), calcium carbonate (1.6), calcium bicarbonate (1.5), sodium chloride (.9), and vitamin/mineral premix (.4). The vitamin/mineral premix contained: 16650 IU/kg vitamin A, 4350 IU/kg vitamin D3, 66.65 mg/kg vitamin E (α‐tocopherol), 120 mg/kg Zn (oxide), 50 mg/kg Mn (oxide II), 27.5 mg/kg Cu (sulfate), 7.6 mg/kg Fe (sulfate), 2.0 iodine (potassium iodide), 1.3 mg/kg Co (carbonate), and .5 mg/kg Se (sodium selenite).
Treatment Allocation
Animals were randomly allocated to treatment or control groups using the random function of Excel.1 A blood sample was collected 15 ± 2 days before expected calving by coccygeal venipuncture into evacuated tubes without anticoagulant,2 and animals in the supplementation group subsequently received an IM injection of a commercial product3 at a dosage of 6 mg/kg body weight (BW) of DL‐alpha‐tocopheryl acetate (equivalent to 6 IU/kg BW of vitamin E) and 0.06 mg/kg BW of sodium selenite, whereas cows in the control group were injected with isotonic sterile saline solution.4 BWs for dose calculation were adjusted estimating the weight of the conceptus according to NRC16 using an estimated calf birth weight of 40 kg. The farm personnel, but not the investigators, were blinded to group allocation. Because of longer gestation lengths than expected, the interval between treatment and calving ranged from 9 to 19 days (mean ± SD: 16 ± 4.76).
Intravenous Glucose Tolerance Test
Between days 3 to 7 after calving, animals in both groups were subjected to IVGTT around 3:00 pm, thereby allowing 6 hour between when the ration was offered and the infusion of glucose to decrease any potential interference in blood metabolite clearance patterns. Cows were restrained in the feedbunk headlocks and the feed was removed from their access. A 14‐gauge × 8 cm catheter with a 250 mL/min capacity5 was inserted in either the right or the left jugular vein. Cows were allowed to rest for 15 minute after insertion of the catheter until blood sampling started. Stress was avoided as much as possible and cows generally appeared relaxed and continued to ruminate during the test. Blood samples were collected at −10, −5, 5, 10, 20, 30, 45, 60 and 90 minutes after the infusion of 0.25 g/kg BW of glucose.6 The infusion of glucose was completed in 3 to 4 minutes. After infusion, the catheters were irrigated with 10 mL of sterile salined and the first 5 mL of blood discarded from the first collection. Samples were collected into tubes without anticoagulant and tubes containing fluoride heparin.7
Laboratory Analysis
Samples were transported under refrigeration to the laboratory, where they were centrifuged at 2000 × g for 20 minutes within 2 hour after collection and the supernatant serum or plasma was harvested, aliquoted into 1.7 mL microcentrifuge tubes8 and stored at –80°C pending analysis within 3 months of collection. Commercially available kits were used for analysis. Plasma was analyzed for glucose concentration,9 whereas serum was used to measure the concentration of nonesterified fatty acids10 (NEFA) and beta‐hydroxybutyrate11 (BHBA).
Biomarkers of oxidant status were measured at enrollment into the study and in the basal IVGTT samples. Reactive oxygen species12 (ROS) were quantified in serum samples as markers of pro‐oxidants. The assay employed determines hydroperoxides (breakdown products of lipids and other organic substrates generated by oxidative attack of ROS) through their reaction with the chromogen N,N‐diethylparaphenylenediamine. This assay previously has been validated against electron spin resonance.17 Results are expressed in arbitrary ‘Carratelli units’ (Carr.U), with 1 Carr.U corresponding to the oxidizing power of 0.08 mg H2O2/dL. Total serum antioxidant capacity (SAC) also was quantified using a commercial assay.13 This test exploits the capacity of a concentrated solution of hypochlorous acid (HClO) to oxidize the complete pool of antioxidants in serum (albumin, bilirubin, uric acid, thiol groups, vitamins, glutathione, glutathione peroxidase, superoxide dismutase, catalase, and other compounds). Thus, SAC considers the cumulative action of all the antioxidants present in serum, rather than simply the sum of measurable antioxidants. Results are expressed as μmol HClO/mL. The oxidative stress index (OSi) was calculated as ROS/SAC.11 Thus, an increase in the ratio indicates a higher risk for OS because of an increase in ROS production, defensive antioxidant consumption, or both.
