Abstract
Background
Hypertension and albuminuria often coexist in Greyhounds, suggesting generalized vascular dysfunction that could contribute to the development of a variety of diseases in this breed. Eicosanoid metabolites of arachidonic acid (AA) mediate endothelial function, vascular reactivity, and proteinuria in humans and in rodent models.
Hypothesis
The eicosanoid profile of Greyhounds is shifted toward metabolites that promote vascular dysfunction, hypertension, and proteinuria.
Animals
Healthy Greyhounds (n = 20) and non‐Greyhound (n = 20) dogs that were consecutively enrolled in a blood donor program.
Methods
Prospective study. Plasma eicosanoid metabolites were assayed by liquid chromatography/electrospray ionization mass spectrometry (LC/ESI/MS) and compared to systolic blood pressure (SP) measurements and urine albumin concentration.
Results
Isomers of hydroxyeicosatetraenoic acid (HETE) were higher in Greyhounds than non‐Greyhounds (median, range in pmol/mL: 5(S)HETE 19.82, 8.55–32.95 versus 13.54, 4.33–26.27, P = .033; 8(S)HETE 9.39, 3.28–19.84 versus 5.80, 2.25–17.66, P = .002; 9(S)HETE 9.46, 2.43–13.79 versus 5.82, 1.50–17.16, P = .026; 12(S)HETE 10.17, 3.81–40.06 versus 7.24, 2.9–16.16, P = .022). Dihydroxyeicosatrienoic acid (DHET) isomers also were higher in Greyhounds compared to non‐Greyhounds (mean ± SD in pmol/mL: 8,9DHET 5.78 ± 2.13 versus 4.03 ± 1.36, P = .004; 11,12DHET 11.98 ± 2.86 versus 8.90 ± 3.48, P = .004; 14,15DHET 7.23 ± 2.19 versus 5.76 ± 1.87, P = .028). Albuminuria correlated with total DHET (rs = 0.46, P = .003). SP was positively correlated with 11,12EET (rs = 0.42, P = .006) and 20(S)HETE (rs = 0.38, P = .017). SP and 8,9EET were inversely correlated (rs = −0.49, P = .001).
Conclusions and Clinical Importance
Plasma eicosanoid profile in Greyhounds was consistent with activation of metabolic pathways known to promote vascular dysfunction and might contribute to higher blood pressures and albuminuria. Inhibition of these eicosanoid pathways should be evaluated as therapeutic targets in Greyhounds.
Keywords: Arachidonic acid, Cytochrome P450, Hypertension, Microalbuminuria
Abbreviations
- AA
arachidonic acid
- CRGV
cutaneous and renal glomerular vasculopathy
- CYP450
cytochrome P450
- DHET
dihydroxyeicosatrienoic acid
- EET
epoxyeicosatrienoic acid
- HETE
hydroxyeicosatetraenoic acid
- Hode
hydroxyoctadecadienoic acid
- HUS
hemolytic uremic syndrome
- LC/ESI/MS
liquid chromatography/electrospray ionization mass spectrometry
- LOX
lipoxygenase
- MRM
multiple reaction monitoring
- sEH
soluble epoxide hydrolase
- SP
systolic blood pressure
Greyhounds have an increased prevalence of conditions in which vascular dysfunction is part of disease pathogenesis. Greyhounds are predisposed to ischemic stroke and have an increased prevalence of postsurgical bleeding unassociated with apparent primary or secondary hemostatic defects.1, 2 Cutaneous and renal glomerular vasculopathy (CRGV) in Greyhounds is a form of thrombotic microangiopathy characterized by initial vascular lesions that include endothelial cell swelling, detachment, and microthrombosis.3 Interestingly, kidney disease accounts for approximately 8% of deaths in retired racing Greyhounds, with proteinuria and hypertension being common.4, 5
Vascular dysfunction is characterized by impaired vasomotor response, increased vascular permeability, endothelial cell proliferation, inflammation, and platelet adhesion and aggregation.6 Hypertension and loss of glomerular permselectivity are recognized manifestations of generalized endothelial dysfunction in people and are predictors of increased risk for cardiovascular dysfunction, progressive renal disease, ischemic heart disease, stroke, and thrombotic microangiopathy.7, 8, 9, 10 In dogs, hypertension and albuminuria simultaneously can occur with a variety of diseases, and albuminuria is a marker of glomerular disease,11 whereas, hypertension could be both as a consequence and cause of kidney dysfunction.12, 13, 14 Greyhounds have higher blood pressures15, 16, 17, 18 and exhibit a tendency to develop albuminuria,5 consistent with generalized vascular dysfunction. Given these potentially vascular‐associated abnormalities in Greyhounds, altered baseline concentrations of vasoactive or vasoprotective mediators should be considered as possible predisposing factors to vascular‐based diseases in this breed.
Arachidonic acid (AA) metabolites modulate endothelial function and vascular reactivity in naturally occurring human cardiovascular and renal diseases and their corresponding rodent models. Epoxyeicosatrienoic acids (EET) are synthesized from AA by endothelial cytochrome P450 (CYP450) expoxygenases.19, 20 Dihydroxyeicosatrienoic acids (DHET) are in turn produced when EETs are hydrolyzed by soluble epoxide hydrolase (sEH) (Fig 1).19, 20 EETs have vasoprotective properties, including vasodilatory, antihypertensive, anti‐inflammatory, proangiogenic, and renoprotective effects, whereas DHETs are less protective.19 An alternative CYP450 hydrolase pathway produces 20‐hydroxyeicosatetraenoic acid (20(S)HETE), a potent vasoconstrictor, regulator of glomerular function and prohypertensive agent.20 AA also can be metabolized by lipoxygenase pathways, resulting in the production of additional HETE isomers, which are vasoconstrictive and proinflammatory, and have been implicated in renal and cerebrovascular disease and the development of colon, prostate, and lung cancer.21, 22 Little information regarding AA metabolic pathways exist in the Greyhound; however, slower drug metabolism in Greyhounds might relate to breed specific differences in CYP450 activity.23, 24
Figure 1.
