Abstract
Background
Ketones, including beta hydroxybutyrate (BHB), are produced in conditions of negative energy balance and decreased glucose utilization. Serum BHB concentrations in cats are poorly characterized in diseases other than diabetes mellitus.
Hypothesis
Serum BHB concentrations will be increased in cats with chronic kidney disease (CKD), hyperthyroidism (HT), or hepatic lipidosis (HL).
Animals
Twenty‐eight client‐owned cats with CKD, 34 cats with HT, and 15 cats with HL; 43 healthy cats.
Methods
Prospective observational study. Serum BHB concentrations were measured at admission in cats with CKD, HT, and HL, for comparison with a reference interval established using healthy cats. Results of dipstick urine ketone measurement, when available, were compared to BHB measurement.
Results
Beta hydroxybutyrate was above the reference interval (<0.11 mmol/L) in 6/28 cats (21%) with CKD, 7/34 cats (20%) with HT, and 11/15 cats (73%) with HL, significantly exceeding the expected 2.5% above the reference interval for healthy cats (P < .001 for all groups). Elevations were mild in CKD and HT groups (median BHB 0.1 mmol/L for both groups, 80th percentile 0.12 and 0.11 mmol/L, respectively), but more marked in HL cats (median BHB 0.2 mmol/L, 80th percentile 0.84 mmol/L). None of 11 cats with increased serum BHB concentration having urine dipstick analysis performed within 24 h of sampling for BHB were ketonuric.
Conclusions and Clinical Importance
Increases in serum BHB concentrations occur in cats with CKD, HT, and HL, and might provide an useful index of catabolism.
Keywords: Ketonuria, Ketosis, Metabolism, Reference intervals
Abbreviations
- BHB
beta hydroxybutyrate
- CKD
chronic kidney disease
- DKA
diabetic ketoacidosis
- DM
diabetes mellitus
- HT
hyperthyroidism
- HL
hepatic lipidosis
Ketones (beta hydroxybutyrate (BHB), acetoacetate, and acetone) are produced in states of negative energy balance/catabolism or decreased glucose utilization, and detection in the serum or urine is routinely associated with diabetes mellitus (DM) in both humans and animals. While BHB and acetoacetate are normally present in roughly equal concentrations in serum, in human patients BHB is the predominant ketone associated with prolonged fasting and diabetic ketoacidosis (DKA).1 Serum BHB is routinely measured in human medicine as a more specific indicator of DKA in hyperglycemic emergency room patients,2 to improve monitoring of DKA in children,3 and to reduce the need for hospitalization and emergency room visits when monitored at home by type I diabetics.4 While measurement of BHB has become standard in the monitoring of human diabetic patients, semi‐quantitative measurement of acetoacetate by urine dipstick is still the predominant diagnostic test used to assess ketone status in veterinary medicine. However, as measurement of serum BHB becomes more available, an understanding of its diagnostic utility in veterinary medicine is important to establish. Also, because ketones reflect utilization of fat as an energy source and can reflect energy needs that exceed intake, serum BHB measurement has the potential to be used as a marker of caloric stress and the need for nutritional support in veterinary patients.
While there is a frequent clinical presumption that ketosis equates to DM in companion animals, increased serum BHB in cats can be associated with other conditions. Twenty‐six and a half percent of 215 ill cats had an increased serum BHB;5 and while diabetic cats had the highest BHB, cats with hepatic lipidosis (HL) were also found to have significantly higher BHB than cats with other conditions.5 Several older studies demonstrate increased BHB in both naturally occurring and starvation‐induced HL in cats.6, 7, 8 However, no prospective studies have been done evaluating BHB in clinically relevant numbers of cats with other conditions, and no recent prospective studies of BHB in HL cats have been performed.
The aim of this observational study was to evaluate serum BHB in cats with one of three feline diseases: chronic kidney disease (CKD), hyperthyroidism (HT), or hepatic lipidosis (HL) to determine if BHB was significantly increased in these groups of cats. These conditions were selected as they are common conditions that might result in a catabolic state in cats. We hypothesized that serum BHB would be increased in cats with CKD, HT, and HL.