These analytical determinations were performed in duplicate on a biochemistry autoanalyzer14 calibrated against a multipoint calibrator.15 Physiologic16 and pathologic17 control sera, as well as an in‐house reference sample, were analyzed alongside the samples for quality control. Duplicated serum samples also were analyzed for insulin using a bovine‐specific ELISA kit,18 which has a limit of detection of 0.025 μg/L. Two samples fell below this limit and were assigned a concentration of 0.025 μg/L. The intra‐assay coefficients of variation for all the determinations were below 5%, with all samples analyzed in the same run.
IVGTT Data Processing
Basal concentrations for the studied analytes were determined as the mean concentration of the 2 blood samples taken before glucose infusion (−10 and −5 minutes samples). The area under the curve (AUC) of glucose, insulin, NEFA, and BHBA were computed with the trapezoidal method as the total increment of these metabolites above (below for NEFA and BHBA) basal concentrations during the 90 minutes after infusion. Peak and nadir concentrations of these analytes also were determined. Elimination rates and times to reach half‐maximal (T1/2) and basal (Tbasal) concentrations for glucose, insulin, NEFA, and BHBA were computed with the following formulas, as previously described18:
In these formulas, [t a] is the concentration of the metabolite at time a (t a) and [t b] is the concentration of metabolite at time b (t b).
Statistical Analyses
No assumptions for normality of data were made because of small sample size. All variable concentrations were analyzed with the Mann–Whitney U‐test using SPSS software19 and expressed as medians. Statistical significance was declared at P < .05, and values of P between .05 and .10 were considered a trend toward significance.
Results
No statistically significant differences between the control and supplemented groups were observed for the distribution of parity (mean ± SD: 2.4 ± 0.54 and 2.8 ± 1.07, respectively, P = .48), the number of days open before conception (123.2 ± 32.78 and 127.8 ± 85.37, P = .88), or adjusted BW at treatment allocation (637.4 ± 29.40 and 639.20 ± 37.55 kg, respectively, P = .91). Milk yields in the day preceding the IVGTT were similar between both groups (28.7 ± 4.47 vs. 29.6 ± 7.45 L, P = .37), as were the means ± SD of days postpartum at the time of IVGTT (4.8 ± 0.98 vs. 5.0 ± 1.09, P = .69). No differences between groups in the concentration of the studied analytes were found at the time of enrollment (Table 2), whereas during the IVGTT basal measurements, only the SAC and OSi differed, being higher and lower, respectively, in the supplemented group (Table 2), thereby indicating a decreased risk for OS.
Table 2.
Concentration of the studied serum/plasma analytes at the different time points of the study
| Variable | Units | Enrolment (2 weeks before expected calving) | Basal Measurements at IVGTT (3 to 7 DIM) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control Group (n = 5) | Supplemented Group (n = 5) | P‐Value | Control Group (n = 5) | Supplemented Group (n = 5) | P‐Value | ||||||
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | ||||
| ROS | Carr.U. | 97.4 | 51.1 | 104.2 | 52.5 | NS | 100.6 | 21.9 | 106.4 | 25.6 | NS |
| SAC | μmol HClO/mL | 222.4 | 217.6 | 327.1 | 208.8 | NS | 208.7 | 51.8 | 401.1 | 62.7 | <.01 |
| OSi | — | 0.47 | 0.29 | 0.38 | 0.34 | NS | 0.44 | 0.09 | 0.26 | 0.19 | .036 |
| Glucose | mg/dL | 76.2 | 23.4 | 76.7 | 14.7 | NS | 58.7 | 32.2 | 67.9 | 29.3 | NS |
| Insulin | μg/L | 0.46 | 0.39 | 0.29 | 0.17 | NS | 0.20 | 0.61 | 0.21 | 0.25 | NS |
| NEFA | mEq/L | 0.15 | 0.22 | 0.23 | 0.34 | NS | 0.71 | 0.53 | 0.68 | 0.50 | NS |
| BHBA | mg/dL | 8.35 | 7.73 | 8.25 | 8.04 | NS | 13.81 | 10.10 | 10.31 | 10.21 | NS |
ROS, Reactive oxygen species; SAC, Serum antioxidant capacity; OSi, Oxidative stress index; NEFA, nonesterified fatty acids; BHBA, beta‐hydroxybutyrate; IVGTT, intravenous glucose tolerance test; DIM, Days in milk; IQR, interquartile range.
