Abstract
Background
Chronic proton pump inhibitor administration has been associated with electrolyte and cobalamin deficiency, disrupted bone homeostasis, hypergastrinemia, and rebound acid hypersecretion in humans. It is unknown if this occurs in cats.
Objectives
Prolonged oral omeprazole results in altered bone mineral density or content, serum calcium, magnesium, cobalamin, and gastrin concentrations in healthy cats.
Animals
Six healthy adult DSH cats.
Methods
In a within subjects, before and after design, cats received placebo followed by omeprazole (0.83–1.6 mg/kg PO q12h) for 60 days each. Analysis of serum calcium, magnesium, cobalamin, and gastrin concentrations was performed on days 0, 30, and 60. Bone density and content were evaluated on days 0 and 60 of each intervention. Continuous data were analyzed using a two‐way ANOVA (α = 0.006). On day 60 of omeprazole administration, continuous intragastric pH monitoring was performed in 2 cats to evaluate the effects of abrupt withdrawal of omeprazole.
Results
No significant changes were detected between treatments for any variables, except serum gastrin, which was significantly higher during omeprazole treatment in comparison to placebo (P = 0.002). Evidence of gastric hyperacidity was seen in both cats in which intragastric pH monitoring was performed following cessation of omeprazole.
Conclusions and Clinical Importance
Although further studies with larger populations of cats will be needed to draw any definitive conclusions, these preliminary results suggest that prolonged PPI treatment results in hypergastrinemia and abrupt PPI withdrawal might result in RAH in cats.
Keywords: DEXA, Feline, Intragastric pH, Rebound acid hypersecretion
Abbreviations
- BMC
bone mineral content
- BMD
bone mineral density
- DEXA
dual‐energy X‐ray absorptiometry
- MMA
methylmalonic acid
- PPI
proton pump inhibitor
- RAH
rebound acid hypersecretion
Omeprazole, a substituted benzimidazole proton pump inhibitor (PPI), is a potent gastric acid suppressant drug that has proven to be superior to histamine‐2 receptor antagonists in raising intragastric pH in dogs1 and, more recently, cats.2, 3 For this reason, omeprazole is often the first choice acid suppressant prescribed to cats with diseases suspected to result in excessive gastric acidity, ulcerative esophagitis, gastritis, and/or gastrointestinal (GI) bleeding. These diseases include reflux esophagitis, GI and pancreatic cancers (eg, lymphoma, mast cell tumor, carcinoma, or gastrinoma), and hepatic failure among others.
Short‐term administration (<8 days) of omeprazole is well‐tolerated in dogs1 and cats.2, 3 Few adverse effects, with the exception of self‐limiting diarrhea, have been described with short‐term PPI administration.1, 4, 5 However, growing evidence in humans suggests that prolonged omeprazole administration can result in severe adverse effects, including an increased risk of community‐acquired pneumonia and Clostridium difficile infections, undesired drug interactions, and cobalamin deficiency. Disruption of calcium and magnesium homeostasis with subsequent development of osteoporosis and pathologic fractures has also been reported in older humans receiving chronic PPI therapy.6, 7, 8, 9 In addition, a phenomenon known as rebound acid hypersecretion (RAH) has been shown to occur where gastric hyperacidity ensues after abrupt cessation of PPI administration with prolonged treatment.10, 11
Only omeprazole raises feline intragastric pH to a degree associated with healing of acid‐related injury in people.2 Because of the superior efficacy of omeprazole compared with famotidine, omeprazole is the treatment of choice for chronically ill cats with upper GI ulcers, erosions, and bleeding. Despite the increasing evidence that prolonged PPI administration is associated with adverse effects in humans, no studies have investigated the potential for chronic PPI administration to cause adverse effects in cats. Therefore, the purpose of this study was to evaluate the effect of prolonged oral omeprazole administration on serum calcium, magnesium, cobalamin, and gastrin concentrations and on bone mineral density and content in cats. An additional subaim was to evaluate a smaller subset of cats for evidence of RAH following cessation of omeprazole therapy. We hypothesized that continuous administration of omeprazole would result in altered magnesium, calcium, cobalamin, and gastrin concentrations and bone homeostasis in cats.
Materials and Methods
Cats
The Institutional Animal Care and Use Committee at the University of Tennessee approved the protocol for this study (#2312‐0115). The subjects of this study were six healthy adult domestic shorthair cats from a research colony at the University of Tennessee (3 neutered females, 3 neutered males), aged 7–10 years (median, 8 years) and weighing 3.22–5.46 kg (median, 4.14 kg). Adverse effects of PPI administration on bone have been more commonly documented in older humans6, 8, 12, 13; thus, our study was restricted to older adult cats (≥7 years of age). Cats included into the study were deemed healthy on the basis of normal physical examination and blood work (complete blood count [CBC], serum chemistry, TT4, urinalysis) performed within 6 months and day 1 of study entry. Only spayed/neutered cats were used to eliminate the confounding effect of gonadal sex hormones on bone metabolism. To ensure inclusion of healthy cats and to comply with IACUC guidelines, cats were excluded from the study if they developed inappetence for >24 hours, lost >10% of their body weight, developed systemic diseases, and/or required therapeutics that could interfere with any of the parameters being measured (e.g., electrolytes, bone mineral density).
