Abstract
Background
In children, frequent congenital malformations with concomitant agenesis of the corpus callosum are diagnosed by neuroimaging in association with other cerebral malformations, including interhemispheric cysts and ventriculomegaly. Similar studies providing full characterization of brain defects by in vivo magnetic resonance imaging (MRI), and correlations with the pertinent anatomic pathologic examinations are absent in veterinary medicine.
Hypothesis/Objectives
Congenital brain defects underlie the neurologic signs observed in Toyger cats selectively bred for a short ear phenotype.
Animals
Using proper pedigree analysis and genetic evaluations, 20 related Oriental‐derived crossbred Toyger cats were evaluated. Seven clinically healthy (carrier) cats and 13 clinically affected cats that had neurologic signs, short ear phenotype and concomitant complex brain anomalies were studied.
Methods
Complete physical and neurologic examinations and MRI were performed in all clinically healthy and affected cats. Postmortem and histopathologic examinations were performed in 8 affected cats and 5 healthy cats.
Results
Neurologic and MRI investigations confirmed 13 clinically affected cats with structural brain abnormalities. Ventriculomegaly with frequent concomitant supratentorial interhemispheric, communicating ventricular type‐1b cysts and multiple midline and callosal malformations were detected in all cats displaying neurologic signs. Genetic analysis confirmed autosomal recessive mode of inheritance with no chromosomal abnormalities.
Conclusions and Clinical Importance
Neuroanatomic dissections and histopathology were helpful for evaluation of abnormalities in midline brain structures, and for the full characterization of cysts. However, MRI was more sensitive for detection of small cysts. In this feline model, MRI diagnosis had extremely good correlation with pathologic abnormalities noted in the subset of animals that were examined by both modalities.
Keywords: Ependymal cyst, Hydrocephalus, Lesion, Magnetic resonance imaging
Abbreviations
- BAER
brainstem auditory evoked response
- CA
clinically affected
- CBC
complete blood count
- CH
clinically healthy
- CNS
central nervous system
- CSF
cerebrospinal fluid
- GFAP
glial fibrillary acidic protein
- HE/LFB
HE‐luxol fast blue
- HE
hematoxylin and eosin
- IHC
interhemispheric cyst
- MRI
magnetic resonance imaging
- PBS
phosphate buffered saline
- RBC
red blood cells
- TNCC
total nucleated cell count
- UC Davis
University of California – Davis
- URI
upper respiratory infection
- VMTH
Veterinary Medical Teaching Hospital
Malformations of the central nervous system (CNS), which are described sporadically in domestic animals,1 mostly occur in food animals in association with gestational exposure to toxins or infectious agents, with death occurring at an early age. In dogs, congenital malformations of CNS and the calvaria are common but often incidental findings, especially in toy breeds. In cats, they are rare and associated occasionally with in utero parvoviral infections.2
In people, most CNS malformations are thought to have multifactorial etiology that can include underlining familial inheritance of specific DNA variants as well as nutritional, infectious and environmental causes.3 Disorders of prosencephalic midline development such as holoprosencephaly and septo‐optic dysplasia are the most commonly reported malformations in people and include genetic and chromosomal anomalies.4 These complex congenital disorders in humans result in agenesis or dysgenesis of midline structures with variable involvement of the septum pellucidum and optic nerves with hypothalamic‐pituitary dysfunction.4 Midline structures are cortico‐cortical bundles of white matter connecting the cerebral cortex of one hemisphere with the other, and include the corpus callosum, and the anterior (rostral) and hippocampal commissures. Proper regulation of sequential events of formation, cleavage, and development of these midline structures is necessary for normal development of the septum pellucidum, hippocampus and ventricular system in the mammalian brain.4, 5
The advent of modern neuroimaging techniques allows for identification and precise definition of cerebral malformations and their relationship with the remainder of the brain. These techniques also enable accurate assessment of ventricular size, midline structures, and changes in cortical gyral and sulcal structure. Diagnostic neuroimaging techniques have indicated that congenital forebrain anomalies frequently occur with interhemispheric cysts (IHC) or hydrocephalus.4, 6, 7, 8 In domestic cats, inherited anomalies of the forebrain occasionally are suspected based on functional clinical abnormalities or confirmed by structural abnormalities identified by magnetic resonance imaging (MRI) or both. However, the occurrence of concomitant defects of midline structures with IHC and ventriculomegaly in the feline brain has not been documented. Current major distinct IHC entities described in human patients include ventricular neuroepithelial cysts9, 10, 11 and noncommunicating arachnoid cysts.12, 13, 14 In cats, there is a paucity of case reports of ventriculomegaly,15, 16, 17 mostly in association with arachnoid cysts.
Herein, we report an inherited condition affecting a family of related cats with congenital midline brain malformations and concomitant ventriculomegaly and IHC. This study describes the mode of inheritance and clinical presentation of the syndrome, the MRI characterization of structural defects, and the pathologic features in affected cats compared to carrier cats. A hypothesis for the sequential occurrence of these complex congenital malformations is discussed within the context of holoprosencephaly.