Materials and Methods
Animals
Three groups of client‐owned cats were prospectively selected between May 2013 and September 2014. The investigation was conducted in accordance with the guidelines and with the approval of the University of Minnesota Institutional Animal Care and Use Committee. Forty‐three healthy cats were prospectively recruited during April 2014 to generate the reference interval for BHB. These consisted of 31 cats presenting to the General Practice service of the University of Minnesota Veterinary Medical Center (UMN‐VMC) for wellness screening; all cats had normal physical examinations and unremarkable health histories, and CBC and serum biochemical results. Twenty‐eight cats had serum T4 concentrations measured, all of which were within the reference interval. The remaining 12 cats meeting the same criteria were recruited from faculty, staff, and student owners, or were cats that were examined by other UMN‐VMC services. Two had serum T4 concentrations measured and both were within reference intervals.
Study cats in all 3 disease groups had a complete medical record available for review. Age, sex, breed, weight, body condition score (BCS), as well as serum creatinine, urea, glucose, and cholesterol concentrations on presentation to the hospital were recorded. Any cat with a history or diagnosis of DM, a blood glucose >250 mg/dL, or glucosuria on a urine dipstick was excluded, although a urine dipstick was not required for inclusion into the study. The presence or absence of ketonuria on a urine dipstick1 was recorded in all cats having a urinalysis performed at the VMC within 24 h of serum sampling for BHB.
Criteria for enrollment of cats in the CKD group were: serum creatinine concentration above the reference interval (>2.1 mg/dL) and a concurrent urine specific gravity <1.030. Cats with evidence of acute kidney injury (acute exacerbation of previously stable azotemia or new onset of severe azotemia with a creatinine ≥5 mg/dl) were excluded. Cats were enrolled in the HT group based on a total thyroxine (T4) concentration of either >6 mg/dL or above the reference interval (>3.8 mg/dL) with a history of characteristic clinical signs (BCS >5/9 or weight loss of 0.2 kg or more in the last month). Cats in the HT group were excluded if they had a serum creatinine >2.1 mg/dL. Exclusion criteria for both CKD and HT included any other serious clinically apparent complaint, including moderate/severe gastrointestinal disease, heart disease, neoplasia, etc. Criteria for enrollment of cats in the HL group were a compatible clinical history (anorexia, hyporexia) and either a cytologic or histologic diagnosis of HL. To make the HL group size as robust as possible, both cats with primary (i.e., no concurrent or underlying disease detected that was considered likely to have initiated anorexia/hyporexia) and secondary HL were recruited. Comorbidities, serum bilirubin concentration, and serum ALP, ALT, and AST activities were recorded for cats in the HL group. The mean body weight, BCS, age, serum concentrations of creatinine, urea nitrogen, and BHB, and urine specific gravities of cats with presumed primary and secondary HL were compared to establish the validity of aggregating cats with primary and secondary HL.
Beta Hydroxybutyrate Measurement
Samples were analyzed using a Beckman Coulter AU480.2; using the Stanbio β‐Hydoxybutyrate LiquiColor® system and Stanbio TDM/ß‐Hydroxybutyrate Linearity Standards3 , the primary and secondary spectrophotometer readings were taken at 520 and 800 nm, respectively. Within day precision based on 10 replicates of a patient sample was 1.7%, within the manufacturer's recommendations. In most instances, serum BHB was measured using residual sample from blood collected for diagnostic or monitoring purposes unrelated to the study in order to avoid an additional blood collection procedure. In cases when residual serum was not available, informed consent was obtained from the owner. If possible, BHB sampling for cats in the HL group was performed before initiating enteral feeding. Time to measurement of BHB was not standardized; some samples were analyzed immediately after collection; some were refrigerated for up to 7 days before measurement; and a small number were frozen for up to 40 days and thawed before BHB measurement. Based on data from the manufacturer and the published literature, these testing conditions should not have impacted results. Stability of BHB in human patients has been previously established in samples refrigerated at 2–8 °C for up to 7 days (Stanbio Laboratory Beta Hydroxybutyrate Liquicolor product insert), as well as in samples frozen at −80 °C for up to 40 days.9
Statistical Analysis
Chronic kidney disease, HT, and HL cats were analyzed for differences in age, body weight, BCS, serum creatinine, urea nitrogen, glucose, and cholesterol, and urine specific gravity using pairwise t‐tests with P‐values corrected for multiple comparisons using the Bonferroni‐Holm method. There were no visual departures from normality for any variables, though there was an occasional unequal variance between groups; so, the unequal variances unpaired t‐test was used. These groups were also analyzed for differences in sex using a chi‐squared test. The same tests were performed to test for differences in HL cats with and without comorbidities. These variables were also tested for association with BHB concentration, using Kendall's correlation.