Study Design
A within‐subjects, before and after, study was performed in which all cats received treatment for 60 consecutive days with placebo (250 mg lactose1 PO q12h, administered in gelatin capsules) and later 5 mg (0.83–1.6 mg/kg) PO q12h omeprazole.2 The goal of omeprazole treatment was to achieve a dose that approximated as close to 1 mg/kg q12h as possible. Because the degree and duration of effect of omeprazole on bone was unknown, all cats initially received placebo treatment, followed by omeprazole, to eliminate the possibility of carryover effects of omeprazole as a result of an inadequate recovery period. Cats were medicated at 7:30 AM and 6:30 PM daily. This time interval was selected to best mimic the feeding schedule of many client‐owned cats. Cats were fed a maintenance diet3 30 minutes after medicating at 8:00 AM and 7:00 PM daily. Cats were fasted (food withheld) the night prior to each procedure date, but water was always made available. On days 0, 30, and 60 of each phase, 25 mL of blood was drawn via jugular venipuncture following a 12 hours fast for each of the following assays: venous blood gas4 (including ionized calcium and magnesium), serum chemistry profile5 (including total calcium and magnesium), CBC6 (including blood smear review with manual platelet count and evaluation for Heinz body formation), serum cobalamin, methylmalonic acid (MMA), and gastrin concentrations. Serum was collected from blood tubes following centrifugation at 250×g, and the serum was separated and stored in cryovials at −80°C. Following study completion, the serum was shipped on dry ice to the Gastrointestinal Laboratory at Texas A&M University for measurement of cobalamin, gastrin, and MMA concentrations. Serum cobalamin and gastrin concentrations were measured using an automated chemiluminescent, enzyme‐labeled immunometric assay7 as previously described.14 Serum MMA concentrations were measured using a previously described gas chromatography‐mass spectrometry method.14 Evaluation for Heinz bodies was performed with a standardized staining technique using new methylene blue (NMB).15 An equal volume of EDTA‐treated blood was mixed with NMB and allowed to sit at room temperature for 10 minutes. The number of Heinz bodies was then evaluated per high‐power field (HPF), with enough HPFs reviewed to give a total of 1,000 red blood cells (RBCs) counted. Heinz bodies were reported as a percentage out of the counted population [(number of Heinz bodies/1,000 RBCs) × 100]. Urine was collected on days 0, 30, and 60 of each study phase. On days 0 and 60 of each treatment period, cats were sedated with dexmedetomidine8 (0.005 mg/kg), ketamine9 (5 mg/kg), and butorphanol10 (0.4 mg/kg) IM. Cats were positioned in sternal recumbency following sedation to facilitate performance of dual‐energy X‐ray absorptiometry (DEXA11) scans as previously described.16 All cats were reversed with atipamezole12 (0.005 mg/kg) IM. Bone mineral density (BMD) and bone mineral content (BMC) were evaluated at the end of the study period by a single investigator (T.M.) blinded to the cats' treatments. A period of 3 days separated treatment groups, with no medications administered during these days. Appetite, attitude, and episodes of vomiting and/or diarrhea were recorded daily and physical examinations and body weight measurements were performed on each cat weekly. A standardized fecal scoring system13 was used to characterize episodes of diarrhea. For the purposes of this study, diarrhea was defined as a fecal score ≥4.
pH Monitoring
Twenty‐four hours prior to the end of the omeprazole study period (day 60), pH14 capsules were placed in the stomach of a subset of cats (n = 2) in order to provide a preliminary evaluation of the effect of abrupt drug withdrawal on feline gastric pH following prolonged omeprazole administration. Immediately before placement, the pH capsule and 2 receivers were calibrated with commercial buffer solutions (pH 1.07 and 7.01) as previously described.2 Following performance of the DEXA scan, while the cat was still heavily sedated, a pH capsule, preassembled with its catheter delivery system, was introduced blindly into the cat's stomach transorally. Once the desired position of the capsule was confirmed by lateral and ventrodorsal abdominal radiographs, mucosal attachment of the pH capsule was secured with vacuum suction as previously reported2 and the capsule delivery device was removed from the cat. Mucosal attachment of the capsule was again confirmed by radiographic visualization of the capsule within the gastric fundus by one of the authors (L.E.). Intragastric pH recordings were obtained telemetrically at 6‐second intervals for 10 days following capsule placement. The receivers were placed on the front of the cats' cages to ensure proper recording throughout the time period. The pH data from the receivers were uploaded every 48 hours, when the receivers reached maximum data capacity, to the computer via manufacturer software.15 The daily mean intragastric pH was calculated using the computer software. The second receiver previously calibrated to the existing capsule was used to collect the next 48 hours of data. Daily mean intragastric pH from these cats was compared to the mean intragastric pH of 6 placebo‐treated cats from this colony that were measured in a previously published study.2
Statistical Analysis
