Abstract
Background
Dysregulated apoptosis is a hallmark of tumorigenesis, and is also involved in resistance to cytotoxic treatment, and might be relevant in lymphoma in dogs.
Hypothesis/Objectives
That Bcl‐2/Bax expression patterns differ between lymphoma immunophenotypes, and that Bcl‐2/Bax ratio is correlated with prognosis.
Animals
Fifty‐five client‐owned dogs with multicentric lymphoma and 5 healthy dogs.
Methods
Prospective, case–control study. We compared 3 methods (flow cytometry, qRT‐PCR, Western blot) for Bcl‐2 and Bax quantification in a subset of dogs. The effect of time on Bcl‐2/Bax ratios measured by flow cytometry was assessed in lymphoma cell lines. Immunophenotype and Bcl‐2/Bax expression by flow cytometry were determined in LN aspirates from all dogs with multicentric lymphoma compared to healthy dogs. Progression‐free survival (PFS) was retrospectively evaluated in a group of dogs all receiving similar treatment.
Results
Bcl‐2/Bax ratios remain consistent for at least 5 days after sample collection. Bcl‐2/Bax ratio was higher in dogs with T‐cell lymphoma (TCL; median 0.97, range 0.37–1.36) compared to B‐cell lymphoma (BCL; median 0.36, range 0.07–1.45) (P < .0001) and normal dogs (median 0.36, range 0.21–0.48) (P = .0006), respectively. Dogs with Bcl‐2/Bax ratios higher than the median of the group experienced a median PFS of 101 days and dogs with ratios equal and lower than the median had PFS of 130 days (P = .19).
Conclusions and clinical importance
Higher intrinsic resistance to apoptosis following cytotoxic treatment might contribute to the less favorable prognosis associated with multicentric TCL in dogs. Whether Bcl‐2/Bax will be helpful to identify canine BCL and TCL with more aggressive and more indolent behavior, respectively, should be evaluated in larger prospective clinical studies.
Keywords: Canine, Flow cytometry, Lymphosarcoma, Programmed cell death
Abbreviations
- APC
allophycocyanin
- BCL
B‐cell lymphoma
- DLBCL
diffuse large B‐cell lymphoma
- FC
flow cytometry
- FL
follicular lymphoma
- FBS
fetal bovine serum
- FITC
fluorescein isothiocyanate
- Ig
immunoglobulin
- MFI
mean fluorescence intensity
- mRNA
messenger ribonucleic acid
- PBS
phosphate‐buffered saline
- PE
phycoerythrin
- PFS
progression‐free survival
- PTCL
peripheral T‐cell lymphoma
- PVDF
polyvinylidene fluoride
- qRT‐PCR
quantitative real‐time polymerase chain reaction
- SDS‐PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- TCL
T‐cell lymphoma
- TTBS
tris‐buffered saline and tween 20
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
- WB
Western blot
Lymphoma is the most common hematopoietic malignancy in dogs. Multiple studies have investigated prognostic markers in dogs with lymphoma, and tumor immunophenotype remains one of the most critical factors.1, 2, 3 Canine high‐grade TCL is generally associated with a less favorable prognosis compared to most types of BCL.1, 2, 3 The reason for this difference in behavior is largely unknown; although immunophenotype alone might be insufficient for predicting outcome.3, 4, 5, 6, 7, 8, 9
Bcl‐2, an antiapoptotic molecule of the intrinsic apoptotic pathway, inhibits apoptosis by binding to and subsequently inhibiting proapoptotic molecules such as Bax and Bak.10 Dysregulation of this pathway results in imbalances between cell survival, proliferation, and death, which impacts on tumor growth. Therefore, apoptosis serves as a natural barrier to cancer development, especially in tissues with high intrinsic proliferative capacity and cell turnover such as hematopoietic and lymphoid tissue.11, 12 Also, dysregulation of apoptosis plays an important role in tumor progression and in resistance to cytotoxic treatment.11, 13, 14
In humans, a distinct translocation of the Bcl‐2 proto‐oncogene (t(14;18)) causes aberrant Bcl‐2 expression in follicular lymphoma (FL).15 This and other translocations, mutations, and amplification of the Bcl‐2 gene as well as posttranslational mechanisms give rise to increased Bcl‐2 levels in diffuse large BCLs (DLBCL) and in other B‐cell tumors, and increased Bcl‐2 expression also occurs in human peripheral TCL (PTCL).16, 17, 18, 19, 20, 21, 22 In both, Bcl‐2 overexpression, alone or in conjunction with other apoptotic proteins, was associated with worse clinical outcome.18, 19, 20, 21 Likewise, loss of function mutations in Bax, one of the key target genes of the tumor suppressor gene p53, have been identified in human lymphoblastic leukemia and lymphoma, and overexpression of Bax in human DLBCL and PTCL was associated with longer survival.18, 21, 23, 24
In the aforementioned human studies, overexpression was primarily determined by immunohistochemistry of individual proteins. However, it is not only the relative amount of any involved molecule but more importantly the ratio of pro‐ to antiapoptotic proteins is determining sensitivity to apoptotic stimuli.13, 14, 25, 26 More precisely, the ratio of Bcl‐2 to Bax regulates apoptosis.25 In humans, elevated Bcl‐2/Bax ratio, determined by protein microarray analysis, was associated with shorter survival in FL,27 while Bax/Bcl‐2 ratio, measured by flow cytometry (FC), correlated with high‐risk cytogenetics, chemoresistance, and poor clinical outcome in acute myeloid leukemia and chronic lymphocytic leukemia.26, 28
There are only few reports describing the expression of apoptotic molecules in canine tumors.29, 30, 31, 32, 33 One study demonstrated higher expression of the proapopoptic molecule Bad in canine BCL compared to nonneoplastic lymphoid tissue,33 but to the authors' knowledge, the relationship of pro‐ and antiapoptotic molecules in dogs with lymphoma has not been reported.
Flow cytometry (FC) is commonly used to identify lymphoma immunophenotype.8, 9, 34, 35, 36 In addition, FC can also provide additional prognostic information for BCL and TCL, respectively.8, 9, 36 The aim of this study was to prospectively evaluate Bcl‐2 and Bax expression in neoplastic lymphocytes from dogs with previously diagnosed, untreated multicentric lymphoma. We hypothesized that (1) Bcl‐2/Bax expression patterns differs between B‐cell and T‐cell immunophenotypes, and that, (2) Bcl‐2/Bax ratio can predict outcome in a subset of dogs receiving identical treatment.
