Abstract
Background
A novel test using whole‐body barometric plethysmography (WBBP) was developed recently to diagnose brachycephalic obstructive airway syndrome (BOAS) in unsedated French bulldogs.
Hypothesis/Objectives
The hypotheses of this study were: (1) respiratory characteristics are different between healthy nonbrachycephalic dogs and brachycephalic dogs; and among pugs, French bulldogs, and bulldogs; and (2) obesity and stenotic nares are risk factors for BOAS. The main objective was to establish a diagnostic test for BOAS in these 3 breeds.
Animals
A total of 266 brachycephalic dogs (100 pugs, 100 French bulldogs, and 66 bulldogs) and 28 nonbrachycephalic dogs.
Methods
Prospective study. Exercise tolerance tests with respiratory functional grading, and WBBP were performed on all dogs. Data from WBBP were associated with functional grades to train quadratic discriminant analysis tools to assign dogs to BOAS+ and BOAS‐ groups. A BOAS index (0–100%) was calculated for each dog. Receiver operating characteristic (ROC) curves were used to evaluate classification ability.
Results
Minute volume was decreased significantly in asymptomatic pugs (P = .009), French bulldogs (P = .026), and bulldogs (P < .0001) when compared to nonbrachycephalic controls. Respiratory characteristics were different among breeds and affected dogs had a significant increase in trace variation. The BOAS index predicted BOAS status for each breed with 94–97% (95% confidence interval [CI], 88.9–100%) accuracy (area under the ROC curve). Both obesity (P = .04) and stenotic nares (P = .004) were significantly associated with BOAS.
Conclusions and Clinical Importance
The WBBP can be used as a clinical tool to diagnose BOAS noninvasively and objectively.
Keywords: Brachycephalic obstructive airway syndrome, Quadratic discriminant analysis, Respiratory function test, Whole‐body barometric plethysmography
Abbreviations
- AIC
Akaike's information criterion
- BCS
body condition score
- BOAS
brachycephalic obstructive airway syndrome
- BW
body weight
- CI
confidence interval
- EMMS
electromedical measurement systems
- ETT
exercise tolerance test
- MV
minute volume
- OR
odds ratio
- PEF
peak expiratory flow rate
- PENH
enhanced pause
- PIF
peak inspiratory flow rate
- QDA
quadratic discriminant analysis
- ROC
receiver operating characteristic
- RR
respiratory rate
- RT
relaxation time
- SD
standard deviation
- TBFVL
tidal breathing flow volume loops
- Te
expiratory time
- Ti
inspiratory time
- TV
tidal volume
- WBBP
whole‐body barometric plethysmography
Brachycephalic obstructive airway syndrome (BOAS) is common among extremely brachycephalic breeds of dogs.1, 2 Physical examination, history, and lesion assessment under sedation or general anesthesia are used to diagnose BOAS.3 These methods however are either subjective or invasive, which creates difficulty when evaluating disease progression and the effectiveness of treatment in a clinical setting. Hence, development of new methods for non‐invasive and objective measurements of respiratory function in affected dogs is crucial.
The use of pneumotachographs along with analysis of tidal breathing flow volume loops (TBFVL), as well as the forced oscillation technique, have allowed previous measurement of respiratory function in conscious dogs and detection of airway obstructions.4, 5, 6 However, these techniques require use of a tight‐fitting facemask attached to the pneumotachograph, which is particularly difficult to apply to a brachycephalic dog's muzzle without having air leakage or causing stress in untrained dogs. Whole‐body barometric plethysmography (WBBP) is a non‐invasive technique of measuring respiratory function that has been validated and utilized in unrestrained unsedated experimental mice to characterize respiratory patterns during sleep and wakefulness.7, 8, 9, 10 This technique has also been used in experimental and clinical studies using dogs and cats for pharmacological studies and respiratory disorders.11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 Our previous study on French bulldogs showed that respiratory airflow characteristics obtained from WBBP are distinguishable between BOAS‐affected and clinically healthy French bulldogs.18 Affected dogs showed high variations in respiratory parameters caused by dynamic obstructions and multiple lesion sites. Quadratic discriminant analysis (QDA) was developed as a classifier using the respiratory data obtained from WBBP and the BOAS index that was proposed can be used for diagnostic and screening purposes.
In addition to French bulldogs, pugs and bulldogs also are reported to be highly predisposed to BOAS.1, 2, 22, 23 Although the differences in anatomy and BOAS lesion sites among the 3 extremely brachycephalic breeds have been investigated recently,24, 25, 26, 27 studies on respiratory characteristics in different brachycephalic breeds are limited. A previous study investigated the differences in respiratory parameters among healthy nonbrachycephalic dogs, BOAS‐affected and clinically healthy bulldogs and Boston terriers.5 Another study compared respiratory parameters between healthy nonbrachycephalic dogs and BOAS‐affected dogs (bulldogs, pugs, Bordeaux dogs, and Shar‐Pei dogs) before and after upper airway corrective surgery.11 Both studies identified significant differences in respiratory variables between healthy nonbrachycephalic dogs and BOAS‐affected brachycephalic dogs, but did not show differences in most respiratory variables between clinically healthy (or BOAS‐affected dogs postoperatively) and BOAS‐affected brachycephalic dogs. Brachycephalic characteristics that have been associated with BOAS include stenotic nares and obesity.1, 28, 29, 30 Their effects on respiratory function, however, have not yet been assessed.
The questions asked in this study were: (1) whether respiratory characteristics are distinguishable between affected and nonaffected dogs of different breeds, and among the 3 extremely brachycephalic breeds; and (2) whether obesity and stenotic nares are risk factors for BOAS. A major purpose of this study was to develop a noninvasive diagnostic test for BOAS.
Materials and Methods
Animals
Our prospective study included pugs, French bulldogs, and bulldogs referred for upper airway consultation to the Queen's Veterinary School Hospital (QVSH), University of Cambridge (termed “clinical dogs”), as well as pet dogs of the selected 3 breeds that were volunteered by UK owners and breeders between September 2011 and June 2015 (termed “study dogs”). A detailed history of each dog was taken from owners including type, severity, frequency and circumstances of occurrence of respiratory signs. Exclusion criteria included age <1 year, previous upper airway surgery, history and clinical findings of lower airway disease, or some combination of these. Nonbrachycephalic dogs referred to the QVSH for reasons other than respiratory disease, and staff‐owned dogs were included in the study as controls. All control dogs underwent physical examination by the investigators to rule out airway disease. Dogs that were on medications that may change respiratory parameters (eg, prednisolone, other anti‐inflammatory drugs) were excluded from the study. Work was performed under informed ethical consents CR62 and CR63 from the Department of Veterinary Medicine, University of Cambridge.
Methods
Respiratory Functional Grading and Risk Factor Assessments for BOAS
Each dog was graded for functional severity of BOAS using a previously established 4‐point functional grading system (Table 1) based on clinical evaluation before and after a 3‐minute exercise tolerance test (ETT).18 BOAS functional Grade 0 dogs (asymptomatic, BOAS free) and Grade I dogs (mild BOAS, dog shows mild respiratory noise but exercise tolerance is unaffected) were considered clinically healthy for their breed. Grade II dogs (moderate BOAS, dog requires medical attention such as weight control, surgical intervention or both) and Grade III dogs (severe BOAS, dog requires immediate surgical intervention) were considered clinically affected. These results were used in further training of the computational classifier.
Table 1.
