Abstract
Background
This study evaluated the effectiveness and safety of grapiprant for treatment of pain in dogs with osteoarthritis (OA).
Hypothesis/Objectives
Grapiprant will relieve pain as measured by the owner's and veterinarian's evaluation of pain in dogs with OA. Another objective was evaluation of the safety of grapiprant.
Animals
Two hundred and eighty‐five client‐owned dogs with OA were enrolled and treated with grapiprant or placebo with 262 cases (N = 131 in each group) evaluable for the effectiveness analysis.
Methods
In this prospective, randomized, masked, placebo‐controlled study dogs were treated daily with grapiprant (2 mg/kg) per OS or placebo. Owners completed an evaluation using the Canine Brief Pain Inventory (CBPI) on days 0, 7, 14, 21, and 28. Success was defined as improvement in the CBPI. Veterinary assessments were made on screening and days 14 and 28. Safety was evaluated by physical examination, evaluation of clinical pathology results, and owner observations.
Results
Grapiprant treatment improved pain compared to placebo on day 28 (48.1 and 31.3% treatment successes respectively; P = .0315). The pain interference score (PIS) and pain severity score (PSS) improved in the grapiprant group compared to placebo (P = .0029 and 0.0022, respectively). Veterinary assessments were significantly better in the grapiprant‐treated dogs (P = .0086). Grapiprant generally was well tolerated, but a higher percentage of treated dogs (17.02%) had occasional vomiting as compared to the placebo group (6.25%).
Conclusions and Clinical Importance
Grapiprant is an effective treatment for alleviation of pain in dogs with OA, and represents a modality of treatment that may be better tolerated than current options.
Keywords: Anti‐inflammatory, Osteoarthritis, Pain, Piprant
Abbreviations
- Ca
calcium
- CBPI
canine brief pain inventory
- FDA
Food and Drug Administration
- GI
gastrointestinal
- ITT
intention to treat
- NSAID
nonsteroidal anti‐inflammatory drug
- OA
osteoarthritis
- PGE2
prostaglandin E2
- PGI2
prostaglandin I2
- PIS
pain interference score
- PO
per OS
- PPP
per protocol population
- PRA
prostaglandin receptor antagonist
- PSS
pain severity score
- SAE
serious adverse event
- SD
standard deviation
- TOS
total orthopedic score
Grapiprant (AT‐001, CJ‐023,423) is a potent and specific antagonist of the prostaglandin E2 (PGE2) EP4 receptor1. It is a member of a newly defined class of drugs, the piprant class, that block prostaglandin receptors. Grapiprant is an EP4 piprant, also known as an EP4 prostaglandin receptor antagonist (EP4 PRA).2 It binds to human and rat EP4 receptors with high affinity.1 In several rat pain models of inflammatory pain, grapiprant was shown to be effective in pain reduction1 and in a rat model of adjuvant‐induced arthritis, grapiprant was shown to inhibit paw swelling, inflammatory biomarkers, and synovial inflammation.3
Many FDA‐approved drugs are available to treat pain associated with osteoarthritis (OA) in dogs (e.g., carprofen,1 firocoxib,2 meloxicam3 , and deracoxib4), all of which work by inhibiting cyclooxygenase enzymes. The adverse effect profile of the cyclooxygenase‐inhibiting nonsteroidal anti‐inflammatory drugs (NSAIDs) is well established. The Food and Drug Administration (FDA) has required language in the precaution section of the package inserts of these drugs warning that, as a class, they may be associated with renal, gastrointestinal (GI), and hepatic toxicity. Specifically, labels of these drugs warn of “the potential to produce GI ulceration and/or GI perforation.”2 Because grapiprant's mechanism of action targets the EP4 receptor, it does not inhibit the production of prostanoids, which are important in a variety of physiological functions that maintain normal homeostatic functions, including maintenance of GI integrity. Therefore, grapiprant may represent a treatment that could avoid the adverse effects associated with inhibition of cyclooxygenase in dogs.
Grapiprant binds to the canine EP4 receptor with high affinity and has been shown to be extremely well tolerated in a study in which it was given daily for 9 months to laboratory Beagles at doses up to 15 times the therapeutic dose.4 Our study was conducted to evaluate the effectiveness and safety of grapiprant given PO q24h for 28 days in client‐owned dogs with OA. Previous studies have shown that treatment of OA in dogs elicits a large placebo effect,5, 6 and thus appropriate power and use of a placebo were employed in the design of this study, which was conducted using a validated instrument to measure changes in pain, the Canine Brief Pain Inventory (CBPI).7 Care was taken that owners and veterinarians were masked to treatment. In addition to effectiveness, we evaluated safety in this population of predominately older dogs using owner observations, physical examinations, and clinical pathology test results.
Materials and Methods
Study Design
Our study was a prospective, multicenter, masked, randomized, placebo‐controlled parallel study conducted at 16 veterinary hospitals located in Colorado, Florida, Georgia, Illinois, Maine, Michigan, Missouri, New York, Pennsylvania, and Texas. It was conducted according to Good Clinical Practice guidelines.5
Animals
Dogs of any age, breed, or sex were enrolled, but dogs that were pregnant, lactating, or intended for breeding were excluded. To be enrolled, dogs had to weigh ≥3.6 kg.