Materials and Methods
Pedigree Analysis and Genetic Evaluation
DNA was isolated from buccal swabs from 69 cats to develop an extended pedigree of individuals segregating for short ears and neurologic abnormalities. The parentage of affected and nonaffected cats was confirmed using standard microsatellite markers as previously described.18 The genetic analysis software COLONY19 was used to make assignments of parents and offspring. A karyotype was produced for the foundation tom at the University of Milan, Italy, before the cats were transfered to the United States. The G‐banded chromosomes were karyotyped from white blood cells isolated from anticoagulated whole blood using standard methods.20
Animals
The intact male Oriental cat (Fig 1), selected phenotypically for its short and rounded ears, was imported from Italy to the United States for use in a novelty cat‐breeding program. The owner bred this foundation tom to at least 4 mixed breed female domestic cats. All F1 offspring had normal ears. F1 female offspring were backcrossed to the tom, producing litters that had male and female kittens with normal and similar short ears. In addition, the production of F2 litters from brother to sister matings produced additional kittens with both normal and the short ear phenotype (Fig 1). The breeder suspected “clumsiness” in some of the cats with the short and rounded ears and after a fall, a kitten was examined at a specialty referral centre in Fountain Valley, California. After a diagnosis of marked ventricular dilatation was made using MRI, 3 additional adult cats examined by MRI had marked ventricular dilatation and associated anomalies (Table S1). The breeder contacted the investigators at the University of California (UC) – Davis to initiate a clinical, genetic and neuropathologic study of the cats. Cats were donated under approved Institutional Animal Care and Use protocols 11977, 15117, and 16691 for complete clinical evaluations and continued breeding studies. The donated cats included in this study were examined at the UC Davis Veterinary Medical Teaching Hospital between August 2010 and September 2013. Recorded patient data included age, sex, coat color, aural phenotype and clinical physical examination findings. Nineteen cats were referred alive and 1 cat was submitted after euthanasia along with accompanying imaging and clinical data.
Figure 1.

Phenotypic appearance of Oriental‐derived cats that are genetic carriers, and those that are affected with congenital brain abnormalities. (A) Foundation tom (No. 9 in Table S1; No. 10455 in Fig S1) imported from Italy to produce a breeding program of small‐eared cats. (B) and (C) oriental shorthair cats with a variety of backcross and F2 offspring that consistently presented with abnormal pinnae and domed craniums. (C) Offspring to the left are carrier individuals (Nos. 18310 and 18311 in Fig S1) that present with normal long pinnae and angular craniums. Offspring to the right are affected individuals (Nos. 18307, 18308 and 18309 in Fig S1) that present with abnormal short pinnae and domed craniums.
Laboratory Testing
Assessment of clinical status for the clinically healthy (CH) and clinically affected (CA) cats included physical and neurologic criteria, laboratory data based on results of CBC, serum biochemical profile, MRI, cerebrospinal fluid (CSF) analysis, and brainstem auditory evoked response (BAER) tests. Cerebrospinal fluid collected from the cisterna magna was evaluated for red blood cells (RBC), total nucleated cell count (TNCC), morphology, and protein concentration (mg/dL). A Nicolet Viking™ IV instrument1 was used to perform BAER tests on cats.
Magnetic Resonance Imaging
Magnetic resonance imaging was performed on 13 CA cats and compared to 6 CH cats. MRI was performed with a 1.5T GE Signa.2 Transverse T1W pre‐ and postcontrast3 T2W, and FLAIR sequences also were obtained as well as sagittal T1W pre‐ and postcontrast and T2W images. Magnetic resonance images were studied and assessed according to the following criteria: (1) normal gyral patterns and pachygyria (present, absent); (2) sulcal depth (normal, shallow, absent); (3) ventricular size of lateral, third and fourth ventricles (normal, mildly enlarged, moderately enlarged, markedly enlarged) and size of mesencephalic aqueduct (normal, stenotic, absent); (4) corpus callosum, interthalamic adhesion, septum pellucidum, hippocampus (normal, hypoplastic, absent); and (5) cystic structures (present, absent). Structural changes of CA cats were compared to CH cats, which were within normal limits on clinical and imaging evaluation, and served as normal controls.
Postmortem and Neuropathologic Examinations
Cats were humanely euthanized by barbiturate overdose immediately after diagnostic testing or 24–30 months later, after repeated diagnostic testing that included physical and neurologic examinations, MRI, and CSF analysis. A complete postmortem examination was performed immediately after euthanasia, and representative sections of extraneural tissue and the whole brain were immersion fixed in 10% buffered formalin for at least 24 hours and 7 days, respectively. For the middle and inner ear, the petrous portion of the temporal bone, tympanic bulla and portions of the occipital bone were dissected, and then decalcified in 15% formic acid. Brains were serially sectioned transversely at 3 mm intervals to provide slices corresponding with those visualized from MRI, except for 1 brain from an affected animal (cat # 3; Table S1) that was sagittally sectioned. Standardized tissues from the brain and other non‐neural tissues were processed routinely for paraffin embedding, sectioned at 5 μm, and evaluated after staining with hematoxylin and eosin (HE).
Gross and subgross sections of brains of the CA cats were studied and compared to those of the CH cats. Structural changes were assessed as follows: (1) normal gyral and sulcal patterns (present, absent); (2) ventricular size of lateral, third and fourth ventricles (normal, mildly enlarged, moderately enlarged, markedly enlarged); (3) corpus callosum, interthalamic adhesion, septum pellucidum, hippocampus, hippocampal commissure, fornix, fimbria, and cingulate gyrus (normal, absent, hypoplastic with or without segmental dygenesis); and (4) cystic structures (present, absent).
