Abstract
Background
Heaves is a severe debilitating condition of horses, characterized by lower airway inflammation and permanent structural changes of the bronchial wall. Chronic inflammation promotes the formation of new vessels, a phenomenon known as angiogenesis. Narrow band imaging (NBI) endoscopy is a noninvasive technique that enhances the visualization of submucosal vessels, and commonly is employed for the study of angiogenesis in human patients.
Objectives
Using NBI, we aimed to determine whether or not the central airways of horses with heaves undergo angiogenesis.
Animals
Horses with heaves during exacerbation of the disease (n = 5) and healthy controls (n = 6).
Methods
A library of NBI images was established from previously recorded videoendoscopies. Images were acquired by an operator blinded to horse ID. Images were obtained from 3 sites: 130 from the trachea (14 ± 9.3 [mean ± SD] images per horse with heaves and 10 ± 5.4 from controls; P = .45), 58 from the carina (5.4 ± 3.2 from horses with heaves and 5.2 ± 2.8 fromn controls; P > .99) and 167 from the intermediate bronchi (17.8 ± 6.7 from horses with heaves and 13 ± 5.6 from controls; P = .17). Using dedicated stereology software (NewCAST, Visiopharm; Denmark), the volume density of superficial and deep vessels was calculated blindly by point counting at each site for all horses.
Results
In the trachea, the volume density of superficial vessels was increased in horses with heaves compared to controls (P = .02). No difference was found between groups for the volume density of both superficial and deep vessels at the carina or intermediate bronchi.
Conclusion and Clinical Relevance
NBI imaging of the airways was easily performed in standing sedated horses.
Keywords: Blood vessels, Equine Asthma, Recurrent airway obstruction
Abbreviations
- NBI
narrow band imaging
- ROI
region of interest
Heaves, also known as recurrent airway obstruction, is a disease of horses characterized by episodes of respiratory distress at rest accompanied by neutrophilic pulmonary inflammation during exacerbations. This condition affects approximately 10–15% of adult horses >5–7 years of age in the northern hemisphere.1 Exacerbations are triggered by exposure to environmental antigens, and can be reversed by enhancing environmental control or using steroids, bronchodilators or both.
Airway remodeling1, 2, 3 leads to the thickening of the bronchial wall, contributing to airflow obstruction in heaves. Hyperplasia and hypertrophy of airway myocytes and fibrosis of the lamina propria are mainly involved in this process.2 However, angiogenesis develops concurrently with inflammation and remodeling in human with asthma,4 increasing bronchial wall thickness, and therefore could be present in horses with heaves given the similarities between these conditions.1 If present, targeting new blood vessel formation could represent a possible strategy to control inflammation and remodeling in heaves.
Angiogenesis is observed both in physiological (eg, embryonic development, exercise, scarring) and pathological (eg, tumor growth, inflammation) conditions.4, 5, 6, 7 It has been quantified using several methods. Among these, narrow band imaging (NBI) is a recently developed, noninvasive technique allowing an enhanced visualization of submucosal vessels during endoscopy. It employs filters producing light of specific blue and green wavelengths instead of the white light used during conventional endoscopy, which correspond to the peak light absorption of hemoglobin.5 This technique facilitates visualizing blood vessels because they appear as contrasting structures (black or dark green, depending on their depth within the bronchial tissue) beneath the clear bronchial mucosa.
Despite the availability of new imaging techniques and the likelihood of angiogenesis contributing to remodeling in heaves, no study has attempted to quantify airway blood vessels in this condition. We hypothesized that NBI would allow identification of an increased density of blood vessels (ie, angiogenesis) in the airways of horses with heaves compared to controls.