Inclusion and Exclusion Criteria
Client‐owned dogs that were candidates for enrollment were presented by the owner with clinical signs of OA, confirmed by radiographs of at least 1 appendicular joint taken within 60 days before enrollment. The veterinarian also confirmed OA by evaluating weight bearing on the affected limb, lameness at walking and trotting, willingness to raise the contralateral limb, joint swelling, pain or resistance on palpation, forced movement and range of motion of the joint (veterinary assessment) and being in general good health or stabilized for chronic conditions and able to complete the study in the opinion of the veterinarian, as assessed by physical examination, medical history, and clinical pathology evaluations (CBC, serum biochemistry profile, and urinalysis including sediment examination).
Dogs were evaluated at day 0 (first day of treatment) by the owners using the CBPI.6 The CBPI is a 2‐part instrument6: the pain severity score (PSS) is the arithmetic mean of 4 items scored on an 11‐point (0–10) numerical scale, and the pain interference score (PIS) is the mean of 6 items scored similarly. In addition, the CBPI includes a single question for the owner to rate his or her overall impression of the dog's quality of life over the last 7 days using the following terms (owner to choose 1): poor, fair, good, very good, or excellent.
To be enrolled, each dog had to have a PSS ≥ 2 and a PIS ≥ 2 (each score representing the mean of the questions in each section, with a total possible score of 10). Owners were unaware of these criteria for inclusion so as not to bias their scoring. Dogs that had clinically relevant abnormal clinical pathology findings, spinal orthopedic abnormalities, or neurologic abnormalities that affected gait were excluded. Other exclusion criteria were concomitant autoimmune disorders that may have affected evaluations for the study, and concomitant disease at the site of the OA, such as infections, parasitic or immunological arthritis, or neoplasia. Dogs that had had major surgery within 1 month, or cruciate ligament surgery within 3 months were excluded, as were dogs that had surgeries that could confound the detection of OA pain and inflammation. To ensure that no other medications that could confound results were being used during the study, the following medications were restricted: cyclooxygenase‐inhibiting NSAIDs (including aspirin), polysulfated glycosaminoglycan6 injections, or laser treatment on the joints within 7 days before day 0; short‐acting steroids, oclacitinib7 , or other immunomodulatory drugs within 30 days before day 0; mid‐ to long‐acting steroids within 60 days before day 0; nutraceuticals for joint pain or OA within 7 days before day 0; or, a diet containing nutraceuticals for <30 days before day 0. Dogs that were on a diet containing a nutraceutical for joints for ≥30 days before day 0 remained on that diet.
Sample Size Calculation
An evaluable sample size of 138 dogs per treatment group was targeted to provide 90% power to detect a difference of 20% in success rates between the grapiprant‐treated group and the placebo group, assuming a 60% success rate in the grapiprant‐treated group and a 40% success rate in the placebo group. This assumption was based on results from a pilot field study using a dosage of 2 mg/kg (data on file). To account for a rate of attrition of approximately 10%, up to 300 subjects (150 per treatment group) could be enrolled.
Screening
Owners signed an owner consent form that had been reviewed and approved by the Center for Veterinary Medicine at the FDA. This form was signed before any study activities, and then dogs were screened to see if they met the inclusion and exclusion criteria, blood was drawn to evaluate standard serum biochemistry and hematology variables, and urine was collected for urinalysis. The CBPI was completed by the owner and veterinary assessment was completed by the veterinarian.
During the study, owners completed the CBPI on day 0 (baseline), day 14, and day 28 in the clinic, and at home after a telephone call on days 7 and 21. Veterinarians completed physical examinations and the veterinary assessment of the total orthopedic score (TOS) on days 14 and 28. Blood and urine were collected for clinical pathology testing at day 28 or, if a dog was withdrawn before day 28, on the day the dog was withdrawn from the study.
Randomization
Dogs were randomly assigned to 1 of 2 treatments based on the order of entry into the study at the site, using a unique randomization table provided to each site with randomization in blocks of 4. The program used to randomly assign treatments to dogs was SAS Proc Plan.8 To maintain masking with 2 treatment groups and equal allocation, the bottles containing placebo or grapiprant were labeled with treatment codes A, B, C, or D with bottles containing grapiprant assigned to B and D, and bottles containing placebo assigned to A and C.
Masking
Grapiprant and placebo were matched with regard to tablet size, shape, and color. The owners, veterinarians, and all clinic personnel who participated in evaluations were masked to treatment group. The treatment code (A, B, C, and D), but not treatment group, was known only to the study monitor and personnel at the clinic that assigned dogs to treatment group (dispenser).
Treatment
Each dog was treated once daily with either placebo or grapiprant at a dosage of 2 mg/kg using 20, 60, and 100 mg whole or half tablets. Placebo tablets were matched to grapiprant tablets, but without the active drug. Owners were instructed to give the dose at approximately the same time each day. Compliance with dosing was evaluated by a pill count for each dog, reconciling the returned pills with what was dispensed, and supported by a daily owner diary indicating administration of the dose.
Outcome Measures
Owner Assessment of Pain Associated with OA (CBPI)
The CBPI was completed by the owner at screening, day 0, 7, 14, 21, and 28 or, if possible, upon withdrawal or removal of the dog from the study. The owner did not have access to the scores of previous assessments when completing each CBPI. The format and content of the CBPI were presented to the owner in the form in which it was validated.6
Veterinarian Assessment of OA
Using criteria such as abnormal gait, pain on palpation, joint swelling, abnormal extension, or range of motion, the appendicular joint showing the most severe signs of OA was chosen by the veterinarian for evaluation throughout the study. This same joint was evaluated throughout the study, regardless of whether another joint also was affected by OA. The joint chosen could not be an intervertebral joint.