The sample size estimates were based on a paired means test, assuming that the paired measurements were moderately correlated at r = 0.50. A significance level of alpha = 0.05 was used for all estimates as was power of 80%. The SAS power analysis procedure (proc power) was used for all calculations. If the % change to be detected in bone assays were 20% in the omeprazole group compared with the placebo group and there was a common standard deviation (SD) of 10% (numbers based on historical data in humans17) at 80% power, 5 cats would be required to find that the 2 means were significantly different. A total of 6 study cats were chosen to account for drop out of a study cat if necessary. Variables demonstrated to be affected by chronic omeprazole treatment in humans, including biochemical indices (i.e., serum total calcium, magnesium, ionized magnesium, cobalamin, MMA, and gastrin concentrations) and markers of bone homeostasis (i.e., BMD and BMC), were compared between treatment groups. Continuous data were analyzed using a two‐way repeated‐measures analysis of variance (ANOVA) and tested to account for the effects of phase of treatment, time of measurement, and the interaction of phase and time. The interaction of treatment phases and time was tested to determine if significant differences between phases, when found, also differed significantly by time of measurement. A Shapiro‐Wilk W statistic was used to evaluate normality of ANOVA residuals, with the assumption of equal variances tested using the Levene's F test. Log transformations of serum gastrin, BMD, and BMC were required for the data to meet the ANOVA normality assumption. To reduce the possibility of type I error due to analysis of multiple study variables, the Bonferroni correction was applied, with a new alpha level of 0.006. Other measured biochemical variables that were not statistically analyzed were provided as descriptive data. Commercially available software was used to perform all data analyses and generate all descriptive statistics.16
Results
Effect of Omeprazole on Hematologic and Biochemical Variables
Serum electrolytes, platelet count, and Heinz bodies are presented in Table 1. Since the generic omeprazole product used by many private practitioners and in this study contains propylene glycol, a blood smear evaluation for Heinz body formation was also performed at each time point. Serum total calcium was corrected for serum albumin using the equation (total serum calcium [mg/dL] + 4)—serum albumin (g/dL) prior to statistical analysis. In order to prevent the chance of a type I or II error, statistical analyses were not performed on the remaining electrolytes shown to be inconsistently or not affected by prolonged omeprazole treatment in humans. No significant differences were found between treatment phases (P = 0.10 with a mean difference between phases of −0.16 [95% CI: −0.42–0.11] and 0.27 with a mean difference between phases of −0.01 [95% CI: −0.04–0.01], respectively) or time of measurement (P = 0.012 and 0.28, respectively) for serum total calcium or ionized magnesium. The interaction of treatment phases and time of measurement was also not significant for these measurements, with p‐values of 0.93 for serum total calcium or ionized magnesium, respectively. No significant differences were found between treatment phases for serum total magnesium (P=0.29 with a mean difference between phases of −0.02 [95% CI: −0.06–0.02]). No differences were observed in CBC, serum chemistry, urine specific gravity, urine pH, or venous blood gas between treatment phases or days. Heinz body counts remained below 0.4% in all cats throughout the study.
Table 1.
Serum clinicopathologic values in 6 cats before and after administration of omeprazole. Data is represented by either the mean ± standard deviation for parametric data or the median and range (in parentheticals) for all nonparametric data in cats receiving omeprazole or placebo. An asterisk (*) denotes nonparametric datasets. The mean differences between treatment phases and 95% confidence intervals for the mean differences are reported for parametric data. P values are only reported for variables that were statistically analyzed. Reference ranges were not available for variables generated by the venous blood gas analyzerd
| Analyte | Placebo Treatment Phase | Omeprazole Treatment Phase | Mean Difference (95% CI) | RR | P‐value |
|---|---|---|---|---|---|
| Serum | |||||
| Total calcium (mg/dL) | |||||
| Day 0 | 10.2 ± 0.2 | 10.3 ± 0.6 | 9.5–11.2 mg/dL | 0.10 | |
| Day 30 | 10.6 ± 0.3 | 10.8 ± 0.1 | −0.2 (95% CI of mean diff: −0.4–0.1) | ||
| Day 60 | 10.4 ± 0.5 | 10.5 ± 0.3 | |||
| Ionized calcium (mmol/L) | |||||
| Day 0 | 1.35 (1.27–1.40) * | 1.35 (1.25–1.39) * | — | — | — |
| Day 30 | 1.37 (1.33–1.43) * | 1.39 (1.31–1.45) * | — | ||
| Day 60 | 1.29 (1.18–1.30) * | 1.29 (1.25–1.31) * | — | ||
| Total magnesium (mmol/L) | |||||
| Day 0 | 0.87 ± 0.05 | 0.88 ± 0.04 | 0.7–1.0 mmol/L | 0.29 | |
| Day 30 | 0.81 ± 0.04 | 0.91 ± 0.08 | −0.02 (95% CI of mean diff: −0.06–0.02) | ||
| Day 60 | 0.91 ± 0.04 | 0.85 ± 0.06 | |||
| Ionized magnesium (mmol/L) | |||||
| Day 0 | 0.58 ± 0.02 | 0.56 ± 0.04 | — | 0.27 | |
| Day 30 | 0.57 ± 0.04 | 0.58 ± 0.06 | −0.01 (95% CI of mean diff: −0.04–0.01) | ||
| Day 60 | 0.53 ± 0.03 | 0.57 ± 0.01 | |||
| Sodium (mEq/L) | |||||
| Day 0 | 151 ± 1 | 152 ± 1 | 148–155 mEq/L | — | |
| Day 30 | 153 ± 3 | 154 ± 2 | 1 (95% CI of mean diff: ‐1 – 3) | ||
| Day 60 | 150 ± 1 | 151 ± 1 | |||
| Potassium (mEq/L) | |||||
| Day 0 | 3.6 ± 0 | 4.2 ± 0.4 | 2.8–4.8 mEq/L | — | |
| Day 30 | 3.9 ± 0.3 | 4.1 ± 0.4 | −0.3 (95% CI of mean diff: −0.6–0) | ||
| Day 60 | 3.7 ± 0.3 | 3.8 ± 0.2 | |||
| Chloride (mEq/L) | |||||
| Day 0 | 115 ± 2 | 119 ± 2 | 113–123 mEq/L | — | |
| Day 30 | 117 ± 3 | 118 ± 2 | −2 (95% CI for mean diff: −4–0) | ||
| Day 60 | 116 ± 2 | 117 ± 2 | |||
| Blood | |||||
| Platelets (×103/uL) | |||||
| Day 0 | 365 ± 81 | 250 ± 22 | — | — | |
| Day 30 | 277 ± 109 | 256 ± 121 | 38 (95% CI for mean diff: −37–113) | ||
| Day 60 | 246 ± 65 | 269 ± 70 | |||
| Heinz bodies (%) | |||||
| Day 0 | 0.1 (0–0.2) * | 0.1 (0–0.3) * | — | — | — |
| Day 30 | 0.05 (0–0.2) * | 0.2 (0–0.4) * | — | ||
| Day 60 | 0.0 (0–0.1) * | 0.15 (0–0.4) * | — | ||
Effect of Omeprazole on Bone Homeostasis
For BMD, no significant mean differences were found between treatment phase or time of measurement, with P‐values of 0.52 (and mean difference between phases of 0.004 [95% CI: −0.02–0.03]) and 0.95, respectively (Fig 1). The interaction of treatment phase and time was also not significant (P = 0.24). The BMC was lower in all 6 cats after the 60‐day omeprazole treatment; however, this difference was not statistically significant (P = 0.092 with a mean difference between phases of 7.78 [95% CI: −20.27–35.82]) nor were there any significant differences between time of measurement or interaction of phase and time of measurement for BMC, with P‐values of 0.10 and 0.08, respectively.
