Abstract
Background
Noninvasive methods of quantitating exercise tolerance in dogs with neuromuscular disease are needed both for clinical and research use. The 6‐minute walk test (6MWT) has been validated as a reliable test of exercise tolerance in dogs with pulmonary and cardiac disease, but not in dogs with neuromuscular disease.
Hypothesis/Objectives
Distance walked and number of steps taken during 6MWT will differ between Labrador retriever dogs with centronuclear myopathy (CNM) and control (ie, healthy) littermates.
Animals
Eight purebred Labrador retrievers were drawn from a purpose‐bred research colony (status: 3 clear, 2 carrier, and 3 homozygous mutants for the protein tyrosine phosphatase‐like A (PTPLA) gene mutation associated with CNM).
Methods
Pilot, prospective, Case–controlled study. Researchers were blinded to disease status. Each dog was leash‐trained and acclimatized to the testing area (length, 12.8 m). At the start of testing, each animal was fitted with a pedometer, a timer was started, and dogs were allowed to walk at their own pace for 6 minutes. Distance walked and pedometer readings were recorded.
Results
Degree of paresis varied among affected dogs, and was reflected by significant differences in distance walked between CNM‐affected dogs and those with clear and carrier genotypes (P = .048). Pedometer readings did not vary according to genotype (P = .86).
Conclusions
The 6MWT appears to differentiate between the ambulatory capacity of normal and CNM‐affected dogs. Additional studies are needed to confirm this relationship in a larger number of dogs, and to evaluate the ability of the 6MWT to differentiate between dogs with variable severity of neuromuscular disease‐associated exercise intolerance.
Keywords: Ambulatory capacity, Exercise intolerance, Labrador retriever, Myopathy, Paresis, Weakness
Abbreviations
- 6MWT
6‐minute walk time
- CNM
centronuclear myopathy
- PTPLA
protein tyrosine phosphatase‐like A
Canine centronuclear myopathies (CNM) occur primarily in the Labrador retriever breed, and are inherited either as an autosomal recessive trait with full penetrance or as an X‐linked condition.1, 2, 3, 4, 5, 6 As with most neuromuscular diseases in dogs, paresis is the primary clinical manifestation of CNM. Clinical signs develop within the first 1–7 months of life, and are characterized by generalized weakness and exercise intolerance, generalized muscle atrophy, and abnormal spinal reflexes.2, 7, 8, 9, 10, 11, 12 The severity of signs and the rapidity of progression differs between the autosomal recessive and X‐linked conditions; dogs affected with the latter typically develop signs earlier in life, progress more rapidly and develop more severe signs than those with the autosomal recessive form of the disease.1, 4, 7, 8, 9, 11, 12 Both groups develop characteristic muscle pathology, including an increased number of centralized nuclei within myofibers, atrophy of type II myofibers, and mitochondrial aggregates.1, 4, 9, 13, 14
The clinical and pathological characteristics of CNM in dogs closely resemble those of CNM in humans and affected dogs represent a useful large‐animal model for the human condition, with the goal of testing gene and stem cell therapies.2, 4, 11, 14, 15 Forms of CNM in humans manifest with varying degrees of exercise intolerance, ranging in severity from generalized muscular weakness, atrophy, and physical disability appearing in youth to young adulthood (autosomal recessive and autosomal dominant forms, respectively) to some combination of severe hypotonia, ventilator dependence and perinatal death (X‐linked form).16, 17, 18, 19, 20 Treatment of CNM in humans currently is limited to symptomatic care.15
For CNM in dogs to serve as a large‐animal model for its human counterparts, specific, and reproducible measures of disease severity are needed.2, 11 Given that the most common manifestation of both conditions is decreased exercise tolerance, researchers using the dog model need objective methods to assess the severity of exercise impairment. Currently, few techniques are available to quantitate exercise tolerance in individual dogs. Gait analysis can differentiate between normal dogs and those with neuromuscular disease based on the short‐strided gait of affected dogs,11 but, it does not permit quantitative comparisons of exercise tolerance among dogs and has not been validated as a quantitative longitudinal assessment tool in dogs.