Comparison of serum BHB concentrations among CKD, HT, and HL cats was performed using pairwise Wilcoxon tests, with P‐values corrected for multiple comparisons again using the Bonferroni‐Holm method, because of non‐normality caused by the analytical detection limit of 0.1 mmol/L. For consistency, all serum BHB measurements reported to be below this limit were considered to have a BHB of 0.1 mmol/L for statistical analyses. As above, this test was also performed to screen for differences in HL cats with and without comorbidities. Because of the skewness of the data and compression of values at the lower end of the reference interval, the median and 80th percentile values of BHB for each group are reported to better show differences among the groups at the higher end of the range of values.
Using the normal cats, a 95% reference interval was calculated for serum BHB concentration, with confidence intervals calculated using the bootstrap method. Univariate proportion tests were then used to test if the proportion of cats in each group with a BHB above the upper limit of the reference range was different from 0.025, the proportion of healthy cats expected to be above the reference range.10, 11 In addition, pairwise proportion tests were used to test if proportions in each group were different from each other.
Results
Twenty‐eight cats were enrolled in the CKD group, 34 in the HT group, and 15 in the HL group. Cats in the HT group had a median T4 value of 10.3 μg/dL (range 4.0–34.4, reference interval 1.5–3.8). Twelve of 28 (43%) CKD cats were in IRIS stage II, whereas 16/28 (57%) were in IRIS stage III. Thirteen of 15 HL cats were diagnosed with HL based on fine needle aspiration cytology of the liver, 1 based on an ultrasound‐guided biopsy, and 1 at necropsy. The median serum total bilirubin was 6.6 mg/dL (range 0.2–25.8, reference interval 0.0–0.3), median serum ALP activity was 354 U/L (range 27–951, reference interval 27–951), and median serum ALT activity was 262 U/L (range 64–583, reference interval 16–127) in HL cats. No associated disease was found based on the clinical evaluation performed at the discretion of the attending clinician in 8 cats, designated primary HL. Another diagnosis was reached in the remaining 7 cats (designated secondary HL), although a causal relationship with HL could not necessarily be established. The diagnoses included: pancreatitis (4 cats), biliary cystadenoma (1), biliary adenocarcinoma with carcinomatosis (1), and a mass in the common bile duct suspected to be a biliary carcinoma (1). Diagnostic testing done for HL cats varied depending on what was considered indicated by the attending clinician. One cat was diagnosed on necropsy only, whereas the remaining 14/15 cats had a full abdominal ultrasound performed at the time of liver sampling. Five cats had a prothrombin time (PT) and partial thromboplastin time (PTT) performed; 3 had infectious disease testing for feline immunodeficiency virus (FIV) and feline leukemia virus (FeLV); 3 had chest radiographs; and 2 had total T4 measurement. One cat had blood measurement of trypsin‐like immunoreactivity (TLI), pancreatic lipase immunoreactivity (PLI), cobalamin, and folate; and 1 each had a bone marrow aspirate and biopsy, urine culture, liver culture, bile culture, and abdominal radiographs. Five of the 15 HL cats ultimately had a necropsy performed. There were no statistically significant differences between HL cats with and without comorbidities in weight, BCS, age, serum creatinine, and urea, or serum BHB concentrations (data reported in aggregate); however, HL cats without comorbidities had significantly more concentrated urine than cats with comorbidities (mean USG 1.047 and 1.022, P = .01, respectively).