Functional grading system of brachycephalic obstructive airway syndrome (BOAS) based on respiratory signs before and after an exercise tolerance test (ETT)
| Respiratory Noisea | Inspiratory Effortb | Dyspnea/Cyanosis/Syncopec | ||
|---|---|---|---|---|
| Grade 0 | Pre‐ETT | Not audible | Not present | Not present |
| Post‐ETT | Not audible | Not present | Not present | |
| Grade I | Pre‐ETT | Not audible or mild | Not present | Not present |
| Post‐ETT | Mild | Not present to mild | Not present | |
| Grade II | Pre‐ETT | Mild to moderate | Mild to moderate | Not present |
| Post‐ETT | Moderate to severe | Moderate to severe | Mild dyspnea; cyanosis or syncope not present | |
| Grade III | Pre‐ETT | Moderate to severe | Moderate to severe | Moderate to severe dyspnea; may or may not present cyanosis. Inability to exercise. |
| Post‐ETT | Severe | Severe | Severe dyspnea; may or may not present cyanosis or syncope. | |
The grading system was established previously.18 The clinical grading was based on respiratory signs before (pre‐ETT) and immediately after a 3‐minute exercise tolerance test (post‐ETT) with trotting speed of approximately 4–5 miles/hour performed by the study investigators. Presentation of at least one sign in the highest grade determines the final grading result.
aRespiratory noise was diagnosed by pharyngolaryngeal auscultation. Mild: only audible under auscultation; moderate: intermittent audible noise that can be heard without stethoscope; severe: constant audible noise that can be heard without stethoscope.
bAn abnormal respiratory cycle characterized by evidence of increased effort to inhale the air with the use of diaphragm and/or accessory muscles of respiration and/or nasal flaring with an increase in breathing rate. Mild: regular breathing patterns with minimal use of diaphragm; moderate: evidence of use of diaphragm and accessary muscles of respiration; severe: marked movement of diaphragm and accessary muscles of respiration.
cDogs that have had episodes of syncope and/or cyanosis as documented by owner's report are classified into Grade III without ETT. Mild dyspnea: presents sign of discomfort; moderate dyspnea: irregular breathing, signs of discomfort; severe dyspnea: irregular breathing with signs of breathing discomfort and difficulty in breathing.
In addition to body weight measurement, a standard assessment of body fat (body condition score [BCS] on a 1–9 point scale)31 was performed on each dog. A BCS ≥7 was categorized as obese. Severity of nostril stenosis was examined. Open or mild stenotic nares were considered normal for the breeds, whereas moderate or severe stenosis was defined as “stenotic nares” (Fig 1).
Figure 1.
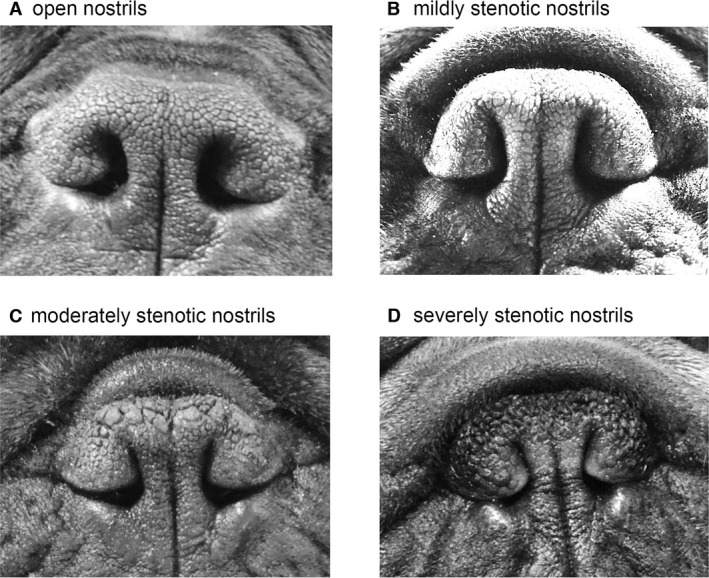
Definition of the degree of nostril stenosis in brachycephalic dogs. Representative nostrils of French bulldogs with different degrees of stenosis. (A) Open nostrils: nostrils are wide open; (B) Mild stenotic nostrils: slightly narrowed nostrils but the lateral nostril wall does not touch the medial nostril wall. Immediately after the exercise tolerance test (ETT), the nostril wings should move dorsolaterally to open on inspiration. (C) Moderately stenotic nostrils: the lateral nostril wall touches the medial nostril wall at the dorsal part of the nostrils and the nostrils are only open at the bottom. Immediately after the ETT, the nostril wings are not able to move dorsolaterally and there may be nasal flaring (ie, muscle constraction around the nose trying to enlarge the nostrils); (D) Severely stenotic nostrils: nostrils are almost closed. The dog may switch to oral breathing from nasal breathing with stress or very gentle exercise such as playing.
Non‐Invasive Respiratory Function Test Using Whole‐Body Barometric Plethysmography
Whole‐body barometric plethysmography was performed using 2 barometric chambers: ElectroMedical Measurement Systems (EMMS) model PLY370 (inner volume 175 L) for small dog breeds and model PLY360 (inner volume 280 L) for middle‐sized to large breeds.1 The chambers were equipped with 4 ports (pneumotachographs) on the upper surface. A balanced bias airflow of room air (20 L/min) was supplied (bias flow regulator BFL0404, EMMS) to maintain the O2 concentration and prevent CO2 accumulation. A CO2 concentration sensor was equipped at the side of the chamber. One pole of a pressure transducer (TRD5701, EMMS) was open to the main chamber and the other pole was open to a reference chamber (chamber PLY370) or to the exterior room environment (chamber PLY 360). Transducer signals were amplified using a strain gauge amplifier and were sampled by a commercial software.2 Calibration of the chamber pressure signal was performed dynamically before each test by injecting 50 mL of room air into the chamber and integrating under the resultant flow curve. The flow reading was tested by continuous cycles of injecting and withdrawing 10 mL, 20 mL, 30 mL of air into and from the chamber. The calibration procedure was repeated if tidal volume reading errors exceeded ±10%. The flow was measured via pseudoflow, with the animal completely unrestrained inside the chamber. The pressure transducer measured the pressure increase caused by the added temperature and humidity of the inspired air on inspiration, and the pressure decrease caused by cooling on expiration (minus any small changes caused by oxygen, CO2, and water vapor exchange across the lung surface). This pressure difference, again measured via a reference environment, was directly proportional to flow. The pseudoflow signals were analyzed to obtain respiratory parameters including: respiratory rate (RR, breaths/minute), inspiratory time (Ti, s), expiratory time (Te, s), tidal volume (TV, mL), minute volume (MV = TV × RR, mL), peak inspiratory flow rate (PIF, mL/s), peak expiratory flow rate (PEF, mL/s), relaxation time (RT, time point when 65% of TV was expired), pause ([Te‐RT]/RT), and enhanced pause (PENH = [PEF/PIF] × pause).
Detailed protocols, data acquisition of variables, and data processing using WBBP for unrestrained, unsedated conscious dogs have been described previously.18 Briefly, after clinical examination, each dog was placed in the chamber. Acclimatization (5–10 minutes) was followed by a recording period of 20 minutes. Dogs that were intolerant to the procedure (ie, showed signs of anxiety) after 2 attempts were excluded from the study. Each breath cycle was recognized automatically by the eDacq software.2 All respiratory cycles that had differences >20% between inspiratory and expiratory volumes were excluded automatically. Periods of body movement, sniffing, or vocalization that caused artifacts were identified using the recorded surveillance video and eliminated manually. Twenty representative, consecutive, resting breaths during wakefulness were collected for further data analysis. An ETT for functional grading was performed after WBBP testing to avoid possible alterations of baseline respiratory function after exercise.
Classifier Design for Discrimination between Grade 0/I and Grade II/III Dogs
The classifier design was based on the model established for French bulldogs in our previous study.18 Briefly, the 4‐point functional grades of dogs were used as classes in training the QDA‐based classifier.32 The QDA generates a quadratic decision surface in the feature space to separate classes. In a probabilistic setting where 4‐class QDA corresponds to minimum‐error‐probability classification of new samples into four multivariate Gaussian classes, this value reflects the relative prior probabilities of the 4 classes. A predictive index, the BOAS index, was generated by modeling the caudal probabilities obtained from a 4‐class QDA model using 6 variables: means and standard deviations (SDs) of 3 ratios of WBBP parameters (ie, Te/Ti_m; Te/Ti_sd; PEF/PIF_m; PEF/PIF_sd; MV/BW_m and MV/BW_sd) for the 20 breaths obtained from each dog.