Figure 1.
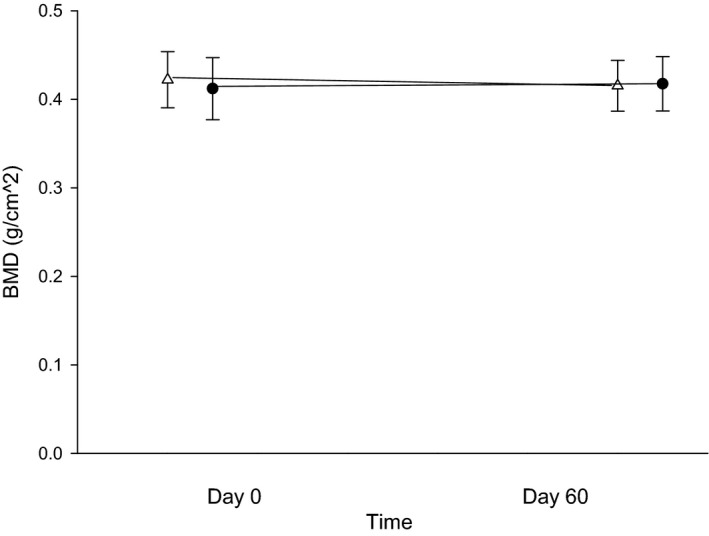
Bone mineral density (BMD) in g/cm2 between days 0 (before treatment) and 60 (after 8 weeks of treatment with either 250 mg lactose PO q12h or 5 mg omeprazole PO q12h). White triangles (placebo) and black circles (omeprazole) represent the mean ± SD of BMD for days 0 or 60 with each treatment arm.
Figure 2.
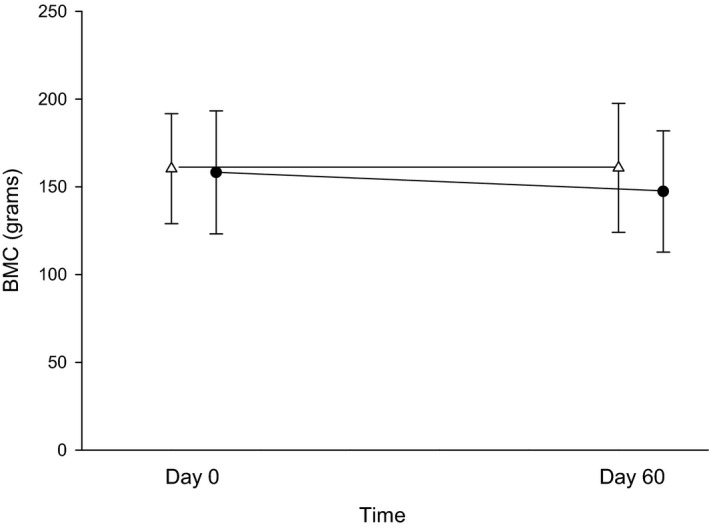
Bone mineral content (BMC) in grams between days 0 (before treatment) and 60 (after 8 weeks of treatment with either 250 mg lactose PO q12h or 5 mg omeprazole PO q12h) of each treatment arm. White triangles (placebo) and black circles (omeprazole) represent the mean ± SD of BMC for days 0 or 60 with each treatment arm.
Effect of Omeprazole on Serum Cobalamin
No significant changes were found between treatment phase and time of measurement for cobalamin (P = 0.12 with a mean difference between phases of 117 [95% CI: −259–493] and 0.95) or MMA (P = 0.60 with a mean difference between phases of −6.9 [95% CI: −28.6–14.9] and 0.26); Figs 3 and 4). The interaction of treatment phase and time for both variables did not reach significance (P = 0.01 day 30 for cobalamin and MMA, respectively).
Figure 3.