Differences between groups were assessed using the Mann–Whitney U‐test. NS, Nonsignificant (P > .10).
Responses to the IVGTTs are quantified in Table 3. Cows supplemented with vitamin E and selenium showed a smaller glucose AUC, lower nadir concentration, and shorter half‐life. There was no difference between groups in maximum concentration during IVGTT (Fig 1A, P = .64) or Tbasal (P = .77) for glucose during IVGTT. Similar to the changes observed in glucose, the insulin AUC, insulin minimum concentration, and insulin half‐life were decreased in supplemented cows. Insulin secretion in response to glucose infusion (peak concentration) was not affected by treatment (Fig 1B, P = .29), but supplemented cows had more rapid insulin clearance (elimination rate) and a shorter Tbasal, requiring only 44% of the time required by nonsupplemented animals to reach basal insulin concentration after glucose infusion. Differences in fatty acid metabolism also were observed between groups (Fig 1C). Supplemented cows had larger NEFA AUC, a faster NEFA elimination rate, and a decreased NEFA half‐life. However, neither the peak nor nadir concentrations of NEFA were different between groups. In addition, the metabolism of ketones was similar between the 2 groups (Fig 1D), where only the nadir concentration of BHBA tended to be lower in supplemented animals (P = .086).
Table 3.
Comparison of the response to the IVGTT between supplemented and nonsupplemented animals
| Control Group (n = 5) | Supplementation Group (n = 5) | P‐Value | |||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| Area under the curve | |||||
| Glucose (mg/dL × 90 min) | 6615.6 | 1401.6 | 4047.6 | 1897.2 | <.01 |
| Insulin (μg/L × 90 min) | 108.4 | 31.8 | 74.9 | 44.8 | .043 |
| NEFA (mEq/L × 90 min) | −16.9 | 17.0 | −42.2 | 28.7 | .041 |
| BHBA (mg/dL × 90 min) | −181.4 | 323.7 | −203.1 | 348.4 | NS |
| Peak concentration | |||||
| Glucose (mg/dL) | 243.6 | 106.8 | 278.4 | 200.4 | NS |
| Insulin (μg/L) | 3.30 | 2.06 | 3.49 | 1.83 | NS |
| NEFA (mEq/L) | 0.47 | 0.63 | 0.50 | 0.35 | NS |
| BHBA (mg/dL) | 17.32 | 10.31 | 11.34 | 8.35 | NS |
| Nadir concentration | |||||
| Glucose (mg/dL) | 81.6 | 52.8 | 45.6 | 8.4 | .029 |
| Insulin (μg/L) | 0.74 | 0.32 | 0.22 | 0.10 | <.01 |
| NEFA (mEq/L) | 0.21 | 0.38 | 0.17 | 0.19 | NS |
| BHBA (mg/dL) | 12.37 | 7.63 | 7.53 | 5.57 | .086 |
| Elimination rate (%/min) | |||||
| Glucose | 1.83 | 1.05 | 2.50 | 0.91 | .080 |
| Insulin | 1.48 | 1.79 | 3.36 | 1.43 | <.01 |
| NEFA | 1.38 | 1.25 | 2.82 | 1.84 | .047 |
| BHBA | 0.56 | 0.42 | 0.84 | 0.53 | NS |
| Time to reach half‐maximal concentration (min) | |||||
| Glucose | 38.6 | 28.7 | 25.8 | 17.1 | .040 |
| Insulin | 46.7 | 92.0 | 20.6 | 6.03 | <.01 |
| NEFA | 40.6 | 21.3 | 24.2 | 10.8 | .032 |
| BHBA | 124.0 | 92.2 | 98.0 | 63.7 | NS |
| Time to reach basal concentration (min) | |||||
| Glucose | 70.0 | 30.0 | 85.0 | 37.5 | NS |
| Insulin | 85.0 | 7.5 | 70.0 | 20.0 | <.01 |
BHBA, beta‐hydroxybutyrate; IQR, interquartile range; NEFA, nonesterified fatty acids.