The six‐minute walk test (6MWT) has been used to quantitate exercise tolerance in dogs with systemic disease,21, 22, 23, 24 but has not been evaluated in those with neuromuscular disease. This test measures the linear distance walked by individual dogs when dogs are allowed to walk at their own pace for 6 minutes. Using this test, statistically significant differences in walking distance have been found between control dogs and dogs with pulmonary fibrosis, heart failure, and obesity.21, 22, 23, 24 In these studies, the 6MWT was reported as a low cost, reproducible, and easy‐to‐implement test of exercise tolerance.21, 22, 23, 24 Similarly, the test has been used to assess exercise capacity in humans with chronic disease (eg, cardiac dysfunction,25 multiple sclerosis26), and in those recovering from critical illness,27 and is used routinely to assess ambulatory capacity in children affected by Duchènne's muscular dystrophy.28, 29, 30, 31 In the latter, the 6MWT was established internationally as the outcome measure of choice to assess ambulatory capacity in clinical trials of ambulatory patients, because of its ease of use, low cost, and ability to provide functional assessment of weakness.28, 29, 30, 31, 32, 33 Validation of the 6MWT for use in dogs with neuromuscular disease could prove similarly useful in clinical and research settings. For research, quantitation of neuromuscular function is essential in therapeutic trials involving canine models of human neuromuscular disease, as a measure of response to treatment. In the clinical setting, the 6MWT could serve as an objective measure of the degree of clinical improvement of dogs with neuromuscular disease.
Pedometers also have been validated as a low cost, accurate, measure of steps taken in humans and dogs, and are used in both species to track the relationship between obesity and exercise,34, 35, 36 but their use has not been evaluated in the neuromuscular disease setting.
Methods
Our study aimed to evaluate the 6MWT and pedometer as non‐invasive measures ambulatory capacity using dogs with autosomal recessive CNM as a model of generalized neuromuscular disease. We hypothesize that dogs with CNM would walk shorter distances and have lower pedometer readings after 6MWT than their nondiseased littermates.
Dog Characteristics and Source
Eight purpose‐bred Labrador retriever dogs were used for our pilot study. All dogs were housed in a research facility as part of a research colony and were managed in accordance with Cornell University's Care and Animal Care and Use Committee. Although dogs were genotyped at birth, study personnel were blinded to the genotype throughout the study. A neurologic and physical examination was performed on each dog once monthly as part of each dog's routine care protocol. Orthopedic examination did not identify evidence of orthopedic disease. Dogs' body weights ranged from 8 to 36.4 kg as they aged throughout the evaluation period.
Six‐minute Walk Test
All dogs were leash‐trained, and acclimatized to people and to the testing area before the start of testing, as previously described.21, 22, 23, 24 The testing area consisted of a quiet, straight hallway, free from the passage of people or dogs, with a premeasured length of 12.8 m. The temperature, lighting, and appearance of the testing area were maintained constant as part of the routine maintenance of the research facility. To minimize the potential impact of travel or environmental novelty on test results, dogs were housed and tested in the same facility, because CNM‐associated paresis can worsen when affected dogs undergo stress or excitement, or when they are in cold environments.14 Pedometer testing was done using an Accusplit AE120XL1 pedometer mounted on a bungee‐cord collar (3/8th inch diameter cord), attached using a zip‐locking cable tie and a threaded eye screw. This methodology has been validated for the measurement of step counts in Labrador retriever dogs.35
Before each 6MWT, a pedometer was zeroed and placed on each dog immediately before the start of walking. A timer then was started, and each dog was walked back and forth in a straight line within the predetermined test area for a period of 6 minutes. Dogs were allowed to set their own pace, while an observer counted the number of laps walked within the 6‐minute period, in accordance with reported testing methods.22, 23, 24 If the final lap was only partially completed, the completed length was measured and included in the distance walked. The pedometer was removed immediately after completion of the 6‐minute period and the step count was recorded. The number of laps walked was multiplied by the length of the walking area to determine the total distance walked. The researchers also subjectively evaluated the quality of the gait, including the presence or absence of paresis and exercise intolerance. Each 6MWT was video‐recorded to facilitate gait evaluation.
