Abstract
Background
Dilated cardiomyopathy (DCM) is a common cause of morbidity and mortality in the Irish Wolfhound (IW). However, the benefit of medical treatment in IW dogs with preclinical DCM, atrial fibrillation (AF), or both has not been demonstrated.
Objectives
Compare the time to develop congestive heart failure (CHF) or sudden death in IW dogs with preclinical DCM, AF, or both receiving monotherapy with pimobendan, methyldigoxin, or benazepril hydrochloride.
Animals
Seventy‐five client‐owned IW dogs.
Methods
Irish Wolfhound dogs were prospectively randomized to receive pimobendan (Vetmedin®)1, benazepril HCl (Fortekor®)2, or methyldigoxin (Lanitop®)3 monotherapy in a 1:1:1 ratio in a blinded clinical trial. The prospectively defined composite primary endpoint was onset of CHF or sudden death. To assure stringent evaluation of treatment effect, data from dogs complying with the study protocol were analyzed.
Results
Sixty‐six IW fulfilling the study protocol included 39 males, 27 females; median (interquartile range) age, 4.0 years (3.0–5.0 years) and weight, 70.0 kg (63.0–75.0 kg). Primary endpoint was reached in 5 of 23 (21.7%) IW receiving pimobendan, 11 of 22 (50.0%) receiving benazepril HCl, and 9 of 21 (42.9%) receiving methyldigoxin. Median time to primary endpoint was significantly longer for pimobendan (1,991 days; 65.4 months) compared to methyldigoxin (1,263 days; 41.5 months; P = .031) or benazepril HCl‐(997 days; 32.8 months; P = .008) treated dogs.
Conclusions and Clinical Importance
In IW dogs with preclinical DCM, AF or both, pimobendan monotherapy significantly prolonged time to onset of CHF or sudden death than did monotherapy with benazepril HCl or methyldigoxin.
Keywords: Dog, Heart disease, Heart failure, Occult cardiomyopathy, Survival, Treatment
Abbreviations
- ACEI
angiotensin‐converting enzyme inhibitor
- AF
atrial fibrillation
- CHF
congestive heart failure
- CI
confidence interval
- DCM
dilated cardiomyopathy
- FAS
full analysis dataset
- HCl
hydrochloride
- ITT
intention to treat population
- IW
Irish Wolfhound
- MMVD
chronic myxomatous valve disease
- PPS
per protocol set
- SD
sudden death
Myocardial disease is an important cause of morbidity and mortality in large and giant breed dogs. The Irish Wolfhound (IW) breed has been noted for its high prevalence of dilated cardiomyopathy (DCM), atrial fibrillation (AF), or both.1, 2, 3, 4, 5 , 4, 5, 6 In 1 large study of 1,038 IW dogs prospectively screened between 1990–2004, approximately 29% were affected,7 and in selected populations of IW dogs, AF has been reported in up to 21% of screened animals.4, 5, 6, 7, 4, 7 Moreover, AF may develop before structural or functional changes become apparent, and some affected dogs may progress to DCM and congestive heart failure (CHF).6, 7, 8 , 5, 7
The effects of cardiac drugs on morbidity and mortality in humans have received much attention. Indeed, outpatient management guidelines for heart failure patients emphasize a systematic approach, incorporate heart failure performance measures, and stress evidence‐based treatment which includes angiotensin‐converting enzyme inhibitors and digitalis glycosides.9, 10, 11 Clinical trial data are limited in dogs, and the majority of studies have focused on treatment to manage chronic myxomatous valvular disease (MMVD). Trials using enalapril and conducted largely in populations of dogs with advanced heart disease but not heart failure have reported limited benefit or no benefit in these populations.12, 13 Digitalis traditionally has been used to manage supraventricular tachyarrhythmias14 and dilated cardiomyopathy in dogs,14, 15, 16 but evidence for clinical efficacy is sparse. A positive effect on left ventricular systolic function was identified in digoxin‐treated dogs with experimentally decreased left ventricular ejection fraction,17 and in a small retrospective study of dogs with idiopathic DCM receiving digoxin, decreased heart rate was identified with less pronounced benefit on left ventricular fractional shortening.18 Pimobendan, a calcium‐sensitizing drug with both inotropic and vasodilatory properties, is commonly used to manage CHF in dogs with MMVD and DCM.19, 20 Little data exist, however, comparing cardiac drugs for managing dogs with preclinical DCM. Delayed onset of CHF or sudden death was recorded in pimobendan‐treated Doberman pinscher dogs with preclinical DCM compared to placebo in a randomized, multicenter trial,21 and benazepril administered to Doberman pinschers with asymptomatic DCM prolonged the time to develop CHF in 1 retrospective report.22 Few original studies, however, have attempted to identify whether treatment improves survival in giant breed dogs with preclinical DCM or AF. Thus, the present study aimed to compare the effects of pimobendan, benazepril, or methyldigoxin monotherapy on long‐term clinical outcome in asymptomatic IW dogs with preclinical DCM, AF, or both.