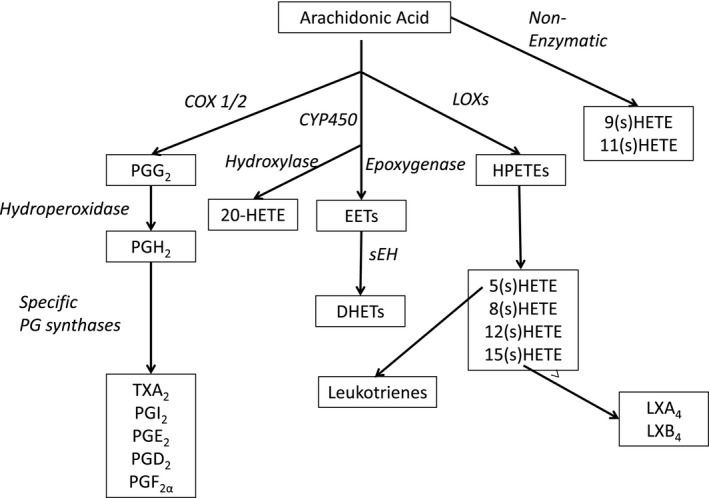
Pathways of arachidonic acid metabolism. COX, cyclooxygenase; LX, lipoxin; PG, prostaglandin; for other abbreviations, please see abbreviation list.
The objectives of this study were to evaluate AA metabolic products, blood pressure, and urine albumin concentration in a group of retired, nonracing Greyhounds versus a control group of non‐Greyhound dogs enrolled in a blood donor program. We hypothesized that the eicosanoid metabolite profile of Greyhounds would be consistent with a shift away from vasoprotective metabolites. Higher concentrations of AA metabolites associated with inflammation, endothelial dysfunction, hypertension, and proteinuria could partially explain the predisposition to develop diseases associated with vascular dysfunction observed in the breed.
Materials and Methods
The study was conducted in accordance with the guidelines of the Animal Care and Use Committee of The Ohio State University and with informed consent of the owners. Dogs consecutively enrolled in The Ohio State University Veterinary Medical Center Animal Blood Bank donor program over a 2‐month period were eligible for inclusion. Eligibility for the blood donor program was limited to dogs between 1 and 7 years of age, weighing >25.0 kg, and which tested negative for blood‐borne diseases; dogs were excluded if they had ever received a blood transfusion. No attempt was made to control for diet. Owners were instructed to fast dogs for 12 hours before evaluation. All dogs underwent complete physical exams, CBC1 with a manual differential white blood cell count, serum biochemistry profile,2 and urinalyses. Dogs were excluded if any clinically relevant abnormalities were detected or if insufficient volume of blood or urine was collected for all required tests.
Blood Pressure Measurement
Dogs were acclimated to the clinic environment for a minimum of 5 minutes before performing blood pressure measurement, and measurements were taken before physical examination or other sample collection. Measurements were obtained with dogs lightly restrained in right lateral recumbency using an oscillometric blood pressure monitor3 with a cuff size approximately 40% of limb circumference on the left pelvic limb, as previously reported.25 Blood pressure values were determined by averaging 5 systolic (SP), diastolic, and mean arterial oscillometric blood pressure readings.
Urine Collection and Evaluation
Midstream voided urine samples were collected after blood pressure measurement. A minimum of 7 mL were required for analysis. Routine urinalysis was first performed on 1‐mL aliquots; dogs were excluded from study enrollment if >3 leukocytes or >3 red blood cells per high‐power field were noted on sediment examination. The remaining 6 mL of urine were centrifuged to remove sediment and frozen at −80°C. Urine albumin concentration was determined by a commercial laboratory.4
Blood Collection and Eicosanoid Analysis
Six milliliter of lithium heparin anticoagulated blood was collected by jugular venipuncture with a butterfly catheter for AA metabolite assays. Blood tubes were placed on ice immediately after collection, and plasma separated within 1 hour of collection. All plasma samples were stored at −80°C until time of analysis.
Plasma eicosanoid concentrations were measured by liquid chromatography/electrospray ionization mass spectrometry (LC/ESI/MS)5 as previously described.26, 27, 28 Dog plasma (200 μL) was added to 700 μL of buffer (0.1 m NaH2PO4, 0.9% NaCl, 2.5 mm deferoxamine, pH 5) plus 100 μL of 0.1% butylated hydroxytoluene, then spiked with a deuterated internal standard (0.5 ng/μL × 10 μL). The samples were extracted by the Bligh‐Dyer technique, dried under a stream of nitrogen, and reconstituted in 100‐μL ethanol. A binary system set at a flow rate of 0.3 mL/min executed a gradient elution with 8.3 mm acetic acid, pH 5.7 with ammonium hydroxide (mobile phase A), and acetonitrile:2‐propanol (50 : 50) (mobile phase B) as follows: 3 minutes hold at 15% B, 10 minutes linear to 55% B, 15 minutes linear to 80% B, 5 minutes wash at 100% B, 7 minutes re‐equilibration at 15% B on a Zorbax SB‐C18 Narrow Bore column (2.1 × 100 mm, 5 micron) with a corresponding guard column at 40°C. The injection volume was 20 μL. Analysis was performed using multiple reaction monitoring (MRM). Individual calibration curves were generated for each analyte, and sample concentrations quantified by calculations from an external standard curve ranging from 0 to 2 ng/μL.
