Abstract
Background
Dog breeds with the ABCB1‐1Δ mutation have substantially truncated nonfunctional P‐glycoprotein. Dogs homozygous for this mutation (mut/mut) are susceptible to the toxic adverse effects of ivermectin, loperamide, and vincristine. Anecdotal reports suggested ABCB1 mut/mut dogs showed increased depth and duration of acepromazine sedation.
Hypothesis/Objectives
That ABCB1 mut/mut dogs have increased depth and duration of sedation after acepromazine IV compared to normal dogs (nor/nor).
Animals
Twenty‐nine rough‐coated collies were divided into 3 groups of dogs based on their ABCB1 genotype: 10 mut/mut, 10 mut/nor, and 9 nor/nor.
Methods
Dogs were given 0.04 mg/kg of acepromazine IV. Level of sedation, heart rate, respiratory rate, and blood pressure were recorded for 6 hours after acepromazine administration. Area under the curves (AUCs) of the normalized sedation score results were calculated and compared.
Results
The median sedation scores for ABCB1 mut/mut dogs were higher than nor/nor dogs at all time points and were higher in mut/nor dogs for the first 2 hours. These differences were not found to be significant for any individual time point (P > .05). The median sedation score AUC for mut/mut dogs was significantly higher than nor/nor dogs (P = .028), but the AUC for mut/nor dogs was not (P = .45). There were no significant differences between groups for heart rate, respiratory rate, and blood pressure (P > .05).
Conclusions and Clinical Importance
In ABCB1 mut/mut dogs acepromazine dose rates should be reduced and careful monitoring performed during sedation.
Keywords: Collie, MDR1, P‐glycoprotein
Abbreviations
- AUC
area under the curve
- P‐gp
p‐glycoprotein
The ABCB1 gene (previously known as the MDR1 gene) codes for P‐glycoprotein (P‐gp), a transmembrane ATPase that transports small molecules from inside to outside of the cell membrane in an energy dependent process.1 P‐gp is also a major component of the blood barrier influencing the exposure of the central nervous system to certain drugs.2 A 4‐bp mutation (ABCB1‐1Δ) has been identified in dogs that results in premature stop codons and a nonfunctional P‐gp.3, 4 Dogs that are homozygous for this mutation (ABCB1 mut/mut) have no functional P‐gp resulting in an increased sensitivity to a variety of xenobiotics including ivermectin, vincristine, doxorubicin, loperamide, and others.5, 6, 7, 8, 9, 10 Dogs that are heterozygotes have shown increased sensitivity to some of these drugs (vincristine, ivermectin) compared to ABCB1 nor/nor dogs. In particular, the increased sensitivity to ivermectin in ABCB1 mut/mut dogs is thought to result from a build‐up of the drug in the central nervous system. This increased concentration causes an ivermectin toxicosis that can lead to coma and death at doses that would be safe in an ABCB1 nor/nor dog.11
The ABCB1‐1Δ mutation is thought to have originated in an ancestral herding dog during the 19th century in England before breed standards were introduced.12 Collies show the highest frequency of the mutation with approximately 75% of dogs from this breed carrying at least 1 copy. Other breeds that also commonly carry the mutation include the Australian shepherd, longhaired whippet, McNab, silken windhound, Shetland sheepdog, English shepherd, and old English sheepdog.12
Many drugs are transported by P‐gp and the exact structure–function relationship between substrates has been hard to fully identify.13 Several drugs that are commonly used in veterinary sedation and analgesia of dogs are thought to be transported by P‐gp including acepromazine, morphine, butorphanol, and fentanyl.1 Routinely used sedatives and analgesics have not been investigated to determine if their actions are potentiated in dogs with the ABCB1‐1Δ mutation. However, there are anecdotal reports in the literature that dogs that are homozygous for the ABCB1‐1Δ mutation show increased sensitivity to these drugs.11
The aim of the current study was to determine if dogs that were homozygous for the ABCB1‐1Δ mutation (ABCB1 mut/mut) would show increased and prolonged sedation after administration of acepromazine compared to heterozygous mutants (ABCB1 nor/mut) and homozygous normal (ABCB1 nor/nor) dogs. Our null hypothesis was the dogs that were ABCB1 mut/mut would not show greater sedation than dogs with nor/nor or nor/mut genotypes.
Materials and Methods
Animals
All dogs enrolled in this study were client‐owned animals that were returned to their owners at the completion of the study. Owners gave signed, informed consent for study enrollment and animal ethics approval for the study was obtained from the Massey University Animal Ethics Committee (MUAEC No. 12/63).