To characterize brain malformations histologically, additional histochemical stains (HE‐luxol fast blue [HE/LFB], Masson's trichrome, Bielschowsky's silver impregnation) were performed in selected cats, and immunohistochemical evaluation also was performed as follows: 4 μm sections were processed using primary antibodies specific for glial fibrillary acidic protein (1 : 600 dilution, GFAP, rabbit polyclonal antibody4), pan cytokeratin (1 : 100 dilution, Lu5, mouse monoclonal antibody5; 1 : 300 dilution, AE1/AE3, mouse monoclonal antibody6), and vimentin (1 : 300 dilution, 3B4, mouse monoclonal antibody7). Briefly, after antigen retrieval, slides were rinsed in deionized H2O and placed in 0.1 m phosphate‐buffered saline (PBS), pH 7.4 or TRIS‐buffered saline, pH 7.6. The antibody diluent was PBS‐Tween 20 (0.02%) and the blocking reagent was either PBS‐Tween 20 (0.2%) and 10% normal horse serum or TBS‐Tween 20 (0.025%) and 3% normal goat serum. Sections were blocked for 20 minutes. After blocking, the primary antibody was applied without rinsing and incubated for 1 hour. Antibody‐enzymatic binding reactions for AE1/AE3 and GFAP were detected with the Dako Envision System‐HRP mouse K4001‐Ms Env and rabbit K4003‐Rb Env, respectively. Biocare Medical 4Plus Detection System (mouse link HM606‐Ms LL) immunolabeling was used instead for Lu5 and vimentin with secondary biotinylated horse antimouse antibodies, and subsequently a streptavidin complex staining method.8 Final detection of any peroxidase immunoreactivity was visualized with NovaRed9 after the manufacturer's instructions. Slides were counterstained with Mayer's hematoxylin. Our standard laboratory positive control tissues for each of the above antigenic markers were tested in parallel to provide consistency of results. Negative controls consisted of omission of primary antibody and substitution with PBS‐Tween 20 (0.02%).
Results
Pedigree Analysis and Genetic Evaluation
From the breeder's breeding trials of the foundation tom to at least 4 females of nonoriental breed and domestic shorthair cats, no F1 offspring had the similar short ear phenotype of the tom, thereby excluding the trait as Y‐linked because offspring were not affected. An X‐linked dominant transmission also was excluded because female F1 offspring were CH. The F1 female offspring were backcrossed to the foundation tom producing litters that had male and female kittens with both normal and short ears. Although approximately 50% of the kittens had the short ear phenotype, actual litter sizes and sex distribution were not available. In addition, the production of F2 litters from brother to sister matings produced additional kittens with the short ear phenotype, but less frequently than observed in the backcross generation. Because the female cats were unrelated and the backcrossing to the foundation tom produced kittens with the same phenotype, the syndrome was concluded to have an autosomal recessive mode of inheritance.
The DNA from 66 of 69 submitted samples produced genotypes adequate for parentage analyses (Table S2). Fifty cats formed an extended pedigree, which required the inclusion of 10 additional unknown parentage cats that had not been sampled. Parentage analysis assigned 32 cats, whereas 18 cats represented controlled matings. All affected cats were descendants of the foundation tom, but not all outcross cats or cats completing all generations were available for analysis. Two CA cats (#'s 2 and 13; Table S1) could not be assigned parentage as well as 1 CH cat. Many of the parentage assignments failed because both parents were not represented in the sample set. The cats included in the clinical examinations and histopathology studies are presented on the pedigree (Fig S1).
The foundation tom (cat # 9; Table S1) had a normal male karyotype of 38, XY (Fig S2). No gross translocations or insertions and deletions were observed.
Clinical Examination
Clinically healthy cats included 7 cats (3 female; 4 male) ranging in age from 9 months to 4 years. All CH carrier cats had elongate (normal) ear morphology (Fig 1) and normal physical and neurologic examinations. All had signs of chronic upper respiratory infection (URI) of varying severity and bilateral ear mite infestation.
Clinically affected cats included 13 cats (6 female; 7 male), aged 6 months to 4 years that had abnormalities in ear morphology and neurologic examination. All CA cats had chronic URI of variable severity and bilateral ear mite infestation. All cats had short, rounded pinnae (Fig 1), stenotic horizontal ear canals, and moderate to severe bilateral tarsal valgus. On neurologic examination, all cats with abnormal pinnae had quiet but appropriate mentation. Cranial nerve examination was within normal limits for all CA cats. Visual gait evaluation indicated that all CA had variable degrees of increased tarsus valgarum and mild plantigrade stance in the pelvic limbs when standing or walking slowly. Five CA (#'s 2, 3, 4, 12, and 13; Table S1) cats had mild generalized ataxia when walking but all were able to jump on and off of chairs and counters in their environment. Postural reactions were evaluated with proprioceptive positioning tests, hopping reactions, and visual and tactile placing reactions in 12/13 CA. Mild to marked postural reactions deficits were seen in the pelvic limbs in 11/13 CA and 4 of the 11 also had postural reaction deficits in the thoracic limbs. One cat could not be adequately evaluated and another cat had no convincing postural deficits. Segmental reflexes were within normal limits in all CA cats. There was no apparent cranial or spinal pain elicited by palpation of the head and vertebral column and no cranial or spinal structural abnormalities were suspected except in 1 CA cat. In this cat, doming of the calvarium, palpable fontanelles, depressed frontal bone and facial asymmetry were observed. In addition, CA females never became pregnant and never produced kittens when bred to CA, CH carrier cats and unrelated normal males. The females had normal displays of estrus and were receptive to all males.
Laboratory Testing
Routine laboratory tests, including CBC and serum biochemical profile, were performed in 5 of 7 CH cats. Moderate leukocytosis, considered consistent with severe URI, was present in 2 of 5 cats. Serum biochemistry results were within normal limits. Cerebrospinal fluid analysis was performed on 5/7 CH cats and all results were within normal reference ranges. Four CH cats had BAER tests performed and all results were within normal limits.
Complete blood counts and serum biochemical profiles were performed on 9/13 CA cats. Moderate leukocytosis, consistent with severe, chronic URI was found in 2 of 9 cats. Eleven CA cats had normal CSF results. Five CA cats had BAER performed, and all results were within normal limits.