Materials and Methods
Patient Selection
The LN aspirate samples and demographic data were prospectively acquired from client‐owned dogs diagnosed with multicentric lymphoma. Samples were submitted to North Carolina State University (NCSU) College of Veterinary Medicine Clinical Immunology Laboratory for immunophenotyping of lymphoma from referring veterinarians and from NCSU Veterinary Hospital (VH) Oncology Service. All dogs had cytologic or histologic confirmation of lymphoma. Dogs with multicentric lymphoma of any clinical stage and which received no prior treatment including glucocorticoids were included. Signalment, date of lymphoma diagnosis, stage, and substage of lymphoma (determined based on physical exam, CBC, biochemistry, urinalysis, abdominal ultrasound, and 3‐view thoracic radiographs), date of sample collection, and age of sample at analysis were entered into a searchable database using information provided by the submitting veterinarian and by the NC State VH electronic database. For NCSU VH Oncology patients, additional information included date of first treatment, type of treatment, response to treatment, date of disease progression, and date of death.
Bcl‐2 and Bax Expression
For 5 lymphoma patients, 3 methods were used to determine Bcl‐2 and Bax in peripheral LN aspirate samples. These included Bcl‐2 and Bax protein expression determined by FC and Western blot (WB), and Bcl‐2 and Bax mRNA levels determined by quantitative RT (qRT)‐PCR. For each dog, the same cell populations were used for all 3 methods and divided into 3 aliquots. The results of different methods were compared to determine the most efficient and reliable method of Bcl‐2 and Bax quantitation.
Flow Cytometry
Cells from peripheral LN aspirates submitted in sterile tubes without additives in RPMI 1640 cell culture medium1 or isotonic saline supplemented with 5–10% patient serum were suspended in 5 mL RPMI 1640/10% FBS1, refrigerated, and analyzed by FC within 48 hours of sample submission. Samples were washed with PBS1, spun for 10 minutes at 500 x g, and the supernatant was discarded. The pellet was resuspended in PBS and cells were stained with monoclonal antibodies that react with canine Bcl‐22 and Bax,3 respectively, and treated with permeabilization reagents4 following the manufacturer's instructions. The combination of antibodies in each tube was as follows: anti‐Bcl‐2 (duplicate), anti‐Bax (duplicate), secondary fluorescein isothiocyanate (FITC)‐conjugated antibody,5 and a “cells only” control. Following incubation, data were acquired on a FACSCalibur analyzer,6 with a minimum of 15,000 events collected and analyzed using BD CellQuest software6. The Bcl‐2/Bax ratios were determined by dividing the relative mean fluorescence intensity (MFI) of Bcl‐2 staining by the relative MFI of Bax staining (Fig 1). Relative MFI was determined by dividing the MFI of the staining with the monoclonal antibody by the MFI of the secondary antibody5 alone. Staining for Bcl‐2 and Bax was performed in duplicate, and final Bcl‐2/Bax ratio was determined by the mean of both data.
Figure 1.
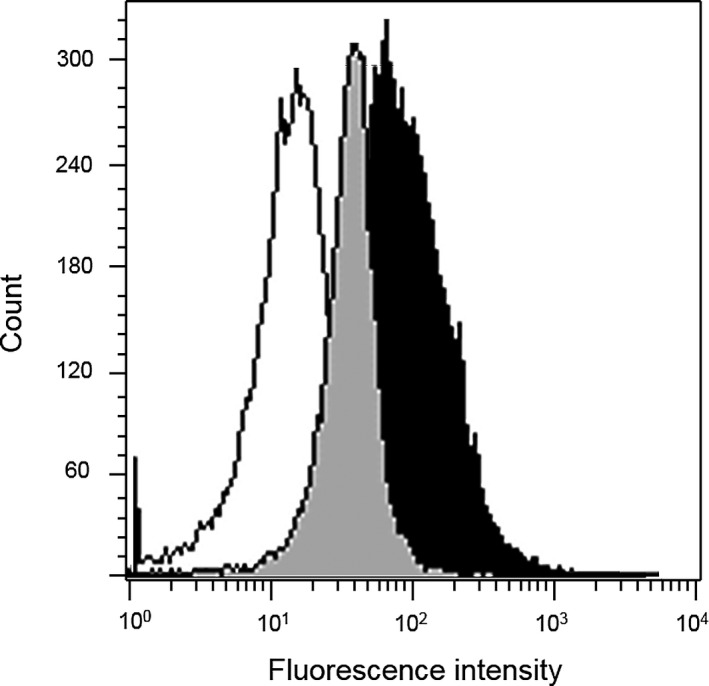
Representative flow cytometric histograms of Bcl‐2 and Bax expression. Lymph node aspirate from a dog with multicentric lymphoma. Cases were analyzed after exposure to anti‐bcl‐2 (clone7/Bcl‐2), anti‐bax (clone 3/Bax), and a FITC‐conjugated secondary antibody (fragment of rabbit antimouse immunoglobulin), in conjunction with immunophenotyping of lymphoma (not shown here). Lymphocytes were gated based on forward versus side light scatter characteristics (not shown). Total cell count is depicted on the y‐axis. Secondary antibody alone (solid white), Bcl‐2 (solid gray), and bax (solid black) histograms show the distribution of fluorescence intensity (x‐axis), reported as arithmetic means. Bcl‐2/Bax ratio was determined by dividing the relative mean fluorescence intensity (MFI) of Bcl‐2 staining by the relative MFI of Bax staining. Relative MFI was determined by dividing the MFI of Bcl‐2 staining and Bax staining by the MFI of the secondary antibody alone. FITC: fluorescein isothiocyanate.
Western blot Analysis
Total protein was isolated using standard techniques and quantitated. Briefly, equivalent amounts of buffered protein samples were electrophoresed in SDS‐PAGE gels and electroblotted (Invitrogen X Cell Gel Box and X cell Blotting Module) onto PVDF membranes (Millipore Immobilon Transfer Membranes). The transfer membrane was blocked (5% milk in TTBS), incubated with primary antibody (anti‐Bcl22, anti‐Bax3), washed (TTBS), incubated with secondary IgG:HRP antibody,7 and washed (TTBS). Antibodies were diluted in 1% bovine serum albumin in TTBS. For protein blot visualization, the membrane was incubated with chemiluminescent substrate (Millipore Immobilon Western Chemiluminescent HRP Substrate). Signal strength densitometry was performed for Bcl‐2 and Bax protein quantification using standard techniques with normalization to the β‐actin signal strength.