Materials and Methods
Study population
Client‐owned, purebred, IW dogs were recruited between October 2001 and October 2008 through cardiovascular screening clinics in Belgium, Germany, and the Netherlands. Each dog had a complete physical examination, 6‐lead electrocardiogram (ECG) recorded in right lateral recumbency, right lateral thoracic radiography, CBC with differential count, and a serum biochemistry profile performed. The laboratory tests included measurement of total serum protein, creatinine, urea, and potassium concentrations, and alanine aminotransferase activity. Two‐dimensional (2D) and M‐Mode echocardiographic examination was performed by 1 cardiologist (ACV). From the right parasternal 4‐chamber view, left ventricular chamber, and wall dimensions were determined by M‐mode and 2D measurements of both atria made at their maximal diameter according to published guidelines.23 Measurements for each parameter were averaged from 4 to 6 cardiac cycles in dogs with sinus arrhythmia, and from 10 cardiac cycles in dogs with AF. Criteria to designate DCM were applied from published values.24, 25
Study design
Our study was a prospective, blinded clinical trial. Parallel treatment groups were randomized for 1:1:1 allocation using computer generated random blocks of 3. Dogs received oral pimobendan, benazepril, or methyldigoxin PO. The investigator, study monitor, and sponsor remained blinded until each animal reached the primary endpoint or until study termination. Blinding was ensured by participation of a technician not involved in study procedures who performed randomization and dispensed study medications.
Inclusion Criteria
Preclinical DCM, AF, or both was diagnosed on the basis of medical history and complete physical examination; ECG; and echocardiographic evidence of left ventricular dilatation and decreased left ventricular shortening fraction (SF) based upon reference values in the IW breed.24, 25 Absence of pulmonary edema or vascular congestion was confirmed for each dog at enrollment by evaluating right lateral thoracic radiographs.
Exclusion Criteria
Dogs were excluded if they had congenital heart disease, coughing, exercise intolerance, tachypnea, systemic disease judged to be clinically severe, or if they were pretreated with any cardiovascular drug before enrollment.
Study treatments and monitoring
Each dog was randomly assigned to receive 1 of 3 PO‐administered treatments: pimobendan5 (0.24–0.26 mg/kg PO q12h approximately 1 hour before feeding), benazepril HCl6 (0.25–0.5 mg/kg PO q24h), or methyldigoxin7 (0.005 mg/kg PO q12h). Serum digoxin trough concentrations were measured after at least 7 days of methyldigoxin administration and, if necessary, the dose was modified to achieve serum concentrations between 1–2 ng/mL. Concomitant treatment with diltiazem8 sustained release formulation (1.5–3 mg/kg PO q12h) was permitted if needed to control ventricular heart rate in dogs with AF. Each enrollee received sufficient quantity of medication to treat until the next scheduled visit. At each visit, the remaining number of study medication pills was counted by a technician to assess treatment compliance. Dogs were re‐examined 3 months after enrollment and every 6 months thereafter, or when cardiac decompensation was suspected by the owner or primary veterinarian. For all follow‐up examinations, each dog had a medical history, complete physical examination, ECG, and echocardiographic examination performed. Thoracic radiographs were taken if there was suspicion of CHF based upon history, physical examination, or echocardiographic examination. The dogs were followed until their discontinuation in the study, or until the study ended on 31 March, 2011.
Outcome measures
The primary study endpoint was defined as first onset of CHF or SD. Diagnosis of CHF was supported by evidence from history, physical examination, and thoracic radiography indicating changes consistent with pulmonary edema or pleural effusion. Death was defined as SD when witnessed by the owner as a sudden collapse and death. When unwitnessed, the designation of SD required the owner to have observed the dog to be in normal health within 8 hours before to it was found dead, and with no history or indication of other disease or condition that may have caused death.
Statistical analysis
Dogs included in efficacy analysis were required to fulfill the inclusion criteria and to comply with the study protocol. The study, which required at least 1 follow‐up examination after enrollment, was designated as the per protocol dataset (PPS). The full‐analysis‐set (FAS) followed the intention‐to‐treat principal and was defined as all dogs randomized to the study and having received at least 1 dose of study medication.26 Continuous data were described using median and interquartile range (IQR) and categorical variables were described by percentage. Treatment groups were compared with the F‐test for continuous variables or with Fisher's exact test for categorical variables. The primary endpoint was prospectively defined as a composite comprising CHF or sudden cardiac death. Efficacy was defined as the time interval from study initiation to primary endpoint as estimated using the Kaplan–Meier survival method. Survival estimates were plotted for the 3 treatment groups. Two‐sided log‐rank tests were performed for pairwise comparisons of the time to primary endpoint curves for pimobendan and benazepril as well as for pimobendan and methyldigoxin, followed by the Bonferroni–Holm procedure for multiple comparisons. Median values and IQR are reported. Hazard ratios (HR) for pimobendan versus benazepril HCI and for pimobendan versus methyldigoxin were calculated using proportional hazard models with treatment as factors. Dogs were right‐censored if they were euthanized or died for noncardiac reasons, or were lost to follow‐up. Analyses were performed using commercially available statistical software.9 A P value < .05 was considered statistically significant.