Magnetic Resonance Imaging
Magnetic resonance imaging was performed on 6/7 CH cats and 13/13 CA cats. Structural changes were detected in all CA only, with no structural abnormalities identified in CH cats. The spatial distribution of changes involved the telencephalon, diencephalon and mesencephalon, and the associated ventricular system (lateral, third, and mesencephalic aqueduct; Table S1). Abnormalities in gyral patterns (Fig 2) were present in 9 of the 13 affected cats; in 2 cats the patterns could not be assessed because of severe thinning and flattening of the cerebral cortices; and in 2 cats they were normal. Shallow sulcal depth was present in 10 of 13 CA cats; sulci were absent in 2 CA cats and 1 cat was considered normal.
Figure 2.

Encephalic malformations evaluated by magnetic resonance imaging (MRI) and macroscopic examination. Brains of clinically healthy carrier cats (top panel) compared to mild and severely‐affected cats. These series of brain images include gross specimens [fresh, dorsal view] and transversal sections of formalin‐fixed brains at telencephalic and diencephalic levels, and their corresponding coronal sections of T2W MRI images. Compared to clinically healthy brains (A), note the enlargement and rounding of the occipital lobes of affected cats (B and C). The precruciate gyri, cruciate sulci, and postcruciate gyri are discernable in the unaffected and mildly affected brains, but there is generalized asymmetric disorganization and often widening of gyri of the occipital, parietal, and temporal lobes of the mildly affected brain (B, E and H), and nearly complete loss of discernable sulci and gyri in the severely affected brain (C, F and I). There is moderate to severe dilation of the lateral (Lat) and third ventricles (T) in mild and severely affected cat, respectively, with corresponding thinning of the cerebral cortex. The rostral white commissure (F, blue arrowhead) remains present in all cat's categories. However, abnormalities of other midline structures are present: (1) thinning of the corpus callosum (F, blue arrow), (2) loss of the interthalamic adhesion (I, red arrow) and (3) septum pellucidum (E, purple arrow). The parahippocampal gyri (I, black asterisk), and both septal and temporal hippocampal formation are remarkably reduced in size only in affected animals. Note that T2W MRI images of affected cats (K and L) have various degrees of abnormal gyral patterns with shallow sulcal depth, lack of the interthalamic adhesion and ventriculomegaly when compared to clinically healthy animals (J).
Midline structures (Fig 2), including the corpus callosum, interthalamic adhesion and septum pellucidum, were abnormal in all CA cats. The corpus callosum was hypoplastic in 3 cats and absent in 10 cats. The interthalamic adhesion was abnormal in all CA cats (characterized as hypoplastic in 4 cats, questionably present in 3 cats, and absent in 6 cats). The septum pellucidum was abnormal in all CA cats (hypoplastic in 8 cats and absent in 5 cats). In addition, the hippocampus was normal in only 1 cat, hypoplastic in 11 cats, and not visualized in 1 cat.
Ventricular dilatation was present in all CA cats (Figs 2, 3). The lateral ventricles were moderately dilated in 4 cats and markedly dilated in 9 of the CA cats. The third ventricle was assessed as normal in 1 cat, mildly dilated in 4 cats, moderately dilated in 3 cats, and markedly dilated in 5 cats. The fourth ventricle was assessed as normal in 12 CA cats and mildly dilated in 1 cat. The mesencephalic aqueduct was abnormal in all CA cats (stenotic in 6 cats and absent in 7 cats).
Figure 3.
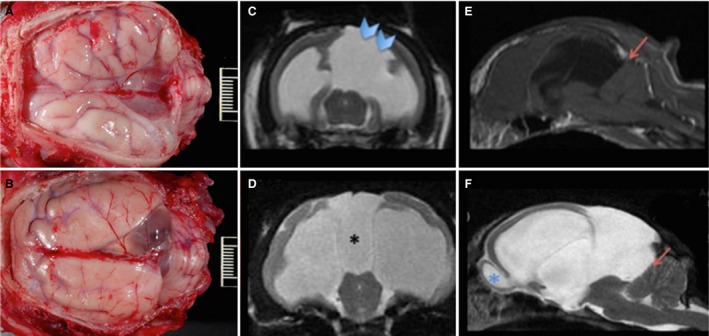
Communicating interhemispheric cysts. Sequence of images of 2 feline brains with moderate (A, C) and severe (B, D–F) ventriculomegaly and cyst formation, evaluated grossly (A, B), by coronal (C, D) T2W magnetic resonance imaging (MRI) and by axial T1W (E) and T2W (F) MRI images of the brain in B. Cysts (A, B) communicate with the lateral ventricles (C, D) and displaced the occipital lobes and cerebellum (E, F; red arrows). The cyst wall in the cat with the unilateral cyst is faintly perceptible on the coronal section (C, blue arrow heads). In some cats, a third large cyst was observed communicating with the suprapineal recess (D, black asterisk), resting on the midbrain and tentorium cerebelli and displacing the cerebellum caudally (E, F, red arrows). There is prominent ventriculomegaly in both cats (C, D). In the cat more severely affected, there is enlargement of the rostral recess of the lateral ventricle at the level of the olfactory bulb (F, blue asterisk).
In 11 CA cats, the suprapineal recess was dilated. In addition, a midline interhemispheric cystic dilatation of the lateral ventricle at the occipital lobe was present in 11 CA cats (Fig 3). The cyst was present on the left side in 4 cats, and on the right side in 7 cats.
Histopathological Correlation with MRI Findings
A complete neuropathologic examination performed in 5 CH cats identified no macro‐ or microscopic brain abnormalities. Similar examinations of 8 CA cats confirmed abnormalities affecting the prosencephalon (telencephalon and diencephalon) and mesencephalon, and the associated ventricular system. More specifically, these malformations included dilated ventricles, abnormal midline and limbic structures, and hemispheral cysts with or without a suprapineal cyst (Table S1). Overall, lesions detected by MRI imaging were confirmed by histopathology except in those 5 CA cats for which brains were not available Table S1.