Quantitative RT‐PCR
Quantitative RT‐PCT was performed essentially as previously descrived.37 Briefly, primers for Bcl‐2 (forward 5′‐ATCCAGGACAACGGAGGCTG‐3′, reverse 5′‐CAGATAGGCACCCAGGGTGA‐3′) and Bax (forward 5′‐GATCGAGCAGGGCGAATG‐3′, reverse 5′‐CATCTCAGCTGCCACTCG‐3′) were designed (PrimerSelect, DNASTAR) based on the published canine Bcl‐2 and Bax sequences, with reaction efficiencies ranging from 90 to 105%. Total RNA was isolated from >106 cells per dog (RNeasy Plus Mini Kit, Qiagen, Valencia, CA, USA). About 500 ng of RNA was reverse transcribed using Oligo(dT) primers according to the manufacturer's instructions (Reverse Transcription System, Promega, Madison, WI, USA). Products were assessed in duplicate for the Bcl‐2 or Bax mRNA in separate reactions (Quantitec RT‐PCR Kit, Qiagen), using a Bio‐Rad icycle quantitative PCR thermocycler. Relative expression was calculated using the 2−ΔΔCT method with GAPDH as the control gene.38
Flow Cytometry for Immunophenotyping and Bcl‐2 and Bax Expression
Immunophenotype and Bcl‐2/Bax ratio was determined by FC from peripheral LN aspirates obtained from all study dogs with lymphoma. Data from LN aspirates from 5 healthy research dogs collected at the time of euthanasia were obtained as controls. Preanalytical processing of cells was identical as described above. Cells were stained with a panel of antibodies (anti‐CD45,8 anti‐CD3,9 anti‐CD21,10 anti‐CD4,11 anti‐CD8,12 anti‐B5 antigen,13 mouse IgG114 ) after counting. The combination of antibodies in each tube for surface immunophenotypic LN analysis was as follows: CD4/CD8/CD45, CD21/CD3/CD45, CD4/CD3/CD45, CD8/CD3/CD45, B5/CD45, CD14/CD45, secondary antibody/CD45, isotype control antibodies, and a “cells only” control. For intracellular staining (anti‐CD3 antibody, clone: CD3‐12h; anti‐CD79b, clone: AT107‐2h), cells were treated with permeabilization reagents4 following the manufacturer's instructions. Antibodies were directly conjugated to FITC, phycoerythrin (PE), or allophycocyanin (APC). Clinical samples from animals with confirmed lymphoma were stained with propidium iodide (PI) to assess the average percentage of dead cells in clinical samples, which was approximately 20%. Data were acquired on a FACSCalibur analyzer, FACSCalibur System6; 15,000 events were acquired and stored for analysis using BD CellQuest software6. Cells were gated using forward versus side light scatter to identify lymphocytes, monocytes, and neutrophils, with side scatter versus CD45 staining to confirm these populations. Lymphocyte gating was used to evaluate the surface and intracellular markers described above for immunophenotyping (B‐cell or T‐cell). Reference values were used as previously reported.29 Samples that displayed ambiguous staining patterns not definitively consistent with B cell or T cell, and dual immunophenotype were excluded. Bcl‐2 and Bax staining and analysis was similar as described above.
Time Course Study
Cells from 3 canine B‐cell lines (GL‐1, 17‐71, CLBL‐1) and 2 canine T‐cell lines (CL‐1, OSW), and 1 human BCL cell line (Ramos) were grown and kept in cell culture media (RPMI 1640/10% FBS with 1% L‐glutamine and 0.2% primocin) in a 37°C incubator until a concentration of approximately 1 × 106/mL was reached. Six mL of the cell line suspension was transferred from the cell culture flask to a sterile tube without additives and analyzed by FC for intracellular Bcl‐22 and Bax3 staining as described above after being stored in the refrigerator at 4°C. The same cell populations were used throughout the time course study period and aliquots were analyzed at the following time points: immediately after obtaining cells (d0), 24 hours (d1), 48 hours (d2), 72 hours (d3), 96 hours (d4), and 120 hours (d5) following collection. Data were acquired on a LSR II Flow Cytometer (BD Biosciences) with BD FACSDiva software; with a minimum of 20,000 events collected and analyzed using BD CellQuest software6. Analyses were done in duplicate for both Bcl‐2 and Bax.
Outcome Analysis
In a subset of lymphoma study dogs treated at NCSU VH Oncology Service, progression‐free survival (PFS) was calculated retrospectively from the time of treatment initiation until progressive disease or death of any cause. Staging was similar as for the other dogs included in the study and consisted of physical examination including caliper measurements of enlarged peripheral LNs, CBC, chemistry panel, urinalysis, thoracic radiographs, and abdominal ultrasound. In addition, pathologist blood smear review and bone marrow aspiration cytology was performed based on clinician's discretion if blood or bone marrow involvement was suspected (eg, circulating blasts, peripheral cytopenias). Response was evaluated by repeat measurements of clinical detectable disease at each visit and always included caliper measurement of peripheral LNs and a CBC, thoracic and abdominal imaging if internal organs were involved at baseline, and a biochemistry panel including total calcium if hypercalcemia or other abnormalities were present at diagnosis or during the course of treatment. Initial staging was repeated after the full course of treatment. Follow‐up visits were scheduled once a month at either the referring veterinarian or at NCSU, and included at least, depending on clinical presentation and clinician's discretion, a physical examination with evaluation of peripheral LNs. Disease progression was defined as an at least 20% increase in size of all measurable tumors or the appearance of new lesions and confirmed by cytology or histopathology. All dogs were treated with a 12‐week multiagent protocol (L‐asparaginase, cyclophosphamide, doxorubicin, vincristine, prednisone) followed by cranial and caudal half‐body irradiation (4 Gy on 2 consecutive days, 3 weeks apart).
Statistical Analysis
Bcl‐2/Bax ratio determined by protein expression (FC, WB), and by mRNA levels (qRT‐PCR) were compared by one‐way analysis of variance (ANOVA). Bcl‐2/Bax ratios between normal LN samples and lymphoma LN samples were compared using a paired two‐sample t‐test. Normal distribution was assessed visually in a graph. Correlation of other patients' variables with Bcl‐2/Bax ratio were not assessed. Longitudinal analysis with repeated‐measures ANOVA was used to compare Bcl‐2/Bax ratio over time in lymphoma cell lines kept at 4°C from d0 to d5, and also to compare Bcl‐2/Bax between BCL and TCL cell lines from d0 to d5 or within each day.