Results
Study population
Seventy‐seven dogs were randomized to 1 of 3 treatment groups (Fig 1). The FAS consisted of 75 dogs (all enrolled animals excluding the 2 that never were given a dose of study medication). Sixty‐six dogs comprised the PPS efficacy analysis for the following reasons: 2 dogs were excluded because owners did not administer the study medication; 9 additional dogs did not satisfy the study protocol (3 in the benazepril HCl group [1 experienced SD before the first reexamination visit; 1 died from noncardiac cause; and 1 received additional nonpermitted cardiac treatment]); 2 dogs in the pimobendan group were lost to follow‐up; and, 4 dogs in the methyldigoxin group (1 lost to follow‐up, 2 withdrawn from the study because of gastrointestinal disease on days 58 and 511 after enrollment, and 1 dog that received additional nonpermitted cardiac treatment).
Figure 1.
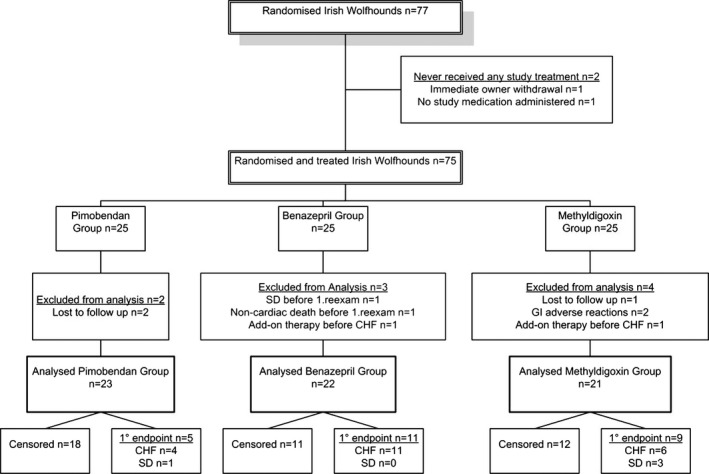
Flow diagram of patient allocation. SD, sudden death; CHF, congestive heart failure; GI, gastrointestinal, (1°endpoint = primary endpoint).
Baseline parameters
The 3 treatment cohorts for the 66 PPS dogs included pimobendan (n = 23), benazepril (n = 22) and methyldigoxin (n = 21; Table 1). There were 39 males (all intact) and 27 females (24 intact, 3 neutered). Dogs (median [IQR]) were 4.0 years (3.0–5.0 years) old and weighed 70.0 kg (63.0–75.0 kg). Twenty of these 66 dogs had AF as the only detected cardiac abnormality (8 in the pimobendan group, 6 in the benazepril HCl group, and 6 in the digoxin group). Forty‐six of the 66 dogs had DCM of which 30 had AF (9 dogs in the pimobendan group, 12 in the benazepril group, and 9 in the methyldigoxin group) and 16 of which had sinus arrhythmia (6 dogs in the pimobendan group, 4 in the benazepril HCl group, and 6 in the methyldigoxin group). Diltiazem was given to control ventricular heart rates for AF in 8 dogs with persistently increased mean ventricular heart rates that varied between 168–211 bpm during physical examination, ECG recordings, or echocardiography. These were 2 dogs in the pimobendan group, 3 dogs in the benazepril group, and 3 dogs in the methyldigoxin group. In 5 of these dogs, diltiazem was prescribed at the time of inclusion and in 3 dogs after tachycardia was detected during reexaminations (1 at day 81 in a dog treated with benazepril, the others at days 419 and 582 in dogs treated with methyldigoxin and pimobendan, respectively). The dogs that were given methyldigoxin had trough serum digoxin concentrations between 1.2 and 1.8 ng/mL, assessed 7 to 14 days after initiation of treatment or after dose adjustment.
Table 1.