The parameters were used in the following Equation (1):
| (1) |
where Σk are the covariance matrices, μk are the parameter means and πk are the prior probabilities for the different grades in the training set (data published in Data S1). The matrix x contains the measured parameters of the actual dog so that the equation results in a matrix δk (x) containing logarithmic probabilities. This matrix then is corrected to contain normal probability values.
| (2) |
The final caudal probabilities are calculated using the normalized values in δk (x)corr.
| (3) |
The final caudal probability values (p k) are calculated in percentage format for the 4 grades (p 0 + p I + p II + p III = 100%). The caudal probabilities are weighed to suggest relative severity of the disease. For this study, the interval of disease severity was assumed to be equal. Hence, the BOAS index (IBOAS) is derived as:
| (4) |
Note that p 0 is omitted from the equation because it is multiplied by 0 during the weighting.
The BOAS index ranges from 0 to 100%. The initial cut‐off point of BOAS index was set at 50% to discriminate between BOAS− (ie, BOAS index <50%) and BOAS+ (ie, BOAS index ≥50%) after a binary classification. The cut‐off values then were refined based on receiver operating characteristic (ROC) curves. Three breed‐specific models and 1 general model were built:
Model (PD): breed‐specific model based on 100 pugs
Model (FB): breed‐specific model based on 100 French bulldogs
Model (BD): breed‐specific model based on 66 bulldogs
Model (PFB): general model based on 266 brachycephalic dogs (100 pugs, 100 French bulldogs, and 66 bulldogs).
Statistical Analysis
Differences in subject characteristics among groups were tested using nonparametric Mann–Whitney tests. Associations of age, sex, obesity, and nostril stenosis with BOAS status (normal and clinically affected) were assessed using forward stepwise logistic regression. For each WBBP parameter, means and SDs of 20 representative breaths from each dog were calculated. The normality of the data within groups was assessed using descriptive statistics and the Kolmogorov–Smirnov test. Homogeneity of variance was evaluated using Levene's test. Variables of the nonbrachycephalic controls and Grade 0 brachycephalic dogs were compared using nonparametric Mann–Whitney tests. A simple 3‐way QDA was used to discriminate the respiratory characteristics (Te/Ti_m, Te/Ti_sd, PEF/PIF_m, PEF/PIF_sd, MV/BW_m, and MV/BW_sd) among the 3 Grade 0 brachycephalic breeds. To compare WBBP data obtained for the 2 groups of BOAS functional Grade 0/I and Grade II/III dogs, a multi‐level mixed linear regression model was used. Breed was included as a random effect encompassing both body size and morphometric differences among breeds. Model fit was assessed using Akaike's information criterion (AIC). Other predictors such as obesity (yes/no; yes = BCS 7–9; no = BCS 3–6), age (years), and sex (male/female) were tested as fixed effects or covariates. Bonferroni correction was used for adjustment for multiple comparisons except in table 3, where raw P values are indicated. The statistic analyses were performed with a commercial statistical software.3 Significance was set at P < .05.
Table 3.
The baseline respiratory parameters in nonbrachycephalic control dogs and Grade 0 pugs, French bulldogs, and bulldogs
| Nonbrachycephalic controls (n = 28) | Grade 0 pugs (n = 7) | Grade 0 French bulldogs (n = 10) | Grade 0 bulldogs (n = 10) | |
|---|---|---|---|---|
| Obesity (%) | 0% | 28.57% | 0% | 20% |
| Stenotic nares (%) | 0% | 14.29% | 0% | 0% |
| RR_m | 20.81 (16.95–25.02)‡ | 22.50 (16.81–24.1) | 23.16 (21.82–29.85)* | 23.46 (19.81–28.13) |
| TV/BW_m | 11.64 (9.71–12.85)‡‡ , §§§ | 10.17 (8.55–13.11)§§ | 8.59 (7.83–10.32)** | 6.66 (6.05–8.18)*** , †† |
| MV/BW_m | 233.42 (224.56–254.69)†† , ‡ , §§§ | 217.57 (190.57–219.95)** , §§ | 211.14 (190.33–235.11)* | 176.97 (140.52–183.51)*** , †† , ‡‡ |
| Te/Ti_m | 1.37 (1.28–1.55)‡‡‡ , §§§ | 1.29 (0.83–1.66) | 0.90 (0.82–1.12)*** | 0.95 (0.81–1.20)*** |
| PIF/BW_m | 16.40 (13.72–18.11)‡‡‡ , §§§ | 13.68 (12.55–15.92)§§ | 11.90 (10.07–12.80)*** | 9.83 (8.10–10.56)*** , †† , ‡ |
| PEF/BW_m | 13.42 (10.92–14.75)§§§ | 12.15 (10.99–14.84)§§ | 11.92 (11.19–13.46) | 8.38 (7.07–9.94)*** , †† , ‡‡ |
| PEF/PIF_m | 0.83 (0.75–0.89)‡‡‡ , § | 0.89 (0.81–1.12) | 1.02 (0.96–1.10)* | 0.90 (0.83–0.98)* , ‡ |
| RR_sd | 2.14 (1.49–2.76) | 2.85 (2.09–3.79) | 2.37 (2.05–3.09) | 2.73 (1.38–3.27) |
| TV/BW_sd | 1.45 (1.22–1.82)§§ | 1.73 (1.17–1.85)§ | 1.35 (1.06–1.62) | 0.82 (0.69–1.06)** , † , ‡ |
| MV/BW_sd | 24.64 (15.09–31.19)§ | 23.95 (13.92–28.10) | 25.23 (16.32–37.02) | 14.52 (11.31–22.56)* , ‡ |
| Te/Ti_sd | 0.24 (0.17–0.32)‡ , § | 0.32 (0.22–0.36)‡ , § | 0.16 (0.13–0.24)* , †† | 0.14 (0.11–0.22)* , † |
| PIF/BW_sd | 1.89 (1.51–2.65)‡ , §§ | 1.85 (1.40–2.70)§ | 1.42 (1.20–1.86)* | 1.05 (0.79–1.74)** , † |
| PEF/BW_sd | 1.93 (1.48–2.55)§ | 2.39 (1.90–2.72)§ | 1.92 (1.57–2.41) | 1.33 (0.86–1.92)* , † |
| PEF/PIF_sd | 0.11 (0.09–0.12)† | 0.19 (0.08–0.21)* | 0.13 (0.12–0.17) | 0.11 (0.08–0.14) |
Data are presented as median with interquartile range.
RR = respiratory rate (breath/minute); Te/Ti = expiratory time (s)/inspiratory time(s); PEF/PIF = peak expiratory flow rate (ml/s)/peak inspiratory flow rate (mL/s); MV/BW = minute volume (mL)/body weight (kg); m = mean of the parameter calculated from the 20 breaths of each dog; sd = standard deviation of the parameter calculated from the 20 breaths of each dog.
*Significantly different from the non‐brachycephalic controls P(raw) < .05; **P(raw) < .01; ***P(raw)< .0001.
†Significantly different from the Grade 0 pugs at P(raw) < .05; †† P(raw) < .01.
‡Significantly different from the Grade 0 French bulldogs at P(raw) < 0.05; ‡‡ P(raw) <0.01; ‡‡‡ P(raw) < 0.001.
§Significantly different from the Grade 0 bulldogs at P(raw) < 0.05; §§ P(raw) <0.01; §§§ P(raw) < 0.001.
The diagnostic value of the BOAS index was assessed by calculating the area under each ROC curve. Performance metrics were computed over 2,000 bootstrap samples of the whole dataset to generate 95% CI to delineate the expected range of classifier performance. Diagnostic accuracy was estimated using sensitivity, specificity, positive and negative predictive values, and positive and negative likelihood ratios. The cut‐off values selected from the ROC curves were those that best identified BOAS+ animals where the sensitivity and specificity were approximately equal. Computations for data processing, feature extraction, QDA, bootstrap resampling, and ROC construction were implemented using the packages “MASS,” “verification,” and “caret” available in the R Project.4
Results
Subjects and Clinical Assessments
Characteristics of the subjects are summarized in Table 2. In total, 294 dogs were recruited including 28 nonbrachycephalic controls and 266 brachycephalic dogs (100 pugs, 100 French bulldogs, and 66 bulldogs). Of the 266 brachycephalic dogs, 44 were “clinical dogs,” and the remainders were “study dogs.” Body condition score (BCS) was found to be significantly higher in pugs (P < .0001) and bulldogs (P = .003) compared to nonbrachycephalic controls. This was particularly so in pugs, where 62% of dogs were diagnosed as obese (ie, BCS ≥7). Stenotic nares were found in >50% of pugs and French bulldogs, and approximately 40% of bulldogs. The prevalence of Grade II/III BOAS among the “study dog” population was 60% of pugs, 46% of French bulldogs, and 40% of bulldogs.