Metabolites measured included EET and DHET isomers, leukotrienes (B4, C4, D4, E4), lipoxin A4, prostaglandins (F2‐α, E2, and D2), thromboxane B2, 8‐iso‐prostaglandin F2‐α, hydroxyoctadecadienoic acids (9S‐ and 13(S)Hode), and hydroxyeicosatetraenoic acids (5(S)‐, 8(S)‐, 9(S)‐, 11(S)‐, 12(S)‐, 15(S)‐, and 20(S) HETE). A positive control standard solution was prepared containing all analytes at a concentration of 1 ng/μL in ethanol. Calibration standards were prepared at a range of 0.0–2.0 ng/μL. Similarly, a mixed deuterated internal standard was prepared at 0.5 ng/μL in ethanol, and was spiked into both samples and standards for a final concentration of 0.05 ng/μL. The limit of quantitation was 0.05 pmol/mL. Recovery based on spiking with deuterated arachidonic acid in plasma was 88%. Recovery for other metabolites in the internal standard mixture were as follows: TXB2‐d4 60.55%, PGF2α‐d4 97.97%, PGD2‐d4 84.42%, LTB4‐d4 66.7%, 5(S)HETE‐d8 66.36%, 13(S)Hode‐d4 70.36%, 5,6DHET‐d11 64.84%, and 5,6EET‐d11 88.33%. Intra‐assay reproducibility coefficient of variations ranged from 7.1 to 11.7% and interassay reproducibility ranged from 11.4 to 15.7%.
Statistical Analysis
Statistical analyses were performed using commercial software.6 Normality for each analyte was evaluated by the Shapiro–Wilk test. Groups were compared by t‐test and data expressed as mean ± SD for normally distributed data. Nonparametric data were compared by Mann‐Whitney test and data are expressed as median and range. The Benjamini‐Hochberg procedure was used to control for false positives with multiple tests from a single sample using a false discovery rate of 10%, resulting in P < .033 considered significant for AA metabolites.29 Statistical significance was set at P < .05 for all other comparisons. Spearman rank correlations (rs) were used to examine associations between AA metabolites and either SP or urine albumin concentration.
Results
Forty‐four dogs were evaluated for possible inclusion in this study. Two non‐Greyhound dogs were excluded because of leukocytes in the urine sediment. One Greyhound and 1 non‐Greyhound dog were excluded because of failure to collect urine. The final study population of 40 dogs consisted of 20 Greyhounds and 20 non‐Greyhound dogs. The 20 Greyhounds included 10 spayed females and 10 neutered males. The 20 non‐Greyhound dogs included 15 mixed breed and 5 purebred dogs (2 Boxers, 1 Standard Poodle, 1 German Shepherd, and 1 Great Dane) of which 8 were spayed females and 12 were neutered males. There was no significant difference in mean age between the 2 groups (Greyhounds, 5.3 ± 1.6 years, range 3–8 years; non‐Greyhounds 3.8 ± 1.6 years, range 1–7 years; P = .066).
SP was significantly higher in Greyhounds compared to non‐Greyhounds (152 ± 14 versus 143 ± 11 mmHg, respectively, P = .030). There was no significant difference in diastolic blood pressure (Greyhounds, 87.5 ± 13.2 mmHg; non‐Greyhounds, 88.4 ± 10.2 mmHg; P = .816) or mean arterial pressure (Greyhounds, 107.6 ± 12.3 mmHg; non‐Greyhounds, 106.0 ± 10.1 mmHg; P = .656) between the 2 groups.
Median urine albumin concentration was significantly greater in Greyhounds than in non‐Greyhounds (Fig 2, P = .006). Ten of 20 (50%) Greyhounds (6 males and 4 females) had urine albumin concentrations greater than 1.0 mg/dL, whereas only 2 of 20 (10%) non‐Greyhounds had urine albumin concentrations greater than 1.0 mg/dL.
Figure 2.
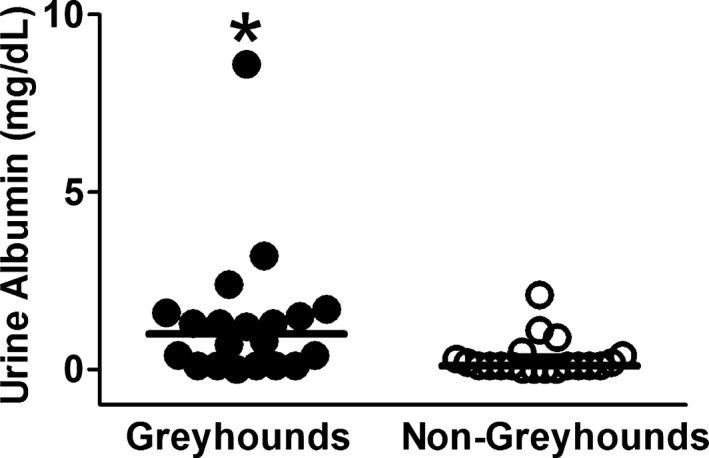
Urinary albumin concentration in Greyhounds and non‐Greyhound dogs. The horizontal bars represent the medians. Filled circles are Greyhounds. Open circles are non‐Greyhounds. Significant difference indicated with (*P < .05).