The selected joint was evaluated using the veterinary assessment (VA) which included 7 components: (1) lameness at a trot, (2) lameness at a walk, (3) weight bearing on the affected limb, (4) willingness to raise the contralateral limb, (5) abnormal extension or range of motion, (6) pain or resistance on palpation and forced movement of the joint, and (7) swelling. Each component was rated on a scale of 0–4 (unremarkable, slightly affected, moderately affected, very affected, severely affected). Dogs were assigned a TOS which was the sum of the scores for each of the 7 components and could range from 0 to 28. Assessments were carried out during the screening visit (from 1 to 7 days before day 0) and on days 14 and 28, or at any time a dog was withdrawn from the study.
Safety
Safety was evaluated by recording adverse events that occurred throughout the 28 days of dosing. An adverse event was defined as any observation, undesirable experience, or reaction in animals that is unfavorable and unintended and occurred after the use of grapiprant or placebo, whether or not considered to be product related. A serious adverse event (SAE) was defined as any adverse event that results in death, is life‐threatening, or results in persistent or substantial disability or incapacity and occurs after the use of grapiprant or placebo, whether or not considered to be product related.
Statistical Analysis
Each dog served as the experimental unit. Statistical significance was evaluated at a 2‐sided α = 0.05. The analysis for safety evaluation was performed on a population that included all dogs that were randomized and received at least 1 dose of the study medication: the safety or intent to treat (ITT) population. Each site needed a minimum of 3 evaluable cases in each treatment group for those cases to be included in the effectiveness evaluation. The primary evaluation of the effectiveness variable was conducted on a Per Protocol Population (PPP). The PPP was a subset of the ITT population and comprised those dogs without substantial protocol violations or missing assessments and as agreed to, based on a blinded review meeting with Center for Veterinary Medicine at the FDA (Fig 1).
Figure 1.
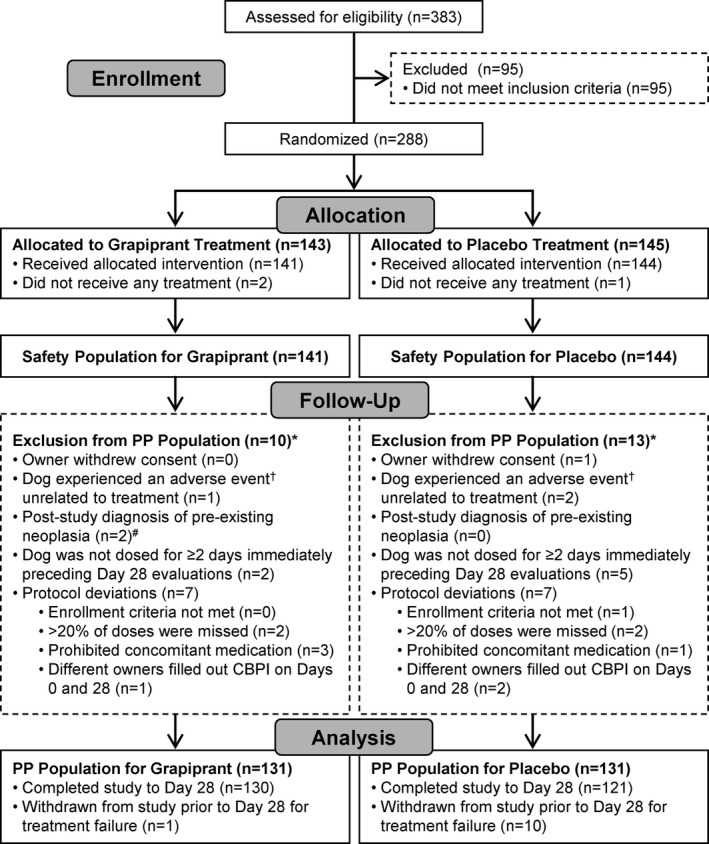
Case flow indicating how the safety (intent to treat) and per protocol populations were identified for the grapiprant and placebo treatment groups. *Some dogs were excluded for more than one reason. †Grapiprant group: ruptured cranial cruciate ligament. Placebo group: toe infection and fell down stairs. #Grapiprant group: histiocytic sarcoma and osteosarcoma (none in placebo group).
The primary effectiveness variable was defined as the CBPI score on day 28 in comparison to the CBPI score at day 0. A predefined criteria of success was used to classify each dog as either a treatment success or failure. A treatment success was defined as a dog that had a PSS that was decreased by ≥1, a PIS that was decreased by ≥2, and with the overall impression question rated as the same or better at day 28 compared to day 0. Any dog not defined as a treatment success using these criteria was defined as a treatment failure.
Animals withdrawn from the study at the request of owner or investigator because of lack of effectiveness in controlling pain also were considered treatment failures. No imputations were performed for missing values. Dogs missing the day 28 assessment were considered unevaluable.
The number (%) of dogs exhibiting success was presented by treatment group. The primary effectiveness variable (treatment success or failure) was analyzed using a generalized linear mixed model assuming a binomial distribution and using a logit link. The model included treatment group as a fixed effect, and site and treatment by site interaction as random effects. Pairwise comparisons were assessed comparing the grapiprant group to placebo at a 2‐sided 0.05 significance level.