Table 2.
Characteristics of the study subjects (n = 294)
| Pugs | French Bulldogs | Bulldogs | Nonbrachycephalic Controlsa | |
|---|---|---|---|---|
| Dog number | 100 | 100 | 66 | 28 |
| Clinical dogsb/study dogsc | 18/82 | 20/80 | 6/60 | 0/28 |
| Female %/intact % | 54%/76% | 62%/68% | 65.2%/94% | 60.71%/60.71% |
| Age (years) | 3.13 [1–12.25] | 2.5 [1–10.5] | 1.83 [1–10.5] | 2.75 [1–12] |
| Body weight (kg) | 8.4 [4.6–14.4] | 11.5 [8–17] | 24.9 [15–32] | 11.62 [6–27] |
| BCS (1–9), Obesityd% | 7 [4–9]ee, 62% | 5 [3–7], 13% | 6 [4–8]e, 36.4% | 5 [4–6], 0% |
| Stenotic nares % | 58.2%ee | 66.7%ee | 40.9%ee | 0% |
| Functional Grade | Grade 0: 7% | Grade 0: 10% | Grade 0: 15.2% | Grade 0: 100% |
| Grade I: 26% | Grade I: 34% | Grade I: 40.9% | ||
| Grade II: 50% | Grade II: 41% | Grade II: 28.8% | ||
| Grade III: 17% | Grade III: 15% | Grade III: 15.2% | ||
| Prevalence of BOAS in study dogs | 59.8% (CI95: 48.9–69.7%) | 46.3% (CI95: 35.8–57.1%) | 40% (CI95: 28.6–52.6%) | 0% |
Data are presented as median [minimum–maximum]. CI95 = 95% confidence interval.
aBreeds of control dogs: Border collie (n = 1), Cairn terrier (n = 1), Cross (n = 4), Jack Russell terrier (n = 4), King Charles spaniel (n = 1); Springer spaniel (n = 2); Beagle (n = 6); West Highland white terrier (n = 1); miniature Schnauzer (n = 2); King Charles spaniel (n = 1); Labrador retriever (n = 3); American bullterrier (n = 1); Dachshund (n = 1).
bClinical dogs: dogs that were referred to the Queen's Veterinary School Hospital for upper airway corrective surgery.
cStudy dogs: dogs from owners who participated in the study voluntarily. The dogs may or may not present clinical signs of brachycephalic obstructive airway syndrome (BOAS).
dBCS = body condition score; obesity defined here as BCS ≥7.
eSignificantly different from nonbrachycephalic control dogs at P < .01 or at ee P < .001.
Comparison of Respiratory Parameter Measurements and Risk Factor Analysis
Respiratory parameters from nonbrachycephalic controls and Grade 0 brachycephalic dogs are presented in Table 3 and Fig 2A.
Figure 2.
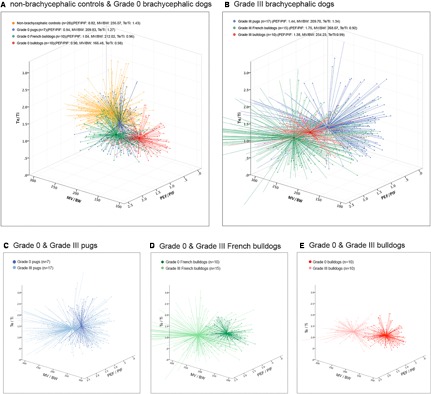
Breaths plotted against three selected respiratory parameters. Twenty representative breaths per dog (represented as crosses), assigned colors for each group with a marked centroid (co‐ordinates of each axis). (A) Nonbrachycephalic control dogs vs Grade 0 brachycephalic dogs; (B) Grade III brachycephalic dogs to be compared with Grade 0 groups in (A); (C)–(E), breed‐specific comparisons between Grade 0 and Grade III dogs. PEF/PIF = peak expiratory flow rate (mL/s)/peak inspiratory flow rate (mL/s); MV/BW = minute volume (mL)/bodyweight (kg); Te/Ti = expiratory time (s)/inspiratory time (s).
In general, MV/BW_m in all the Grade 0 brachycephalic breeds was significantly decreased (P = .009 in pugs; P = .026 in French bulldogs; P < .0001 in bulldogs) when compared to nonbrachycephalic controls. Te/Ti_m was significantly lower in Grade 0 French bulldogs (P < .0001) and bulldogs (P < .0001), but not in pugs (P = .284), when compared to nonbrachycephalic controls. RR_m was significantly higher (P = .043) in Grade 0 French bulldogs when compared to controls.
Most of the individual respiratory variables in Grade 0 bulldogs were significantly different from those of the other 2 brachycephalic breeds, whereas respiratory characteristics in Grade 0 pugs and Grade 0 French bulldogs were more similar. Only Te/Ti_sd was significantly different (P = .005). Altogether, the classification results for respiratory characteristics from 3‐class QDA using 6 variables (ie, Te/Ti_m, Te/Ti_sd, PEF/PIF_m, PEF/PIF_sd, MV/BW_m, and MV/BW_sd) show that the Grade 0 dogs of the 3 breeds display distinct respiratory characteristics. All dogs could be accurately classified into the correct breed (Data S2) using the QDA tool. Obesity was found in 29% and 20% of the Grade 0 pugs and bulldogs, respectively. None of the nonbrachycephalic controls and Grade 0 French bulldogs was obese. Stenotic nares only were found in 1 of the Grade 0 pugs. None of the Grade 0 French bulldogs and bulldogs had stenotic nares.
Figure 3 illustrates representative examples of baseline WBBP flow traces in different breeds with varying BOAS grades. The WBBP flow traces were clearly distinguishable between Grade 0/I and Grade II/III brachycephalic dogs. Grade 0/I dogs had smoother flow traces with relatively equal inspiratory and expiratory phases (Fig 3B). In Grade II/III dogs, the waveforms varied among breeds and also among the affected individuals within breeds (Fig 3C). Affected bulldogs commonly had dynamic obstructions with high frequency airflow fluctuations over respiratory cycles. Affected French bulldogs showed a range of different waveforms, but most of them had additional clearly identifiable waveforms where the inspiratory phase was extremely restricted with noticeable airflow fluctuations and a high peak at the early stage of expiration, followed by a gradual tapering (Fig 3C, French bulldog Type B).
Figure 3.
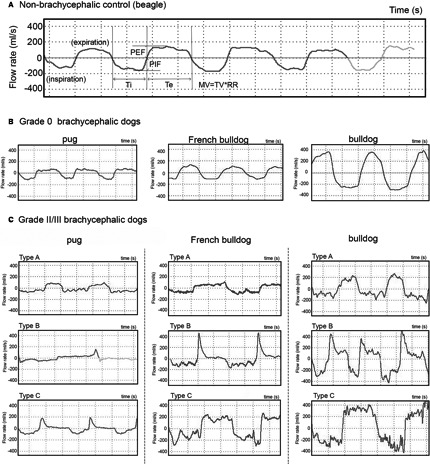
Respiratory flow trace samples. (A) Flow traces of a nonbrachycephalic control; (B) Flow trace samples of Grade 0 brachycephalic dogs; (C) Flow trace samples of Grade II/III brachycephalic dogs, showing variations in amplitude, flow pattern, and noise within the trace. Take French bulldog as an example, Type A shows extremely low amplitude when compared to Type B and C; however, the peak flow rates of inspiration and expiration are equal while they are significantly different in Type B. Noise, low amplitude high frequency fluctuations can be seen in all three types mainly during inspiration.