The breed distribution for the CKD group was: 17 Domestic, 6 Siamese, 3 Maine Coon, and 1 each of Egyptian Mau and Himalayan. For the HT group, breed distribution was 27 Domestic, 4 Siamese, 2 Maine Coon, and 1 Russian Blue. For the HL group, breed distribution was 13 Domestic, and 2 Ragdoll cats. Sex distribution and ages are reported in Table 1. Cats in the HL group were significantly younger than cats in either the CKD (P = .01) or HT (P = .006) groups, but there were no differences in sex distribution, body weight, or BCS among groups. Serum cholesterol concentration was significantly higher in CKD cats compared with HT and HL cats (P ≤ .01), although the means of all 3 groups fell within the reference range. Serum glucose was significantly higher in HL cats than in HT cats (P = .04), not in HL cats when compared to CKD cats (P = .06). The mean serum glucose in HL cats was slightly above the reference interval at 145 mg/dL, whereas the mean glucose in both HT and CKD cats was within the reference interval. As expected, CKD cats had significantly higher serum creatinine and urea concentrations than the other groups (P < .0001 CKD versus HL and CKD versus HT) but HL and HT were not different from each other. Hyperthyroidism cats had significantly higher urea concentrations than HL cats despite the lack of differences between the two groups in creatinine; however, the means of both groups fell within the reference interval. Urine specific gravity was significantly lower in the CKD group than in the other two groups, not in HT cats when compared to HL cats (P = .06), reflecting the known tendency for polyuria in hyperthyroidism. Serum concentrations of cholesterol and glucose were not correlated with BHB, either across or within groups.
Table 1.
Population characteristics and selected clinicopathologic data for cats with chronic kidney disease (CKD), hyperthyroidism (HT), and hepatic lipidosis (HL)
| CKD (n = 28) | HT (n = 34) | HL (n = 15) | |
|---|---|---|---|
| Sex distribution | 13 male, 15 female | 18 male, 16 female | 7 male, 8 female |
| Age (years) | 13.0 (4.5) | 13.1 (2.8) | 9.1 (3.7)* |
| Weight (kg) | 4.3 (1.1) | 4.4 (1.2) | 4.5 (1.5) |
| BCS (9 point scale) | 4.7 (1.9) | 4.9 (1.7) | 5.0 (1.8) |
| Creatinine (mg/dL) RI 0.5–2.1 | 3.1 (0.8)* | 1.1 (0.4) | 1.2 (0.5) |
| BUN (mg/dL) RI 12–39 | 55 (23)* | 28 (7)* | 18 (9)* |
| Glucose (mg/dL) RI 74–143 | 115 (24) | 110 (24)** | 145 (47)** |
| Cholesterol (mg/dL) RI 56–226 | 220 (60)* | 154 (41) [31 cats] | 163 (60) |
| Urine SG | 1.014 (0.005)* | 1.027 (0.011) [29 cats] | 1.037 (0.015) [10 cats] |
Values are reported as mean (standard deviation). RI = reference interval and *indicates a statistically significant difference from the other two groups and **indicates that two groups are different from each other but not the third group at P < .05. Data are reported for all cats unless indicated in brackets.
Serum BHB values for CKD, HT, and HL cats are represented in Fig 1. The bootstrap reference interval was 0–0.11 mmol/L. The upper limit of the reference interval and the confidence intervals (0.10–0.12) are shown in the figure since the lower limit of the reference interval was below the linearity of the assay; thus, limits and confidence intervals could not be calculated. The median serum BHB in CKD and HT cats fell within the reference interval at 0.1 mmol/L for both. The median BHB value for HL cats was significantly higher than both other groups and exceeded the reference interval at 0.20 mmol/L). As illustrated by the representation of individual cat values and emphasized by the demarcation of the 80th percentile for each group in Fig 1, the range and distribution of the values in HL demonstrated the increased frequency of values exceeding the reference interval in this group. Comparison of the proportion of each group of cats falling above the reference interval revealed that all groups exceeded the statistically anticipated 2.5%, with approximately 20% of CKD and HT cats having increased serum BHB concentrations (P = .003 and P = .016, respectively), whereas the proportion of HL cats exceeding the reference interval was significantly higher at almost 75% (P < .01). Of the 6/28 cats of the CKD group with an increased BHB, 50% were in stage II and 50% were in stage III.