Population characteristics of IW dogs summarized by treatment. Data are reported in frequencies or medians (interquartile range)
| Variable | Treatment Groups (PPS n = 66) | P‐value | ||
|---|---|---|---|---|
| Pimobendan (n = 23) | Benazepril (n = 22) | Methyldigoxin (n = 21) | ||
| Age (years)* | 4.5 (3.3,5.8) | 3.4 (2.5,5.0) | 4.0 (2.8,4.5) | .257 |
| Sex (F/FC/M/MC) | 11/1/11/0 | 7/0/15/0 | 6/2/13/0 | .365 |
| Body weight (kg)* | 70.0 (62.0,75.0) | 68.5 (62.0,74.0) | 70.0 (63.0,75.0) | .782 |
| Diltiazem (n) | 2 | 3 | 3 | .805 |
| AF (%) | 8 (34.8%) | 6 (27.3%) | 6 (28.6%) | .843 |
| DCM (%) | 15 (65.2%) | 16 (72.7%) | 15 (71.4%) | .843 |
| DCM with AF (%) | 9 (60.0%) | 12 (75.0%) | 9 (60.0%) | .59 |
| DCM with sinus arrhythmia (%) | 6 (40.0%) | 4 (25.0%) | 6 (40.0%) | .59 |
PPS, per protocol dataset; F, female; FC, female neutered; M, male; MC, male castrated; AF, atrial fibrillation; DCM, dilated cardiomyopathy; *Median (interquartile range).
Outcomes
Twenty‐five of the 66 dogs (37.9%) reached a primary study endpoint (CHF [21/25] and sudden death [4/25]). Median time to reach primary endpoint was 1,576 days (51.8 months). Thirty‐seven of the 66 dogs (56%) died or were euthanized as a consequence of noncardiac causes (Table 2). Four of the 66 dogs (6.1%) were alive at the end of study. When reviewing treatment allocations of the 25 dogs that reached primary endpoint, 5 of 23 dogs (21.7%) were randomized to receive pimobendan, 11 of 22 (50%) were randomized to receive benazepril HCl, and 9 of 21 (42.9%) were randomized to receive methyldigoxin. The median time to reach primary endpoint was significantly longer for dogs that received pimobendan (1,991 days; 65.4 months) compared to those that received benazepril HCl (997 days; 32.8 months; P = .008), or for pimobendan compared to methyldigoxin (1,263 days; 41.5 months; P = .031; Fig 2). The HR for the primary efficacy variable indicated that the risk for reaching the primary endpoint was significantly higher for dogs that received benazepril (HR, 4.26; 95% CI, 1.33, 13.60; P = .014) and for dogs that received methyldigoxin (HR, 3.27; 95% CI, 1.06 10.09; P = .04) compared to dogs that received pimobendan. Both pimobendan and benazepril were well tolerated. Two dogs in the methyldigoxin group were withdrawn because of gastrointestinal signs.
Table 2.
Causes for right censoring per treatment group (Per Protocol Set)
| Reason for Censoring | Pimobendan (n = 23) | Benazepril (n = 22) | Methyldigoxin (n = 21) | Total | ||
|---|---|---|---|---|---|---|
| Euthanized | Most prominent finding | 13 | 7 | 8 | 28 | 42.4% |
| Osteosarcoma, Lymphoma, Tumor | 5 | 3 | 5 | 13 | ||
| Paraparesis, Tetraparalysis | 6 | 1 | 0 | 7 | ||
| Pain, Fever | 1 | 1 | 0 | 2 | ||
| Aggression | 0 | 1 | 0 | 1 | ||
| Renal failure | 0 | 0 | 1 | 1 | ||
| Gastric torsion | 0 | 1 | 0 | 1 | ||
| Diabetes mellitus, anorexia | 0 | 0 | 1 | 1 | ||
| Vomiting | 0 | 0 | 1 | 1 | ||
| Pneumonia | 1 | 0 | 0 | 1 | ||
| Died | 3 | 3 | 3 | 9 | 13.6% | |
| Pneumonia | 2 | 2 | 0 | 4 | ||
| Gastric torsion | 1 | 0 | 1 | 2 | ||
| Shock | 0 | 1 | 0 | 1 | ||
| Splenic torsion | 0 | 0 | 1 | 1 | ||
| Vomiting | 0 | 0 | 1 | 1 | ||
| Noncardiac death‐total | 16 | 10 | 11 | 37 | 56.1% | |
| Alive at end of study‐total | 2 | 1 | 1 | 4 | 6.1% | |
| Censored‐total | 18 | 11 | 12 | 41 | 62.1% | |
Figure 2.
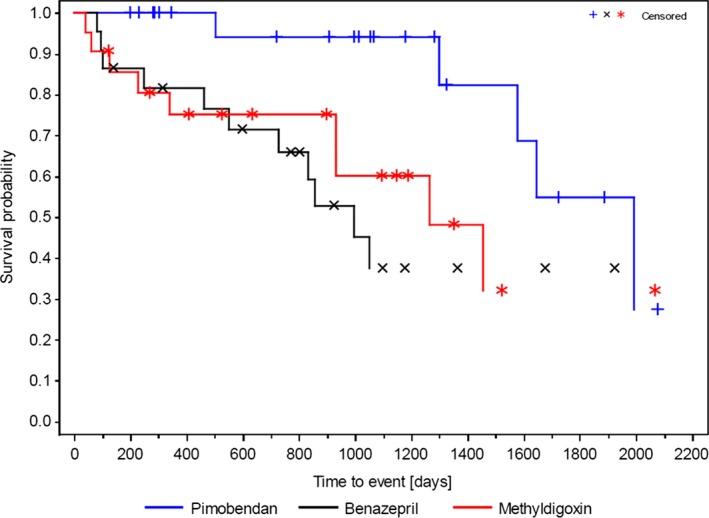
Kaplan–Meier survival curves for time to primary endpoint (CHF, SD), (Per Protocol Set, n = 66). Comparison by log‐rank test between pimobendan and benazepril HCl (P = .008) and between pimobendan and methyldigoxin (P = .031) were statistically significant, respectively. Because of right censoring, the 25% quartile could not be calculated.