Comparisons between groups were made using the Mann–Whitney U‐test. NS, Not significant (P > .10).
Figure 1.
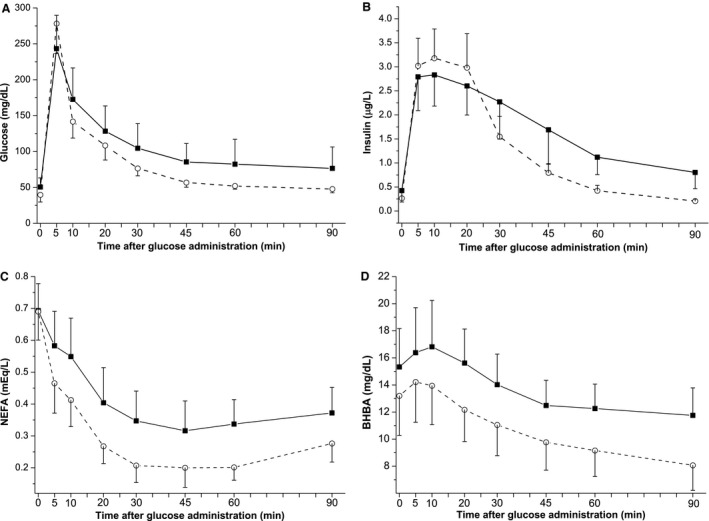
Mean serum/plasma concentration of (A) glucose, (B) insulin, (C) NEFA, and (D) BHBA during the IVGTT (0.25 g glucose/kg BW) performed between 3–7 days in milk. (‐■‐), Control group; (‐○‐), Supplemented group. Cows in the supplemented group received a parenteral supplementation containing vitamin E and selenium 15 ± 2 days before expected calving date. Graphs represent the mean ± SD. Time point 0 represents the baseline concentration (average of samples taken 10 and 5 minutes before glucose infusion). BHBA, beta‐hydroxybutyrate, IVGTT, intravenous glucose tolerance test; NEFA, nonesterified fatty acids.
Discussion
In humans suffering from diabetes, OS plays a causal role in the development of IR,7, 19 decreasing insulin biosynthesis and release.8 However, this direct relationship has hitherto not been proven in periparturient cattle, although from epidemiologic data we recently reported a significant association between markers of OS and IR in these animals.14 OS is well known in cattle as an underlying cause of dysfunctional inflammatory and host immune responses around the time of calving, thereby increasing cows’ susceptibility to health disorders.20 Indeed, antioxidant supplementation has shown an overall beneficial effect on the health status and performance of cows.13 OS links nutrient metabolism with inflammatory responses in transition cattle12 and therefore, supplementation with vitamin E and selenium precalving has the potential to alter the metabolic response of the animals to an IV infusion of glucose. Vitamin E (α‐tocopherol) is a potent lipid‐soluble, chain‐breaking antioxidant,21 and selenium also exerts antioxidant functions both directly and as a cofactor for selenoproteins.22 Hence, the parenteral administration of these 2 compounds increased the SAC of the animals (Table 2), thereby decreasing the risk for OS in the supplemented animals when they underwent IVGTT, as shown by the lower OSi values. Also, more individual variability was observed in SAC before treatment application than at IVGTT. Cows managed under identical conditions show high individual variability in their physiologic adaptation to metabolic stress around calving.23 Yet, cows at the onset of lactation typically show decreased antioxidant capacity,13 which could explain the decreased variability in control cows in the first week of lactation. On the other hand, supplemented cows all received the same dose of vitamin E and selenium at a similar time point, which contributes to a similar total antioxidant potential.