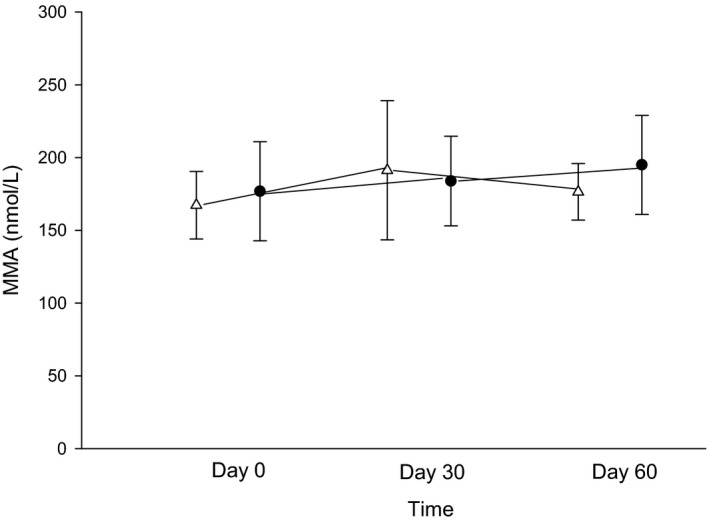
Serum methylmalonic acid concentrations (MMA; nmol/L) between days 0 (before treatment), 30 (after 4 weeks of treatment with either 250 mg lactose PO q12h or 5 mg omeprazole PO q12h), and 60 (after 8 weeks of treatment) of each treatment arm. White triangles (placebo) and black circles (omeprazole) represent the mean ± SD of serum MMA concentrations for days 0, 30, or 60 with each treatment arm.
Figure 4.
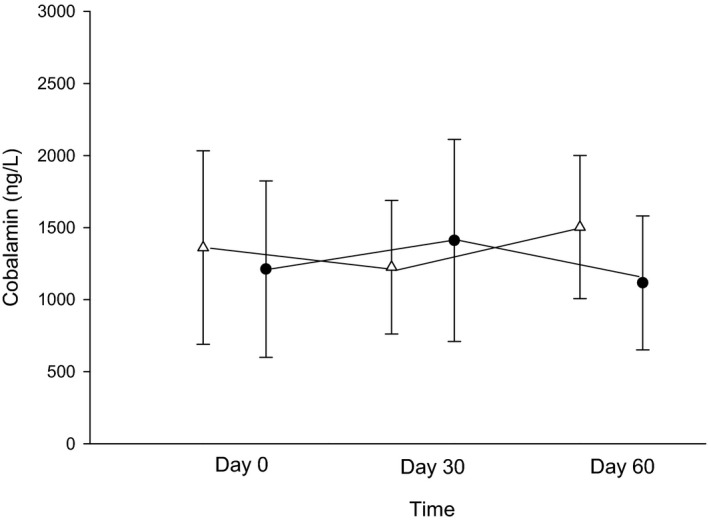
Serum cobalamin concentrations (ng/L) between days 0 (before treatment), 30 (after 4 weeks of treatment with either 250 mg lactose PO q12h or 5 mg omeprazole PO q12h), and 60 (after 8 weeks of treatment) of each treatment arm. White triangles (placebo) and black circles (omeprazole) represent the mean ± SD of serum cobalamin concentrations for days 0, 30, or 60 with each treatment arm.
Effect on Gastrin and Rebound Gastric Hyperacidity
The mean serum gastrin concentration during the omeprazole treatment phase (ie, days 30 and 60 of omeprazole treatment) was significantly higher than the mean serum gastrin concentration during the placebo phase (P = 0.002 with a mean difference between phases of −30 [95% CI: −57.3–2.6]; Fig 5).
Figure 5.
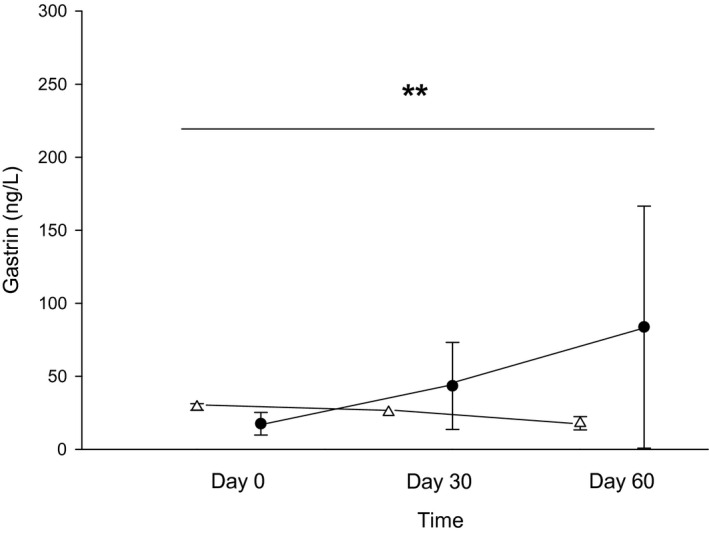
Serum gastrin concentrations (ng/L) between days 0 (before treatment), 30 (after 4 weeks of treatment with either 250 mg lactose PO q12h or 5 mg omeprazole PO q12h), and 60 (after 8 weeks of treatment) of each treatment arm. White triangles (placebo) and black circles (omeprazole) represent the mean ± SD of gastrin for days 0, 30, or 60 with each treatment arm. **Omeprazole (black circles) results in a significant increase in serum gastrin compared with placebo (white triangles) (P = 0.002).