Greyhounds had significantly higher levels of the following AA metabolites than non‐Greyhounds: 5(S)HETE (P = .033), 8(S)HETE (P = .002), 9(S)HETE (P = .026), 12(S)HETE (P = .022), 8,9DHET (P = .004), 8,9DHET/EET (P = .016), 11,12DHET (P = .004), 14,15DHET (P = .028), 14,15EET (P = .005), and Total DHET (0.005) (Figs 3, 4). There were no significant differences between groups for the other measured metabolites (Table 1). Urinary albumin concentration was correlated with total DHET (rs = 0.46, P = .003). There was a weak inverse correlation between 8,9EET and SP (rs = −0.49, P = .001). SP was positively correlated with 11,12EET (rs = 0.42, P = .006) and 20(S)HETE (rs = 0.38, P = .017).
Figure 3.
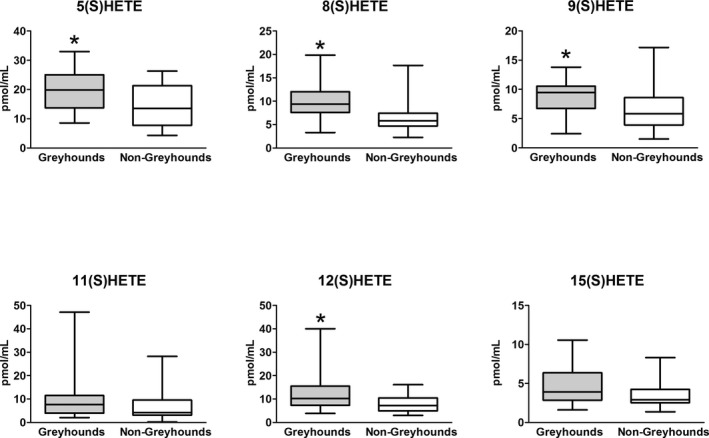
Lipoxygenase metabolites (HETEs) of AA in Greyhounds and non‐Greyhound dogs. The boxes represent the interquartile intervals from the 25 to 75th percentile, the solid horizontal line through the box represents the median, and the whiskers represent the minimum and maximum values. Significant difference between groups indicated with (*P < .033).
Figure 4.
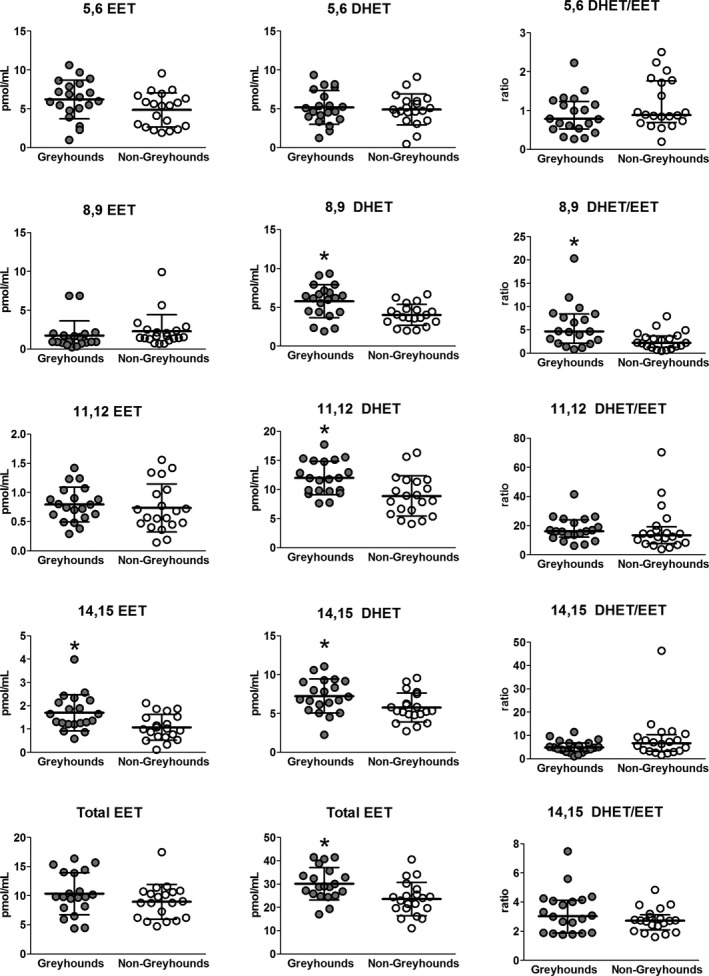
CYP450 epoxygenase metabolites of arachidonic acid (epoxyeicosatrienoic acids, EET) and their soluble expoxide hydrolase metabolites (dihydroxyeicosatrienoic acids, DHETs) in Greyhounds and non‐Greyhound dogs. Filled circles are Greyhounds. Open circles are non‐Greyhounds. The horizontal bars represent the mean ± SD for the EETs and DHETs. Horizontal bars represent the median and interquartile range for the ratios. Significant difference indicated with (*P < .033).
Table 1.