Materials and Methods
Animals and Study Design
A library of NBI images was established from videoendoscopies previously recorded. Additional details are provided elsewhere.3 Eleven horses were studied (5 with heaves and 6 controls of similar age and weight) using an observational study design. Horses with heaves were part of our research herd and had a documented history of abnormal lung function when exposed to hay. The control horses were part of the teaching herd at our faculty. They were determined to be healthy based on history, physical examination and endoscopic evaluation of the respiratory tract. All horses were stabled and exposed to hay starting 2 weeks before the study period, in order to induce exacerbations of the disease in affected animals.
Bronchoscopy
Horses were sedated using detomidine (0.012 mg/kg IV) and butorphanol (0.01 mg/kg IV). An endoscope (13 mm Ø, CF‐H180AL1) was passed through the ventral meatus of a nostril. Once past the larynx, lidocaine solution (0.5%) was instilled as needed to topically anesthetize the airways and prevent coughing episodes. Lower airway endoscopy of 1 randomly selected lung was performed in each horse. Endoscopies were performed using the NBI mode while advancing from the larynx toward the most distal bronchial site, and recorded on a computer in order to be analyzed retrospectively.
Selection of Images
Images of the bronchial mucosa (tiff files, 1280 × 800 pixels) were acquired by an operator blinded to horse ID. Images were acquired when the submucosal vessels were clearly visible. Images in which mucus covered a considerable part of the bronchial mucosa were excluded. Images were obtained from 3 sites (Fig 1A): trachea, carina and between the 2nd and 9th branches of a main caudal bronchus). Each image was renamed and the order of analysis mixed by someone different from the person who conducted the morphometry using the stereology software.
Figure 1.
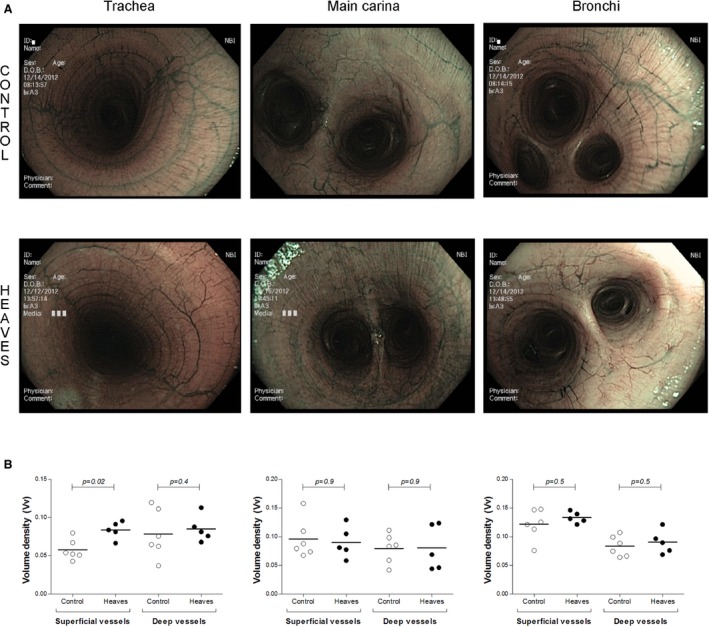
(A) Narrow Band Imaging images of the trachea, the main carina and bronchi of control (upper panels) and affected horses (lower panels). (B) Vessel area densities in the trachea (left), the main carina (center) and the bronchi (right). Each diagram has been divided into superficial and deep vessels.
Angiogenesis Assessment
Vascularity was blindly assessed with NewCast software version 4.5.1.324.2 A region of interest (ROI) was defined for each image by someone who was blinded to the group in order to exclude the darkest area of the image and the regions where accumulated mucus prevented a reliable assessment of submucosal vascularity. Superficial and deep vessels were differentiated based on their color. Superficial vessels appeared black, whereas deep vessels appeared dark green during NBI endoscopy. Vascular density was assessed by point counting using grids with 256 crosses per screen, because this point density allowed reliable estimation of the vascular density in endoscopic images (data not shown). Blood vessel density was calculated for each horse as follow: Vvessel = ΣPvessel/ΣPbronchial wall, where ΣPvessel indicates the sum of the points falling onto the vessels and ΣPbronchial wall the sum of the points falling onto the bronchial wall in each image.