Figure 1.
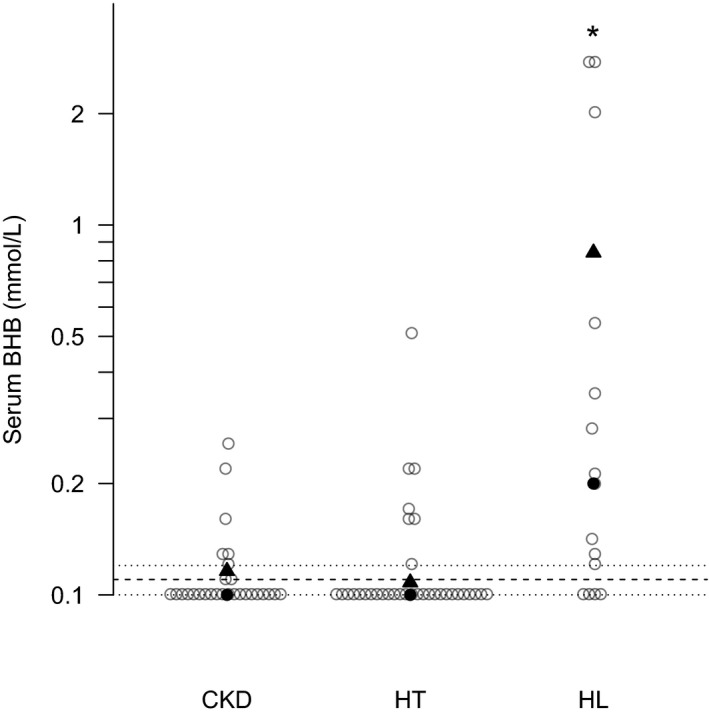
Serum beta hydroxybutyrate (BHB) concentrations in cats with chronic kidney disease (CKD, n = 28), hyperthyroidism (HT, n = 34), and hepatic lipidosis (HL, n = 15). Individual cats are represented by open circles; the median for each group by solid circles; and the 80th percentile by solid triangles. The bootstrap reference interval for serum BHB was <0.11 mmol/L; the upper limit of the reference interval is indicated by the dashed lines; and the confidence intervals of that limit (0.10–0.12) are represented by the dotted lines. The lower limit could not be calculated because it fell below the linearity of the assay. * indicates the median serum BHB concentration was significantly higher for the HL cats compared with CKD and HT (P < .05).
Forty‐one of the 72 cats enrolled in the study (14/28 CKD, 21/34 HT, and 6/15 HL cats) had a urinalysis performed within 24 h of sampling for BHB measurement, and 35 of these cats had a urinalysis performed simultaneously with BHB sampling. All 41 cats were negative for ketones on the urine dipstick. Of these 41 cats, 11 had a BHB concentration above the upper limit of the reference range (0.11 mmol/L) including 3/14 CKD cats (21%), 3/21 HT cats (14%), and 5/6 HL cats (83%). Two of the HL cats had markedly increased BHB concentration (>2 mmol/L).
Discussion
This study revealed that a significantly greater proportion of cats with CKD, HT, and HL had increased serum BHB concentrations than expected in a population of healthy cats. This difference was most dramatic in the HL group, in which almost 75% of cats had a BHB concentration above the reference interval, including values as high as 2.78 mmol/L (over 25 times the upper limit of reference interval). By contrast, about 20% of cats in the CKD and HT groups had modestly increased serum BHB concentrations, although this was still higher than the expected 2.5% in a healthy population. These differences in the proportion of cats with ketonemia were observed despite the lack of differences among the groups in body weight or BCS. This study demonstrates that BHB can be increased in disease conditions other than DM in cats, presumably because of negative energy balance associated with catabolism.