To provide a preliminary evaluation for the possibility of rebound gastric hyperacidity following rapid omeprazole withdrawal, a sequela demonstrated to occur in humans following chronic PPI usage, continuous intragastric pH monitoring was performed in a subset of cats (n = 2) starting on the final day (day 60) of the omeprazole treatment phase. Continuous intragastric pH was measured for 5 days in one cat and 10 days in the second cat before the capsules passed uneventfully into the small intestine and eventually into the feces. In the previously published study of 6 healthy cats from the same colony, the daily mean ± SD intragastric pH was 2.0 ± 0.5.2 The lowest minimum pH in that study was 0.6. A rapid decline in mean intragastric pH sustained below what is observed for normal cats was detected on day 3 in cat 1 and day 2 in cat 2 (Fig 6). Moreover, both cats experienced a minimum intragastric pH (0.06 and 0.02, respectively) lower than that reported from cats in the previous study.
Figure 6.
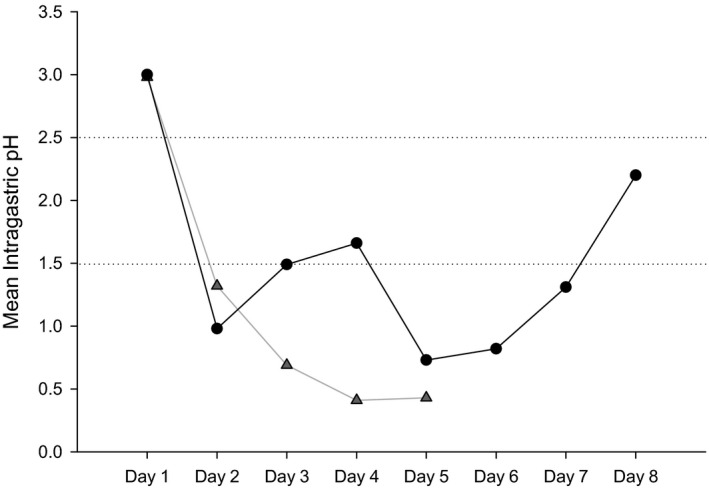
Intragastric pH in 2 cats (denoted by lines connected by gray triangles or black circles, respectively) for 8 and 5 days following cessation of omeprazole therapy. The mean ± SD intragastric pH (represented by the horizontal dashed lines) from cats (n = 6) in this colony receiving placebo in a previous study was 2.0 ± 0.5.2 Note that from days 2–5, cat 1 (gray triangles) had a mean intragastric pH more acidic than a normal cat and on days 2, 5, 6, and 7 the mean pH of cat 2 (black circles) was also below 1.5. The pH capsule passed from the stomach of cat 1 on day 6, so readings past day 5 were not evaluated.
Adverse Effects of Omeprazole Administration
No cats developed adverse effects during the placebo phase of the study. Clinical adverse effects of prolonged omeprazole administration in study cats were also uncommon. Only one cat developed intermittent hyporexia for 3 days and diarrhea (3 episodes of a fecal score13 of 5 and one episode of fecal score of 6) while receiving omeprazole treatment. Both adverse effects resolved with supportive care.
Discussion
Long‐term omeprazole treatment alters bone mineral homeostasis in middle‐aged to older patients (i.e., especially osteoporotic women).6, 7, 8 Although omeprazole is thought to be specific for inhibition of gastric parietal H+/K+‐ATPase pumps, evidence exists that PPIs may also exert inhibitory effects on bone and renal medullary ATPases, leading to altered bone homeostasis.18 In this study, we used DEXA technology to evaluate the effect of prolonged omeprazole treatment on BMD and BMC in cats. Although there were no significant differences detected between treatment arms for BMD or BMC, it is notable that all 6 study cats exhibited >1% decrease in BMC following a 60‐day course of omeprazole. Furthermore, more than half of the cats (n = 2 spayed females and 2 castrated males) exhibited a >8% decrease in their BMC following omeprazole treatment. Although BMD is the most common variable used to evaluate altered bone homeostasis in humans,19, 20 it could be that BMC could represent an earlier marker of altered bone homeostasis. Human studies evaluating the variability within and between DEXA machines have found low coefficients of variability (CV) for both BMD and BMC when looking at intra‐individual variability in repeated measures of a machine.21 A CV of <1% was found for both variables, with pooled averages of 0.42% and 0.4% across 9 readings, respectively. Although no similar studies have been performed evaluating the precision of DEXA in cats, it is reasonable to propose that values >1% might represent a real change in BMC (i.e., not attributable to machine or analyzer variability) following omeprazole administration. Given the small number of cats in this study, the lack of a statistically significant change in either BMD or BMC could represent a type II error. Further studies investigating markers of bone homeostasis in a larger population of cats receiving omeprazole are warranted.
Previously asymptomatic human patients who have received prolonged omeprazole therapy, ranging in duration from 8 weeks to 1 year, have experienced RAH and clinical signs of dyspepsia following abrupt PPI withdrawal.22, 23 This condition results in excess secretory capacity of hydrochloric acid from gastric parietal cells following abrupt withdrawal of acid suppressant therapy. The proposed mechanism behind an increased secretory capacity of parietal cells is that, during PPI therapy, an increased intragastric pH stimulates hypergastrinemia, which then leads to hyperplasia of the enterochromaffin‐like (ECL) cells.10, 11, 23 In turn, these ECL cells secrete increased amounts of histamine, which stimulates release of gastric acid from parietal cells. In our study, omeprazole treatment resulted in a significant increase in serum gastrin compared with placebo. Five of the six study cats had an increase in serum gastrin concentration by day 30 of omeprazole treatment. Moreover, all 6 study cats had an increased serum gastrin concentration by day 60 of omeprazole treatment. As intragastric pH monitoring was not part of the original study design and we did not have enough receivers to measure the effects of omeprazole withdrawal in all 6 cats, we only evaluated for the possible effect of hypergastrinemia and abrupt omeprazole withdrawal on pH in 2 study cats. However, the mean intragastric pH in both study cats dropped and was maintained below what has been reported for normal cats for several days after withdrawal of omeprazole. Although further studies with larger populations of cats will be needed in order to draw any definitive conclusions, these preliminary results suggest that prolonged PPI treatment results in hypergastrinemia and abrupt PPI withdrawal may result in RAH in cats.