Secondary outcome variables included the percentage changes in PSS and PIS scores from day 0 to day 28. Descriptive statistics (number of subjects, mean, standard deviation (SD), minimum, median, and maximum values) were presented for day 0, day 28 and for the percentage changes from day 0 to day 28. Analysis of variance modeling was employed to assess possible differences between treatment groups. The model contained terms for treatment, site, and treatment by site interaction.
Additional secondary outcome variables included the success rates and percentage changes in PSS and PIS scores from day 0 to days 7, 14, and 21. The success rates were presented and analyzed as described for the primary outcome variable. The percentage changes were analyzed as described for the percentage change from day 0 to day 28.
For the veterinary assessment, the TOS was analyzed across time (at screening, day 14 and day 28) and treatment groups using a longitudinal model with baseline age and weight as covariates. In the case of missing postbaseline assessments a Last Observation Carried Forward method was used. This analysis also was carried out on the subset of dogs with TOS >10 to assess the effect of grapiprant in the more severely affected dogs in the study.
Serum biochemistry, hematology and urinalysis data, physical examination data, and adverse event data were summarized and evaluated by treatment group using descriptive statistics.
Results
Study Population
Of the 383 dogs screened at the 16 sites, 288 cases were enrolled in the study, 3 of which were randomized but not treated. Out of this population, 285 dogs were included in the safety population and 262 in the PPP, with an equal number of dogs in the PPP treated with grapiprant or placebo (N = 131 per group; Fig 1). In the safety (ITT) population, dogs ranged in age from 6 months to >16 years of age, with a median age of 9.92 years in the grapiprant group, and 10.04 years in the placebo group. The majority of dogs were spayed or castrated, with approximately equal numbers of males and females in the population (148 females, 137 males). Body weights ranged from 4.10 to 70.40 kg with median weights in the grapiprant and placebo groups of 30.45 and 29.15 kg, respectively (Table 1).
Table 1.
Population demographics at screening of the intention to treat population
| Characteristic | Grapiprant | Placebo |
|---|---|---|
| Age (years) | ||
| N | 141 | 144 |
| Mean (SEM) | 9.44 (0.24) | 9.80 (0.24) |
| Median | 9.92 | 10.04 |
| Min, Max | 2.00, 16.75 | 0.50, 16.42 |
| Sex | ||
| N | 141 | 144 |
| Female (%) | 2 (1.4) | 5 (3.5) |
| Female spayed (%) | 67 (47.5) | 74 (51.4) |
| Male (%) | 1 (0.7) | 9 (6.3) |
| Male Castrated (%) | 71 (50.4) | 56 (38.9) |
| Weight (kg) | ||
| N | 140 | 144 |
| Mean (SEM) | 29.03 (1.03) | 28.86 (1.09) |
| Median | 30.45 | 29.15 |
| Min, Max | 4.10, 59.60 | 5.10, 70.40 |
Owner Assessment of Effectiveness (CBPI)
Eleven dogs (10 from the placebo group and 1 from the grapiprant group) were removed from the study before day 28 because of owner‐perceived lack of therapeutic effectiveness and were defined as treatment failures. Results from the CBPI were examined at each of the 4 time points after baseline (days 7, 14, 21, and 28). At day 28, the success rates were 48.1% (63/131) for the grapiprant‐treated dogs and 31.3% (41/131) for the placebo‐treated dogs. This difference of 16.8% was statistically significant (P = .0315).
For dogs that were deemed treatment failures before day 28, the failure was carried forward to all subsequent time points. At each time point (days 7, 14, and 21) more dogs in the grapiprant treatment group were classified as treatment successes compared to the placebo treatment group, and this treatment effect was statistically significant (P < .05) at all time points (Table 2).
Table 2.
Percentage of dogs treated with either grapiprant or placebo classified as treatment success comparing CBPI scores on Day 0 to scores on Days 7, 14, and 21
| Timepoint | Treatment Success | P value | |
|---|---|---|---|
| Grapiprant N (%) | Placebo N (%) | ||
| Day 7 | 40 (30.5) | 21 (16.0) | .0154 |
| Day 14 | 54 (41.2) | 37 (28.2) | .0442 |
| Day 21 | 61 (46.6) | 43 (32.8) | .0443 |
The parts of the CBPI (PSS and the PIS) were examined independently. The mean percentage change (±SD) at days 7, 14, 21, and 28 in the PSS and PIS are shown in Fig 2.
Figure 2.