Cats in the CKD group had an increased serum cholesterol compared to HT and HL cats. Although the mean cholesterol in the CKD group was still within the reference interval at 220 mg/dL, 8/28 cats in the CKD group were hypercholesterolemic; whereas only 1 cat each in the HT and HL groups had increases in serum cholesterol. The importance of this finding is unclear, although it did not appear to affect ketone status in our population given the lack of correlation between cholesterol and BHB concentrations. Hypercholesterolemia is a well‐documented complication of CKD in dogs, with 55% of dogs with CKD having increased serum cholesterol levels.12 Dyslipidemia is a common finding in human patients with CKD of various etiologies, although this does not always result in an increased total serum cholesterol, but rather changes in the distribution of high, low, and very low density lipoproteins.13 Further studies could be indicated to determine if hypercholesterolemia is a repeatable finding in cats with CKD since information in the literature is limited for this species.
Hepatic lipidosis cats had higher serum glucose concentrations than HT cats, and exhibited higher glucose concentrations than CKD cats that almost achieved statistical significance. Eight per 15 HL cats were hyperglycemic, compared with 6/28 CKD and 3/34 HT cats. The cause of this difference is unknown, although both stress hyperglycemia and peripheral insulin resistance in HL cats are considerations. Either of these could have been more common in the HL cats given the generally more severe nature of their clinical disease compared with CKD and HT cats. Although over half of HL cats were hyperglycemic, this was generally mild; the highest glucose concentration documented in this group was 231 mg/dL. BHB was not correlated with serum glucose concentration in any group or across groups, indicating that hyperglycemia did not seem to influence ketone status in this population of cats, although the potential to identify a correlation could have been negatively influenced by the number of cats falling below the linear range of the assay. This finding is similar to that of a study done by using a similar assay methodology; BHB correlated with blood glucose concentration in diabetic cats; however, BHB did not correlate with blood glucose in sick nondiabetic cats. Furthermore, there were no significant differences in BHB when comparing sick nondiabetic cats that were normo‐ or hyperglycemic.14
In diabetic animal and human patients, serum BHB concentration varies greatly depending on the metabolic status of the patient. In one study of newly diagnosed pediatric diabetics, 83% had a BHB >0.6 mmol/L, and the median BHB for those patients who were not ketoacidotic on presentation was still markedly increased at 2.45 mmol/L.15 The median BHB of 37 diabetic cats was 1.47 mmol/L, with a range of 0.06 to 17.01 mmol/L,14 and BHB differed significantly depending on whether cats were classified as nonketotic, ketotic, or ketoacidotic diabetics on the basis of urine dipstick methodology and venous blood gas assessment.16 In this instance, median BHB concentrations were 0.1, 1.7, and 7.9 mmol/L in nonketotic, ketotic, and ketoacidotic diabetic cats, respectively, and all cats with DKA had BHB concentrations exceeding 3.8 mmol/L. Neither of these studies established a reference interval for serum BHB; however, in both studies, BHB in healthy control cats did not exceed the upper limit of the reference interval of 0.1 mmol/L. Although this study did not evaluate BHB in diabetic cats, comparison of serum BHB levels in the CKD, HT, and HL cats of this study with BHB in diabetic cats of the previous studies using a methodology employing identical biochemical reactions suggests the potential for significant overlap between uncomplicated or ketotic diabetic cats and HL cats, and even some overlap between uncomplicated diabetic cats and CKD or HT cats. Therefore, when hyperglycemia is noted in a sick cat, especially when HL is a consideration, increased serum BHB concentration should not prompt the clinician to establish a diagnosis of DM without further supporting evidence.