Statistical Analysis
Analyses were performed using Prism 5.3 Nonparametric Mann–Whitney tests were used to compare the 2 groups. The level of statistical significance was set at P < .05.
Results
A total of 130 images for the trachea (14 ± 9.3 [mean ± SD] images per horse with heaves and 10 ± 5.4 for controls; P = .45), 58 images for the carina (5.4 ± 3.2 for horses with heaves and 5.2 ± 2.8 for controls; P > .99), and 167 images for intermediate bronchi (17.8 ± 6.7 for horses with heaves and 13 ± 5.6 for controls; P = .17) were obtained. The number of images analyzed in the 2 groups was similar (mean ± SD, 37 ± 17.9 images per horse with heaves and 28 ± 13.3 in the control group; P = .62) and for each site.
No significant differences (Fig 1B) were observed for the number of deep vessels in trachea (P = .43), carina (P = .93), and bronchi (P = .53) between the 2 groups. Superficial vessel density was not different between the 2 groups at the level of the carina (P = .93) and the bronchi (P = .53). Horses with heaves, however, had significantly increased tracheal superficial vessel density (P = .02) compared to controls.
Discussion
Angiogenesis consists of the formation of new vessels (especially venules) from the pre‐existing vascular tree, forming a capillary‐like network. Many pathological conditions are associated with angiogenesis, presumably to provide adequate amounts of oxygen to tissues with increased metabolism, such as growing, inflamed, or neoplastic tissues. Contrary to what we hypothesized, no increase in vascular density of the bronchial tree was observed in heaves‐affected horses compared to controls using NBI, whether evaluating superficial or deep vessels. The only significant difference observed was for superficial vessels of the trachea. These results suggest that either angiogenesis is only a tracheal feature in horses with heaves or that NBI is not sufficiently sensitive to evaluate angiogenesis occurring in the bronchi of affected horses.
Angiogenesis has been quantified using several methods. Unfortunately, most of these assays have limited clinical application because of their cost and invasiveness. Airway angiogenesis was first studied in endobronchial biopsies of asthmatic patients in research settings. It was shown to be associated with disease severity in children with asthma.4, 6 In recent years, several noninvasive imaging techniques have been developed and used as tools to evaluate angiogenesis.8 Of particular interest, high magnification endoscopy provides an adequate assessment of the submucosal vascular network in asthmatic patients.7 Narrow band imaging allows differentiation of blood vessels based on their depth, by making superficial blood vessels appear as brown or black and deep vessels as blue or green structures under the bronchial mucosa. It is used primarily in gastroenterology (eg, adenomas in laparoscopy, inflammatory bowel disease, Barrett's esophagus), urology (eg, bladder tumors, cystitis), and less frequently in pneumology5 (during thoracoscopy for assessment of angiogenesis in lung tumors).
In this study, NBI was easily performed on sedated standing horses during routine bronchoscopy. Our results identified an increase in tracheal superficial vascularity in horses with heaves compared to controls. A similar finding has been reported in human asthmatic patients,7 but the clinical relevance of this observation is uncertain. The presence of luminal tracheal inflammatory mediators in heaves possibly could contribute to the increased vascularity we observed in these animals.
Administration of α2‐agonists results in activation of peripheral receptors (α1 and α2) on the blood vessels leading to vasoconstriction, thus possibly affecting NBI measurements. However, although these effects were reported to be ≤ 8 minutes in duration,9 NBI was performed between 8 and 10 minutes after the first injection, thus likely minimizing this phenomenon. Also, differences between groups were observed only in the trachea, and we would have expected to see an effect at all sites if the sedation had an impact on blood vessel measurements.
An increase in the vascular network density in the bronchi of horses with heaves was not observed, unlike what is reported in human medicine.4, 6, 7 Alternatively, angiogenesis might be present only during the establishment of the disease or a period of 2 weeks of antigen exposure may not have been long enough to generate angiogenesis. Finally, edema in the respiratory tract, bronchospasm, and mucus might have prevented optimal visualization of bronchial blood vessels in these horses.