Statistical Analyses
Dogs heterozygous for the PTPLA mutation associated with autosomal recessive CNM do not manifest clinical evidence of disease.4, 5, 14 These dogs therefore were grouped with homozygous wild type dogs and considered unaffected for purposes of this study. To determine whether test acclimatization affected findings over time #bib6MWT data were evaluated longitudinally for each of the phenotypically unaffected dogs (ie, CNM carrier and clear dogs). Only a single affected dog could be followed over time because of limitations in colony expansion (see Table 1). Consequently, test results comparing affected and unaffected dogs were treated as independent entities, rather than longitudinal comparisons.
Table 1.
Six‐minute walk test and pedometer findings reported by genotype. P‐values assess the relationship between test outcomes (ie, step count, distance walked, and time walked) and genotype. Statistically significant relationships are listed in bold text
| Test | Pedometer (# Steps) | Distance Walked (m) | Time Walked (minutes) |
|---|---|---|---|
| Affected | 994, 82 | 299.0, 60.8 | 6, 0 |
| Carrier/clear | 997.6, 53 | 453.8, 83.0 | 5.8, 0.45 |
| P‐value | .4286 | .0476 | .7143 |
The relationship between pedometer readings and genotype was evaluated at approximately 11 weeks of age in all dogs using a Wilcoxon exact test. Distances and time walked also were compared between affected and unaffected dogs at this age using a Wilcoxon exact test. One‐tailed comparisons were used for the above analyses, testing the hypothesis that distance walked and number of steps would be smaller in affected (ie, homozygous mutant) dogs as compared to unaffected (ie, carrier and homozygous wild type) dogs. For all tests, values of P < .05 were considered significant.
Results
Dogs 1 through 6 and dog number 7 were repeatedly evaluated between 9 and 50 weeks of age (Table 1). Dog 1 was euthanized at 12 weeks of age because of refractory status epilepticus without previous clinical evidence of disease. Necropsy disclosed hepatic microvascular dysplasia. Although systemic disease could have affected this dog's 6MWT results before the onset of seizures, this was considered unlikely due to the absence of evidence of systemic disease on regular physical examinations performed at the time of each walk test. Dog 8 was added to the colony as a mature Labrador retriever to act as a sire for expansion of the CNM colony as part of a separate study. A 6MWT was performed on this dog after a period of 2 months of acclimatization to its environment, to decrease the likelihood that stress related to environmental change would affect the test results.22, 24
Physical examination findings were unremarkable in clear and carrier dogs. Conversely, CNM‐affected dogs had absent patellar reflexes and moderate to severe generalized muscle atrophy that was most severe in the temporal and caudal thigh muscles. The gaits of affected and unaffected (ie, clear and carrier) dogs were indistinguishable from their affected littermates within the first month of life. By the second month of age, affected dogs developed progressively more severe paresis and exercise intolerance, cervical ventroflexion, and a persistently kyphotic posture.
Six‐minute Walk Test
Step counts, distance walked, and time walked did not differ significantly by sex, and did not reflect acclimatization over time (ie, findings did not change over time in clear and carrier dogs). All but 1 of the clear and carrier dogs were able to complete the 6MWT. Dog 1 refused to walk beyond 5 minutes on 1 occasion, and this appeared to be for behavioral reasons rather than exercise intolerance because the dog was able to walk to its kennel immediately after the test without evidence of weakness or abnormalities in its gait. Dogs with CNM, however, walked with variable severity of paresis (Fig 1); 2 were unable to complete the test because of the severity of their exercise intolerance. Specifically, dog 2 was unable to continue the test in 3 instances, whereas dog 8 was unable to walk longer than 1 minute. In these cases, the dogs became progressively paretic, with short strides, a crouched body posture and low head carriage, until they sat. Even when allowed to rest, they were unable to take more than a few short‐strided steps before sitting. Further testing of dog 8 was not pursued because of the severity of its exercise intolerance, which prevented the dog from walking for longer than 1–2 minutes at any time.
Figure 1.