Kaplan–Meier plot was used to evaluate PFS, and log rank analysis was used to assess differences in outcome according to a set threshold of Bcl‐2/Bax. Dogs were censored from analysis if progression had not occurred and if they were lost to follow‐up at time of analysis. A statistical power analysis was run to estimate the number of patients needed to allow determination a cutoff point for Bcl‐2/Bax ratio which subsequently could be used as a candidate prognostic marker. Statistical analyses were performed using SAS 9.4 software and P values < .05 were considered significant. All statistical analyses were performed in consultation with NCSU statistical consulting group (Dr E. Griffith).
Results
Comparison of Methods and Time Course Study
In a subset of 5 dogs (all diagnosed with multicentric BCL), there was no difference in Bcl‐2/Bax ratios determined by FC, WB and qRT‐PCR (P = .30) (Fig 2).
Figure 2.
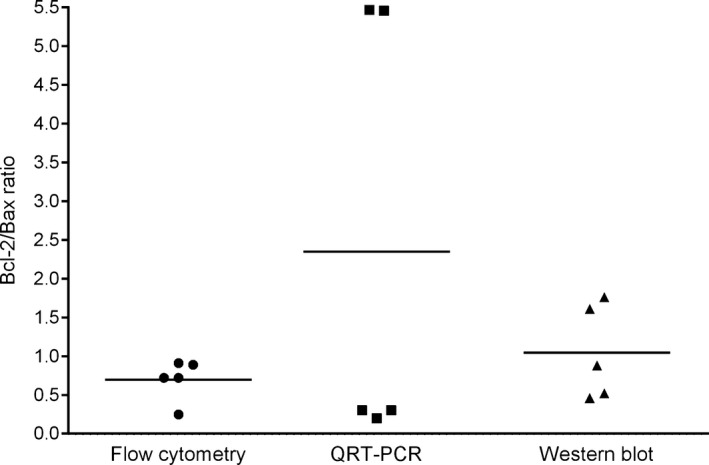
Flow cytometry is a rapid, reliable method for measuring Bcl‐2/Bax ratios. Bcl‐2 and Bax expression determined for 5 patients was not different (P = .30) by flow cytometry (mean ± standard deviation, 0.7 ± 0.27), quantitative RT‐PCR (QRT‐PCR) (2.35 ± 2.85), and Western blot (1.05 ± 0.61), but flow cytometry offers a rapid measurement and revealed less deviation of results. Solid line represents the mean.
Given that FC is a readily clinical applicable method, it was subsequently used for the remainder study patients.
The NCSU College of Veterinary Medicine Clinical Immunology Laboratory analyzes lymphoma samples from in‐house patients and from referring veterinarians. In‐house samples are usually analyzed within 24 hours of sample collection. Samples from referring veterinarians are shipped on ice packs overnight and analyzed within 24–48 hours of sample collection. However, samples are sometimes shipped over the weekend or a holiday, which may extend the period until analysis. In order to analyze the effect of transport and storage time on Bcl‐2/Bax ratios, a time course study on lymphoma cell lines was conducted and revealed that Bcl‐2/Bax ratios remained consistent for 5 days when stored at 4°C (Fig 3). There were also no differences between BCL and TCL cell lines over time (d0–d5) and within each day, respectively (adjusted univariate P value for H‐F correction factor = 0.1882). Based on these results we included samples from our patient population up to 5 days following collection in our Bcl‐2/Bax analysis.
Figure 3.
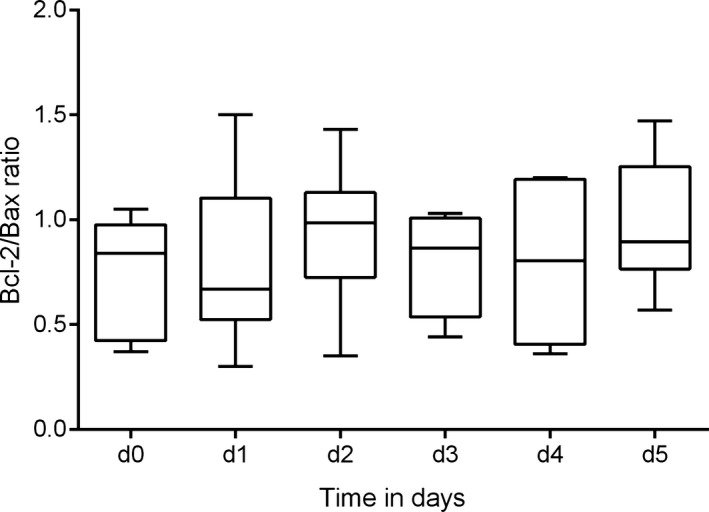
Bcl‐2/Bax ratios remain consistent after 5 days of refrigeration. Time course study evaluating Bcl‐2/Bax expression measured by flow cytometry in duplicates immediately following collection (d0) and on 5 consecutive days using 6 different lymphoma cell lines stored at 4°C. The mean Bcl‐2/Bax ratio (solid line within the box) remained between 0.7 and 1.0 for all 6 measurements, and univariate approach to within‐subject repeated‐measures analysis of variance (ANOVA) revealed no significant change in the Bcl‐2/Bax between days (adjusted univariate P value for Huynh‐Feldt Epsilon (H‐F) correction factor = 0.9509). d: day.
Dog Demographics and Lymphoma Immunophenotype
Fifty‐five dogs with multicentric lymphoma were recruited. Eighteen cases were submitted by the NCSU VH Oncology Service, and 37 cases were submitted by referring veterinarians. Patient demographics were not reported for all animals in the study. Median age at diagnosis was 9.0 years (n = 27, range 2.3–15.0 years). Fourteen dogs were neutered female, and 15 cases were male (1 intact, 14 neutered). Golden and Labrador Retriever dogs were the most frequently represented breed (each n = 5/29; 17%), followed by mixed breed dogs (n = 3/29; 10%), German Shepherd dogs (n = 2/29; 7%), West Highland White Terriers (n = 2/29; 7%), Shi Tzu (n = 2/29; 7%), and there was one each (3.5%) Welsh Pembroke Corgi, Malinois, Basset Hound, German Shorthair Pointer, Schnauzer, Shar‐Pei, Mastiff, Boxer, Pomerian, and Basenj. Three of 17 dogs (18%) presented in stage 3, 7/17 dogs (41%) presented in stage 4, and 7/17 (41%) presented in stage 5 multicentric lymphoma. Five dogs (29%) had substage a, and 12 dogs (71%) had substage b. Flow cytometry analysis was performed within 48 hours after collection in 41/55 cases, on day 3 in 9/55, on day 4 in 3/55, and on day 5 in 2/55 following sample collection. Immunophenotyping by FC revealed 42 (76%) cases with BCL and 13 (24%) dogs with TCL, respectively. Among the 13 dogs with TCL, there were 3 dogs with CD3+/CD45+ TCL, 3 dogs with CD3‐positive TCL identified by intracellular staining only, 3 dogs with CD3+/CD4+/CD45+ TCL, 2 dogs with CD3+/CD4+/CD45‐ TCL with CD21‐coexpression (T‐zone lymphoma)8, 36, and 1 dog each with CD3+/CD4+/CD8+/CD45+ and CD3+/CD8+/CD45+ TCL.