Airways have a tubular shape, and endoscopic images were obtained with the camera oriented longitudinally to their axis. Some area of the tracheobronchial mucosal might have been analyzed twice on different images, introducing a bias in our results. However, this is unlikely to have occurred in our study for 2 reasons. First, when generating the library, care was taken to avoid selecting images in which overlapping mucosal fields could be identified (for trachea and bronchi). Second, during analysis, only the mucosal areas proximal to the endoscope had adequate conditions of brightness and contrast and therefore were included in the ROI. An additional bias could have resulted from the analysis of a 3‐dimensional image using markers arranged in a bidimensional grid that does not take into account the image perspective. We believe that the bias introduced by our approach, if it occurred, would have been similar in both groups because airway anatomy (dimensions) was very similar among the horses we studied.
In conclusion, evaluation of airway vascularity with NBI technology is easily performed in standing sedated horses. The clinical relevance of the increased tracheal vascularity observed in heaves‐affected horses remains to be ascertained.
Acknowledgments
Funding sources: The videoendoscopies analyzed in this study originated from horses enrolled in a previous study that investigated the physiological and technical variables affecting the quality of equine endobronchial biopsy samples (see Reference 3).
This study was supported by grants from the Canadian Institutes of Health Research (#MOF102751) and Canadian Foundation for Innovation (#29172).
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Footnotes
Olympus; Richmond Hill, ON, Canada
NewCAST, Visiopharm; Denmark
GraphPad Software Inc; CA, USA
References
- 1. Leclere M, Lavoie‐Lamoureux A, Lavoie JP. Heaves, an asthma‐like disease of horses. Respirology 2011;16:1027–1046. [DOI] [PubMed] [Google Scholar]
- 2. Leclere M, Lavoie‐Lamoureux A, Gelinas‐Lymburner E, et al. Effect of antigenic exposure on airway smooth muscle remodeling in an equine model of chronic asthma. Am J Respir Cell Mol Biol 2011;45:181–187. [DOI] [PubMed] [Google Scholar]
- 3. Bullone M, Chevigny M, Allano M, et al. Technical and physiological determinants of airway smooth muscle mass in endobronchial biopsy samples of asthmatic horses. J Appl Physiol (1985) 2014;117:806–815. [DOI] [PubMed] [Google Scholar]
- 4. Li X, Wilson JW. Increased vascularity of the bronchial mucosa in mild asthma. Am J Respir Crit Care Med 1997;156:229–233. [DOI] [PubMed] [Google Scholar]
- 5. Zaric B, Perin B, Stojsic V, et al. Relation between vascular patterns visualized by Narrow Band Imaging (NBI) videobronchoscopy and histological type of lung cancer. Med Oncol 2013;30:374. [DOI] [PubMed] [Google Scholar]
- 6. Barbato A, Turato G, Baraldo S, et al. Epithelial damage and angiogenesis in the airways of children with asthma. Am J Respir Crit Care Med 2006;174:975–981. [DOI] [PubMed] [Google Scholar]
- 7. Tanaka H, Yamada G, Saikai T, et al. Increased airway vascularity in newly diagnosed asthma using a high‐magnification bronchovideoscope. Am J Respir Crit Care Med 2003;168:1495–1499. [DOI] [PubMed] [Google Scholar]
- 8. Rissanen TT, Korpisalo P, Karvinen H, et al. High‐resolution ultrasound perfusion imaging of therapeutic angiogenesis. JACC Cardiovasc Imaging 2008;1:83–91. [DOI] [PubMed] [Google Scholar]
- 9. Clarke KW, Paton BS. Combined use of detomidine with opiates in the horse. Equine Vet J 1988;20:331–334. [DOI] [PubMed] [Google Scholar]