Analysis of respiratory parameter measurements of Grade 0/I and Grade II/III dogs is shown in Table 4. The relationship between BOAS status and respiratory parameters varies significantly across the different breeds as shown by a decrease in AIC index when the effect of breed was added into the models. TV/BW_m, MV/BW_m, PIF/BW_m, PEF/BW_m, and PEF/PIF_m were significantly greater in Grade II/III dogs compared to the Grade 0/I dogs. SDs of all respiratory parameters were greater in Grade II/III dogs. This is reflected by the much larger scatter of breath parameters for grade III dogs, which is graphically illustrated in Fig 2B when compared to Fig 2A as well as intrabreed comparisons in Fig 2C–E. RR_m and Te/Ti_m were not significantly different between Grade 0/I and Grade II/III dogs.
Table 4.
The differences in respiratory parameters between BOAS functional Grade 0/I and Grade II/III brachycephalic dogs, and the effect of obesity on respiratory parameters
| Grade 0/I Brachycephalic Dogs (n = 114)a | Grade II/III Brachycephalic Dogs (n = 152)b | |
|---|---|---|
| Obesity (%) | 28.95% | 43.42% |
| Stenotic nares (%) | 31.37% | 74.65% |
| RR_m | 22.46 ± 5.03 | 22.12 ± 4.57 |
| TV/BW_m | 9.25 ± 2.49 | 10.28 ± 3.33** , † |
| MV/BW_m | 195.81 ± 31.73 | 218.60 ± 67.18*** , †† |
| Te/Ti_m | 1.08 ± 0.27 | 1.10 ± 0.31 |
| PIF/BW_m | 11.77 ± 2.52 | 13.58 ± 4.46*** , †† |
| PEF/BW_m | 11.17 ± 2.66 | 17.58 ± 8.46** , †† |
| PEF/PIF_m | 0.96 ± 0.14 | 1.32 ± 0.43* |
| RR_sd | 2.74 ± 0.94 | 3.12 ± 1.16** |
| TV/BW_sd | 1.44 ± 0.58 | 1.95 ± 0.82*** |
| MV/BW_sd | 22.87 ± 8.49 | 35.15 ± 15.09*** |
| Te/Ti_sd | 0.21 ± 0.10 | 0.39 ± 0.15*** |
| PIF/BW_sd | 1.64 ± 0.76 | 2.39 ± 1.25*** |
| PEF/BW_sd | 1.79 ± 0.74 | 3.78 ± 2.14** |
| PEF/PIF_sd | 0.13 ± 0.06 | 0.30 ± 0.16** |
A linear mixed model was used with level 1 individual dog and level 2 breeds (random effect). Data are presented as mean ± standard deviation.
RR = respiratory rate (breath/minute); Te/Ti = expiratory time (s)/inspiratory time(s); PEF/PIF = peak expiratory flow rate (mL/s)/peak inspiratory flow rate (mL/s); MV/BW = minute volume (mL)/body weight (kg); m = mean of the parameter calculated from the 20 breaths of each dog; sd = standard deviation of the parameter calculated from the 20 breaths of each dog.
aGrade 0/I pugs = 33, Grade 0/I French bulldogs = 44, Grade 0/I bulldogs = 37.
bGrade II/III pugs = 67, Grade II/III French bulldogs = 56, Grade II/III bulldogs = 29.
*Significantly different from the BOAS− dogs at P < .05; **P < .01; ***P < .001.
†Obesity has a significant negative effect on the respiratory parameter at P < .05; †† P < .01.
Among the three brachycephalic breeds, obesity (β = 0.64, odds ratio [OR] = 1.90, 95%CI = 1.03–3.50, P = .04), male sex (β = 0.90, OR = 2.47, 95%CI = 1.35–4.53, P = .004), and stenotic nares (β = 1.81, OR = 6.09, 95%CI = 3.40–10.89, P < .0001) were positively associated with BOAS Grade II/III. There were no interactions between any of the factors. Obesity had a negative effect on the means of all the volume‐related respiratory parameters (ie, TV/BW_m, MV/BW_m, PIF/BW_m, PEF/BW_m), but had no significant effect on the SD of any respiratory parameters. Age was not significantly associated with BOAS status. Stenotic nares were significantly associated with BOAS status in Pugs (β = 1.46, OR = 4.3, 95%CI = 1.69–10.97, P = .002), French bulldogs (β = 2.97, OR = 19.56, 95%CI = 5.48–69.80, P < .0001), and bulldogs (β = 1.15, OR = 3.147, 95%CI = 1.05–9.45, P = .011). After adjusting for the stenotic nares, male dogs were more likely to be affected if they were French Bulldogs (β = 1.82, OR = 6.17, 95%CI = 1.68–22.67, P = .006) or Bulldogs (β = 1.50, OR = 4.49, 95%CI = 1.44–14.05, P = .01), but not if they were pugs.
Quadratic Discriminant Analysis (QDA) to Classify BOAS Status
Classification results using the BOAS index for each model are presented in Table 5 and Data S3. The ROC curves derived from each BOAS index are shown in Fig 4. Each of the 3 breed‐specific models, Model (PD), Model (FB) and Model (BD), had better classification results than the general model, Model (PFB). Nevertheless, positive predictive values in all models were >90%, and all final ROC curves had good or excellent discrimination: area under the curve (AUC) = 91.2% (95%CI: 87.5–94.8%) for Model (PFB), AUC = 93.9% (95%CI: 88.9–98.9%) for Model (PD), AUC = 97.2% (95%CI: 94.1–100%) for Model (FB), and AUC = 97.0% (95% CI: 91.2–100%) for Model (BD). The best cut‐off points for the breed‐specific models (BOAS index = 55.38% in pugs, 49.41% in French bulldogs, and 43.53% in bulldogs) were similar to the original classification setting (BOAS index = 50%), whereas the cut‐off points on Model (PFB) for each breed varied (49.90% in pugs, 66.37% in French bulldogs, 31.11% in bulldogs). The overlap of BOAS index between Grade 0/I and Grade II/III dogs in this model can be clearly seen in Data S3, but is decreased by specifying different cut‐off points for each breed.
Table 5.
Classification results of BOAS− and BOAS+ in pugs, French bulldogs, and bulldogs using quadratic discriminant analysis (QDA)
| Model (PD) | Model (FB) | Model (BD) | Model (PFB) | |
|---|---|---|---|---|
| Prevalence | 67% (CI95: 56.88–76.08%) | 56% (CI95: 45.72–65.92%) | 43.94% (CI95: 31.74–56.70%) | 57.14% (CI95: 50.96–63.17%) |
| Sensitivity | 88.06% (CI95: 77.82–94.70%) | 94.64% (CI95: 85.13–98.88%) | 89.66% (CI95: 72.65–97.81%) | 80.92% (CI95: 73.76–86.83%) |
| Specificity | 93.94% (CI95: 79.77–99.26%) | 93.18% (CI95: 81.34–98.57%) | 100% (CI95: 90.51–100%) | 92.98% (CI95: 86.64–96.92%) |
| Positive predictive value | 96.72% (CI95: 88.65–99.60%) | 94.64% (CI95: 85.13–98.88%) | 100% (CI95: 86.77–100%) | 93.89% (CI95: 88.32–97.33%) |
| Negative predictive value | 79.49% (CI95: 63.54–90.70%) | 93.18% (CI95: 81.34–98.57%) | 92.50% (CI95: 79.61–98.43%) | 78.52% (CI95: 70.63–85.12%) |
| Positive likelihood ratio | 14.53 (CI95: 3.78–55.83) | 13.88 (CI95: 4.65–41.46) | N/A* | 11.53 (CI95: 5.89–22.59) |
| Negative likelihood ratio | 0.13 (CI95: 0.07–0.24) | 0.06 (CI95: 0.02–0.17) | 0.10 (CI95: 0.04–0.30) | 0.21 (CI95: 0.15–0.29) |
Model (PD): breed‐specific model based on 100 Pugs. Model (FB): breed‐specific model based on 100 French Bulldogs. Model (BD): breed‐specific model based on 66 Bulldogs. Model (PFB): general model based on 266 brachycephalic dogs (100 Pugs, 100 French Bulldogs, and 66 Bulldogs).