Bcl‐2/Bax Ratio by FC in Normal Dogs and Dogs with Multicentric Lymphoma
We first asked if Bcl‐2/Bax ratios differed between healthy dogs and dogs with lymphoma. There was no statistically significant difference in Bcl‐2/Bax ratios between normal dogs (median 0.36, range 0.21–0.48) and all dogs with lymphoma (median 0.45, range 0.07–1.45) (P = .43) (Fig 4). There was no statistically significant difference in Bcl‐2/Bax ratio between normal dogs and dogs with BCL (median 0.36, range 0.07–1.45) (P = .85). However, there was a statistically significant difference in the Bcl‐2/Bax ratio between normal dogs and dogs with TCL (median 0.97, range 0.37–1.36) (P = .0006) and between BCL (median 0.36, range 0.07–1.45) and TCL (median 0.97, range 0.37‐‐1.36) dogs (P < .0001), respectively (Fig 4).
Figure 4.
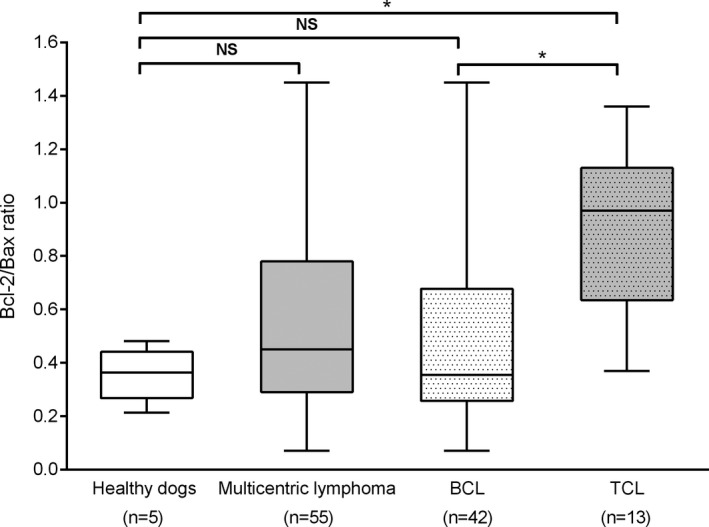
T‐cell lymphoma exhibits the highest Bcl‐2/Bax ratio. Bcl‐2/Bax ratio determined by flow cytometry from lymph node aspirates from dogs with multicentric lymphoma compared to healthy dogs. Paired two‐sample t‐test analyses revealed no difference of Bcl‐2/Bax ratios between healthy dogs and all dogs with lymphoma (P = .43) and between healthy dogs and dogs with B‐cell lymphoma (BCL) (P = .85). Bcl‐2/Bax ratio was significantly different between healthy dogs and dogs with T‐cell lymphoma (TCL) (P = .0006) and between BCL and TCL (P < .0001). BCL: dogs with B‐cell lymphoma. TCL: dogs with T‐cell lymphoma. n: number of dogs. NS: not significant. * indicates P < .05.
Effect of Bcl‐2/Bax Ratio on Outcome
Finally we asked if Bcl‐2/Bax ratio was associated with PFS. Unfortunately only 12 of 55 dogs had the same treatment regimen and available outcome data. All 12 dogs were clinically staged and treated at the same institution (NCSU VH Oncology Service). Median age of this group of dogs at diagnosis was 8.2 years (range 4.9–14.5 years). Six dogs were neutered female, and 6 dogs were male (1 intact, 5 neutered). There were 3 Labrador Retrievers, 2 Golden Retrievers, and 1 dog each of the following breeds: Basset Hound, German Shorthair Pointer, German Shepherd Dog, Pomeranian, and Miniature Schnauzer. Two of 12 dogs presented in stage 3a, 2/12 in stage 4a, 4/12 in stage 4b, and 4/12 dogs in stage 5b. Nine dogs had BCL and 3 dogs had TCL, respectively. Among the dogs with TCL, there were 2 dogs with CD3+/CD4+/CD45+ and 1 dog with CD3+/CD8+/CD45+ TCL. All 12 dogs received a multiagent chemotherapy protocol followed by half‐body radiation treatment, and median PFS for these dogs was 130 days (range, 20–322 days). None of the dogs were censored from analysis. Using the median Bcl‐2/Bax ratio (0.60, range 0.26–0.91) as a cutoff value as previously described,27, 28 dogs with higher ratios had a median PFS of 101 days (4 dogs with BCL, 2 dogs with TCL), while dogs with equal and lower ratios had median PFS of 130 days (5 dogs with BCL, 1 dog with TCL) (P = .194) (Fig 5).
Figure 5.
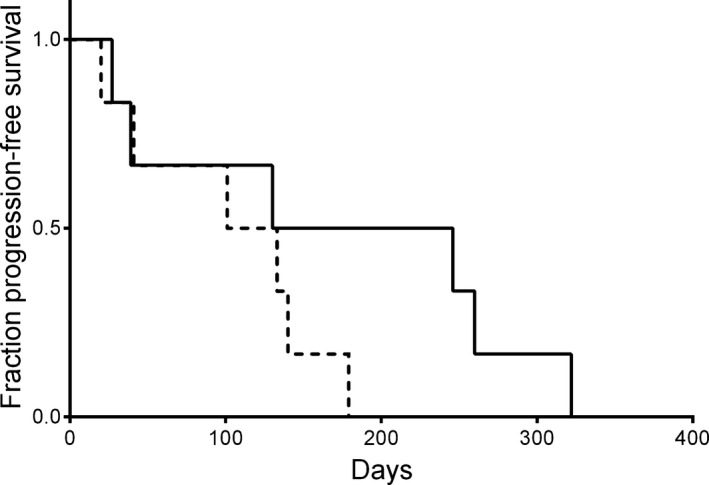
Kaplan–Meier curve depicting progression‐free survival (PFS) of dogs with multicentric lymphoma with identical treatment. PFS was discriminated based upon this group's median Bcl‐2/Bax ratio (0.6, range 0.26–0.91). Dogs with higher ratios (dotted line) had a median PFS of 101 days (4 dogs with BCL, 2 dogs with TCL). Dog with equal and lower ratios (solid line) had median PFS of 130 days (5 dogs with BCL, 1 dog with TCL) (P = .194).