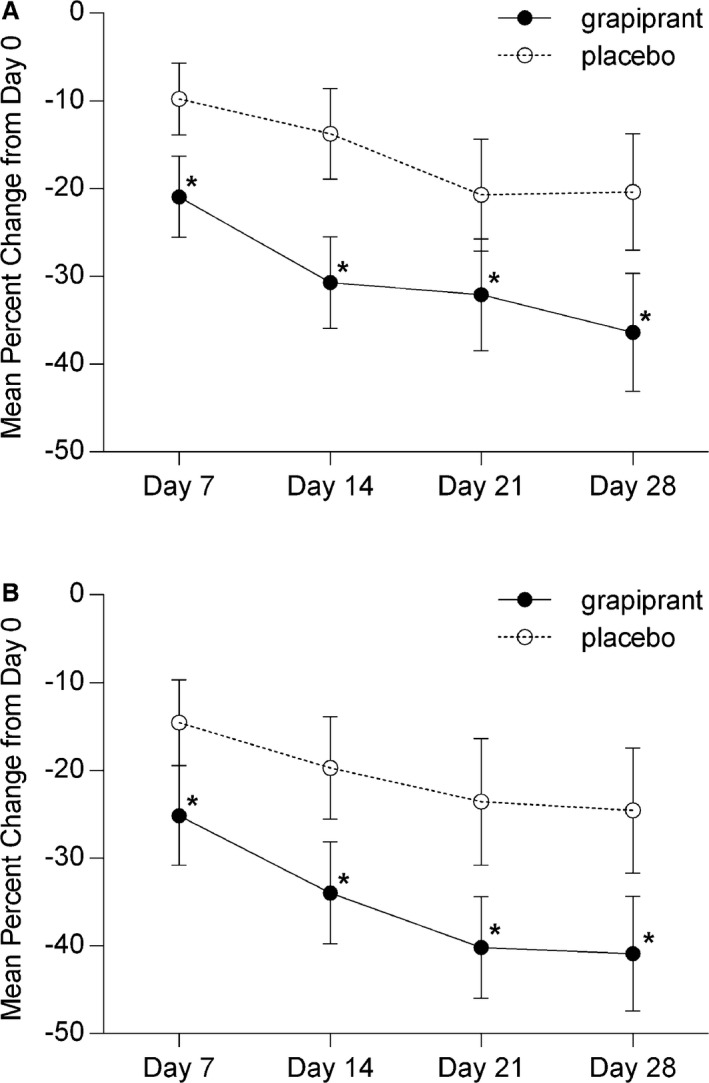
Mean percentage change (with 95% confidence intervals) in (A) pain severity score and (B) pain interference score scores from Day 0 to Days 7, 14, 21, and 28 in dogs treated with grapiprant (n = 131) or placebo (n = 131). *Denotes statistical significance (P < .05).
Veterinarian Assessment of Effectiveness
At baseline in the PPP, the mean TOS (±SD) was not statistically different between groups. The TOS was evaluated at each of the 2 clinic visits (days 14 and 28) after study initiation. At both of these time points, the mean scores in the grapiprant group were significantly improved (decreased) compared to those in the placebo‐treated group (Table 3). As in the full population, the analysis of the TOS in the subset of dogs with TOS >10 showed dogs treated with grapiprant had a significantly greater decrease in TOS than did dogs treated with placebo (Table 4).
Table 3.
Mean (±standard deviation) total orthopedic scores in the PPP at baseline, days 14 and 28 in dogs treated with grapiprant or placebo; comparison between treatment groups at each time point
| Total Orthopedic Score (Mean ± SD) | P value | ||
|---|---|---|---|
| Grapiprant (n = 131) | Placebo (n = 131) | ||
| Baseline | 10.41 ± 4.36 | 10.83 ± 4.61 | .6259 |
| Day 14 | 6.75 ± 4.20 | 8.59 ± 4.91 | .0029 |
| Day 28 | 6.48 ± 4.75 | 8.26 ± 5.02 | .0086 |
Table 4.
Mean (±standard deviation) total orthopedic scores in the subpopulation of dogs with TOS >10 at baseline, days 14 and 28; comparison between treatment groups at each time point
| Total Orthopedic Score (Mean ± SD) | P value | ||
|---|---|---|---|
| Grapiprant (n = 59) | Placebo (n = 63) | ||
| Baseline | 14.25 ± 2.89 | 14.75 ± 3.22 | .7428 |
| Day 14 | 8.93 ± 4.39 | 11.56 ± 4.81 | .0047 |
| Day 28 | 8.12 ± 4.91 | 10.62 ± 5.27 | .0172 |
Safety
The safety (ITT) population included 285 dogs that had received at least 1 dose of grapiprant or placebo. One SAE occurred during the study: a dog in the placebo group was diagnosed with diabetes mellitus based on day 28 clinical pathology results. One dog in the grapiprant group developed immune‐mediated hemolytic anemia that was discovered on the day 28 clinical pathology results. The dog responded to standard treatment. The adverse event was not considered serious and a cause was not identified. Table 5 summarizes the frequency of adverse events seen in ≥5% of the dogs in the study and potentially related to treatment (adverse reactions). Table 6 gives additional details on the duration of adverse events related to the GI system. The majority of the diarrhea or soft stool adverse events in both treatment groups was mild, lasted a few days, and resolved without treatment. The reported vomiting was, for the most part, a single occurrence, and classified as mild. In 1 dog, vomiting was classified as moderate for 2 episodes of vomiting. Two dogs were treated for vomiting with maropitant9 (2 doses) or a kaolin, pectin, probiotic gel.10 Seven dogs in the study were reported to have both diarrhea or soft stool and vomiting, 2 in the placebo group, and 5 in the grapiprant‐treated group. No adverse events prompted any owner or veterinarian to remove a dog from the study.
Table 5.
Adverse reactions in dogs treated with either placebo or grapiprant for 28 days
| Adverse reactiona | Grapiprant N = 141 N (%) | Placebo N = 144 N (%) |
|---|---|---|
| Vomiting | 24 (17.02) | 9 (6.25) |
| Diarrhea, soft stool | 17 (12.06) | 13 (9.03) |
| Anorexia, inappetence | 9 (6.38) | 7 (4.86) |
| Lethargy | 6 (4.26) | 2 (1.39) |
| Buccal ulcer | 1 (0.71) | 0 |
| Immune‐mediated hemolytic anemia | 1 (0.71) | 0 |
Dogs may have experienced more than one type or occurrence during the study.