CI95 = 95% confidence interval.
*Not calculable as specificity = 1.
Figure 4.
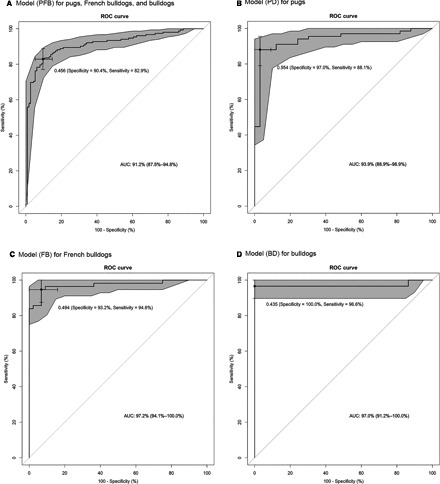
Receiver operating curves (ROC) assess the classification accuracy on diagnostic models for brachycephalic obstructive airway syndrome. The area between 95%CI for the curve is shaded. A cross and the associated values show the cut‐off values chosen for use in the diagnostic test. AUC, area under the curve.
Discussion
We have developed a tool that allows quantification of BOAS severity utilizing the respiratory variables obtained from WBBP in unrestrained brachycephalic dogs. The baseline respiratory characteristics were significantly different among asymptomatic pugs, French bulldogs, bulldogs, and nonbrachycephalic breeds. In addition, the proposed BOAS index can distinguish BOAS‐affected and clinically healthy brachycephalic dogs using breed‐specific models. Obesity and stenotic nares in brachycephalic dogs are highly associated with BOAS.
Ours is the first study on respiratory function in BOAS‐predisposed breeds that recruited a large number of both affected and clinically healthy dogs. BOAS has a high prevalence in all 3 breeds, but it often is unrecognized. In this study, approximately 40–50% of the pet dogs in the study group that were moderately or severely affected had not been treated for the disease. French bulldogs, pugs, and bulldogs recently have become extremely popular worldwide and all were listed among the top 10 breeds registered with the United Kingdom Kennel Club in 2014. These breeds have experienced a great increase in annual registration in the last 10 years with 343% increase in pugs, 2,985% increase in French bulldogs, and 199% increase in bulldogs.33 Respiratory function in bulldogs and French bulldogs has been characterized in a small number of dogs previously.5, 18 However, pugs have not been investigated and these 3 extremely brachycephalic breeds have not been compared.
Respiratory Characteristics Are Different among NonBrachycephalic Dogs, Pugs, French Bulldogs, and Bulldogs
In this study, Grade 0 bulldogs and French bulldogs showed a decrease in mean MV/BW and mean Te/Ti. They also had higher PEF/PIF compared to nonbrachycephalic dogs. Similar findings were obtained when breathing patterns in healthy nonbrachycephalic dogs and clinically healthy bulldogs and Boston terriers were investigated.5 Grade 0 pugs showed relatively similar respiratory characteristics to nonbrachycephalic controls. By contrast, Grade 0 bulldogs showed changes in respiratory characteristics that indicate upper airway restrictions and dynamic obstructions. We looked further at the differences among the 3 brachycephalic breeds. A multivariable classification method using QDA showed that the respiratory characteristics are clearly distinguishable. Grade 0 bulldogs showed lower minute volume and slower flow rate during both inspiration and expiration when adjusted for weight, and relatively higher variations in most of the variables. Differences in respiratory characteristics reflect the anatomical differences among skull types and among brachycephalic breeds. Therefore, breed‐specific models were developed in this study. Clinically, these Grade 0 dogs are considered “asymptomatic for BOAS.” However, there is very limited evidence about whether the respiratory changes seen in clinically healthy brachycephalic dogs will cause long‐term secondary effects on health such as gastrointestinal disorders, metabolic changes, or other problems.34, 35 Large studies on gastrointestinal disorders in healthy brachycephalic dogs are needed. Hypomagnesemia and hypercoagulation were found in clinically healthy bulldogs compared to nonbrachycephalic control dogs and boxers.36, 37 Hypertension also was reported in systemically healthy pugs, Boston terrier, French bulldogs, and bulldogs.38 These findings support the argument that the bulldog is a natural model for sleep apnea and hypopnea syndromes in humans with similar metabolic changes.29
Respiratory Characteristics in Grade II/III pugs, French Bulldogs, and Bulldogs
Grade II/III dogs are “awake snorers” with increased respiratory noise after exercise and labored breathing. Loss of constancy in the breathing pattern is an obvious change in respiration in BOAS (Fig 3C). In Grade II/III dogs, the breathing appears more chaotic and requires continuous adjustment, which contrasts with the consistent airflow patterns seen in Grade 0/I dogs (Fig 3B). The TBFVL of BOAS‐affected bulldogs has been recorded.5 The study commented that the most common loop shape in brachycephalic dogs was characterized by a flattened (fixed‐type obstruction) or a rounded expiratory phase (nonfixed‐type) with a flattened inspiratory phase. The loop often contained bursts of high frequency flow oscillations during inspiration and occasionally during expiration. The WBBP flow traces of a bulldog were collected before and after surgery,11 and were similar to the Type B Bulldog BOAS traces in this study. So far, interpretation of the flow waveforms in BOAS‐affected dogs remains unclear. As can be seen in Fig 3C, flow waveforms of Grade II/III dogs in all 3 breeds are not uniform. Nevertheless, 1 of the advantages of using QDA is that its quadratic boundary allows inclusion of different types of traces into the models.
The BOAS Index is an Useful Tool to Discriminate Objectively between Affected and Clinically Healthy Pugs, French Bulldogs, and Bulldogs
Marked variations of respiratory characteristics in Grade II/III dogs, not only among breeds but also within breeds, were observed in this study. Such variations were taken into account in the QDA classifier, which finds a novel application in characterizing respiratory flow traces. The QDA is a classic classifier with a quadratic decision surface, generated by fitting class conditional densities to the data and using Baye's rule to perform predictions.32 In our previous study on French bulldogs, a training group, approximately 60 dogs, was evaluated by a test dataset and found to have good sensitivity and specificity.18 We thus used a minimal number of 60 dogs to train the QDA classifier. We further used the caudal probabilities generated from QDA to calculate a predictive BOAS index. The BOAS index proposed here is a numeric scale to quantify the relative severity of BOAS. Our previous study of 89 French bulldogs showed that once the model is trained, QDA could accurately classify new dogs.18 After a preliminary test using a test dataset (20% of the total dog number), an internal permutation test was performed to validate the final models presented in this study. The finding that the discriminant performance is better when we use breed‐specific instead of general models is consistent with the differences that exist in the respiratory pattern and anatomy of the 3 breeds. Positive predictive values are all >94% in the 3 breed‐specific models. The protocol to collect WBBP data, process the data, and generate the BOAS index is straightforward and can be widely used in general practice with minimal staff training.
Obesity and Stenotic Nares are Associated with BOAS
Obese brachycephalic dogs have a higher risk of being BOAS‐affected, and obesity has a significant effect on decreasing TV/BW and MV/BW as well as PIF/BW and PEF/BW. Flow limitation during both inspiratory and expiratory phases suggests that obesity worsens respiration in brachycephalic dogs. An increase in soft tissue abutting the fixed bony structures results in a decreased airway lumen and increased stiffness of the respiratory system, which limits lung expansion.39, 40 Similarly, experimental beagles had significantly decreased TV/BW and significantly increased RR after being fed to obesity.19 The TV/BW often is decreased in severely obese humans, and breathing follows a rapid, shallow pattern with significant decreases in PEF/BW.39, 41 We have not separated the effects of obesity and other conformational effects associated with brachycephaly on respiratory function because there were insufficient obese Grade 0 dogs in each breed. Therefore, further study into changes in respiratory function after weight loss in brachycephalic dogs is warranted. In this study, obesity was defined based on BCS. In human medicine, in addition to the use of body mass index as an indication of obesity when investigating associations between obesity and obstructive sleep apnea, measurements of waist circumference (central obesity), neck circumference, deposition of fat around specific parts of the body such as neck or the base of the tongue were reported.42, 43, 44, 45, 46 Additional studies on the predictive obesity‐related parameters that may increase the risk of developing BOAS are required.