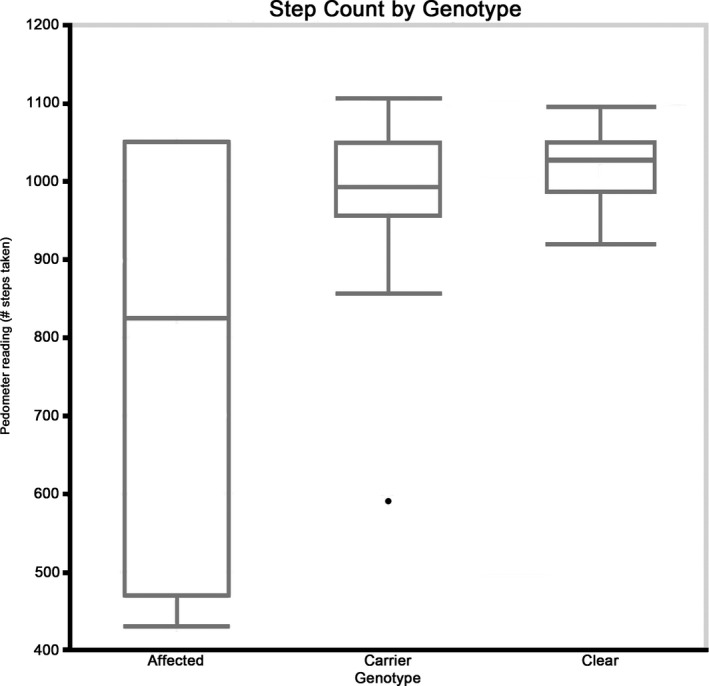
Step count for each genotype group. Although a significant difference was not found between genotype groups, centronuclear myopathy (CNM)‐affected dogs showed more variability in their step counts in comparison to CNM‐carrier and clear dogs.
Genotype did not affect pedometer readings (P = .43); Table 1, but affected dogs differed from carrier and clear dogs in variability in the number of steps taken. As a result of their pronounced paresis, affected dogs took a larger number of short‐strided steps but covered less distance than did their unaffected littermates. This resulted in high pedometer readings concurrent with significantly shorter distances walked in affected dogs as compared to unaffected dogs (P = .048) (Fig 2).
Figure 2.
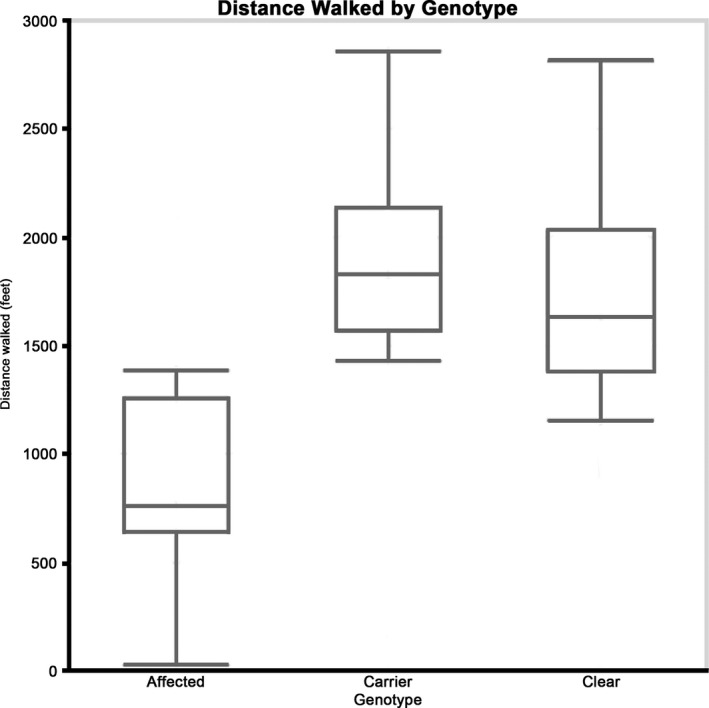
Distance walked during 6‐minute walk time for each genotype group. Distance walked was significantly shorter in centronuclear myopathy‐affected dogs in comparison to their carrier and clear littermates.