The data in the presented study suggests that serum BHB is a more sensitive indicator of ketosis than measurement of acetoacetate in urine via dipstick. In this study, 12 cats that had increased serum BHB concentrations also had a urinalysis performed within 24 h, yet none of these cats had ketonuria as assessed by urine dipstick. Although most of these cats had only mild increases in BHB, two cats in the HL group had a serum BHB >2.0 mmol/L without evidence of ketonuria, indicating that even marked ketonemia may be missed when evaluating urine ketones exclusively. The increased sensitivity of serum BHB is likely due, at least in part, to acetoacetate being the only ketone body measured by urine dipstick. If BHB is the more abundant ketone body in cats, as has been found in human diabetics, acetoacetate measurement may underestimate the degree of ketosis present. Serum BHB is also a more immediate reflection of the ketone status of an animal, as development of ketonuria depends on excretion of ketones in the urine and thus might not reflect very acute increases in serum ketones. Consistent with this, almost half of diabetic cats classified as nonketotic on the basis of a negative urine dipstick had increases in serum BHB, supporting the hypothesis that serum BHB is more sensitive for detecting ketosis in cats.16 Similarly, nearly 2/3 of diabetic dogs classified as nonketotic on a urine dipstick had a BHB above their reference range (>0.15 mmol/L).17 However, the true prevalence of BHB versus acetoacetate in diabetic dogs is unclear, as BHB was found to make up a higher percentage of the total ketones (60%) in dogs with lower blood ketones, but a lower percentage (20%) in those with higher blood ketones.18 This result is opposite of what has been observed in people, where ratios of BHB to acetoacetate can rise to as high as 10 : 1 in the context of diabetic ketoacidosis. The relative concentrations of BHB and acetoacetate for diabetic ketoacidosis in cats are not known.
This study has several limitations. Most cases were examined for the first time at the VMC when the sample for serum BHB was drawn; so, an accurate body weight or body condition history was not consistently available. Historical information regarding appetite was insufficient to permit accurate assessment of energy balance. In addition, only 57% of cats had urine sample results available from within 24 h of collection of the serum sample used for BHB analysis and the numbers of cats with very high serum BHB concentrations was limited; so, further assessment of the relative sensitivity of serum BHB concentrations and urine ketone dipstick measurement requires a future study. Another limitation is that we could not definitively determine if HL cats were affected with primary or secondary HL, and the diagnostics available for each cat varied based on the discretion of the attending clinician and owner. However, other diagnoses that were made in the HL cats were recorded in an attempt to identify cases of secondary HL and significant differences were not detected between primary and secondary cases in other metabolic indicators. Two of the cats in the HL group were diagnosed (one definitively, one presumptively) with cancer, which could have influenced the metabolic status caused by cancer cachexia. One of these cats had a normal serum BHB concentration and one was only mildly increased at 0.13 mmol/L. Because the diagnostic evaluation for each patient was based on clinical presentation and the discretion of the attending clinician, it is not possible to completely exclude comorbidities, especially subclinical conditions. Likewise, the statistical power to detect differences between primary and secondary HL was limited by small numbers.
In summary, over 70% of cats with HL had serum BHB measurements above the reference range, and HL cats had the most marked increases of the 3 groups of cats in the study. Approximately 20% of cats with CKD or HT had mildly increased serum BHB concentrations. None of the cats in the study in which urine was available for evaluation were positive for urine ketones by dipstick, although 12 of these cats were ketonemic. HL, HT, and CKD are important differential diagnoses for ketonemia in cats, and increases in serum BHB concentrations must be interpreted with caution to avoid overdiagnosis of DM. Further studies may permit evaluation of serum BHB concentration in a variety of feline diseases as an objective marker of energy balance and recent nutritional status.
Acknowledgments
Funding: This research was supported by a University of Minnesota Small Companion Animal Research Grant. University of Minnesota College of Veterinary Medicine Small Companion Animal Grant, (Grant/Award Number).
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This research was supported by a University of Minnesota Small Companion Animal Research Grant.