CYP450 hydroxylase, lipoxygenase, and cyclooxygenase metabolites of AA in Greyhounds and non‐Greyhounds measured by LC/ESI/MS
| Greyhound | Non‐Greyhound | P‐Value | |||
|---|---|---|---|---|---|
| Median | Range | Median | Range | ||
| 20(S)HETE | 47.24 | 15.16–136.0 | 47.74 | 20.41–120.4 | .989 |
| 9(S)Hode | 384.8 | 104.6–735.2 | 184.7 | 126.8–1241.0 | .756 |
| 13(S)Hode | 433.7 | 120.1–816.2 | 432.0 | 159.2–1315 | .989 |
| 8‐iso‐PGF2α | 9.31 | 4.21–25.84 | 12.87 | 2.72–26.85 | .245 |
| TXB2 | 2.17 | 0.69–17.06 | 2.13 | 0.73–95.55 | .661 |
| PGD2 | 45.96 | 19.29–336.5 | 33.76 | 6.13–161.7 | .190 |
| PGE2 | 2.14 | 0.33–6.13 | 2.10 | 0.37–12.03 | .989 |
| PGF2α | 1.42 | 0.34–5.12 | 1.87 | 0.77–12.41 | .177 |
| LTB4 | 5.32 | 0.89–12.90 | 3.87 | 1.06–28.29 | .298 |
| LTC4 | ND | ND | |||
| LTD4 | 0.18 | 0.05–0.50 | 0.18 | 0.05–0.64 | .491 |
| LTE4 | 0.23 | 0.06–0.93 | 0.19 | 0.07–0.48 | .596 |
| LXA4 | 0.83 | 0.15–3.59 | 0.70 | 0.05–2.94 | .448 |
LT (leukotrienes), LX (lipoxin), PG (prostaglandins), TX (thromboxane), Hode (hydroxyoctadecadienoic acid), HETE (hydroxyeicosatetraenoic acid). Data are expressed as median and range. ND indicates not detectable.
Discussion
Endothelial dysfunction is characterized by impaired vasomotor response, cell proliferation, platelet aggregation, altered vascular permeability, and interactions between leukocytes and endothelial cells that contribute to vascular inflammation.6, 7 Both hypertension and albuminuria are considered indicators of generalized vascular dysfunction and risk markers for development of renal and cardiovascular disease.6, 7 Similar to previous reports, the Greyhounds in this study had higher SP and urinary albumin concentrations compared to the non‐Greyhound dogs.5, 18 Previous studies have reported that Greyhounds have SP of 10–20 mmHg higher on average than that of mixed breed dogs,15, 16, 17, 18 which is similar to the approximate 9 mmHg difference observed in the Greyhounds in this study. Hypertension has been linked to target organ damage in dogs, and has been suggested to contribute to stroke2 and albuminuria5 in Greyhounds. High SP was associated with more severe proteinuria and renal histologic lesions in dogs with surgically induced renal failure30 and with naturally occurring chronic kidney disease.31 While not directly correlated with SP, 50% of the Greyhounds in this study had urinary albumin concentrations >1.0 mg/dL and 10% had concentrations >2.5 mg/dL, considered to be significant albuminuria. This is in contrast to the non‐Greyhound dogs in which 90% had urine albumin concentrations <1.0 mg/dL and none exceeded 2.5 mg/dL. The Greyhounds tended to be older than the non‐Greyhounds, which could contribute to these findings. However, taken together, the presence of both higher blood pressure and albuminuria in Greyhounds compared to non‐Greyhounds is consistent with alterations in vascular function in the breed.
Certain AA metabolites have been increasingly associated with endothelial health and dysfunction. EETs, products of CYP450 expoxygenases, have been shown to mitigate endothelial dysfunction by promoting vasodilation, inhibiting platelet aggregation, and having anti‐inflammatory and antiapoptotic effects in vivo.19, 20 Activity of sEH, which hydrolyzes EETs to their less protective diols (DHETs), is associated with higher blood pressures and loss of vasoprotective effects. Alterations in the relative proportions of EETs and DHETs can result from CYP450‐mediated production of EETs or conversion of EETs to DHETs by sEH. There is some evidence that Greyhounds differ from other dog breeds in activity of some CYP450 enzymes. Greyhounds exhibit slower drug clearance and longer anesthetic recovery times compared to other dog breeds, consistent with lower activity of some hepatic CYP450 hydrolases.32, 33 The concentrations of 5,6‐, 8,9‐, and 11,12EETs were similar and 14,15EET was increased in Greyhounds compared to non‐Greyhounds. This suggests that there was either no difference or possibly an increase in CYP450 epoxygenase‐mediated production of these EET isomers in the Greyhounds versus non‐Greyhounds. That Greyhounds were found to have significantly higher levels of multiple DHET regioisomers suggests that they have increased activity of sEH.
Deletion of the gene that encodes for sEH (Ephx2) or inhibition of sEH decreases blood pressure, attenuates renal inflammation, and helps prevent glomerular injury in rodent models.34, 35, 36 It has been suggested that inhibitors of sEH could offer a novel approach for the treatment of hypertension and end‐stage renal disease in people. By decreasing hydrolysis of EETs to their less active diols, sEH inhibitors reduce vasoconstriction and thus decrease renin‐angiotensin II‐aldosterone‐dependent hypertension.37 Increasing the ratio of EETs to their less active diols could offer a new strategy for promoting cardiovascular health and reducing the progression of renal disease in dogs with comparatively lower EETs/DHETs ratios, clinical hypertension, and albuminuria. Further study is needed to determine whether there is a significant difference in sEH activity between Greyhounds and non‐Greyhound dogs. Given the higher levels of DHETs, treatment with sEH inhibitors could be of benefit in ameliorating hypertension, albuminuria, and development of renal disease in this breed.