Discussion
The 6MWT effectively differentiated between dogs with CNM and control dogs in this study, despite limitations in the number of dogs evaluated. The distance walked by clear and carrier dogs resembled that of children28, 33 and healthy adult dogs.21, 22, 23, 24 Reported distances walked during the 6‐minute test period in healthy dogs range from 492 m24 to 611 m.21 In contrast, distances walked by CNM‐affected dogs were lower than those reported for dogs suffering from congestive heart failure,22 pulmonary fibrosis,23, 24 and obesity.21
The ability of the 6MWT to differentiate between CNM‐clear and CNM‐affected dogs on the basis of their exercise intolerance expands previous findings utilizing the use of motion capture and instrumented carpet technology to evaluate differences in the spatiotemporal parameters of dogs with and without neuromuscular disease, especially in dogs affected with the canine equivalent of X‐linked myotubular myopathy.11 In contrast to the instrumentation needed to carry out those tests, the 6MWT does not require any equipment beyond a timer and the ability to measure the length of the area traversed. Thus, the 6MWT is an accessible and easy‐to‐learn technique for both research and clinical settings.21, 22, 23, 24 The 6MWT has been used successfully to evaluate exercise tolerance in dogs of different sizes (eg, beagle,21 West Highland White terrier,24 Labrador retriever, hound22), has been shown to be reproducible in pet dogs21, 23 and in the research setting,22 and requires less acclimatization time than do treadmill evaluations.22 In contrast to the reported increase in 6MWT distances over time in healthy children as they develop between 5 and 12 years of age,33 control dogs did not have significant changes in 6MWT findings as they aged.
The 6MWT has been reported as an accurate technique that evaluates a dog's ability to undertake daily physical activity in a more natural way than treadmill evaluations, and requires a shorter period of acclimatization.22Acclimatizing Labrador retriever dogs to a treadmill has not only been shown to take a longer time compared to other breeds but also the stress and the excitement associated with treadmill use could impact test results because stress and excitement worsen clinical signs in CNM‐affected dogs.
In this study, once they became minimally ambulatory, affected dogs were unable to conclude testing due to the severity of their paresis. In these dogs, the test evaluated maximal rather than submaximal exercise tolerance. Similarly, in children with Duchènne's muscular dystrophy, the 6MWT has been validated as an effective measure of quality of life only while they retain ambulatory status.28, 31, 32 Thus, this test may not be a suitable assessment tool for dogs requiring assistance walking, such as those with X‐linked CNM beyond the initial disease period. Additional studies are needed to confirm the ability of the 6MWT to differentiate between normal dogs and those with neuromuscular disease of variable severity in a larger group of dogs.
To the authors' knowledge, pedometers have not been used in dogs as a measure of ambulatory capacity, although their use has been described in relation to obesity as a method to quantitate exercise in dogs.35, 36 The pedometer protocol has been validated as an accurate measure of the number of steps taken in medium‐ and large‐breed dogs, including the Labrador retriever breed.35 However, in our study, pedometers did not appear to serve as an accurate measure of ambulatory capacity in dogs with CNM, likely as a consequence of the short‐strided gait with rapid cadence characteristic of dogs with neuromuscular weakness, resulting in a shorter distance covered as compared to their unaffected counterparts.11
Conclusions
Distance measurements utilizing the 6MWT appear to permit differentiation between CNM‐affected and unaffected dogs, but larger studies are needed to confirm these findings. Pedometers, in contrast, did not appear to be an accurate measure of submaximal exercise tolerance as a consequence of the shorter strided, more rapid cadence of CNM‐affected dogs, which may lead to an overestimation of exercise tolerance. Additional studies are needed to evaluate the 6MWT as a measure of disease progression over time.
Supporting information
Table S1. Cohort characteristics, including dogs' signalment, coat color, and genotype.
Table S2. Six‐minute walk test findings, listed as mean and standard deviation (SD). Distance walked is reported in meters. Affected dogs are highlighted by bolded text.
Acknowledgments
The authors acknowledge Drs Meyers‐Wallen, Wakshlag, Mudrak, Greenbaum, and Kornegay for their help in this project.
Grant Support: This study was funded by a grant from the Cornell University Collaborative Research Grants Program (Cornell University).
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
The work described in this manuscript was performed at the College of Veterinary Medicine of Cornell University, Ithaca, NY.