Subanalysis of the 46 Dogs with DCM
Forty‐six of the 66 dogs had DCM and 16 of these 46 dogs (34.8%) reached primary endpoint. Median time to achieve primary endpoint based on the Kaplan–Maier procedure could not be estimated for all dogs within the 9.5 year study time frame because of right censoring. Of these 46 dogs, primary endpoint was reached in 2 of 15 dogs in the pimobendan group (1 developed CHF and the other experienced SD), in 7 of 16 dogs in the benazepril group (all developed CHF), and in 7 of 15 dogs in the methyldigoxin group (4 developed CHF and SD occurred in 3).
Subanalysis of the 20 Dogs with AF Only
Twenty of the 66 dogs had AF as the only detected cardiac condition initially. Congestive heart failure occurred in 9 (45%) of these 20 dogs, and 4 died from SD later (1 each in the pimobendan and methyldigoxin groups and 2 in the benazepril group). In the pimobendan group 3 of 8 dogs developed CHF (median interval, 1,737 days [57.1 months]), whereas 5 dogs died of noncardiac causes (median interval, 1,014 days [33.8 months]). In the benazepril group, CHF occurred in 4 of 6 dogs (median interval, 522 days [17.2 months] and 3 died of noncardiac causes (median interval, 802 days [26.7 months]). In the methyldigoxin group, 2 of 6 dogs developed CHF (median interval, 1,359 days [44.7 months]), whereas 4 dogs died of noncardiac causes (median interval 1,363 days [45.4 months]). The small number of affected dogs did not permit statistical comparisons.
Full‐analysis‐set (FAS) of 75 Dogs
The median time to primary endpoint of the FAS population (n = 75) was 1,454 days (47.8 months). Consistent with the analysis of the PPS cohort, the median time to primary endpoint was significantly longer for pimobendan‐treated dogs compared to benazepril‐ or methyldigoxin‐treated dogs (pimobendan, 1,991 days [65.4 months]) versus benazepril (858 days [28.2 months], P = .002; pimobendan versus methyldigoxin (1,263 days [41.5 months], P = .019).
Discussion
We prospectively compared long‐term outcomes in IW with preclinical DCM, AF, or both that were treated with 3 commonly used cardiac drugs. Substantial benefit was identified for the cohort that received pimobendan compared with those that received either an ACEI or cardiac glycoside. Pimobendan significantly prolonged the time to onset of CHF or SD by 33.1 months compared to benazepril HCl and by 24.3 months compared to methyldigoxin. Thus, benazepril or methyldigoxin monotherapy were comparatively less effective than pimobendan. These findings complement those observed in the PROTECT study21 that identified improved survival in Doberman pinscher dogs with preclinical DCM that received pimobendan compared with placebo. Others have reported short‐term hemodynamic and neuroendocrine benefits in pimobendan‐treated dogs with CHF associated with MMVD compared with benazepril‐treated dogs, including larger decreases in heart rate, left atrial size, systolic and diastolic left ventricular diameters and volumes, and increased ejection fraction.27 Superiority of pimobendan compared to ACEI treatment has been reported in dogs with CHF associated with MMVD,28, 29 and in DCM dogs with CHF in which pimobendan was added to conventional treatment.30, 31 Pimobendan is a benzimidazolpyridazinone drug with potent positive inotropic and vasodilatory effects.32, 33 Whether positive inotropy, preload, or afterload reduction, or decrease in cardiac size and filling pressures facilitated prolonged preclinical duration in our study warrants further investigation.