Discussion
In our study we determined the ratio of antiapoptotic Bcl‐2 to proapoptotic Bax in neoplastic and normal lymphocytes. To the authors' knowledge there is no established gold standard to determine expression of these markers. Immunohistochemistry is commonly used in human lymphoid malignancies, largely because it can be performed on formalin‐fixed, paraffin‐embedded tissue, which is readily available for routine lymphoma diagnosis in humans.16, 18, 19, 20, 21, 22 Histopathology is considered the gold standard for characterization of lymphoma in dogs, but is more commonly reserved for cases where cytology is inconclusive. Recent studies have demonstrated that FC of LN aspirates can provide correlates with histopathology, such as determination of immunophenotype and expression of antigens associated with prognosis in subtypes of canine BCL and TCL.8, 9, 35, 36 For example, low levels of MHC II expression were predictive of poor outcome in dogs with BCL.9 Although no difference in Bcl‐2/Bax quantitation by FC, qRT‐PCR and WB analysis could be demonstrated in our study, results in Fig 2 demonstrate that FC revealed more consistency in Bcl‐2/Bax levels. In addition, upregulation of Bcl‐2 in human B‐cell tumors does not only occur by different genomic alterations of the Bcl‐2 gene but also by altered regulation by microRNAs and by diminished ubiquitin‐mediated Bcl‐2 turnover.16, 17, 39 Therefore, a protein‐based assay appears more adequate than an mRNA‐based assay. Finally, FC is a practical, quantitative, easily applicable, and cost‐effective method in a clinical setting, and Bcl‐2/Bax is likely a useful adjunct to the standard immunophenotyping panel.
Guidelines suggest that FC should be performed on clinical specimens within 48 hours following collection.34 In reality, shipment and delivery can occasionally extend this period for specimens shipped long distances or delayed in transport. Our time course study showed that there was no significant difference in Bcl‐2 and Bax expression among different lymphoma cell lines over the observed period of up to 5 days when stored under similar conditions. Based upon these findings, we included specimens up to 5 days post collection. These results also suggest Bcl‐2/Bax ratios may be useful for clinical specimens in which immunophenotype is inconclusive because of the age of the specimen as a higher Bcl‐2/Bax ratio in a sample with ambiguous or lack of antigen expression will more likely suggest T‐cell origin.
Dogs with multicentric lymphoma in our study had similar clinical characteristics, lymphoma stage, substage, and immunophenotype at presentation as previously reported.1 As described in Fig 4, the most striking finding was the difference in Bcl‐2/Bax ratios between BCL and TCL. Although other markers of apoptosis were not assessed in this study, this altered pro‐ to antiapoptotic ratio suggests that neoplastic T cells exhibit a higher intrinsic resistance to apoptosis compared to BCL and normal lymphocytes. Although the numbers of dogs evaluated are somewhat small, especially for the TCL group, it does reflect the general distribution of clinically observed B‐cell and T‐cell tumors.1, 3 Importantly, our results are supported by findings of other studies in multiple species. High levels of Bcl‐2 expression were also demonstrated in cats with TCL when compared to B‐cell immunophenotype,40 and it was shown in dogs that neoplastic T cells have a greater than 300% increase in microRNAs associated with increased expression of the antiapoptotic oncogene oncomir‐1.41 A recent abstract suggested that apoptotic activity in canine small‐ and intermediate‐sized lymphoma types correlated with tumor cell lineage. Dogs with TCL had much lower apoptotic activity (13%) based on Annexin‐V positive staining by FC, compared to dogs with BCL (56%).42 In dogs with multicentric lymphoma, Bcl‐2 mRNA expression was not correlated with resistance to chemotherapeutic agents at relapse, but protein levels were not evaluated in this study.32 In contrast, in mice, introduction of bcl‐2 into the normally vulnerable, cortical thymic T cells protected the cells from apoptotic stimuli including glucocorticoids, radiation and anti‐CD3 treatment.10, 25 In human PTCLs, apoptotic rate correlated inversely with Bcl‐2 expression and it was concluded that expression of Bcl‐2 and other Bcl‐2 family proteins may explain the poor response of many PTCL types to chemotherapy,22 while the presence of Bax correlated with longer survival.21 Altogether, it is likely that the higher Bcl‐2/Bax ratio contributes to a higher resistance to cytotoxic treatment, earlier relapse, and a more aggressive clinical behavior in canine TCL.1, 2, 3, 14
In our study 12 dogs were available for follow‐up (Fig 5). Dogs with higher Bcl‐2/Bax ratios experienced shorter PFS (101 days) than dogs with low ratios (130 days). This difference was not statistically significant, likely related to the small sample size. Interestingly, results of another study targeting Bcl‐2 by scintigraphy in dogs with BCL suggested that the amount and intensity of radiolabeled conjugate uptake in neoplastic lymph nodes was negatively correlated with outcome.31 In humans, elevated Bcl‐2/Bax ratio was associated with early death from disease in FL,27 and lower Bax/Bcl‐2 ratio was an independent prognostic indicator in AML and CLL.26, 28 Follicular lymphoma is rare in dogs, but Bcl‐2 overexpression by various mechanisms including t(14;18) also occurs in DLBCLs and other B‐cell tumors in humans, and was negatively associated with outcome.15, 16, 17, 18, 19, 27, 39 DLBCL is the most common lymphoma subtype in the dog, and not only shares morphological but also molecular similarities with human DLBCL variants,3, 6 but if similar mechanisms in regard to Bcl‐2 play a role in dogs with lymphoma still needs further investigation.43 Collectively, these findings support that Bcl‐2/Bax ratio might be a useful prognostic marker for dogs with lymphoma. However, based on the relatively low numbers of dogs available for outcome analysis, this conclusion is considered preliminary. If Bcl‐2/Bax ratio could aid in the identification of risk groups needs to be evaluated prospectively in a larger numbers of dogs with lymphoma. It is also noteworthy that a selective Bcl‐2 inhibitor (ABT‐199) is currently under investigation in phase I, II, and III trials in humans with various B‐cell neoplasms, solid tumors, and AML, with promising results especially in relapsing and refractory lymphoid tumors.39
This study has several limitations. Based upon the results of the small sample size in Fig 5, a larger number of patients is needed for outcome assessment. Also, complete signalment information and clinical stage of lymphoma was not available for all send‐in cases submitted for flow cytometry. Furthermore, other proteins of the Bcl‐2 family and additional upstream and downstream molecules of apoptosis, such as p53 and cytochrome c, were not assessed in this study. However, we decided to focus on the relationship of 2 main pro‐ and antiapoptotic proteins, rather than to look at absolute levels of any additional up‐ and downstream molecules. Several previous human publications postulated that the prognostic discrimination offered by the ratio of Bcl‐2 and Bax is greater than the protein levels alone.13, 14, 25, 26, 28 In addition, other markers of apoptosis, such as immunolabeling for active caspases or TUNEL assay may also have provided additional information. It would have also been interesting to correlate Bcl‐2/Bax with different lymphoma WHO subtypes in dogs. Unfortunately, lymph node histopathology is not routinely performed as part of the diagnostic workup for most dogs with lymphoma.