Sulcal and gyral cerebral profiles and the sulcal depth of the cerebral cortex were abnormal in all CA cats examined (Fig 2). Abnormalities included generalized asymmetry and variable widening of gyri and sulci that were most severe in the occipital, temporal and parietal lobes. In the majority of cats, the cruciate sulcus was prominent and easily visualized. In severely affected animals, however all other gyri and sulci were blunted, irregularly oriented and ill‐defined (Fig 2). All CA cats had some degree of defect in midline structures, which included the corpus callosum, interthalamic adhesion, septum pellucidum, and the hippocampal commissure, fornix, and fimbria. The rostral white commissure, a midline structure, was present and within normal limits in all cats. The severity of the midline abnormalities ranged from absence of structures to hypoplasia to hypoplasia with segmental dygenesis (Fig 2). In cats with hypogenesis of the corpus callosum, the genu and rostral body were thin, and there was complete absence of the splenium. In the regions of dysgenesis, there was continuity and connection of the cerebral cortex and cingulate gyrus with the septum pellucidum or the fornix and fimbria. Consistently, the cingulate gyrus and hippocampal formation were hypoplastic in all CA cats. Moreover, the hippocampus was small and displaced caudally, and in 5 cats (#'s 2, 3, 4, 6, 7), the cingulate gyrus was rotated medially.
All CA cats had dilatation of the lateral ventricles. Moreover, 7 cats had ventriculomegaly of the third ventricle, and 1 cat (# 2) had mild enlargement of the fourth ventricle. In the most severely affected cats (#'s 1, 8), dilatation of the ventricular rostral recess at the level of the olfactory bulb was observed, there was broad communication of the lateral ventricles, and a poorly developed interhemispheric fissure was observed. Moreover, these cats also had partial dysgenesis of the septum pellucidum, thus contributing to the lateral ventricular fusion. Cat # 1 also had broad communication of the lateral ventricles with the third ventricle.
As a consequence of the ventriculomegaly, the gray and white matter of the cerebral cortices of all cats was variably attenuated, correlating with the severity of ventricular dilatation. No lesions consistent with cortical dysplasia were found in any CA cats based on examination of HE and histochemically stained sections or those stained with silver impregnation (not shown). In addition, no clinically relevant abnormalities were noted within the pituitary gland, but extensive and systemic evaluation of this structure was not performed.
Neuropathologic Characterization of Concomitant Interhemispheric Cysts
Seven of 8 CA cats had a midline cyst that was contiguous with 1 of the lateral ventricles. Cysts were unilateral and right‐sided in 6 cats, and bilateral in 1 cat (Fig 3). Cysts protruded caudally, compressing the ipsilateral occipital lobes, which were decreased in size when compared to the contralateral side. Based on HE examination, cysts were lined by variably ciliated and polygonal ependymal‐like cells as confirmed by their strong immunoreactivity to GFAP (Fig 4, left image) and vimentin (not shown), and lack of immunoreactivity to the pan cytokeratin antigenic markers AE1/AE3 (not shown) and Lu5 (Fig 4, right image). The outer layer of the cyst, which was subjacent to the GFAP‐positive ependymal lining, was immunonegative for GFAP, and consistent with a fine fibrovascular stroma based on HE examination. In the 4 least affected cats, the hippocampus unilaterally was caudally displaced as described above. By contrast, in the 3 most severely affected cats, the occipital lobe was displaced laterally, and the hippocampus and cerebellum were displaced caudally. In these extremely affected cats, the cyst protruded dorsally with bulging of the leptomeninges, and was visible in the intact entire brain before serial trimming (Fig 3). In 1 cat (# 2; Table S1), the IHC ruptured. Moreover, 6 CA cats had cystic dilatation of the suprapineal recess. In the most severely affected cats, this recess enlargement resulted in dorsoventral compression of the tectum of the mesencephalon, creating a concave “U” shape (Fig 3).
Figure 4.
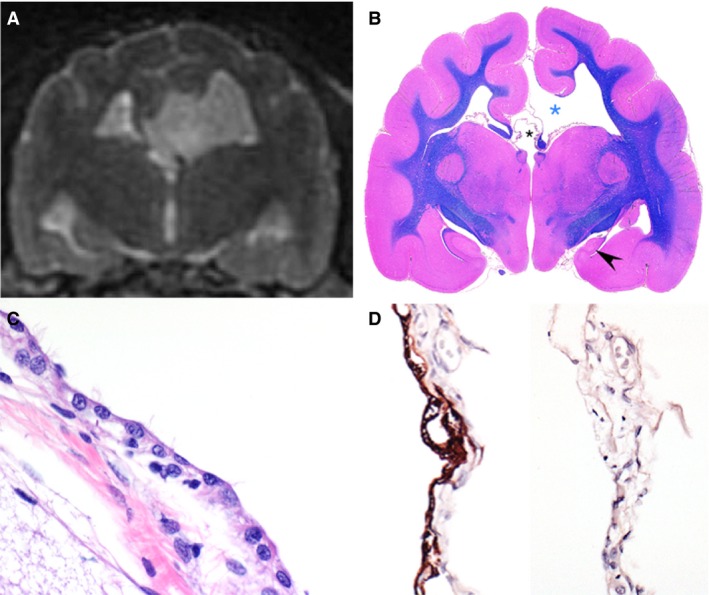
Brain of 1 affected cat illustrating 2 communicating cysts. Coronal T2W magnetic resonance image (A), subgross image of the corresponding transversal section stained with luxol fast blue/hematoxylin and eosin (B), hematoxylin and eosin (HE) of cyst lining (C), and glial fibrillary acidic protein (GFAP; left) and pancytokeratin (Lu5; right) immunohistochemistries (D). The first cyst is a distended thin membrane that communicates with the lateral ventricle and is bordered by the cerebral cortex and white matter consistent with fimbria (B, blue asterisk), and causes a midline shift noted on imaging. The second cyst is a dilation of the suprapineal recess and communicates with the third ventricle (B, black smaller asterisk). The hippocampus in affected cats is often hypoplastic and displaced caudally. As noted in this animal, the septal hippocampus is absent and there is severe hypoplasia of the temporal hippocampus (B, black arrowhead) in the affected hemisphere (right side). Also note that the cingulate is rotated medially (just above the blue asterisk). Also notable is the lack of corpus callosum (interrupted by the enlarged pineal recess, black asterisk) and interthalamic adhesion. The cyst is linked by membrane of variably ciliated polygonal cells (C) that are GFAP positive and cytokeratin negative, and rest upon a GFAP negative fibrovascular membrane consistent with an ependymal cyst (D). (C) HE, ×600. (D) Immunohistochemistry for GFAP [left] and pancytokeratin Lu5 [right], ×400.