Footnote
Accusplit Eagle 120XL with JW200 Pedometer Engine™; Accusplit, Inc.; Livermore, CA #bib94551.
References
- 1. Beggs AH, Bohm J, Snead E, et al. MTM1 mutation associated with X‐linked myotubular myopathy in Labrador Retrievers. Proc Natl Acad Sci USA 2010;107:14697–14702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grange RW, Doering J, Mitchell E, et al. Muscle function in a canine model of X‐linked myotubular myopathy. Muscle Nerve 2012;46:588–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gentilini F, Zambon E, Gandini G, et al. Frequency of the allelic variant of the PTPLA gene responsible for centronuclear myopathy in Labrador Retriever dogs as assessed in Italy. J Vet Diagn Invest 2011;23:124–126. [DOI] [PubMed] [Google Scholar]
- 4. Maurer M, Mary J, Guillaud L, et al. Centronuclear myopathy in Labrador retrievers: A recent founder mutation in the PTPLA gene has rapidly disseminated worldwide. PLoS ONE 2012;7:e46408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pele M, Tiret L, Kessler JL, et al. SINE exonic insertion in the PTPLA gene leads to multiple splicing defects and segregates with the autosomal recessive centronuclear myopathy in dogs. Hum Mol Genet 2005;14:1417–1427. [DOI] [PubMed] [Google Scholar]
- 6. Tiret L, Blot S, Kessler JL, et al. The cnm locus, a canine homologue of human autosomal forms of centronuclear myopathy, maps to chromosome 2. Hum Genet 2003;113:297–306. [DOI] [PubMed] [Google Scholar]
- 7. Gortel K, Houston DM, Kuiken T, et al. Inherited myopathy in a litter of Labrador retrievers. Can Vet J 1996;37:108–110. [PMC free article] [PubMed] [Google Scholar]
- 8. Kramer JW, Hegreberg GA, Bryan GM, et al. A muscle disorder of Labrador retrievers characterized by deficiency of type II muscle fibers. J Am Vet Med Assoc 1976;169:817–820. [PubMed] [Google Scholar]
- 9. Bley T, Gaillard C, Bilzer T, et al. Genetic aspects of Labrador Retriever myopathy. Res Vet Sci 2002;73:231–236. [DOI] [PubMed] [Google Scholar]
- 10. Eminaga S, Cherubini GB, Shelton GD. Centronuclear myopathy in a Border collie dog. J Small Anim Pract 2012;53:608–612. [DOI] [PubMed] [Google Scholar]
- 11. Goddard MA, Burlingame E, Beggs AH, et al. Gait characteristics in a canine model of X‐linked myotubular myopathy. J Neurol Sci 2014;346:221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Snead EC, Taylor SM, van der Kooij M, et al. Clinical phenotype of X‐linked myotubular myopathy in labrador retriever puppies. J Vet Intern Med 2015;29:254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McKerrell RE, Braund KG. Hereditary myopathy in Labrador retrievers: A morphologic study. Vet Pathol 1986;23:411–417. [DOI] [PubMed] [Google Scholar]
- 14. Green SL, Tolwani RJ, Varma S, et al. Absence of mutations in the survival motor neuron cDNA from labrador retrievers with an inherited myopathy. Vet Rec 2005;157:250–254. [DOI] [PubMed] [Google Scholar]
- 15. Childers MK, Joubert R, Poulard K, et al. Gene therapy prolongs survival and restores function in murine and canine models of myotubular myopathy. Sci Transl Med 2014;6:220ra10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Colombo I, Scoto M, Manzur AY, et al. Congenital myopathies: Natural history of a large pediatric cohort. Neurology 2015;84:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Herman GE, Finegold M, Zhao W, et al. Medical complications in long‐term survivors with X‐linked myotubular myopathy. J Pediatr 1999;134:206–214. [DOI] [PubMed] [Google Scholar]
- 18. Bitoun M, Maugenre S, Jeannet PY, et al. Mutations in dynamin 2 cause dominant centronuclear myopathy. Nat Genet 2005;37:1207–1209. [DOI] [PubMed] [Google Scholar]
- 19. Jeannet PY, Bassez G, Eymard B, et al. Clinical and histologic findings in autosomal centronuclear myopathy. Neurology 2004;62:1484–1490. [DOI] [PubMed] [Google Scholar]
- 20. Mora M, Morandi L, Merlini L, et al. Fetus‐like dystrophin expression and other cytoskeletal protein abnormalities in centronuclear myopathies. Muscle Nerve 1994;17:1176–1184. [DOI] [PubMed] [Google Scholar]