Genotyping
The ABCB1 genotyping was performed by obtaining buccal samples from 31 rough‐coated collies for DNA testing. Genotyping was performed by the Veterinary Clinical Pharmacology Laboratory at Washington State University, USA (www.vcpl.vetmed.wsu.edu).
Physical Exam and Blood Testing
One week before sedation scoring, each dog was brought into the clinic and a physical examination performed that included heart and lung auscultation, temperature, pulse rate, abdominal palpation and mucus membranes evaluation. Blood and urine samples were also collected at this time. Complete blood count (CBC) and serum chemistry panels were performed on the blood samples (New Zealand Veterinary Pathology Limited). A urinary test strip (Combur‐Test Strips; Roche, Auckland, New Zealand) and refractometer (Leica 10436 Veterinary Refractometer) were used to perform an in‐house urinary analysis. Dogs were included in the study if they were within 1–10 years of age and the physical examination was considered normal and the CBC, serum chemistry and urinalysis results were within established reference ranges.
Sedation Testing
Dogs were sedation scored in groups of no more than 4 dogs per day. All dogs were brought into the Massey University Veterinary Teaching Hospital early in the morning and moved to a quiet consultation room for the duration of the study. Dogs were fasted overnight but were given free access to water throughout the study and were monitored constantly. The level of sedation was scored at 0, 0.5, 1, and 2 hours before IV administration of acepromazine. After IV dosing dog sedation was evaluated at 30, 60, and 90 minutes and 2, 2.5, 3, 4, and 6 hours. Heart rate and respiratory rate were also measured by palpation and by visual observation. Blood pressure (systolic, mean, and diastolic) was measured with a noninvasive oscillometric blood pressure monitor (SurgiVet V9000). One minute after the 2‐hour sedation score was performed all dogs received 0.04 mg/kg acepromazine via slow intravenous injection into the cephalic vein. The 2‐hour sedation score was considered the baseline.
Sedation scoring was carried out by the same person (DD) who was blinded to the genotype results of the dogs. The sedation score was based upon a previously published sedation scoring system with small modifications.14 This provided a detailed scoring system that assessed several behavioral variables. Using this scoring system, normal alert dogs would score approximately 0, excited dogs would have negative scores, and dogs showing increasing levels of sedation would have positive scores. The sedation scale recorded 7 variables (Table 1). For variable 4—response to restraint, dogs were assessed while having heart rate, respiratory rate and blood pressure taken. For variable 5—response to noise, a handclap was used to generate the noise. For variable 6—gait, this was assessed by walking the dog on a leash in the same room. Variable 7—posture, was assessed 1 minute after assessing the gait.
Table 1.
Sedation scoring system
| Observation | Description | Score |
|---|---|---|
| 1. Vocalization | Quiet | 0 |
| Whining softly but quiets with soothing touch | −1 | |
| Whining continuously | −2 | |
| Barking continuously | −3 | |
| 2. Appearance | Eyes sunken, glazed, or unfocused; ventromedial rotation | 3 |
| Eyes glazed but follow movement | 2 | |
| Protrusion of nictitating membrane; normal visual responses | 1 | |
| Normal appearance | 0 | |
| Pupils dilated; abnormal facial expression | −1 | |
| 3. Interactive behavior | Recumbent; no response to voice or touch | 3 |
| Recumbent; lifts head in response to voice or touch | 2 | |
| Recumbent but stands in response to voice or touch | 1 | |
| Moves toward/away from voice or touch; appears anxious | 0 | |
| Wags tail/excited/growls or hisses when approached or touched | −1 | |
| Very excited/jumps/bites or swats when approached | −2 | |
| 4. Response to restraint | Lies on floor, no restraint required | 2 |
| Lies on floor with light restraint of head or neck | 1 | |
| Sits up on floor; with light restraint | 0 | |
| Requires regular correction | −1 | |
| Struggles continuously against restraint | −2 | |
| 5. Response to noise | No response to a hand clap | 3 |
| Minimal response to a hand clap | 2 | |
| Slow or moderate response to a hand clap | 1 | |
| Brisk response to a hand clap; raises head with eyes open | 0 | |