20(S)HETE is a product of CYP450 hydrolase metabolism of AA and is recognized as a potent vasoconstrictor that plays a role in maintenance of hypertension and albuminuria in rodent models and humans.20 While Greyhounds did not differ in the amount of 20(S)HETE compared to non‐Greyhound dogs, 20(S)HETE did correlate with SP. Further study is needed to evaluate the role of this metabolite in the development of hypertension and vascular disease in the dog.
Our results also indicate that Greyhounds have higher levels of certain regioisomers of HETEs than non‐Greyhounds, which could contribute to vascular dysfunction through a variety of mechanisms. Inhibition of vasodilatory prostaglandin production by 5(S) HETE, 12(S) HETE, and 15(S)HETE results in vasoconstriction38 and 12(S) HETE specifically produces vasoconstriction in isolated dog renal arcuate arteries.39 12(S)HETE has been shown to mediate the hypertrophic effects of angiotensin II, to have direct growth‐promoting effects, to increase levels of fibronectin, and to induce inflammatory cell chemotaxis.40 15(S)HETE has been implicated in vascular wall remodeling through stimulation of smooth muscle migration and neointima formation.41 Thus, 9(S)HETE, 12(S)HETE, and 15(S)HETE are thought to play a role in the development of vascular inflammation and atherosclerosis in people.42 Increased production of 12(S)HETE has been suggested as an early predictor for development of albuminuria in diabetic people with incipient renal disease.43 In order to determine a direct cause and effect relationship between the various HETE isomers, albuminuria, hypertension, and vascular disease in Greyhounds, a prospective study would be needed to evaluate the effects of either blocking endogenous lipoxygenase enzymes or giving exogenous HETEs.
CRGV is a thrombotic microangiopathy of Greyhounds that was identified as early as 1985.44 The pathogenesis of CRGV is incompletely understood; however, administration of Shiga toxin to Greyhounds can reproduce the vascular lesions.45 Alterations in vascular AA metabolism have been suggested in hemolytic uremic syndrome (HUS) and in response to Shiga toxin.46, 47, 48 Shiga toxin increases production of 12(S)HETE by rat glomerular endothelial cells. Treatment of glomerular endothelial cells with either Shiga toxin or with 12(S)HETE alone mimics early endothelial changes observed in HUS and CRGV, including endothelial cell retraction and decreased cell adherence to fibronectin and laminin.48 In our study, Greyhounds were found to have increased plasma concentration of 12(S)HETE compared to non‐Greyhound dogs. Further studies will be needed to determine if increased production of 12(S)HETE plays a role in vasculopathies such as HUS or CRGV in Greyhounds.
The role of angiogenesis and endothelial function is recognized in the development and metastasis of tumors.21, 22 Lord et al. reported a high incidence of cancer in Greyhounds, the reasons for which are probably multifactorial.4 Some HETE isomers such as 5(S)HETE, 8(S)HETE, and 12(S)HETE have been implicated in promoting tumorigenesis and metastasis.21, 49, 50 Further research is needed to determine if these HETE isomers have similar actions in dogs to those observed in humans and rodent models and if altered production of AA metabolites might be implicated in carcinogenesis in Greyhounds.
In conclusion, the plasma eicosanoid profile in Greyhounds differs from non‐Greyhound dogs and is consistent with profiles seen in association with vascular dysfunction, hypertension, albuminuria, and renal disease in humans and in rodent models. Further study is needed to evaluate activity and regulation of specific enzyme pathways involved in the metabolism of AA in Greyhounds compared to non‐Greyhound dogs. This might define early markers that could help predict development of albuminuria, hypertension, and vascular disease. Use of inhibitors of either the sEH or LOX pathways might provide targeted therapeutic options for treatment in the Greyhound breed.
Acknowledgments
Dr Martinez was funded in part through the Ohio State University Veterinary Scholar Summer Research Program.
Conflict of Interest Declaration: The authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Footnotes
Advia 2120, Siemens Medical Solutions, Malvern, PA
Cobas 6000 c501, Roche, Indianapolis, IN
Cardell 9402 BP/SpO2, Sharn Veterinary INC, Tampa, FL
Canine urine microalbumin, Antech Diagnostics, Southhaven, MS
ABI/Sciex 4000 QTrap with a Shimadzu HPLC and Analyst 1.4.2 software, SCIEX, Framingham, MA
GraphPad Prizm, version 5.04, GraphPad Software, La Jolla, CA
References
- 1. Lara‐Garcia A, Couto CG, Iazbik MC, Brooks MB. Postoperative bleeding in retired racing Greyhounds. J Vet Intern Med 2008;22:525–533. [DOI] [PubMed] [Google Scholar]
- 2. Kent M, Glass EN, Haley AC, et al. Ischemic stroke in Greyhounds: 21 cases (2007–2013). J Am Vet Med Assoc 2014;245:113–117. [DOI] [PubMed] [Google Scholar]