- 21. Manens J, Ricci R, Damoiseaux C, et al. Effect of body weight loss on cardiopulmonary function assessed by 6‐minute walk test and arterial blood gas analysis in obese dogs. J Vet Intern Med 2014;28:371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boddy KN, Roche BM, Schwartz DS, et al. Evaluation of the six‐minute walk test in dogs. Am J Vet Res 2004;65:311–313. [DOI] [PubMed] [Google Scholar]
- 23. Swimmer RA, Rozanski EA. Evaluation of the 6‐minute walk test in pet dogs. J Vet Intern Med 2011;25:405–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lilja‐Maula LI, Laurila HP, Syrja P, et al. Long‐term outcome and use of 6‐minute walk test in West Highland White Terriers with idiopathic pulmonary fibrosis. J Vet Intern Med 2014;28:379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brooks GC, Vittinghoff E, Iyer S, et al. Accuracy and usability of a self‐administered 6‐minute walk test smartphone application. Circ Heart Fail 2015;8:905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pearson M, Dieberg G, Smart N. Exercise as a therapy for improvement of walking ability in adults with multiple sclerosis: A meta‐analysis. Arch Phys Med Rehabil 2015;96:1339–1348. e7. [DOI] [PubMed] [Google Scholar]
- 27. Alison JA, Kenny P, King MT, et al. Repeatability of the six‐minute walk test and relation to physical function in survivors of a critical illness. Phys Ther 2012;92:1556–1563. [DOI] [PubMed] [Google Scholar]
- 28. McDonald CM, Henricson EK, Han JJ, et al. The 6‐minute walk test as a new outcome measure in Duchenne muscular dystrophy. Muscle Nerve 2010;41:500–510. [DOI] [PubMed] [Google Scholar]
- 29. McDonald CM, Henricson EK, Abresch RT, et al. The 6‐minute walk test and other endpoints in Duchenne muscular dystrophy: Longitudinal natural history observations over 48 weeks from a multicenter study. Muscle Nerve 2013;48:343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McDonald CM, Henricson EK, Abresch RT, et al. The 6‐minute walk test and other clinical endpoints in duchenne muscular dystrophy: Reliability, concurrent validity, and minimal clinically important differences from a multicenter study. Muscle Nerve 2013;48:357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Henricson E, Abresch R, Han JJ, et al. The 6‐minute walk test and person‐reported outcomes in boys with duchenne muscular dystrophy and typically developing controls: Longitudinal comparisons and clinically‐meaningful changes over one year. PLoS Curr 2013;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pane M, Mazzone ES, Sivo S, et al. Long term natural history data in ambulant boys with Duchenne muscular dystrophy: 36‐month changes. PLoS ONE 2014;9:e108205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goemans N, Klingels K, van den Hauwe M, et al. Six‐minute walk test: Reference values and prediction equation in healthy boys aged 5 to 12 years. PLoS ONE 2013;8:e84120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brown DC, Michel KE, Love M, et al. Evaluation of the effect of signalment and body conformation on activity monitoring in companion dogs. Am J Vet Res 2010;71:322–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Warren BS, Wakshlag JJ, Maley M, et al. Use of pedometers to measure the relationship of dog walking to body condition score in obese and non‐obese dogs. Br J Nutr 2011;106(Suppl 1):S85–S89. [DOI] [PubMed] [Google Scholar]
- 36. Chan CB, Spierenburg M, Ihle SL, et al. Use of pedometers to measure physical activity in dogs. J Am Vet Med Assoc 2005;226:2010–2015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Cohort characteristics, including dogs' signalment, coat color, and genotype.
Table S2. Six‐minute walk test findings, listed as mean and standard deviation (SD). Distance walked is reported in meters. Affected dogs are highlighted by bolded text.