Benazepril monotherapy in our study was inferior to pimobendan and methyldigoxin when comparing duration of survival to primary endpoint. Of the IW dogs that reached primary endpoint, benazepril‐treated dogs had median survival of 997 days (32.8 months), which was 994 days (32.7 months) shorter than observed in pimobendan‐treated dogs and 266 days (8.7 months) shorter than observed in methyldigoxin‐treated dogs. Several studies have reported efficacy of ACEI drugs in the management of dogs with heart disease associated with MMVD. Two clinical trials designed to determine whether treatment with enalapril would prevent or delay onset of CHF in small breed dogs with MMVD reported limited12 benefit or lack of clinical effect.13 Benazepril used alone or as an add‐on to standard treatment in MMVD or DCM dogs with CHF extended life span compared to dogs that did not receive benazepril.34 Regarding use of ACEI in dogs with preclinical DCM, a retrospective study comparing the effect of benazepril given to Doberman Pinschers in the preclinical phase of DCM reported a 3 month delay in progression to overt DCM in benazepril‐treated dogs compared to nontreated Doberman Pinscher dogs.22 Some studies of dogs with preclinical DCM have failed to demonstrate rennin‐ angiotensin‐aldosterone activation,35, 36 leading to the possibility of limited ACEI efficacy in some cases.
In this study, survival of the cohort that received methyldigoxin monotherapy to primary endpoint was inferior to survival of pimobendan‐treated dogs. The study groups did not differ significantly with regard to the proportion of AF cases. Cardiac glycosides have long been used in veterinary medicine to treat heart disease, but a clear survival benefit has not been reported, and little data are available regarding its efficacy in dogs with preclinical DCM. Based on the energetic consequences of calcium handling by the myocyte in an experimental animal model, a beneficial effect of digitalis in CHF may depend upon the stage of disease.37 In humans, digoxin did not decrease overall mortality in a large trial of human CHF patients, but did decrease the rate of hospitalization and worsening CHF.38 However, in a meta‐analysis of digoxin use and mortality risk in humans with AF, digoxin use was associated with a greater risk for mortality regardless of concomitant CHF.39 Thus, the role of digitalis in the management of CHF remains controversial and the true effect of digoxin on morbidity and mortality remains unclear. Relatively small numbers of dogs were reported with SD and the proportions among treatment cohorts were relatively similar, especially between the pimobendan and methyldigoxin cohorts. The dogs in this study had trough digoxin concentrations between 1.2 and 1.8 ng/mL measured 7–14 days after starting treatment or after dose adjustment, respectively. However, digoxin monotherapy was substantially inferior to pimobendan with respect to outcome.
An important limitation of this study is that the relationship of AF to the development of DCM or the exacerbation of CHF are uncertain and could have affected outcome in some dogs. Secondly, inasmuch as no placebo group was included, an estimate of time to CHF or SD in untreated IW dogs was not available. Thus, we cannot ascertain whether no treatment would have been equivalent to, or better than, any of the tested treatments. In addition, a high rate of naturally occurring, noncardiac death affected some proportion of IW dogs before they reached the primary cardiac endpoint.
In conclusion, long‐term PO administration of pimobendan to dogs with preclinical DCM, AF or both significantly prolonged time to onset of CHF or SD compared to those treated with benazepril HCl or methyldigoxin. These finding lend credence to the concept that clinical benefit can accompany early disease detection and treatment. Furthermore, the findings support the practice of cardiac screening examinations for breeds of dogs that have a high prevalence of DCM with or without AF.
Acknowledgments
This work was done in Belgium, Germany, and the Netherlands. The study was supported by a grant from Boehringer Ingelheim Vetmedica GmbH. The authors thank the Irish Wolfhound Fanciers and breeders for their support.
Conflict of Interest Declaration: Dr Philip R. Fox: Consulting, IDEXX Laboratories, Merck & Co; Dr Andrea C. Vollmar: Research funding, Boehringer Ingelheim Vetmedica GmbH.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Footnotes
Vetmedin® capsules 1.25 mg, 2.5 mg and 5 mg, Boehringer Ingelheim, Germany
Fortekor® 2.5 mg and 5 mg tablets, Novartis, Germany
Lanitop® E, 0.15 mg tablets, Riemser, Germany
Harpster NK. Cardiac arrhythmias in the Irish wolfhound: preliminary study. Proc. 12th ACVIM Forum 1994;1:17–22
Vollmar C, Keene B, Kohn B, Vollmar A. Long‐term outcome of Irish wolfhounds with lone atrial fibrillation. J Vet Int Med 2015;29:C19
Vollmar C, Keene B, Kohn B, Vollmar A. Irish wolfhounds with dilated cardiomyopathy: causes of death. J Vet Int Med 2015;29:C20
Vollmar AC, Trötschel C, Kleemann R, Fox PR, Keene BW. Cardiomyopathy in Irish wolfhounds. Proc. 23rd ACVIM Forum, Baltimore 2005:66
Dilzem® 60 mg tablets; Cephalon UK Ltd, Welwyn Garden City, UK.
SAS 9.2, SAS Institute Inc, Cary, NC, USA.