Until now, it has been difficult to distinguish in BOAS the functional consequences of each individual anatomic change associated with brachycephaly. Our study shows that severe stenosis of nares in brachycephalic dogs is a very important contributor to (by restricting airflow), or consequence of BOAS (through further collapse after a period of chronic high negative pressure within the airway). For French bulldogs in particular, the risk of BOAS in dogs with stenotic nares increases about 20 times. This breed previously has been shown to be at particular risk of mucosal contact points between the plica recta and nasal septum.28 The Starling resistor model47 equates airway function to a hollow tube with a constriction within the nasal cavity and near the nostrils, and a caudal collapsible segment, the oropharynx. This model predicts that a nose obstruction upstream will generate a negative intraluminal pressure downstream at the oropharynx, resulting in pharyngeal collapse.48 Even when the dog breathes orally, the majority of inspired air passes through the nose, and the expired air goes through the mouth, both during shallow thermal panting and deep panting.49, 50 Therefore, nasal obstructions not only restrict the airflow during nasal breathing at rest but also affect thermal regulation during panting in dogs.
Limitations
This study has several limitations. First, the prevalence of BOAS observed here reflects a mixed group of clinical cases and volunteer dogs and may not represent the true prevalence in the 3 breeds. However, it does not affect the aim of this study because both the numbers of affected dogs and clinically healthy dogs are sufficient for QDA. Second, we have not separated the effects of obesity and BOAS on respiratory function because there were insufficient obese Grade 0 dogs in each breed. Therefore, further study of the changes that occur in respiratory function after weight loss in brachycephalic dogs is warranted. Third, only the clinical dogs had computed tomography scans of the thorax to exclude lower airway disease. Invasive diagnostic assessments (eg, radiography, CBC, and serum biochemistry panel) on volunteer study dogs were not possible because of ethical considerations. For these dogs, lower airway disease was ruled out based on history and chest auscultation. Mild underlying lower airway compromise may affect ventilatory variables. Nevertheless, the proposed classifier closely reflects overall functional respiration as seen in the ETT, and so is of considerable value to the owners of tested animals. Fourth, we deliberately chose dogs that were ≥1 year old after consideration of maturity in terms of body dimension and respiratory physiology. However, there are limited studies on changes in respiratory physiology with age in dogs. Therefore, whether the model can be applied to puppies will require a cohort investigation. Last, only 3 brachycephalic breeds were included in the study. Classification models for other commonly affected breeds such as Pekingese, Japanese Chin, Boston terriers, and Shih‐Tzu might require further validation.
Conclusions and Relevance of the Study
In conclusion, breed‐specific WBBP‐based QDA classification tools for pugs, French bulldogs, and bulldogs showed excellent discrimination accuracy between Grade 0/I and Grade II/III dogs. The use of a BOAS index represents a large advance over the subjective criteria previously used to identify BOAS. This testing has the potential to facilitate clinical practice in monitoring disease progression and evaluating surgical outcome after upper airway surgery.51 The use of QDA may be relevant to other respiratory function studies that require characterization of respiratory flow traces.
Supporting information
Data S1. Input data for QDA models.
Data S2. Classification results of respiratory waveforms in Grade 0 pugs, French bulldogs, and bulldogs using quadratic discriminant analysis (QDA).
Data S3. The positions of all individuals, by breed and BOAS status measured by clinically supervised exercise tolerance test, compared with their caudal probabilities on the breed specific and combined breed BOAS indices..
Acknowledgments
This study is supported by a grant from the Kennel Club Charitable Trust (KCCT), no. RG71960.
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This article has not been previously presented. The authors are grateful to the Cambridge Statistics Clinic, Dr. TJ McKinley, and Dr. Caroline Trotter for their expert advice on data analysis and classifier design, as well as to the dog owners and breeders for their co‐operation with this study.
Footnotes
Whole‐body Barometric Plethysmography PLY 370 and PLY 360, Electro‐Medical Measurement Systems, Bordon, UK
Data Acquisition for Microsoft Windows XP ESS‐102, Electro‐Medical Measurement System, Bordon, UK
SPSS statistics for Mac version 22.0, IBM Corporation, Armonk, NY
R: A Language and Environment for Statistical Computing for Mac OX version 3.1.1. (http://www.R-project.org), R Foundation for Statistical Computing, Vienna, Australia
References
- 1. Oechtering G. Brachycephalic syndrome – new information on an old congenital disease. Vet Rec 2010;20:2–9. [Google Scholar]
- 2. O'Neill D, Jackson C, Guy JH, et al. Epidemiological associations between brachycephaly and upper respiratory tract disorders in dogs attending veterinary practices in England. Canine Genet and Epidemiol 2015;2:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hendricks J. Brachycephalic airway syndrome In: King L, ed. Textbook of Respiratory Disease in Dogs and Cats. St Louis: Saunders; 2004:310–318. [Google Scholar]
- 4. Amis TC, Kurpershoek C. Tidal breathing flow‐volume loop analysis for clinical assessment of airway obstruction in conscious dogs. Am J Vet Res 1986;47:1002–1006. [PubMed] [Google Scholar]
- 5. Amis TC, Kurpershoek C. Pattern of breathing in brachycephalic dogs. Am J Vet Res 1986;47:2200–2204. [PubMed] [Google Scholar]
- 6. Clercx C, Gustin P, Landser FJ, et al. Measurement of total respiratory impedance in dogs by the forced oscillation technique. Vet Res Commun 1993;17:227–239. [DOI] [PubMed] [Google Scholar]
- 7. Hernandez AB, Kirkness JP, Smith PL, et al. Novel whole body plethysmography system for the continuous characterization of sleep and breathing in a mouse. J Appl Physiol 2012;112:671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Friedman L, Haines A, Klann K, et al. Ventilatory behavior during sleep among A/J and C57BL/6J mouse strains. J Appl Physiol 2004;97:1787–1795. [DOI] [PubMed] [Google Scholar]
- 9. Han F, Subramanian S, Price ER, et al. Periodic breathing in the mouse. J Appl Physiol (1985) 2002;92:1133–1140. [DOI] [PubMed] [Google Scholar]
- 10. Nakamura A, Fukuda Y, Kuwaki T. Sleep apnea and effect of chemostimulation on breathing instability in mice. J Appl Physiol 2003;94:525–532. [DOI] [PubMed] [Google Scholar]
- 11. Bernaerts F, Talavera J, Leemans J, et al. Description of original endoscopic findings and respiratory functional assessment using barometric whole‐body plethysmography in dogs suffering from brachycephalic airway obstruction syndrome. Vet J 2010;183:95–102. [DOI] [PubMed] [Google Scholar]
- 12. Hirt R, Leinker S, Mosing M, et al. Comparison of barometric whole body plethysmography and its derived parameter enhanced pause (PENH) with conventional respiratory mechanics in healthy beagle dogs. Vet J 2008;176:232–239. [DOI] [PubMed] [Google Scholar]
- 13. Hirt R, Vondrakova K, de Arespacochaga A, et al. Effects of cadmium chloride inhalation on airflow limitation to histamine, carbachol and adenosine 5′‐monophosphate assessed by barometric whole body plethysmography in healthy dogs. Vet J 2007;173:62–72. [DOI] [PubMed] [Google Scholar]
- 14. Hoffman AM, Dhupa N, Cimetti L. Airway reactivity measured by barometric whole‐body plethysmography in healthy cats. Am J Vet Res 1999;60:1487–1492. [PubMed] [Google Scholar]