Table 6.
Adverse events related to the gastrointestinal system: number of affected dogs and duration of event
| Grapiprant (N = 141) | Placebo (N = 144) | |
|---|---|---|
| Vomiting | ||
| Number of dogs with evaluations | 24 | 9 |
| Mean number of days affected per affected dog | 1.75 | 1.50 |
| Minimum NUMBER OF DAYS | 1 | 1 |
| Maximum number of days | 5 | 3 |
| Diarrhea/soft stool | ||
| Number of dogs with evaluations | 17 | 13 |
| Mean number of days affected per affected dog | 1.94 | 1.69 |
| Minimum number of days | 1 | 1 |
| Maximum number of days | 5 | 4 |
| Anorexia/inappetence | ||
| Number of dogs with evaluations | 9 | 7 |
| Mean number of days affected per affected dog | 2.67 | 2.86 |
| Minimum number of days | 1 | 1 |
| Maximum number of days | 4 | 7 |
No treatment‐related changes in clinical pathology results were identified. The mean and median laboratory values for day 0 and day 28 (or end of study) were within reference ranges across groups, with no trends for increasing or decreasing results and very few adverse events reported for changes in clinical pathology results. Five adverse events were observed describing increased calcium (Ca) concentrations (above the reference range of 8.8–11.2 mg/dL), 1 in the placebo group and 4 in the grapiprant‐treated group. Increases were mild in 3 of the dogs (11.3–11.9 mg/dL). Two grapiprant‐treated dogs had more marked increases; 1 dog had a Ca serum concentration that was increased at baseline (11.9 mg/dL) and further increased to 13.1 mg/dL. This dog was diagnosed with osteosarcoma after completion of the study. A second dog, which also had an increased baseline serum Ca concentration (11.3 mg/dL) that further increased to 12.4 mg/dL, was being treated with a corticosteroid during the study. Because this medication was not allowed during the study, the dog was removed at day 14. The increases in serum Ca concentration were not considered treatment related.
Discussion
Our randomized, placebo‐controlled masked study of a prostaglandin EP4 receptor antagonist for the control of pain and inflammation in dogs with OA showed that grapiprant treatment resulted in a significant number of dogs with decreased PSS and PIS, as evaluated by the owner, and a decrease in the TOS, as evaluated by the veterinarian. In addition, grapiprant used for 28 days in this population of dogs was found to be safe. Adverse events were relatively mild and of short duration. No dog was withdrawn from the study, despite a higher frequency of transient vomiting in the grapiprant‐treated dogs. These findings are important because grapiprant represents the first approach to daily PO treatment of pain and inflammation in dogs with OA with a mechanism of action targeted to binding and antagonizing a prostaglandin receptor (EP4) rather than inhibiting cyclooxygenase enzymes.
To evaluate the effectiveness of a drug for pain in dogs, studies rely on the owner to accurately report clinical signs, and veterinarians to evaluate these clinical signs when dogs are presented to veterinary clinics. In recent years, the FDA has required companies to rely on owner assessments as the primary effectiveness variable in studies evaluating drugs for OA in dogs. Furthermore, instead of comparing treatment groups using median or mean population data, the FDA has required that a predetermined definition of success be used to classify each dog as either a treatment success or failure. Subsequently, the number of dogs in each group that were treatment successes is compared to assess the effectiveness of the drug treatment. This approach makes it difficult to compare data across studies, unless the same definition of treatment success was used, which is seldom the case.
In our study, the definition of treatment success was based on a previously published rigorous evaluation of the power of various possible definitions of treatment success to differentiate between placebo treatment and treatment with carprofen in dogs with OA evaluated by the CBPI.7 Using this analysis, the definition of treatment success was chosen as PSS decreasing ≥1, PIS decreasing ≥2, and the overall impression score as the same or better on day 28 compared to day 0. Each of these scores (PSS and PIS) are mean values of 4 and 6 questions, respectively, and therefore a decrease represents an improvement in several questions in each score. Results of the study show that 48.1% of grapiprant‐treated dogs were classified as treatment successes compared to 31.3% of placebo‐treated dogs, a statistically significant difference (P = .0315). In addition, to be enrolled in the present study, dogs had to have both PIS and PSS ≥2. Only 1 other study has been conducted using the same enrollment and success criteria as its outcome.7 In an analysis of a placebo‐controlled, randomized, masked study of the treatment carprofen daily for 14 days compared to a placebo (n = 116 dogs), similar results were seen, with 45.6% of carprofen‐treated dogs classified as treatment successes, compared to 23.7% of placebo‐treated dogs. Because these 2 studies are the only studies in which these exact success criteria were used (albeit the present study was a 28‐day study, whereas the carprofen study was a 14‐day study) they represent a valid comparison of these 2 treatments for OA in dogs.
Less than 50% of the dogs were considered treatment successes in these studies because the definition of treatment success required a decrease in mean scores ≥1 (pain severity) and ≥2 (pain interference), which is rigorous. A decrease in the mean score requires that the majority of the questions need to improve by ≥1 categories. Decreases in the PIS and PSS by < 1 point each are also meaningful to the owner, but to maximize the power of the analysis, we chose the above definition of treatment success in our study.