In conclusion, we demonstrated that flow cytometry was a reliable method to measure Bcl‐2 and Bax protein, and that dogs with multicentric TCL had statistically significantly higher Bcl‐2/Bax ratios in peripheral lymph node aspirates compared to normal dogs and dogs with BCL. We hypothesize that this difference likely reflects higher intrinsic resistance to apoptotic stimuli of canine TCL, and therefore contributes to the less favorable prognosis following initiation of cytotoxic treatment compared to BCL. As such, we suggest that Bcl‐2/Bax ratios may provide additional important prognostic information for dogs with lymphoid neoplasia in addition to immunophenotype alone. Moreover, we found that Bcl‐2/Bax ratios remain consistent for at least 5 days following sample collection, making its use as a clinically relevant test feasible. A larger clinical study is needed to investigate the correlation of Bcl‐2/Bax with outcome for dogs with BCL and TCL.
Acknowledgments
The study was supported by the NCSU Immunology Program Fund.
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This work was performed at North Carolina State University, College of Veterinary Medicine.
The study was presented as a poster abstract presentation at the North Carolina State University College of Veterinary Medicine Annual Research Day Forum, September 18, 2015 and as an oral abstract presentation at the ACVP/ASVCP/STP Combined Annual Meeting, October 17–21, 2015.
Footnotes
Mediatech, Inc, Herndon, VA
Clone 7/Bcl‐2, BD Biosciences, San Jose, CA
Clone 3/Bax, BD Biosciences, San Jose, CA
LeucoPerm Reagent A + B; Serotec, Raleigh, NC
Jackson ImmunoResearch, West Grove, PA
BD Biosciences, Mountain View, CA
Rabbit F(ab')2 anti Mouse IgG:HRP, Serotec, Raleigh, NC
Clone YKIX716.13, Serotec, Raleigh, NC
Clone CA17.2A12, Serotec, Raleigh, NC
Clone CA21D6, Serotec Raleigh, NC
Clone YKIX302.9, Serotec, Raleigh, NC
Clone 1.140 Tompkins, NCSU, Raleigh, NC
Clone B5, Tompkins, NCSU, Raleigh, NC
Clone 15H6, Southern Biotech, Birmingham, AL
References
- 1. Teske E, van Heerde P, Rutteman GR, et al. Prognostic factors for treatment of malignant lymphoma in dogs. J Am Vet Med Assoc 1994;205:1722–1728. [PubMed] [Google Scholar]
- 2. Marconato L, Stefanello D, Valenti P, et al. Predictors of long‐term survival in dogs with high‐grade multicentric lymphoma. J Am Vet Med Assoc 2011;238:480–485. [DOI] [PubMed] [Google Scholar]
- 3. Valli VE, Kass PH, San Myint M, et al. Canine lymphomas: Association of classification type, disease stage, tumor subtype, mitotic rate, and treatment with survival. Vet Pathol 2013;50:738–748. [DOI] [PubMed] [Google Scholar]
- 4. Ponce F, Magnol JP, Ledieu D, et al. Prognostic significance of morphological subtypes in canine malignant lymphomas during chemotherapy. Vet J 2004;167:158–166. [DOI] [PubMed] [Google Scholar]
- 5. Frantz AM, Sarver AL, Ito D, et al. Molecular profiling reveals prognostically significant subtypes of canine lymphoma. Vet Pathol 2013;50:693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Richards KL, Motsinger‐Reif AA, Chen HW, et al. Gene profiling of canine B‐cell lymphoma reveals germinal center and postgerminal center subtypes with different survival times, modeling human DLBCL. Cancer Res 2013;73:5029–5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johnston SA, Thamm DH, Legutki JB. The immunosignature of canine lymphoma: Characterization and diagnostic application. BMC Cancer 2014;14:657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Avery PR, Burton J, Bromberek JL, et al. Flow cytometric characterization and clinical outcome of CD4+ T‐cell lymphoma in dogs: 67 cases. J Vet Intern Med 2014;28:538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rao S, Lana S, Eickhoff J, et al. Class II major histocompatibility complex expression and cell size independently predict survival in canine B‐cell lymphoma. J Vet Intern Med 2011;25:1097–1105. [DOI] [PubMed] [Google Scholar]
- 10. Cory S. Regulation of lymphocyte survival by the bcl‐2 gene family. Annu Rev Immunol 1995;13:513–543. [DOI] [PubMed] [Google Scholar]
- 11. Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell 2011;144:646–674. [DOI] [PubMed] [Google Scholar]
- 12. Reed JC, Pellecchia M. Apoptosis‐based therapies for hematologic malignancies. Blood 2005;106:408–418. [DOI] [PubMed] [Google Scholar]
- 13. Adams JM, Cory S. The Bcl‐2 apoptotic switch in cancer development and therapy. Oncogene 2007;26:1324–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reed JC. Regulation of apoptosis by bcl‐2 family proteins and its role in cancer and chemoresistance. Curr Opin Oncol 1995;7:541–546. [DOI] [PubMed] [Google Scholar]
- 15. Tsujimoto Y, Cossman J, Jaffe E, Croce CM. Involvement of the bcl‐2 gene in human follicular lymphoma. Science 1985;228:1440–1443. [DOI] [PubMed] [Google Scholar]
- 16. Visco C, Tzankov A, Xu‐Monette ZY, et al. Patients with diffuse large B‐cell lymphoma of germinal center origin with BCL2 translocations have poor outcome, irrespective of MYC status: A report from an International DLBCL rituximab‐CHOP Consortium Program Study. Haematologica 2013;98:255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Correia C, Schneider PA, Dai H, et al. BCL2 mutations are associated with increased risk of transformation and shortened survival in follicular lymphoma. Blood 2015;125:658–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sohn SK, Jung JT, Kim DH, et al. Prognostic significance of bcl‐2, bax, and p53 expression in diffuse large B‐cell lymphoma. Am J Hematol 2003;73:101–107. [DOI] [PubMed] [Google Scholar]