- 15. Kirschvink N, Leemans J, Delvaux F, et al. Non‐invasive assessment of airway responsiveness in healthy and allergen‐sensitised cats by use of barometric whole body plethysmography. Vet J 2007;173:343–352. [DOI] [PubMed] [Google Scholar]
- 16. Kirschvink N, Leemans J, Delvaux F, et al. Non‐invasive assessment of growth, gender and time of day related changes of respiratory pattern in healthy cats by use of barometric whole body plethysmography. Vet J 2006;172:446–454. [DOI] [PubMed] [Google Scholar]
- 17. Lin C‐H, Lee J‐J, Liu C‐H. Functional assessment of expiratory flow pattern in feline lower airway disease. J Feline Med Surg 2013;16:616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu N‐C, Sargan D, Adams V, et al. Characterisation of brachycephalic obstructive airway syndrome in french bulldogs using whole‐body barometric plethysmography. PLoS ONE 2015;10:e0130741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Manens J, Bolognin M, Bernaerts F, et al. Effects of obesity on lung function and airway reactivity in healthy dogs. Vet J 2012;193:217–221. [DOI] [PubMed] [Google Scholar]
- 20. Talavera J, Kirschvink N, Schuller S, et al. Evaluation of respiratory function by barometric whole‐body plethysmography in healthy dogs. Vet J 2006;172:67–77. [DOI] [PubMed] [Google Scholar]
- 21. Bolognin M, Kirschvink N, Leemans J, et al. Characterisation of the acute and reversible airway inflammation induced by cadmium chloride inhalation in healthy dogs and evaluation of the effects of salbutamol and prednisolone. Vet J 2009;179:443–450. [DOI] [PubMed] [Google Scholar]
- 22. Lorinson D, Bright R, White R. Brachycephalic airway obstruction syndrome: A review of 118 cases. Canine Pract 1997;22:18–21. [Google Scholar]
- 23. Asher L, Diesel G, Summers J, et al. Inherited defects in pedigree dogs. Part 1: Disorders related to breed standards. Vet J 2009;182:402–411. [DOI] [PubMed] [Google Scholar]
- 24. Grosso F, Haar G, Boroffka S. Gender, weight, and age effects on prevalence of caudal aberrant nasal turbinates in clinically healthy English bulldogs: A computed tomographic study and classification. Vet Radiol Ultrasound 2015;56:1–8. [DOI] [PubMed] [Google Scholar]
- 25. Caccamo R, Buracco P, La Rosa G, et al. Glottic and skull indices in canine brachycephalic airway obstructive syndrome. BMC Vet Res 2014;10:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Oechtering T, Oechtering G, Noeller C. Computed tomographic imaging of the nose in brachycephalic dog breeds. Tierärztliche Praxis Kleintiere 2007;35:177–187. [DOI] [PubMed] [Google Scholar]
- 27. Ginn J, Kumar M, McKiernan B, et al. Nasopharyngeal turbinates in brachycephalic dogs and cats. J Am Anim Hosp Assoc 2008;44:243–249. [DOI] [PubMed] [Google Scholar]
- 28. Schuenemann R, Oechtering G. Inside the brachycephalic nose: Intranasal mucosal contact points. J Am Anim Hosp Assoc 2014;50:149–158. [DOI] [PubMed] [Google Scholar]
- 29. Hendricks J, Kline LR, Kovalski RJ, et al. The English bulldog: A natural model of sleep‐disordered breathing. J Appl Physiol 1987;63:1344–1350. [DOI] [PubMed] [Google Scholar]
- 30. Packer R, Hendricks A, Tivers M, et al. Impact of facial conformation on canine health: Brachycephalic obstructive airway syndrome. PLoS ONE 2015;10:e0137496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Laflamme D. Development and validation of a body condition score system for dogs. Canine Pract 1997;22:10–15. [Google Scholar]
- 32. James G, Witten D, Hastie T, et al. Classification In: An introduction to Statistical Learning with Applications in R. New York: Springer Science & Business Media; 2013:127–174. [Google Scholar]
- 33. The Kennel Club . UK: Dog breeds‐registration statistics in the UK, 2005. –2014. Available at: http://www.thekennelclub.org.uk/registration/breed-registration-statistics/. Accessed October 9, 2015.
- 34. Poncet C, Dupré G, Freiche V, et al. Prevalence of gastrointestinal tract lesions in 73 brachycephalic dogs with upper respiratory syndrome. J Small Anim Pract 2005;46:273–279. [DOI] [PubMed] [Google Scholar]
- 35. Poncet C, Dupre G, Freiche V, et al. Long‐term results of upper respiratory syndrome surgery and gastrointestinal tract medical treatment in 51 brachycephalic dogs. J Small Anim Pract 2006;47:137–142. [DOI] [PubMed] [Google Scholar]
- 36. Hoareau G, Mellema M. Pro‐coagulant thromboelastographic features in the bulldog. J Small Anim Pract 2015;56:103–107. [DOI] [PubMed] [Google Scholar]
- 37. Mellema MS, Hoareau GL. Hypomagnesemia in brachycephalic dogs. J Vet Intern Med 2014;28:1418–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hoareau GL, Jourdan G, Mellema M, et al. Evaluation of arterial blood gases and arterial blood pressures in brachycephalic dogs. J Vet Intern Med 2012;26:897–904. [DOI] [PubMed] [Google Scholar]
- 39. Salome C, King G, Berend N. Physiology of obesity and effects on lung function. J Appl Physiol 2010;108:206–211. [DOI] [PubMed] [Google Scholar]
- 40. Isono S. Contribution of obesity and craniofacial abnormalities to pharyngeal collapsibility in patients with obstructive sleep apnea. Sleep Biol Rhythms 2004;2:17–21. [Google Scholar]
- 41. Farooq S, Mustafa S, Ahmed A, et al. The effect of obesity markers on peak expiratory flow rate in young saudi adults. Med Res Chron 2015;2:126–134. [Google Scholar]
- 42. Kim AM, Keenan BT, Jackson N, et al. Tongue fat and its relationship to obstructive sleep apnea. Sleep 2014;37:1639–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cizza G, de Jonge L, Piaggi P, et al. Neck circumference is a predictor of metabolic syndrome and obstructive sleep apnea in short‐sleeping obese men and women. Metab Syndr Relat Disord 2014;12:231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Onat A, Hergenç G, Yu ¨ksel H, et al. Neck circumference as a measure of central obesity: Associations with metabolic syndrome and obstructive sleep apnea syndrome beyond waist circumference. Clin Nutr 2009;28:46–51. [DOI] [PubMed] [Google Scholar]
- 45. Hoffstein V, Mateik S. Differences in abdominal and neck circumferences in patients with and without obstructive sleep apnea. Eur Respir J 1992;5:377–381. [PubMed] [Google Scholar]
- 46. Mortimore I, Marshall I, Wraith P, et al. Neck and total body fat deposition in nonobese and obese patients with sleep apnea compared with that in control subjects. Am J Respir Crit Care Med 1998;157:280–283. [DOI] [PubMed] [Google Scholar]
- 47. Smith P, Wise RA, Gold AR, et al. Upper airway pressure‐flow relationships in obstructive sleep apnea. J Appl Physiol 1988;64:789–795. [DOI] [PubMed] [Google Scholar]
- 48. Michels D, Rodrigues A, Nakanishi M, et al. Nasal involvement in obstructive sleep apnea syndrome. Int J Otolaryngol 2014;2014:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schmidt‐Nielsen K, Bretz WL, Taylor CR. Panting in dogs: Unidirectional air flow over evaporative surfaces. Science 1970;169:1102–1104. [DOI] [PubMed] [Google Scholar]
- 50. Goldberg MB, Langman VA, Taylor CR. Panting in dogs: Paths of air flow in response to heat and exercise. Respir Physiol 1981;43:327–338. [DOI] [PubMed] [Google Scholar]
- 51. Liu N‐C, Sargan D, Adams V, et al. Using Whole‐Body Barometric Plethysmography to Evaluate the Effectiveness of Upper Airway Surgery for Brachycephalic Obstructive Airway Syndrome in French Bulldogs. Proceeding of the 24th European College of Veterinary Surgeons Annual Scientific Meeting; 2015 July 2–4; Berlin, Germany. Zurich: ECVS; 2015. p. 201. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Input data for QDA models.
Data S2. Classification results of respiratory waveforms in Grade 0 pugs, French bulldogs, and bulldogs using quadratic discriminant analysis (QDA).
Data S3. The positions of all individuals, by breed and BOAS status measured by clinically supervised exercise tolerance test, compared with their caudal probabilities on the breed specific and combined breed BOAS indices..