Other drugs for the treatment of OA in dogs have been evaluated using a variety of effectiveness measurements, including various owner questionnaires, veterinary assessments, and objective measurements of gait analysis (eg, force plate). Efforts have been made to evaluate the evidence in these studies based on quality of study design, including masking, randomization, number of animals included, and statistical power.8, 9, 10 Many of these clinical studies have been reviewed with the overall conclusion that, regardless of method of evaluation, the cyclooxygenase‐inhibiting drugs show effectiveness for the treatment of pain associated with OA in dogs.9, 10, 11, 12 Specifically, drugs approved by the FDA for treatment of the pain and inflammation associated with OA in dogs have demonstrated effectiveness in randomized clinical studies, either compared to another approved drug or a placebo.
Our study indicates that the EP4 receptor antagonist drug grapiprant showed effectiveness comparable to cyclooxygenase‐inhibiting NSAIDS. Whether the production of PGE2 is inhibited using a cyclooxygenase enzyme inhibitor or the target EP4 receptor responsible for pain and inflammation is blocked, the end clinical effect would likely be comparable.
Cyclooxygenase‐inhibiting drugs used in dogs with OA produce their effect by lowering circulating concentrations of all prostanoids, including PGE2. Grapiprant directly binds the EP4 receptor and blocks PGE2 from exerting its biological effect. By blocking the binding of PGE2 to its receptor, the signaling pathway for pain and inflammation is interrupted. Thus, grapiprant and other EP4 receptor antagonists would be expected to have similar efficacy to appropriate doses of cyclooxygenase‐inhibiting NSAIDs in the treatment of OA in dogs. The approach of specifically targeting the EP4 receptor may result in other benefits, because other off‐target effects caused by a decrease in prostanoids and needed for normal physiological function (as seen with the cyclooxygenase‐inhibiting class of drugs) would not be expected with grapiprant.
Although in most dogs the chronic use of cyclooxygenase‐inhibiting drugs PO at label dosages is well tolerated,9 several adverse effects, some serious, have been associated with these drugs such as GI, renal, and hepatic toxicity.13, 14, 15 The true incidence of cyclooxygenase‐inhibiting NSAID‐induced adverse effects in animals is unknown,14, 15 but likely is underestimated.
In our study, grapiprant treatment resulted in a higher frequency of vomiting compared to placebo treatment. Most occurrences were mild and sporadic, and none prompted owners to remove their dogs from the study. This finding, and the slight increase in diarrhea or soft stool and anorexia or inappetence when compared to the placebo‐treated group, indicates mild effects on the GI system. These mild, transient GI effects were also noted in a 9‐month safety study in which grapiprant was administered daily to Beagle dogs at up to a dosage that was equivalent to 15 times the clinical dosage.4 In this long‐term, high‐dose study, occasional soft‐formed or mucus‐containing stools and rare occurrences of vomiting were observed in all groups, including controls, with a higher incidence in grapiprant‐treated dogs. None of the dogs required treatment for these mild GI signs. In that study, grapiprant did not cause GI ulceration or perforation as has been seen in dogs treated with cyclooxygenase‐inhibiting NSAIDS.13 Grapiprant‐related histopathological changes in this 9‐month study were limited to 1 animal in the 50 mg/kg group (equivalent to 30.5 mg/kg of tablet formulation)16 that had mild regeneration of the mucosal epithelium of the ileum. The lack of GI pathology is not surprising, given that grapiprant blocks only the PGE2 EP4 receptor, leaving the other PGE2 and prostaglandin I2 (PGI2) receptor pathways intact, both of which are important in GI homeostasis.10 In our study as well as the 9‐month high‐dose study, no effects on the liver or kidney were observed in clinical pathology evaluations.
A limitation of this study was that it did not evaluate the effectiveness and safety of grapiprant for >28 days. Additionally, no objective measurement of lameness was conducted, such as force plate analysis. However, the CBPI tool is validated for use by owners to identify changes in pain in dogs with OA, whereas no force plate measurement had been validated for this application. Grapiprant, like the cyclooxygenase‐inhibiting NSAIDS, decreases inflammation, but clinical studies generally do not include a direct measurement of inflammation (eg, histopathological evidence). The only measurement of inflammation in our study was the subjective evaluation by the veterinarian of joint swelling as part of the TOS. A decrease in inflammation would be expected to occur in grapiprant‐treated dogs, based on its mechanism of action, and data seen in rodent models.2, 3 Veterinary assessment was based on the evaluation of a single joint chosen by the veterinarian as the joint that in his or her opinion was most severely affected. Given that OA is most commonly a multijoint condition, choosing only 1 joint for evaluation may have either under‐ or overestimated the effect of grapiprant. However, given the 1 : 1 randomization, this potential bias would be equally likely in both groups. Another limitation of this study was that, given the study size, it is unlikely that rare adverse events would have been detected. Wider clinical use is required to fully evaluate the risk versus benefit of any new therapeutic agent.
Our study clearly indicates that the EP4 prostaglandin receptor antagonist, grapiprant, when given daily at a dosage of 2 mg/kg for 28 days to dogs with OA, results in demonstrable owner‐ and veterinarian‐assessed improvement in clinical signs when compared to placebo. Furthermore, in dogs of different ages and breeds with concomitant clinical conditions and receiving other medications, grapiprant‐related adverse events were mild and transient, and no SAEs were seen. These data, therefore, support the conclusion that the EP4 prostaglandin receptor antagonist, grapiprant, represents a promising new treatment modality for the pain and inflammation associated with OA in dogs.