Extraneural Histopathological Findings
Although the pinnae of the ears in CA cats were shorter and rounded, no structural histologic abnormalities of the middle and inner ear were identified in the examined cats. Many affected cats had multifocal ossification, sometimes with hematopoiesis, of the dura mater, which was considered an incidental finding. Apart from cerebral anomalies, other findings on postmortem examination included upper respiratory infection, bronchopneumonia, gastrotyphlocolitis, enteric and ecto‐parasitism, and otitis externa and media, which affected both CA and CH cats. One CA cat had a bilobed gallbladder and adjacent accessory gallbladder. There were no histologic abnormalities of the reproductive tract in examined cats.
Discussion
This prospective study reports an autosomal recessive neurologic syndrome in Toyger cats that suffered from various brain midline defects with concomitant IHC and ventriculomegaly. Malformations likely resulted from a breeding selection designed to create a tiger‐like physical phenotype characterized by small round ears, because only those cats with small ears had dysgenesis of the septum pellucidum, interthalamic adhesion and all of the midline commissures (with the exception of the rostral white commissure) and hypoplasia of the hippocampus. Overall, these changes closely approximate variants of human holoprosencephaly21, 22 or septo‐optical dysplasia, but lacking characteristic lesions in the falx cerebri, optic nerves, and pituitary gland, as well as the palato‐facial clefts or cortical heterotopias.
This syndrome appears clinically mild but is characterized by marked structural brain malformations unlike what might be expected in children born with such anomalies. The lack of profound clinical signs is not unusual in domestic animals with major structural anomalies of the forebrain, possibly because of the limited importance of the forebrain in accomplishing normal feline activities. The extrapyramidal motor system, which begins in the red nucleus of the midbrain and requires little control from higher centres, regulates control of most of the cat's motor skills and upon which much of the neurologic examination is based. As such, decreased or altered cerebral function is not assessed well on standard neurologic examination of the cat, except for evaluation of a normal state of arousal and environmental interaction. The so‐called “quiet” mental state of these cats was the primary reason for their referral for evaluation. This abnormality may be the equivalent of such structural anomalies in children.
Atypical external ear morphology was consistently noted in the CA cats as compared to the typical long pinnae of the related CH cats. However, examination of internal ear structures identified no overt developmental or functional abnormalities (based on BAER examination). The link between the neural abnormalities and external ear development was not identified, but the appearance of the ears serves as an important phenotypic marker for CA cats.
The major structural malformations of this syndrome were detected in vivo by neuroimaging and further confirmed by postmortem anatomic pathologic investigations. Ventriculomegaly was the most common anomaly identified in CA cats. A common cause of ventriculomegaly is hydrocephalus, a group of disorders in which excessive CSF accumulates within the cerebral ventricles. In acquired conditions in domestic animals, hydrocephalus frequently occurs secondary to obstruction of ventricular outflow of CSF caused by a pathologic process such as infection or tumor and usually is associated with changes in ventricular CSF pressures. However, in congenital forms of the disease, the accumulation of CSF usually is thought to represent an ex‐vacuo type of CSF accumulation caused by decreased formation or loss of brain tissue, which is generally not associated with changes in CSF flow and pressure.23, 24 Although intraventricular pressure was not measured with an intraventricular catheter, no evidence of increased pressure was observed on clinical examination, on MRI, during CSF collection or at necropsy. In addition, several CA cats were followed up for years without changes in any of these variables and without detectable progression of disease clinically or on MRI. Although rarely documented in domestic animals, during development, ventricular stenosis may have resulted in ventricular dilatation with an eventual return to a homeostatic pressure.
The various brain malformations in these cats may have occurred secondary to an initial embryologic aberration of the commissural plate occurring during early brain development. Based on studies of human embryologic development,25 midline cystic malformations can represent an expansion of the roof plate of the brain vesicle itself, and include supratentorial cysts such as communicating IHC with callosal agenesis or dorsal cysts with holoprosencephaly. In these cats, IHC most likely occurred because of expansion of the suprapineal recesses and lateral ventricle, thus contributing to the severe ventriculomegaly. With regard to midline structures, interhemispheric commissures arise embryologically from the commissural plate of the lamina terminalis in the third month of intrauterine life, and develop in a rostrocaudal direction. Sequentially, over the course of 5 months, the rostral white commissure develops; then the hippocampal commissure, followed by the corpus callosum and, finally, the septum pellucidum.5, 26, 27 This temporal sequence suggests that agenesis of the corpus callosum is an epiphenomenon and should not be considered a specific entity, and emphasizes that anomalies during embryogenesis are time‐dependent. In summary, the spectrum of midline anomalies reported in this family of cats involves only those of the commissural plate (agenesis of the septum pellucidum with or without involvement of the corpus callosum) but not those reported in the human literature involving both the commissural and chiasmatic plates (septo‐optic dysplasia) or even more complex anomalies involving the commissural, chiasmatic and hypothalamic plates (septo‐optic‐hypothalamic syndrome).