Data from studies evaluating the potential for adverse effects in humans receiving prolonged omeprazole treatment are heterogeneous, likely as a result of a large variation in subject age, environment and diet, cytochrome P450 genotype, and concurrent medications and diseases. This study included a small group of healthy cats with no known history of GI or systemic disease. All cats lived in the same colony, received a similar maintenance diet, were administered medications at consistent times, and were of the same age subset. Thus, our study design eliminated many variables that are not easily controlled in studies using human subjects. We chose to evaluate the effect of 60 days of treatment as this is the time at which biochemical and bone alterations have been demonstrated in other studies6, 24, 25, 26, 27, 28, 29, 30, 31 and the authors considered this to be an average length of time in which a cat would receive PPI treatment for esophagitis, gastric erosion, and/or gastric ulceration. In this study, no cats had a statistically significant decrease in serum concentrations of magnesium, calcium, cobalamin, MMA, BMD, or BMC after 30 or 60 days of omeprazole treatment. Moreover, only one cat developed intermittent hyporexia and diarrhea that resolved with supportive care. Although the small sample size limits our ability to broadly extrapolate findings to the domestic feline population, results of this pilot study suggest that oral omeprazole, at a median dose of 1.1 mg/kg, might be relatively safe for healthy cats at courses ≤8 weeks in duration.
Acknowledgments
The authors thank Randy Buddington, Dr. Adesola Odunayo, and Dr. Angela Witzel for their technical support. This study was made possible through financial support from the University of Tennessee Fund for Companion Animal Research.
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This work was done at the University of Tennessee, College of Veterinary Medicine in Knoxville, Tennessee.
Footnotes
Lactose 250 mg encapsulated in size #3 gelatin capsule, Spectrum Chemical Mfg Corp, Gardena, CA.
Omeprazole tablet, Dexcel Pharma Technologies Ltd, Yokneam, Israel.
Hill's Science Diet, Hill's Nutrition, Topeka, KS
Stat Profile pHOx Ultra, Nova Biomedical©
Cobas C501 Chemistry Analyzer, Roche Diagnostics, Indianapolis, IN, USA
Advia 120 Hematology System, Siemens, Malvern, PA, USA
Immulite 2000, Siemens Healthcare Diagnostics, Malvern, PA, USA
Dexdomitor 0.5 mg/mL injection, Orion Pharma, Espoo, Finland
Ketacine, 100 mg/mL injection, VetOne, Boise, ID
Torbugesic, 10 mg/mL injection, Fort Dodge Animal Health, Fort Dodge, IA
QDR 4500 Acclaim Series Elite, Hologic, Apex Software Version 2.3, Lawrenceville, GA, USA
Antisedan 5 mg/mL injection, Orion Pharma, Espoo, Finland
Fecal Scoring System Nestle Purina PetCare Company, St. Louis, MO
Bravo pH monitoring system, Given Imaging, Yoqneam, Israel
Polygram Net Software, Given Imaging, Yokneam, Israel
SAS 9.4, SAS Institute Inc, Cary, NC
References
- 1. Tolbert K, Bissett S, King A, et al. Efficacy of oral famotidine and 2 omeprazole formulations for the control of intragastric pH in dogs. J Vet Intern Med 2011;25:47–54. [DOI] [PubMed] [Google Scholar]
- 2. Parkinson S, Tolbert K, Messenger K, et al. Evaluation of the effect of orally administered acid suppressants on intragastric pH in cats. J Vet Intern Med 2014;29:104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sutalo S, Ruetten M, Hartnack S, et al. The effect of orally administered ranitidine and once‐daily or twice‐daily orally administered omeprazole on intragastric pH in cats. J Vet Intern Med 2015;29:840–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Williamson KK, Willard MD, Payton ME, et al. Efficacy of omeprazole versus high‐dose famotidine for exercise‐induced gastritis in racing alaskan sled dogs. J Vet Intern Med 2010;24:285–288. [DOI] [PubMed] [Google Scholar]
- 5. Tolbert MK, Odunayo A, Howell RS, et al. Efficacy of intravenous administration of combined acid suppressants in healthy dogs. J Vet Intern Med 2015;29:556–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maggio M, Lauretani F, Ceda GP, et al. Use of proton pump inhibitors is associated with lower trabecular bone density in older individuals. Bone 2013;57:437–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Targownik LE, Lix LM, Metge CJ, et al. Use of proton pump inhibitors and risk of osteoporosis‐related fractures. Can Med Assoc J 2008;179:319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moberg LM, Nilsson PM, Samsioe G, et al. Use of proton pump inhibitors (PPI) and history of earlier fracture are independent risk factors for fracture in postmenopausal women. The WHILA study. Maturitas 2014;78:310–315. [DOI] [PubMed] [Google Scholar]