Acknowledgments
The authors acknowledge Sean Matthews for assistance with statistical analysis and the staff at AlcheraBio for assistance in conducting the study.
Conflict of Interest Declaration:This clinical study was conducted at 16 veterinary hospitals throughout the United States, and was funded by Aratana Therapeutics in support of the Food and Drug Administration (FDA) approval of grapiprant. Drs. Rausch‐Derra, Jessica Wofford, and Linda Rhodes are employed by Aratana Therapeutics, Inc. Margie Huebner is a paid consultant for Aratana Therapeutics, Inc.
Off‐label Antimicrobial Declaration:Authors declare no off‐label use of antimicrobials.
Footnotes
Rimadyl, Zoetis, Kalamazoo, MI
Previcox, Merial, Duluth, GA
Metacam, Boehringer‐Ingelheim, St. Joseph, MO
Deramaxx, Elanco, Indianapolis
VICH GL9 for Industry, FDA Guidance 85, 2001
Adequan, Luipold Pharmaceuticals, Shirley, NY
Apoquel, Zoetis
SAS, version 9.3.1, SAS Institute Inc., Cary, NC
Cerenia, Zoetis
Pro‐Pectalin, Vetoquinol, Ft. Worth, TX
References
- 1. Nakao K, Murase A, Ohshiro H, et al. CJ‐023,423, a novel, potent and selective prostaglandin receptor antagonist with antihyperalgesic properties. J Pharmacol Exp Ther 2007;322:686–694. [DOI] [PubMed] [Google Scholar]
- 2. Shaw KK, Rausch‐Derra LC, Rhodes L. Grapiprant: An EP4 prostaglandin receptor antagonist and novel therapy for pain and inflammation. Vet Med Sci 2016;2:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Okumura T, Murata Y, Taniguchi K, et al. Effects of the selective EP4 antagonist, CJ‐023, 423 on chronic inflammation and bone destruction in rat adjuvant‐induced arthritis. J Pharm Pharmacol 2008;60:723–730. [DOI] [PubMed] [Google Scholar]
- 4. Rausch‐Derra LC, Huebner M, Rhodes L. Evaluation of the safety of long‐term, daily oral administration of grapiprant, a novel drug for treatment of osteoarthritic pain and inflammation, in healthy dogs. Am J Vet Res 2015;76:853–859. [DOI] [PubMed] [Google Scholar]
- 5. Conzemius MG, Evans RB. Caregiver placebo effect for dogs with lameness from osteoarthritis. J Am Vet Med Assoc 2012;241:1314–1319. [DOI] [PubMed] [Google Scholar]
- 6. Brown DC, Boston RC, Coyne JC, et al. Ability of the Canine Brief Pain Inventory to detect response to treatment in dogs with osteoarthritis. J Am Vet Med Assoc 2008;233:1278–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown DC, Bell M, Rhodes L. Power of treatment success definitions when the Canine Brief Pain Inventory is used to evaluate carprofen treatment for control of pain and inflammation in dogs with osteoarthritis. Am J Vet Res 2013;74:1467–1473. [DOI] [PubMed] [Google Scholar]
- 8. Aragon CL, Hofmeister EH, Budsberg SC. Systematic review of clinical trials of treatments for osteoarthritis in dogs. J Am Vet Med Assoc 2007;230:514–521. [DOI] [PubMed] [Google Scholar]
- 9. Innes JF, Clayton J, Lascelles BDX. Review of the safety and efficacy of long‐term NSAID use in the treatment of canine osteoarthritis. Vet Rec 2010;166:226–230. [DOI] [PubMed] [Google Scholar]
- 10. KuKanich B, Bidgood T, Knesl O. Clinical pharmacology of nonsteroidal anti‐inflammatory drugs in dogs. Vet Anaesth Analg 2012;39:69–90. [DOI] [PubMed] [Google Scholar]
- 11. Papich MG. An update on nonsteroidal anti‐inflammatory drugs (NSAIDS) in small animals. Vet Clin North Am Small Anim Pract 2008;38:1243–1266. [DOI] [PubMed] [Google Scholar]
- 12. Lees P. Analgesic, anti‐inflammatory, antipyretic drugs In: Riviere JE, Papich MG. eds. Vet Pharm and Ther. Ames, IA: Wiley‐Blackwell; 2009:457–492. [Google Scholar]
- 13. Wallace MS, Zawie DA, Garvey MS. Gastric ulceration in the dog secondary to the use of nonsteroidal antiinflammatory drugs. J Am Anim Hosp Assoc 1990;26:467–472. [Google Scholar]
- 14. Bergh MS, Budsberg SC. The coxib NSAIDs: Potential clinical and pharmacological importance in veterinary medicine. J Vet Intern Med 2005;19:633–643. [DOI] [PubMed] [Google Scholar]
- 15. Monteiro‐Steagall BP, Steagall PVM, Lascelles BDX. Systematic review of nonsteroidal anti‐inflammatory drug‐induced adverse effects in dogs. J Vet Intern Med 2013;27:1011–1019. [DOI] [PubMed] [Google Scholar]
- 16. Rausch‐Derra LC, Rhodes L, Freshwater L, Hawks R. Pharmacokinetic comparison of oral tablet and suspension formulations of grapiprant, a novel therapeutic for the pain and inflammation of osteoarthritis in dogs. J. Vet Pharmacol Therap, 2016, doi:10.1111/jvp.12306. [DOI] [PubMed] [Google Scholar]