Conversely, midline cysts can consist of extra‐axial structures such as an arachnoid membrane or migrating ependymal cells and are exemplified by infratentorial cysts such as interhemispheric arachnoid or ependymal cysts with callosal agenesis. The classification of callosal agenesis with cyst formation has always been controversial.26, 28 Briefly, cysts are broadly classified according to (1) anatomy (supratentorial versus infratentorial), (2) their relationship with the ventricular system (communicating versus non‐communicating), and (3) cell of origin (choroid plexus, ependymal or meningeal).28 Thus, type 1 cysts appear to be a diverticulation or an extension of the third or lateral ventricles whereas type 2 cysts are loculated and do not communicate with the ventricular system.26 Furthermore, subtype 1a excludes other cerebral malformations whereas subtypes 1b and 1c include diencephalic malformations and cortical dysplasia, respectively. Therefore, cats affected with this syndrome present with a supratentorial interhemispheric communicating ventricular subtype 1b cyst. In this scenario, the formation of the corpus callosum could have been partially split by the interposition of the cyst between the two hemispheres. Last, pathogenetic mechanisms underlying the ventriculomegaly and IHC formation are suggestive of a defect of the diencephalic roof plate with persistent cava, which by definition results in expansion of the ventricular tela coroidea and a ventricular neuroepithelial cyst.
Another classification of intracranial cysts is based on the cell of origin which is divided into either arachnoid or neuroepithelial types, with the latter representing a heterogenous group that includes ependymal, choroid plexus and glio‐ependymal cell types.29 In all 3 categories, histopathology is similar and characterized by ciliated epithelial‐like cells that are supported by connective tissue. Determination of cyst type is based on immunoreactivity to GFAP, cytokeratin, and less consistently by other markers such as S100. In these cats, cysts were most consistent with an ependymal origin based on the presence of cilia, variably GFAP immunoreactivity of cells lining the inner cystic spaces and not the outer space (which was supported by a fibrous stroma), and lack of immunoreactivity to cytokeratins. Ventricular cysts are described very rarely in domestic animals and have not been reported in cats.17
In summary, this cohort of cats presented with primary abnormalities of the development of the commissural plates of the brain, which resulted in malformations of midline prosencephalic structures, and a defect of the diencephalic roof plate with persistent cava. Full preservation of chiasmatic and hypothalamic plates is suspected in these cats because features such as hemispheric fusion, cerebral heterotopias, optic nerve hypoplasia, or cranio‐fronto‐nasal deformities are lacking. Overall, the syndrome resembles a mild variant of holoprosencephaly in humans. Holoprosencephaly in humans is associated with genetic and chromosomal anomalies. Genetic analyses in this family of cats suggested an autosomal recessive mode of inheritance with complete penetrance, but with a slightly variable presentation. Karyotype analysis of the foundation tom indicated a normal chromosomal complement (38, X,Y) with no gross chromosomal abnormalities. Candidate genes responsible for this autosomal recessive syndrome have not yet been identified.
Supporting information
Fig S1. Pedigree of Oriental shorthair outcrossed cats segregating for cerebral anomalies.
Fig S2. Karyotype of affected Oriental shorthair proband.
Table S1. Characterization by in vivo and postmortem examinations of neuroanatomical location of brain anomalies and type of defects in 13 cats.
Table S2. Short tandom repeat genotypes used to reconstruct the Oriental shorthair pedigree.
Acknowledgments
The authors appreciate access to the cats and discussions with owners and breeders, especially Judy Sugden and initial clinical diagnostic testing at the VCA, All‐Care Center Animal Referral Center (Fountain Valley, CA) with Drs. A. Reed and C. Crosta. Also, we thank Dr. P. Parma of the University of Milan for karyotyping the foundation tom. Thanks to Barry Puget, Kurt Takahashi, Cristopher Kwong and Mike Manzer at UC Davis for their excellent histologic and immunohistochemical technical support. We thank Professor Robert J Higgins for critical reading of the manuscript. This work was supported in part by funding from the National Center for Research Resources R24 RR016094 and is currently supported by the Office of Research Infrastructure Programs/OD R24OD010928.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Footnotes
Nicolet Biomedical In., Madison, WI
Milwaukee, WI
Magnevist, 469.01 mg gadopentate per mL Berlex Laboratories, Wayne, NJ, with a 0.1 mmol/kg dosage
Dako Z0334, Carpinteria, CA
BioCare Medical CM043C, Concord, CA
BioGenex MU0110UC, Fremont, CA
Dako M7020, Carpinteria, CA
4PLUS LL Streptavidin HRP, Biocare Medical, Concord, CA
Vector SK‐4800, Burlingame, CA
References
- 1. Zachary JF. Nervous system In: Zachary JF, McGavin MD, eds. Pathologic Basis of Veterinary Disease, 5th ed St. Louis, MO: Elsevier Health Sciences; 2012:771–870. [Google Scholar]