The dose of glucose administered during the IVGTT differed from some previous studies, which employed larger24 and smaller25 doses than used in this study. We selected a dosage of 0.25 g glucose per kg BW to facilitate the comparison of results, because this was the same or a similar dosage to that used in the majority of previous studies.26, 27, 28
Higher glucose tolerance was found in the supplemented animals, with lower glucose AUC and T1/2. Increased glucose elimination rates, decreased half‐life and decreased AUC are thought to involve increased insulin sensitivity.29 This assumption is further supported by the smaller insulin AUC, a quicker elimination rate, and shorter T1/2 and Tbasal for insulin found in supplemented animals. Similarly, a higher insulin AUC in control animals clearing the same dose of glucose indicates a higher degree of IR.27
In addition, differences in fatty acid metabolism were observed in the response to IVGTT in this study. In accordance with previous studies,27, 28 NEFA concentrations reached their nadir at approximately 45 minutes, representing rapid inhibition of lipolysis by insulin.30 Supplemented cows had a more rapid NEFA elimination rate after glucose infusion (Table 3), higher NEFA AUC, and a shorter NEFA half‐life, thereby suggesting that supplemented cows had lower IR related to lipid metabolism than did nonsupplemented cows. Conversely, regarding the response of NEFA to the IVGTT, no differences in the metabolism of BHBA after glucose infusion were observed between the 2 groups. However, concentrations of NEFA and BHBA do not correlate well,31 because the synthesis of ketone bodies does not depend only on energy balance. Therefore, the greater decrease in serum NEFA may not directly translate to a greater decrease in the concentration of BHBA.
To the best of the authors’ knowledge, ours is the first study to investigate the effect of supplementation with vitamin E and selenium, the most widely used antioxidants included in the diets of dairy cows,13 on glucose tolerance during early lactation. However, 2 previous reports investigated the effect of chromium supplementation, which has some antioxidant effects in cattle,32 on the response to IVGTT in cows.33, 34 These studies found differences in glucose elimination rates, but not in the clearance of NEFA. However, despite the limited antioxidant potential of chromium, its role in metabolism is believed to be through the glucose tolerance factor,35 enhancing glucose uptake by cells. Therefore, it is not surprising that these studies reported improved glucose clearance, but no changes in the NEFA response to the IVGTT.
Inflammation around the time of calving has gained much attention in recent years.36 The nuclear factor kappa B (NF‐κB) pathway is a pro‐inflammatory signaling pathway responsible for provoking IR.37 This pathway can be activated by OS in cattle during times of negative energy balance.38, 39 In addition, endoplasmic reticulum stress, present in the liver of high‐yielding dairy cows,40 also activates inflammation via the NF‐κB pathway.41 Hence, the lower IR observed in supplemented cows may be a consequence of the down regulation of these pathways because of increased antioxidant capacity. However, as a consequence of the tight interplay among nutrient metabolism, OS, and inflammation in dairy cattle,12 several other factors may play key roles in the development of IR in dairy cows, which must also be taken into consideration when designing nutritional interventions to control IR and the associated enhanced lipolysis.
The use of the IVGTT to assess insulin sensitivity implies normal insulin secretion after glucose administration and assumes similar insulin secretion among animals, which may not always be the case.1 The IVGTT, however still, is considered a good method for assessing IR in cattle given its practicality and agreement with the hyperinsulinemic euglycemic clamp, the gold standard test.26 The major limitation of this study was the small sample size, as there were only 5 animals per study group. Nevertheless, this number was sufficient for showing statistical differences in the response to IVGTT, although the basal metabolic status of the animals was not affected by the supplementation. Animals in this study were not supplemented with any dietary antioxidants aside from the limited amount contained in preserved forages,16 and therefore the improved responses observed in this study might also be in part because of some antioxidant deficiency during the dry period. Hence, further studies should investigate whether antioxidant therapy ameliorates the degree of IR beyond the first week postcalving, as well as the impact that antioxidant supplementation can have on the metabolic and health status of cows.
Conclusions
Cows supplemented parentally with antioxidants (vitamin E and selenium) before calving showed improved insulin sensitivity during the first week of lactation, thereby supporting an effect of OS on the development of IR in dairy cows. Further studies should investigate the effects of different supplementation strategies as adjunct therapies to ameliorate the consequences of prolonged IR and its impact on metabolic stress in cows.