- 9. Abrahamsen B, Vestergaard P. Proton pump inhibitor use and fracture risk – effect modification by histamine H1 receptor blockade. Observational case‐control study using National Prescription Data. Bone 2013;57:269–271. [DOI] [PubMed] [Google Scholar]
- 10. Waldum HL, Qvigstad G, Fossmark R, et al. Rebound acid hypersecretion from a physiological, pathophysiological and clinical viewpoint. Scand J Gastroenterol 2010;45:389–394. [DOI] [PubMed] [Google Scholar]
- 11. Fossmark R, Johnsen G, Johanessen E, et al. Rebound acid hypersecretion after long‐term inhibition of gastric acid secretion. Aliment Pharmacol Ther 2005;21:149–154. [DOI] [PubMed] [Google Scholar]
- 12. Shabajee N, Lamb EJ, Sturgess I, et al. Omeprazole and refractory hypomagnesaemia. BMJ (Clinical research ed) 2008;337:a425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buon M, Gaillard C, Martin J, et al. Risk of proton pump inhibitor‐induced mild hyponatremia in older adults. J Am Geriatr Soc 2013;61:2052–2054. [DOI] [PubMed] [Google Scholar]
- 14. Ruaux CG, Steiner JM, Williams DA. Metabolism of amino acids in cats with severe cobalamin deficiency. Am J Vet Res 2001;62:1852–1858. [DOI] [PubMed] [Google Scholar]
- 15. Tvedten H, Moritz A. Reticulocyte and Heinz body staining and enumeration In: Weiss DJ, Wardrop KJ, eds. Schalm's Veterinary Hematology, 6th ed Ames, IA: Wiley‐Blackwell; 2010:1067–1072. [Google Scholar]
- 16. Bartges JW, Kirk CA, Cox SK, et al. Influence of acidifying or alkalinizing diets on bone mineral density and urine relative supersaturation with calcium oxalate and struvite in healthy cats. Am J Vet Res 2013;74:1347–1352. [DOI] [PubMed] [Google Scholar]
- 17. Mizunashi K, Furukawa Y, Katano K, et al. Effect of omeprazole, an inhibitor of H+, K(+)‐ATPase, on bone resorption in humans. Calcif Tiss Int 1993;53:21–25. [DOI] [PubMed] [Google Scholar]
- 18. Mattsson JP, Vaananen K, Wallmark B, et al. Omeprazole and bafilomycin, two proton pump inhibitors: Differentiation of their effects on gastric, kidney and bone H(+)‐translocating ATPases. Biochim Biophys Acta 1991;1065:261–268. [DOI] [PubMed] [Google Scholar]
- 19. Kanis JA, McCloskey EV, Johansson H, et al. A reference standard for the description of osteoporosis. Bone 2008;42:467–475. [DOI] [PubMed] [Google Scholar]
- 20. Kling JM, Clarke BL, Sandhu NP. Osteoporosis prevention, screening, and treatment: A review. J Womens Health 2002;2014(23):563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rencken ML, Murano R, Drinkwater BL, et al. In vitro comparability of dual energy X‐ray absorptiometry (DEXA) bone densitometers. Calcif Tissue Int 1991;48:245–248. [DOI] [PubMed] [Google Scholar]
- 22. Reimer C, Sondergaard B, Hilsted L, et al. Proton‐pump inhibitor therapy induces acid‐related symptoms in healthy volunteers after withdrawal of therapy. Gastroenterology 2009;137:80–87. [DOI] [PubMed] [Google Scholar]
- 23. Waldum HL, Arnestad JS, Brenna E, et al. Marked increase in gastric acid secretory capacity after omeprazole treatment. Gut 1996;39:649–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. O'Connell MB, Madden DM, Murray AM, et al. Effects of proton pump inhibitors on calcium carbonate absorption in women: A randomized crossover trial. Am J Med 2005;118:778–781. [DOI] [PubMed] [Google Scholar]
- 25. Marcuard SP, Albernaz L, Khazanie PG. Omeprazole therapy causes malabsorption of cyanocobalamin (vitamin B12). Ann Intern Med 1994;120:211–215. [DOI] [PubMed] [Google Scholar]
- 26. Melville C, Shah A, Matthew D, et al. Electrolyte disturbance with omeprazole therapy. Eur J Pediat 1994;153:49–51. [DOI] [PubMed] [Google Scholar]
- 27. Shiba S, Sugiura K, Ebata A, et al. Hyponatremia with consciousness disturbance caused by omeprazole administration. A case report and literature review. Dig Dis Sci 1996;41:1615–1617. [DOI] [PubMed] [Google Scholar]
- 28. Davidson S, Seldon M, Jones B. Omeprazole and Heinz‐body haemolytic anaemia. Aust N Z J Med 1997;27:441. [DOI] [PubMed] [Google Scholar]
- 29. Binnetoglu E, Akbal E, Sen H, et al. Pantoprazole‐induced thrombocytopenia in patients with upper gastrointestinal bleeding. Platelets 2015;26:10–12. [DOI] [PubMed] [Google Scholar]
- 30. Kallam A, Singla A, Silberstein P. Proton pump induced thrombocytopenia: A case report and review of literature. Platelets 2015;26:598–601. [DOI] [PubMed] [Google Scholar]
- 31. Epstein M, McGrath S, Law F. Proton‐pump inhibitors and hypomagnesemic hypoparathyroidism. N Engl J Med 2006;355:1834–1836. [DOI] [PubMed] [Google Scholar]