- 2. Tani K, Taga A, Itamoto K, et al. Hydrocephalus and syringomyelia in a cat. J Vet Med Sci 2001;63:1331–1334. [DOI] [PubMed] [Google Scholar]
- 3. Chen CY, Zimmerman RA. Congenital brain anomalies In: Zimmerman RA, Gibby WA, Carmody RF, eds. Neuroimaging: Clinical and Physical Principles. New York: Springer Science & Business Media; 2000:491–530. [Google Scholar]
- 4. Raybaud C. The corpus callosum, the other great forebrain commissures, and the septum pellucidum: anatomy, development, and malformation. Neuroradiology 2010;52:447–477. [DOI] [PubMed] [Google Scholar]
- 5. Yousefi B. Brain commissural anomalies In: Mantamadiotis T, ed. When Things Go Wrong – Diseases and Disorders of the Human Brain. InTech; 2012:69–110. [Google Scholar]
- 6. Uematsu Y, Kubo K, Nishibayashi T, et al. Interhemispheric neuroepithelial cyst associated with agenesis of the corpus callosum. A case report and review of the literature. Pediatr Neurosurg 2000;33:31–36. [DOI] [PubMed] [Google Scholar]
- 7. Barkovich AJ. Congenital malformations of the brain and skull In: Barkovich AJ, ed. Pediatric Neuroimaging, 4th ed London: Lippincott Williams & Wilkins; 2005:291–405. [Google Scholar]
- 8. Griffiths PD, Reeves MJ, Morris JE, et al. A prospective study of fetuses with isolated ventriculomegaly investigated by antenatal sonography and in utero MR imaging. AJNR Am J Neuroradiol 2010;31:106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Young JN, Oakes WJ, Hatten HP. Dorsal third ventricular cyst: an entity distinct from holoprosencephaly. J Neurosurg 1992;77:556–561. [DOI] [PubMed] [Google Scholar]
- 10. Brocklehurst G. Diencephalic cysts. J Neurosurg 1973;38:47–51. [DOI] [PubMed] [Google Scholar]
- 11. Rothner AD, Duchesneau PM, Weinstein M. Agenesis of the corpus callosum revealed by computerized tomography. Dev Med Child Neurol 1976;18:160–166. [DOI] [PubMed] [Google Scholar]
- 12. Coffey RJ, Lunsford LD. Supracallosal interhemispheric arachnoid cyst: resolution after intracystic hemorrhage and infection. Surg Neurol 1988;29:153–158. [DOI] [PubMed] [Google Scholar]
- 13. Mori K. Giant interhemispheric cysts associated with agenesis of the corpus callosum. J Neurosurg 1992;76:224–230. [DOI] [PubMed] [Google Scholar]
- 14. Rizk E, Awad AJ, Tubbs RS, et al. Dorsal third ventricular cysts revisited. Childs Nerv Syst 2013;29:2271–2274. [DOI] [PubMed] [Google Scholar]
- 15. Reed S, Cho DY, Paulsen D. Quadrigeminal arachnoid cysts in a kitten and a dog. J Vet Diagn Invest 2009;21:707–710. [DOI] [PubMed] [Google Scholar]
- 16. Lowrie M, Wessmann A, Gunn‐Moore D, Penderis J. Quadrigeminal cyst management by cystoperitoneal shunt in a 4‐year‐old Persian cat. J Feline Med Surg 2009;11:711–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. MacKillop E. Magnetic resonance imaging of intracranial malformations in dogs and cats. Vet Radiol Ultrasound 2011;52:S42–S51. [DOI] [PubMed] [Google Scholar]
- 18. Lipinski MJ, Amigues Y, Blasi M, et al. An international parentage and identification panel for the domestic cat (Felis catus). Anim Genet 2007;38:371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jones OR, Wang J. COLONY: a program for parentage and sibship inference from multilocus genotype data. Mol Ecol Resour 2010;10:551–555. [DOI] [PubMed] [Google Scholar]
- 20. Seabright M. A rapid banding technique for human chromosomes Lancet 1971;2:971–972. [DOI] [PubMed] [Google Scholar]
- 21. Greenberg MS. Handbook of Neurosurgery, Vol. XIV. Tampa: Thieme; 2010:1337. [Google Scholar]
- 22. Frim DM, Gupta N. Pediatric Neurosurgery. Georgetown: Landes Bioscience; 2006:252. [Google Scholar]
- 23. Summers BA, Cummings JF, DeLahunta A. Veterinary Neuropathology. St Louis: Mosby; 1995:68–94. [Google Scholar]
- 24. De Lahunta A, Glass EN, Kent M. Veterinary Neuroanatomy and Clinical Neurology, 2nd ed Philadelphia: Elsevier Health Sciences; 2014:30–52. [Google Scholar]
- 25. Utsunomiya H, Yamashita S, Takano K, et al. Midline cystic malformations of the brain: imaging diagnosis and classification based on embryologic analysis. Radiat Med 2006;24:471–481. [DOI] [PubMed] [Google Scholar]
- 26. Lena G, van Calenberg F, Genitori L, Choux M. Supratentorial interhemispheric cysts associated with callosal agenesis: surgical treatment and outcome in 16 children. Childs Nerv Syst 1995;11:568–573. [DOI] [PubMed] [Google Scholar]
- 27. Leech RW, Shuman RM. Holoprosencephaly and related midline cerebral anomalies: a review. J Child Neurol 1986;1:3–18. [DOI] [PubMed] [Google Scholar]
- 28. Barkovich AJ, Simon EM, Walsh CA. Callosal agenesis with cyst: a better understanding and new classification. Neurology 2001;56:220–227. [DOI] [PubMed] [Google Scholar]
- 29. Moriyama E, Nishida A, Sonobe H. Interhemispheric multiloculated ependymal cyst with dysgenesis of the corpus callosum: a case in a preterm fetus. Childs Nerv Syst 2007;23:807–813. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Pedigree of Oriental shorthair outcrossed cats segregating for cerebral anomalies.
Fig S2. Karyotype of affected Oriental shorthair proband.
Table S1. Characterization by in vivo and postmortem examinations of neuroanatomical location of brain anomalies and type of defects in 13 cats.
Table S2. Short tandom repeat genotypes used to reconstruct the Oriental shorthair pedigree.