Acknowledgments
The authors gratefully acknowledge Lucia Casanova for her technical assistance with sample analysis and Manuel Vidal Vallejo and his family for allowing us to perform the study on their farm and for their patience and help during the tolerance tests.
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This work was performed at a commercial farm located in Meira (Lugo) and at the Faculty of Veterinary Science of Lugo, Spain.
This work was funded through the award of the 2014 Sir Kenneth Blaxter award by the British Society for Animal Science to A. Abuelo and by a predoctoral FPU grant from the Spanish Ministry of Education (Ref. AP2010‐0013) granted to A. Abuelo.
Data from this study have not been presented at any meetings.
Footnotes
Microsoft Excel 2010, Redmond, MA.
BD Vacutainer; Becton, Dickinson and Company, Plymouth, UK.
Selevit adultos, Laboratorios SYVA, León, Spain.
FisioVet solución para perfusión, B. Braun VetCare SA, Barcelona, Spain.
Intraflon 2 catheter IV, Laboratories Pharmaceutiques Vygon, Ecouen, France.
GlucosaVet 40 g/100 mL, B. Braun VetCare SA, Barcelona, Spain.
2 mL Glucose Fluoride, Sarsted AG & Co, Nümbrecht, Germany.
Sarsted AG & Co, Nümbrecht, Germany.
Glucose‐Hexokinase Gernon, RAL Tecnica para el Laboratorio, Barcelona, Spain.
NEFA H(2) R1+R2 Set, Wako Chemicals GmbH, Neuss, Germany.
BHB, Biochemical enterprise, Milan, Italy.
d‐ROM test, Diacron International, Grosseto, Italy.
OXY‐Adsorbent test, Diacron International, Grosseto, Italy.
CST‐240, DIRUI Industrial Co., Ltd, Changchun, China.
Biocal; RAL Tecnica para el laboratorio S.A., Barcelona, Spain.
Gernorm; RAL Tecnica para el laboratorio S.A., Barcelona, Spain.
Gerpath; RAL Tecnica para el laboratorio S.A., Barcelona, Spain.
Insulin Bovine ELISA; Mercodia AB, Uppsala, Sweden.
SPSS v.20 for Windows, IBM, Chicago, IL.
References
- 1. De Koster JD, Opsomer G. Insulin resistance in dairy cows. Vet Clin North Am Food Anim Pract 2013;29:299–322. [DOI] [PubMed] [Google Scholar]
- 2. Pravettoni D, Doll K, Hummel M, et al. Insulin resistance and abomasal motility disorders in cows detected by use of abomasoduodenal electromyography after surgical correction of left displaced abomasum. Am J Vet Res 2004;65:1319–1324. [DOI] [PubMed] [Google Scholar]
- 3. Stengärde L, Holtenius K, Tråvén M, et al. Blood profiles in dairy cows with displaced abomasum. J Dairy Sci 2010;93:4691–4699. [DOI] [PubMed] [Google Scholar]
- 4. De Koster J, Opsomer G. Are modern dairy cows suffering from modern diseases? Vlaams Diergeneeskd Tijdschr 2012;81:71–80. [Google Scholar]
- 5. LeBlanc SJ. Interactions of metabolism, inflammation, and reproductive tract health in the postpartum period in dairy cattle. Reprod Domest Anim 2012;47(Suppl 5):18–30. [DOI] [PubMed] [Google Scholar]
- 6. Chang YC, Chuang LM. The role of oxidative stress in the pathogenesis of type 2 diabetes: From molecular mechanism to clinical implication. Am J Transl Res 2010;2:316–331. [PMC free article] [PubMed] [Google Scholar]
- 7. Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006;440:944–948. [DOI] [PubMed] [Google Scholar]
- 8. Kaneto H, Nakatani Y, Kawamori D, et al. Role of oxidative stress, endoplasmic reticulum stress, and c‐Jun N‐terminal kinase in pancreatic β‐cell dysfunction and insulin resistance. Int J Biochem Cell Biol 2006;38:782–793. [DOI] [PubMed] [Google Scholar]
- 9. Luzia LA, Rondo PH. α‐tocopherol supplementation, lipid profile, and insulin sensitivity in diabetes mellitus type 2 In: Preedy V, ed. Diabetes: Oxidative Stress and Dietary Antioxidants. San Diego, CA, USA: Academic Press; 2014:67–77. [Google Scholar]