- 3. Hertzke DM, Cowan LA, Schoning P, Fenwick BW. Glomerular ultrastructural lesion of idiopathic cutaneous and renal glomerular vasculopathy of Greyhounds. Vet Pathol 1995;32:451–459. [DOI] [PubMed] [Google Scholar]
- 4. Lord LK, Yaissle JE, Marin L, Couto GC. Results of a web‐based health survey of retired racing Greyhounds. J Vet Intern Med 2007;21:1243–1250. [DOI] [PubMed] [Google Scholar]
- 5. Surman S, Couto CG, Dibartola SP, Chew DJ. Arterial blood pressure, proteinuria, and renal histopathology in clinically healthy retired racing Greyhounds. J Vet Intern Med 2012;26:1320–1329. [DOI] [PubMed] [Google Scholar]
- 6. Savoia C, Schiffrin EL. Vascular inflammation in hypertension and diabetes: molecular mechanisms and therapeutic interventions. Clin Sci 2007;112:375–384. [DOI] [PubMed] [Google Scholar]
- 7. Currie G, Delles C. Proteinuria and its relation to cardiovascular disease. Int J Nephrol Renovasc Dis 2013;7:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chugh A, Bakris GL. Microalbuminuria: what is it? Why is it important? What should be done about it? An update J Clin Hypertens 2007;9:196–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Jong PE, Curhan GC. Screening, monitoring, and treatment of albuminuria: public health perspectives. J Am Soc Nephrol 2006;17:2120–2126. [DOI] [PubMed] [Google Scholar]
- 10. Barbour T, Johnson S, Cohney S, Hughes P. Thrombotic microangiopathy and associated renal disorders. Nephrol Dial Transplant 2012;27:2673–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grauer GF. Canine glomerulonephritis: new thoughts on proteinuria and treatment. J Small Anim Pract 2005;46:469–478. [DOI] [PubMed] [Google Scholar]
- 12. Wehner A, Hartmann K, Hirschberger J. Associations between proteinuria, systemic hypertension and glomerular filtration rate in dogs with renal and non‐renal diseases. Vet Rec 2008;162:141–147. [DOI] [PubMed] [Google Scholar]
- 13. Jacob F, Polzin DJ, Osborne CA, et al. Association between initial systolic blood pressure and risk of developing a uremic crisis or of dying in dogs with chronic renal failure. J Am Vet Med Assoc 2003;222:322–329. [DOI] [PubMed] [Google Scholar]
- 14. Brown S, Atkins C, Bagley R, et al. Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med 2007;21:542–558. [DOI] [PubMed] [Google Scholar]
- 15. Schneider HP, Truex RC, Knowles JO. Comparative observations of the hearts of mongrel and Greyhound dogs. Anat Rec 1964;149:173–179. [DOI] [PubMed] [Google Scholar]
- 16. Cox RH, Peterson LH, Detweiler DK. Comparison of arterial hemodynamics in the mongrel dog and the racing Greyhound. Am J Physiol 1976;230:211–218. [DOI] [PubMed] [Google Scholar]
- 17. Pape LA, Price JM, Alpert JS, Rippe JM. Hemodynamics and left ventricular function: a comparison between adult racing Greyhound and Greyhounds completely untrained from birth. Basic Res Cardiol 1986;81:417–424. [DOI] [PubMed] [Google Scholar]
- 18. Bodey AR, Rampling MW. Comparison of haemorrheological parameters and blood pressure in various breeds of dog. J Small Anim Pract 1999;40:3–6. [DOI] [PubMed] [Google Scholar]
- 19. Elmarakby AA. Reno‐protective mechanisms of epoxyeicosatrienoic acids in cardiovascular disease. Am J Physiol Regul Integr Comp Physiol 2012;302:R321–R330. [DOI] [PubMed] [Google Scholar]
- 20. Imig JD. Epoxyeicosatrienoic acids, 20‐hydroxyeicosatetraenoic acid, and renal microvascular function. Prostaglandins Other Lipid Mediat 2013;104–105:2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schneider C, Pozzi A. Cyclooxygenases and lipoxygenases in cancer. Cancer Metastasis Rev 2011;30:277–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nie D, Honn KV. Cyclooxygenase, lipoxygenase and tumor angiogenesis. Cell Mol Life Sci 2002;59:799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hay Kraus BL, Greenblatt DJ, Venkatakrishnan K, Court MH. Evidence for propofol hydroxylation by cytochrome P4502B11 in canine liver microsomes: breed and gender differences. Xenobiotica 2000;30:575–588. [DOI] [PubMed] [Google Scholar]
- 24. Kukanich B, Coetzee JF, Gehring R, Hubin M. Comparative disposition of pharmacologic markers for cytochrome P‐450 mediated metabolism, glomerular filtration rate, and extracellular and total body fluid volume of Greyhound and Beagle dogs. J Vet Pharmacol Ther 2007;30:314–319. [DOI] [PubMed] [Google Scholar]
- 25. Marino CL, Cober RE, Iazbik MC, Couto CG. White‐coat effect on systemic blood pressure in retired racing greyhounds. J Vet Intern Med 2011;25:861–865. [DOI] [PubMed] [Google Scholar]
- 26. Hevko J, Bowers R, Murphy R. Synthesis of 5‐Oxo‐6,8,11,14‐eicosatetaenoic acid and identification of novel oxidized metabolites in the mouse macrophage. J Pharmacol Exp Ther 2001;296:293–305. [PubMed] [Google Scholar]
- 27. Harkewicz R. Eicosanoids. Presented at LIPID MAPS Lipidomics Workshop, April 28, 2007. http://www.lipidmaps.org/resources/lipidmapspresentations/EB2007.html. Accessed September 27, 2015.