References
- 1. Sisson D, O'Grady MR, Calvert C. Canine dilated cardiomyopathy In: Fox PR, Sisson D, Moise N, ed. Textbook of Canine and Feline Cardiology Principles and Practice. 2nd ed Philadelphia, PA: WB Saunders; 1999: 582–601. [Google Scholar]
- 2. Sisson DD, Thomas WP. Myocardial diseases In: Ettinger SJ, ed. Textbook of Veterinary Internal Medicine. Diseases of the Dog and Cat, 4th ed Philadelphia, PA: WB Saunders; 1995: 995–1032. [Google Scholar]
- 3. Buchanan JW. Causes and prevalence of cardiovascular disease In: Kirk RW, Bonagura JD, eds. Current Veterinary Therapy XI. Philadelphia, PA: WB Saunders; 1992:647–654. [Google Scholar]
- 4. Brownlie SE. An electrocardiographic survey of cardiac rhythm in Irish wolfhounds. Vet Rec 1991;129:470–471. [DOI] [PubMed] [Google Scholar]
- 5. Vollmar AC. The prevalence of cardiomyopathy in the Irish wolfhound: A clinical study of 500 dogs. J Am Anim Hosp Assoc 2000;36:125–132. [DOI] [PubMed] [Google Scholar]
- 6. Menaut P, Bélanger MC, Beauchamp G, et al. Atrial fibrillation in dogs with and without structural or functional cardiac disease: A retrospective study of 109 cases. J Vet Cardiol 2005;7:75–83. [DOI] [PubMed] [Google Scholar]
- 7. Brownlie SE, Cobb MA. Observations on the development of congestive heart failure in Irish wolfhounds with dilated cardiomyopathy. J Small Anim Pract 1999;40:371–377. [DOI] [PubMed] [Google Scholar]
- 8. Westling J, Westling W, Pyle RL. Epidemiology of atrial fibrillation in the dog. Int J Appl Res Vet Med 2008;6:151–154. [Google Scholar]
- 9. Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol 2009;53:e1–e90. [DOI] [PubMed] [Google Scholar]
- 10. Fatkin D; Members of the CSANZ Cardiac Genetic, Diseases Council Writing Group . Guidelines for the diagnosis and management of familial dilated cardiomyopathy. Heart Lung Circ 2011;20:691–693. [DOI] [PubMed] [Google Scholar]
- 11. Ahmed A, Waagstein F, Pitt B, et al. Effectiveness of digoxin in reducing one‐year mortality in chronic Heart failure in the digitalis investigation group trial. Am J Cardiol 2009;103:82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Atkins CE, Keene BW, Brown WA, et al. Results of the veterinary enalapril trial to prove reduction in onset of heart failure in dogs chronically treated with enalapril alone for compensated, naturally occurring mitral valve insufficiency. J Am Vet Med Assoc 2007;231:1061–1069. [DOI] [PubMed] [Google Scholar]
- 13. Kvart C, Häggström J, Pedersen HD, et al. Efficacy of enalapril for prevention of congestive heart failure in dogs with myxomatous valve disease and asymptomatic mitral regurgitation. J Vet Intern Med 2002;16:80–88. [PubMed] [Google Scholar]
- 14. Church DB. Preferences of veterinarians for drugs to treat heart disease in dogs and cats. Aust Vet J 1995;72:401–403. [DOI] [PubMed] [Google Scholar]
- 15. Borgarelli M, Tarducci A, Tidholm A, et al. Canine idiopathic dilated cardiomyopathy Part II: Pathophysiology and therapy. Vet J 2001;162:182–195. [DOI] [PubMed] [Google Scholar]
- 16. Davies T, Everitt S, Cobb M. Variation in the management of congestive cardiac failure in dogs. Vet Rec 2015;176:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sabbah HN, Shimoyama H, Kono T, et al. Effects of long‐term monotherapy with enalapril, metoprolol, and digoxin on the progression of left ventricular dysfunction and dilation in dogs with reduced ejection fraction. Circulation 1994;89:2852–2859. [DOI] [PubMed] [Google Scholar]
- 18. Kittleson MD, Eyester GE, Knowlen GG, et al. Efficacy of digoxin administration in dogs with idiopathic congestive cardiomyopathy. J Am Vet Med Assoc 1985;186:162–165. [PubMed] [Google Scholar]
- 19. Atkins C, Bonagura J, Ettinger S, et al. Guidelines for the diagnosis and treatment of canine chronic valvular heart disease. J Vet Intern Med 2009;23:1142–1150. [DOI] [PubMed] [Google Scholar]
- 20. Boswood A. Current use of pimobendan in canine patients with heart disease. Vet Clin North Am Small Anim Pract 2010;40:571–580. [DOI] [PubMed] [Google Scholar]
- 21. Summerfield NJ, Boswood A, O'Grady MR, et al. Efficacy of pimobendan in the prevention of congestive heart failure or sudden death in Doberman pinschers with preclinical dilated cardiomyopathy (the PROTECT Study). J Vet Intern Med 2012;26:1337–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. O'Grady MR, O'Sullivan ML, Minors SL, et al. Efficacy of benazepril hydrochloride to delay the progression of occult dilated cardiomyopathy in Doberman Pinschers. J Vet Int Med 2009;23:977–983. [DOI] [PubMed] [Google Scholar]
- 23. Thomas WP, Gaber CE, Jacobs GJ, et al. Recommendations for standards in transthoracic two‐dimensional echocardiography in the dog and cat. Echocardiography Committee of the Specialty of Cardiology, American College of Veterinary Internal Medicine. J Vet Intern Med 1993;7:247–252. [DOI] [PubMed] [Google Scholar]
- 24. Vollmar A. Use of echocardiography in the diagnosis of dilated cardiomyopathy in Irish wolfhounds. J Am Anim Hosp Assoc 1999;35:279–283. [DOI] [PubMed] [Google Scholar]
- 25. Vollmar A. Echocardiographic measurements in the Irish wolfhound: Reference values for the breed. J Am Anim Hosp Assoc 1999;35:271–277. [DOI] [PubMed] [Google Scholar]
- 26. European Medicines Agency/Committee for Medicinal Products for Veterinary Use/Efficacy Working Party/81976/2010: Guideline on statistical principles for clinical trials for veterinary medicinal products (pharmaceuticals). 1 Aug 2012.