- 10. Neyestani TR, Vitamin D. Oxidative stress and diabetes In: Preedy V, ed. Diabetes: Oxidative Stress and Dietary Antioxidants. San Diego, CA, USA: Academic Press; 2014:111–120. [Google Scholar]
- 11. Abuelo A, Hernandez J, Benedito JL, Castillo C. Oxidative stress index (OSi) as a new tool to assess redox status in dairy cattle during the transition period. Animal 2013;7:1374–1378. [DOI] [PubMed] [Google Scholar]
- 12. Sordillo LM, Mavangira V. The nexus between nutrient metabolism, oxidative stress and inflammation in transition cows. Anim Prod Sci 2014;54:1204–1214. [Google Scholar]
- 13. Abuelo A, Hernandez J, Benedito JL, Castillo C. The importance of the oxidative status of dairy cattle in the periparturient period: Revisiting antioxidant supplementation. J Anim Physiol Anim Nutr (Berl) 2015;99:1003–1016. [DOI] [PubMed] [Google Scholar]
- 14. Abuelo A, Hernandez J, Benedito JL, Castillo C. Association of oxidative status and insulin sensitivity in periparturient dairy cattle: An observational study. J Anim Physiol Anim Nutr (Berl) 2016;100:279–286. [DOI] [PubMed] [Google Scholar]
- 15. Edmonson AJ, Lean IJ, Weaver LD, et al. A body condition scoring chart for holstein dairy cows. J Dairy Sci 1989;72:68–78. [Google Scholar]
- 16. NRC . Nutrient Requirements of Dairy Cattle, 7th ed. Washington, DC, USA: National Academic Press; 2001:3–213. [Google Scholar]
- 17. Alberti A, Bolognini L, Macciantelli D, Caratelli M. The radical cation of N, N‐diethyl‐para‐phenylendiamine: A possible indicator of oxidative stress in biological samples. Res Chem Intermed 2000;26:253–267. [Google Scholar]
- 18. Pires JA, Souza AH, Grummer RR. Induction of hyperlipidemia by intravenous infusion of tallow emulsion causes insulin resistance in Holstein cows. J Dairy Sci 2007;90:2735–2744. [DOI] [PubMed] [Google Scholar]
- 19. Henriksen EJ, Diamond‐Stanic MK, Marchionne EM. Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radic Biol Med 2011;51:993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sordillo LM, Aitken SL. Impact of oxidative stress on the health and immune function of dairy cattle. Vet Immunol Immunopathol 2009;128:104–109. [DOI] [PubMed] [Google Scholar]
- 21. Traber MG, Stevens JF. Vitamins C and E: Beneficial effects from a mechanistic perspective. Free Radic Biol Med 2011;51:1000–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sordillo LM. Selenium‐dependent regulation of oxidative stress and immunity in periparturient dairy cattle. Vet Med Int 2013;2013:154045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kessel S, Stroehl M, Meyer HHD, et al. Individual variability in physiological adaptation to metabolic stress during early lactation in dairy cows kept under equal conditions. J Anim Sci 2008;86:2903–2912. [DOI] [PubMed] [Google Scholar]
- 24. Kerestes M, Faigl V, Kulcsár M, et al. Periparturient insulin secretion and whole‐body insulin responsiveness in dairy cows showing various forms of ketone pattern with or without puerperal metritis. Domest Anim Endocrinol 2009;37:250–261. [DOI] [PubMed] [Google Scholar]
- 25. Holtenius K, Agenäs S, Delavaud C, Chilliard Y. Effects of feeding intensity during the dry period. 2. Metabolic and hormonal responses. J Dairy Sci 2003;86:883–891. [DOI] [PubMed] [Google Scholar]
- 26. Schoenberg KM, Ehrhardt RM, Overton TR. Effects of plane of nutrition and feed deprivation on insulin responses in dairy cattle during late gestation. J Dairy Sci 2012;95:670–682. [DOI] [PubMed] [Google Scholar]