Footnotes
Multistix® 10SG for Urinalysis, Siemans Healthcare Diagnostics Inc, Tarrytown, NY. Levels 1 and 2 of Microgenics UA controls
Brea, CA
Stanbio Laboratory, Boerne, TX
References
- 1. Laffel L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes/metab Res Rev 1999;15:412–426. [DOI] [PubMed] [Google Scholar]
- 2. Arora S, Henderson SO, Long T, et al. Diagnostic accuracy of point‐of‐care testing for diabetic ketoacidosis at emergency‐department triage: {beta}‐hydroxybutyrate versus the urine dipstick. Diabetes Care 2011;34:852–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Noyes KJ, Crofton P, Bath LE, et al. Hydroxybutyrate near‐patient testing to evaluate a new end‐point for intravenous insulin therapy in the treatment of diabetic ketoacidosis in children. Pediatr Diabetes 2007;8:150–156. [DOI] [PubMed] [Google Scholar]
- 4. Laffel LM, Wentzell K, Loughlin C, et al. Sick day management using blood 3‐hydroxybutyrate (3‐OHB) compared with urine ketone monitoring reduces hospital visits in young people with T1DM: a randomized clinical trial. Diabetes Med 2006;23:278–284. [DOI] [PubMed] [Google Scholar]
- 5. Aroch I, Shechter‐Polak M, Segev G. A retrospective study of serum beta‐hydroxybutyric acid in 215 ill cats: clinical signs, laboratory findings and diagnoses. Vet J 2012;191:240–245. [DOI] [PubMed] [Google Scholar]
- 6. Pazak HE, Bartges JW, Cornelius LC, et al. Characterization of serum lipoprotein profiles of healthy, adult cats and idiopathic feline hepatic lipidosis patients. J Nutr 1998;128:2747S–2750S. [DOI] [PubMed] [Google Scholar]
- 7. Biourge V, Groff JM, Fisher C, et al. Nitrogen balance, plasma free amino acid concentrations and urinary orotic acid excretion during long‐term fasting in cats. J Nutr 1994;124:1094–1103. [DOI] [PubMed] [Google Scholar]
- 8. Blanchard G, Paragon BM, Milliat F, et al. Dietary l‐carnitine supplementation in obese cats alters carnitine metabolism and decreases ketosis during fasting and induced hepatic lipidosis. J Nutr 2002;132:204–210. [DOI] [PubMed] [Google Scholar]
- 9. Fritzsche I, Buhrdel P, Melcher R, et al. Stability of ketone bodies in serum in dependence on storage time and storage temperature. Clin Lab 2001;47:399–403. [PubMed] [Google Scholar]
- 10. International Federation of Clinical Chemistry, Scientific Committee, Clinical Section. Expert Panel on Theory of Reference Values (EPTRV). The theory of reference values. Part 5. Statistical treatment of collected reference values. Determination of reference limits. Clin Chim Acta 1984;137:97F–114F. [PubMed] [Google Scholar]
- 11. Jones RP, Payne RB. Clinical Investigation and Statistics in Laboratory Medicine. London: ACB Venture Publications; 1997;196. [Google Scholar]
- 12. Behling‐Kelly E. Serum lipoprotein changes in dogs with renal disease. J Vet Intern Med 2014;28:1692–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Scarpioni R, Ricardi M, Albertazzi V, et al. Treatment of dyslipidemia in chronic kidney disease: Effectiveness and safety of statins. World J Nephrol 2012;1:184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zeugswetter F, Handl S, Iben C, et al. Efficacy of plasma beta‐hydroxybutyrate concentration as a marker for diabetes mellitus in acutely sick cats. J Feline Med Surg 2010;12:300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Prisco F, Picardi A, Iafusco D, et al. Blood ketone bodies in patients with recent‐onset type 1 diabetes (a multicenter study). Pediatr Diabetes 2006;7:223–228. [DOI] [PubMed] [Google Scholar]
- 16. Weingart C, Lotz F, Kohn B. Measurement of beta‐hydroxybutyrate in cats with nonketotic diabetes mellitus, diabetic ketosis, and diabetic ketoacidosis. J Vet Diagn Invest 2012;24:295–300. [DOI] [PubMed] [Google Scholar]
- 17. Duarte R, Simoes DM, Franchini ML, et al. Accuracy of serum beta‐hydroxybutyrate measurements for the diagnosis of diabetic ketoacidosis in 116 dogs. J Vet Intern Med 2002;16:411–417. [DOI] [PubMed] [Google Scholar]
- 18. Durocher LL, Hinchcliff KW, DiBartola SP, et al. Acid‐base and hormonal abnormalities in dogs with naturally occurring diabetes mellitus. J Am Vet Med Assoc 2008;232:1310–1320. [DOI] [PubMed] [Google Scholar]