- 19. Hasselblom S, Hansson U, Olsson M, et al. High immunohistochemical expression of p‐AKT predicts inferior survival in patients with diffuse large B‐cell lymphoma treated with immunochemotherapy. Br J Haematol 2010;149:560–568. [DOI] [PubMed] [Google Scholar]
- 20. Jung JT, Kim DH, Kwak EK, et al. Clinical role of Bcl‐2, Bax, or p53 overexpression in peripheral T‐cell lymphomas. Ann Hematol 2006;85:575–581. [DOI] [PubMed] [Google Scholar]
- 21. Li HL, Huang XP, Zhou XH, et al. Correlation of seven biological factors (Hsp90a, p53, MDM2, Bcl‐2, Bax, Cytochrome C, and Cleaved caspase3) with clinical outcomes of ALK+ anaplastic large‐cell lymphoma. Biomed Environ Sci 2011;24:630–641. [DOI] [PubMed] [Google Scholar]
- 22. Rassidakis GZ, Jones D, Lai R, et al. BCL‐2 family proteins in peripheral T‐cell lymphomas: Correlation with tumour apoptosis and proliferation. J Pathol 2003;200:240–248. [DOI] [PubMed] [Google Scholar]
- 23. Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 1995;80:293–299. [DOI] [PubMed] [Google Scholar]
- 24. Meijerink JP, Mensink EJ, Wang K, et al. Hematopoietic malignancies demonstrate loss‐of‐function mutations of BAX. Blood 1998;91:2991–2997. [PubMed] [Google Scholar]
- 25. Korsmeyer SJ, Shutter JR, Veis DJ, et al. Bcl‐2/Bax: A rheostat that regulates an anti‐oxidant pathway and cell death. Semin Cancer Biol 1993;4:327–332. [PubMed] [Google Scholar]
- 26. Del Poeta G, Venditti A, Del Principe MI, et al. Amount of spontaneous apoptosis detected by Bax/Bcl‐2 ratio predicts outcome in acute myeloid leukemia (AML). Blood 2003;101:2125–2131. [DOI] [PubMed] [Google Scholar]
- 27. Gulmann C, Espina V, Petricoin E 3rd, et al. Proteomic analysis of apoptotic pathways reveals prognostic factors in follicular lymphoma. Clin Cancer Res 2005;11:5847–5855. [DOI] [PubMed] [Google Scholar]
- 28. Del Principe MI, Bo MD, Bittolo T, et al. Clinical significance of bax/bcl‐2 ratio in chronic lymphocytic leukemia. Haematologica 2016;101:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Strefezzi Rde F, Kleeb SR, Xavier JG, et al. The value of immunohistochemical expression of BAX in formulating a prognosis for canine cutaneous mast cell tumours. J Comp Pathol 2012;146:314–319. [DOI] [PubMed] [Google Scholar]
- 30. Vinothini G, Balachandran C, Nagini S. Evaluation of molecular markers in canine mammary tumors: Correlation with histological grading. Oncol Res 2009;18:193–201. [DOI] [PubMed] [Google Scholar]
- 31. Statham‐Ringen KA, Selting KA, Lattimer JC, et al. Evaluation of a B‐cell leukemia‐lymphoma 2‐specific radiolabeled peptide nucleic acid‐peptide conjugate for scintigraphic detection of neoplastic lymphocytes in dogs with B‐cell lymphoma. Am J Vet Res 2012;73:681–688. [DOI] [PubMed] [Google Scholar]
- 32. Tomiyasu H, Goto‐Koshino Y, Takahashi M, et al. Quantitative analysis of mRNA for 10 different drug resistance factors in dogs with lymphoma. J Vet Med Sci 2010;72:1165–1172. [DOI] [PubMed] [Google Scholar]
- 33. Dettwiler M, Croci M, Vaughan L, et al. Immunohistochemical expression study of proapoptotic BH3‐only protein bad in canine nonneoplastic tissues and canine lymphomas. Vet Pathol 2013;50:789–796. [DOI] [PubMed] [Google Scholar]
- 34. Reggeti F, Bienzle D. Flow cytometry in veterinary oncology. Vet Pathol 2011;48:223–235. [DOI] [PubMed] [Google Scholar]
- 35. Thalheim L, Williams LE, Borst LB, et al. Lymphoma immunophenotype of dogs determined by immunohistochemistry, flow cytometry, and polymerase chain reaction for antigen receptor rearrangements. J Vet Intern Med 2013;27:1509–1516. [DOI] [PubMed] [Google Scholar]
- 36. Seelig DM, Avery P, Webb T, et al. Canine T‐zone lymphoma: Unique immunophenotypic features, outcome, and population characteristics. J Vet Intern Med 2014;28:878–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Seiser EL, Thomas R, Richards KL, et al. Reading between the lines: Molecular characterization of five widely used canine lymphoid tumour cell lines. Vet Comp Oncol 2013;11:30–50. [DOI] [PubMed] [Google Scholar]
- 38. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐Delta Delta C(T)) Method. Methods 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- 39. Correia C, Lee SH, Meng XW, et al. Emerging understanding of Bcl‐2 biology: Implications for neoplastic progression and treatment. Biochim Biophys Acta 2015;1853:1658–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dank G, Lucroy MD, Griffey SM, et al. bcl‐2 and MIB‐1 labeling indexes in cats with lymphoma. J Vet Intern Med 2002;16:720–725. [PubMed] [Google Scholar]
- 41. Uhl E, Krimer P, Schliekelman P, et al. Identification of altered MicroRNA expression in canine lymphoid cell lines and cases of B‐ and T‐Cell lymphomas. Genes Chromosom Cancer 2011;50:950–967. [DOI] [PubMed] [Google Scholar]
- 42. Poggi A, Miniscalco B, Morello E, et al. Combined flow cytometric approach for the characterization of canine small cell lymphomas. Proceedings of the 25th ECVIM‐CA Congress; 2015. Sep 10‐12; Lisbon, Portugal.
- 43. Breen M, Modiano JF. Evolutionarily conserved cytogenetic changes in hematological malignancies of dogs and humans–man and his best friend share more than companionship. Chromosome Res 2008;16:145–154. [DOI] [PubMed] [Google Scholar]