- 27. Zachut M, Honig H, Striem S, et al. Periparturient dairy cows do not exhibit hepatic insulin resistance, yet adipose‐specific insulin resistance occurs in cows prone to high weight loss. J Dairy Sci 2013;96:5656–5669. [DOI] [PubMed] [Google Scholar]
- 28. Mann S, Yepes FA, Duplessis M, et al. Dry period plane of energy: Effects on glucose tolerance in transition dairy cows. J Dairy Sci 2016;99:701–717. [DOI] [PubMed] [Google Scholar]
- 29. Kahn CR. Insulin resistance, insulin insensitivity, and insulin unresponsiveness: A necessary distinction. Metabolism 1978;27:1893–1902. [DOI] [PubMed] [Google Scholar]
- 30. Ruan H, Lodish HF. Insulin resistance in adipose tissue: Direct and indirect effects of tumor necrosis factor‐alpha. Cytokine Growth Factor Rev 2003;14:447–455. [DOI] [PubMed] [Google Scholar]
- 31. McCarthy MM, Mann S, Nydam DV, et al. Short communication: Concentrations of nonesterified fatty acids and beta‐hydroxybutyrate in dairy cows are not well correlated during the transition period. J Dairy Sci 2015;98:6284–6290. [DOI] [PubMed] [Google Scholar]
- 32. Zhang FJ, Weng XG, Wang JF, et al. Effects of temperature‐humidity index and chromium supplementation on antioxidant capacity, heat shock protein 72, and cytokine responses of lactating cows. J Anim Sci 2014;92:3026–3034. [DOI] [PubMed] [Google Scholar]
- 33. Sumner JM, Valdez F, McNamara JP. Effects of chromium propionate on response to an intravenous glucose tolerance test in growing Holstein heifers. J Dairy Sci 2007;90:3467–3474. [DOI] [PubMed] [Google Scholar]
- 34. Hayirli A, Bremmer DR, Bertics SJ, et al. Effect of chromium supplementation on production and metabolic parameters in periparturient dairy cows. J Dairy Sci 2001;84:1218–1230. [DOI] [PubMed] [Google Scholar]
- 35. Toepfer EW, Mertz W, Polansky MM, et al. Preparation of chromium‐containing material of glucose tolerance factor activity from brewer's yeast extracts and by synthesis. J Agric Food Chem 1976;25:162–166. [DOI] [PubMed] [Google Scholar]
- 36. Bradford BJ, Yuan K, Farney JK, et al. Invited review: Inflammation during the transition to lactation: New adventures with an old flame. J Dairy Sci 2015;98:6631–6650. [DOI] [PubMed] [Google Scholar]
- 37. Ogihara T, Asano T, Katagiri H, et al. Oxidative stress induces insulin resistance by activating the nuclear factor‐κB pathway and disrupting normal subcellular distribution of phosphatidylinositol 3‐kinase. Diabetologia 2004;47:794–805. [DOI] [PubMed] [Google Scholar]
- 38. Shi X, Li X, Li D, et al. Beta‐Hydroxybutyrate activates the NF‐kappaB signaling pathway to promote the expression of pro‐inflammatory factors in calf hepatocytes. Cell Physiol Biochem 2014;33:920–932. [DOI] [PubMed] [Google Scholar]
- 39. Shi X, Li D, Deng Q, et al. NEFAs activate the oxidative stress‐mediated NF‐kappaB signaling pathway to induce inflammatory response in calf hepatocytes. J Steroid Biochem Mol Biol 2015;145:103–112. [DOI] [PubMed] [Google Scholar]
- 40. Gessner DK, Schlegel G, Ringseis R, et al. Up‐regulation of endoplasmic reticulum stress induced genes of the unfolded protein response in the liver of periparturient dairy cows. BMC Vet Res 2014;10:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ringseis R, Gessner DK, Eder K. Molecular insights into the mechanisms of liver‐associated diseases in early‐lactating dairy cows: Hypothetical role of endoplasmic reticulum stress. J Anim Physiol Anim Nutr (Berl) 2015;99:626–645. [DOI] [PubMed] [Google Scholar]