- 27. Häggström J, Lord PF, Höglund K, et al. Short‐term hemodynamic and neuroendocrine effects of pimobendan and benazapril in dogs with myxomatous mitral valve disease and congestive heart failure. J Vet Intern Med 2013;27:1452–1462. [DOI] [PubMed] [Google Scholar]
- 28. Häggström J, Boswood A, O'Grady M, et al. Effect of pimobendan or benazepril hydrochloride on survival times in dogs with congestive heart failure caused by naturally occurring myxomatous mitral valve disease: The QUEST study. J Vet Intern Med 2008;22:1124–1135. [DOI] [PubMed] [Google Scholar]
- 29. Lombard CW, Jons O, Bussadori CM. Clinical efficacy of pimobendan versus benazepril for the treatment of acquired atrioventricular valvular disease in dogs. J Am Anim Hosp Assoc 2006;42:249–261. [DOI] [PubMed] [Google Scholar]
- 30. Fuentes VL, Corcoran B, French A, et al. A double‐blind, randomized, placebo‐controlled study of pimobendan in dogs with dilated cardiomyopathy. J Vet Intern Med 2002;16:255–261. [DOI] [PubMed] [Google Scholar]
- 31. O'Grady MR, Minors SL, O'Sullivan ML, et al. Effect of pimobendan on case fatality rate in Doberman Pinschers with congestive heart failure caused by dilated cardiomyopathy. J Vet Intern Med 2008;22:897–904. [DOI] [PubMed] [Google Scholar]
- 32. Honerjäger P, Heiss A, Schäfer‐Korting M, et al. UD‐CG 115‐a cardiotonic pyridazinone which elevates cyclic AMP and prolongs the action potential in guinea‐pig papillary muscle. Naunyn Schmiedebergs Arch Pharmacol 1984;325:259–269. [DOI] [PubMed] [Google Scholar]
- 33. Fujimoto S. Effects of pimobendan, its active metabolite UD‐CG 212, and milrinone on isolated blood vessels. Eur J Pharmacol 1994;265:159–166. [DOI] [PubMed] [Google Scholar]
- 34. BENCH Study Group . The effect of benazepril on survival times and clinical signs of dogs with congestive heart failure: Results of a multicenter, prospective, randomized, double‐blinded, placebo‐controlled, long term clinical trial. J Vet Cardiol 1999;1:7–18. [DOI] [PubMed] [Google Scholar]
- 35. Koch J, Pedersen HD, Jensen AL, et al. Activation of the renin‐angiotensin system in dogs with asymptomatic and symptomatic dilated cardiomyopathy. Res Vet Sci 1995;59:172–175. [DOI] [PubMed] [Google Scholar]
- 36. Tidholm A, Häggström J, Hansson K. Effects of dilated cardiomyopathy on the renin‐angiotensin‐aldosterone system, atrial natriuretic peptide activity, and thyroid hormone concentrations in dogs. Am J Vet Res 2001;62:961–967. [DOI] [PubMed] [Google Scholar]
- 37. Kohlhaas M, Maack C. Adverse bioenergetics consequences of Na+‐CA2+ exchanger‐mediated Ca2+ influx in cardiac myocytes. Circulation 2010;122:2273–2280. [DOI] [PubMed] [Google Scholar]
- 38. Digitalis Investigation Group . The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med 1997;336:525–533. [DOI] [PubMed] [Google Scholar]
- 39. Ouyang AJ, Lv YN, Zhong HL, et al. Meta‐analysis of digoxin use and risk of mortality in patients with atrial fibrillation. Am J Cardiol 2015;115:901–916. [DOI] [PubMed] [Google Scholar]