- 28. Sharkey LC, Radin MJ, Heller L, et al. Differential cardiotoxicity in response to chronic doxorubicin treatment in male spontaneous hypertension‐heart failure (SHHF), spontaneous hypertensive (SHR), and Wistar Kyoto (WKY) rats. Toxicol Appl Pharmacol 2013;273:47–57. [DOI] [PubMed] [Google Scholar]
- 29. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 1995;57:289–300. [Google Scholar]
- 30. Finco DR. Association of systemic hypertension with renal injury in dogs with induced renal failure. J Vet Intern Med 2004;18:289–294. [DOI] [PubMed] [Google Scholar]
- 31. Bacic A, Kogika MM, Barbaro KC, et al. Evaluation of albuminuria and its relationship with blood pressure in dogs with chronic kidney disease. Vet Clin Pathol 2010;39:203–209. [DOI] [PubMed] [Google Scholar]
- 32. Robertson SA, Johnston S, Beemsterboer J. Cardiopulmonary, anesthetic, and postanesthetic effects of intravenous infusions of propofol in greyhounds and non‐greyhounds. Am J Vet Res 1992;56:1027–1032. [PubMed] [Google Scholar]
- 33. Court MH, Hay‐Kraus BL, Hill DW, et al. Propofol hydroxylation by dog liver microsomes: assay development and dog breed differences. Drug Metab Dispos 1999;27:1293–1299. [PubMed] [Google Scholar]
- 34. Manhiani M, Quigley JE, Knight SF, et al. Soluble epoxide hydrolase gene deletion attenuates renal injury and inflammation with DOCA‐salt hypertension. Am J Physiol Renal Physiol 2009;297:F740–F748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Imig JD, Zhao X, Zaharis CZ, et al. An orally active epoxide hydrolase inhibitor lowers blood pressure and provides renal protection in salt‐sensitive hypertension. Hypertension 2005;46:975–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhao X, Yamamoto T, Newman JW, et al. Soluble epoxide hydrolase inhibition protects the kidney from hypertension‐induced damage. J Am Soc Nephrol 2004;15:1244–1253. [PubMed] [Google Scholar]
- 37. Spector AA, Fang X, Snyder GD, Weintraub NL. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog Lipid Res 2004;43:55–90. [DOI] [PubMed] [Google Scholar]
- 38. Gordon EE, Gordon JA, Spector AA. HETEs and coronary artery endothelial cells: metabolic and functional interactions. Am J Physiol Cell Physiol 1991;2612:C623–C633. [DOI] [PubMed] [Google Scholar]
- 39. Ma YH, Harder DR, Clark JE, Roman RJ. Effects of 12‐HETE on isolated dog renal arcuate arteries. Am J Physiol Heart Circ Physiol 1991;261 (Pt 2):H451–H456. [DOI] [PubMed] [Google Scholar]
- 40. Reddy MA, Thimmalapura PR, Lanting L, et al. The oxidized lipid and lipoxygenase product 12(S)‐hydroxyeicosatetraenoic acid induces hypertrophy and fibronectin transcription in vascular smooth muscle cells via p38 MAPK and cAMP response element‐binding protein activation. Mediation of angiotensin II effects. J Biol Chem 2002;277:9920–9928. [DOI] [PubMed] [Google Scholar]
- 41. Singh NK, Wang D, Kundumani‐Sridharan V, et al. 15‐Lipoxygenase‐1‐enhanced Src‐Janus kinase 2‐signal transducer and activator of transcription 3 stimulation and monocyte chemoattractant protein‐1 expression require redox‐sensitive activation of epidermal growth factor receptor in vascular wall remodeling. J Biol Chem 2001;286:22478–22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shishehbor MH, Zhang R, Medina H, et al. Systemic elevations of free radical oxidation products of arachidonic acid are associated with angiographic evidence of coronary artery disease. Free Radic Biol Med 2006;41:1678–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Antonipillai I, Nadler J, Vu EJ, et al. A 12‐lipoxygenase product, 12‐hydroxyeicosatetraenoic acid, is increased in diabetics with incipient and early renal disease. J Clin Endocrinol Metab 1996;81:1940–1945. [DOI] [PubMed] [Google Scholar]
- 44. Carpenter JL, Andelman NC, Moore FM, King NW. Idiopathic cutaneous and renal glomerular vasculopathy of greyhounds. Vet Pathol 1988;25:401–407. [DOI] [PubMed] [Google Scholar]
- 45. Fenwick B, Cowan L. Canine model of hemolytic uremic syndrome In: Kaper J, O'Brien A, eds. Escherichia coli 0157:H7 and Other Shiga Toxin Producing E. coli Strains, 1st ed Washington, DC: American Society for Microbiology; 1998:268–277. [Google Scholar]
- 46. Schmid DI, Kohan DE. Effect of Shigatoxin‐1 on arachidonic acid release by human glomerular epithelial cells. Kidney Int 2001;60:1026–1036. [DOI] [PubMed] [Google Scholar]
- 47. Leyva‐Illades D, Cherla RP, Calindo CL, et al. Global transcriptional response of macrophage‐like THP‐1 cells to shiga toxin type 1. Infect Immun 2010;78:2454–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Adler S, Bollu R. Glomerular endothelial cell injury mediated by shiga‐like toxin‐1. Kidney Blood Press Res 1998;21:13–21. [DOI] [PubMed] [Google Scholar]
- 49. Powell WS, Rokach J. Biosynthesis, biological effects, and receptors of hydroxyeicosatetraenoic acids (HETEs) and oxoeicosatetraenoic acids (oxo‐ETEs) derived from arachidonic acid. Biochim Biophys Acta 2015;1851:340–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fürstenberger G, Marks F, Krieg P. Arachidonate 8(S)‐lipoxygenase. Prostaglandins Other Lipid Mediat 2002;68–69:235–243. [DOI] [PubMed] [Google Scholar]