| 6. Gait | Unable to get up | 5 |
| Sits up but unable to stand, falls when attempting to stand | 4 | |
| Gait very unsteady, standing but ataxic | 3 | |
| Gait clearly unsteady | 2 | |
| Mild aberrations in gait, wobbly | 1 | |
| Walks normally | 0 | |
| 7. Posture | Lateral recumbence | 3 |
| Sternal recumbence | 2 | |
| Sitting or ataxic while standing | 1 | |
| Standing | 0 | |
| Moving continuously | −1 |
Statistical Analysis
The sedation score at each time point was calculated from the summed total of the 7 variables that make up the sedation scale (Table 1). Sedation scores for each time point during the 2‐hour acclimation period were compared for significant differences with the Kruskal–Wallis rank sum test. For each dog the sedation score at 2 hours (just before acepromazine dosing) was used as the dogs baseline sedation score. For the statistical analysis this baseline sedation score was subtracted from all the sedation scores post‐acepromazine for each dog, creating a normalized sedation score. Normalized sedation scores for each time point post‐acepromazine were compared for significant differences with the Kruskal–Wallis rank sum test. For each dog the area under the curve (AUC) post‐acepromazine over the 6 hours was calculated from the normalized sedation scores using R and a linear interpolation (version 3.1.2, R Core Team 2014).15 The AUCs for each genotype were compared using the Wilcoxon rank sum test. For heart rate, respiration rate, and mean arterial blood pressure a one‐way ANOVA was performed for each time point if the data were found to be normally distributed using Levenne's test. If the data for an individual time point were found not to be normally distributed then it was analyzed with Kruskal–Wallis rank sum test. The P‐value was adjusted for multiple comparisons using the Holm‐Bonferroni method. All statistical tests were performed in R (version 3.1.2, R Core Team 2014).
Results
Genotype Results
A total of 31 rough‐coated collies were enrolled in this study and had their ABCB1 genotypes determined. From these 31 dogs, 2 dogs were excluded from sedation. One dog had increased serum BUN, and creatinine concentrations, and lower urine specific gravity suggestive of renal disease. A second dog was excluded as it was greater than 10 years of age when it was available for sedation scoring. Of the remaining 29 dogs there was an almost even mix of the 3 genotypes, with age, weight, and sex distributions similar across the 3 genotypes (Table 2). Several of the dogs were closely related to each other as either siblings or parents and offspring.
Table 2.
Age, sex, weight, and genotype distribution of the 29 rough‐coated collies used for sedation scoring
| ABCB1 nor/nor | ABCB1 nor/mut | ABCB1 mut/mut | |
|---|---|---|---|
| Number of dogs | 9 | 10 | 10 |
| Males | 5 | 5 | 4 |
| Females | 4 | 5 | 6 |
| Age (mean ± SD)a | 3.9 ± 3.8 | 4.3 ± 2.4 | 3.4 ± 3.1 |
| Weight (mean ± SD)a | 18.9 ± 3.4 | 21.5 ± 3.2 | 22.3 ± 4.9 |
No statistical significant differences were identified between groups (P > .05).
Sedation Trial Results
The median sedation score increased for all 3 ABCB1 genotype groups during the 2‐hour acclimation period in the consult room before IV dosing of acepromazine (Table 3). After IV dosing of acepromazine at 0.04 mg/kg sedation scores of all dogs were increased by the 30‐minute time point (Fig 1). Sedation scores then declined over the following 6 hours until the majority of dogs had sedation scores similar to before treatment values. Median normalized sedation scores were highest in the ABCB1 mut/mut dogs and these higher scores persisted longer compared to the ABCB1 nor/mut dogs and ABCB1 nor/nor groups (Fig 1). The ABCB1 nor/mut dogs had increased sedation scores at 1 hour, 90 minutes and 2 hours compared to the ABCB1 nor/nor dogs. However, none of the sedation scores for the 3 genotypes were found to be significantly different at any of the individual time points.
Table 3.
The median dog sedation scores (range) of the 3 ABCB1‐1Δ genotypes over the acclimation period 2 hours before IV acepromazine dosing. No significant differences were found at any time point between groups (P > .05)
| Time (hours) | nor/nor | mut/nor | mut/mut |
|---|---|---|---|
| 0 | 0 (−5, 5) | 0 (−3, 0) | 0 (−4, 2) |
| 0.5 | 0.5 (−1, 1) | 0 (−1, 4) | 0 (−1, 3) |
| 1 | 1 (1, 3) | 1 (−2, 4) | 0.5 (−1, 4) |
| 2 | 2 (0, 5) | 1.5 (−2, 4) | 1 (−1, 4) |
Figure 1.
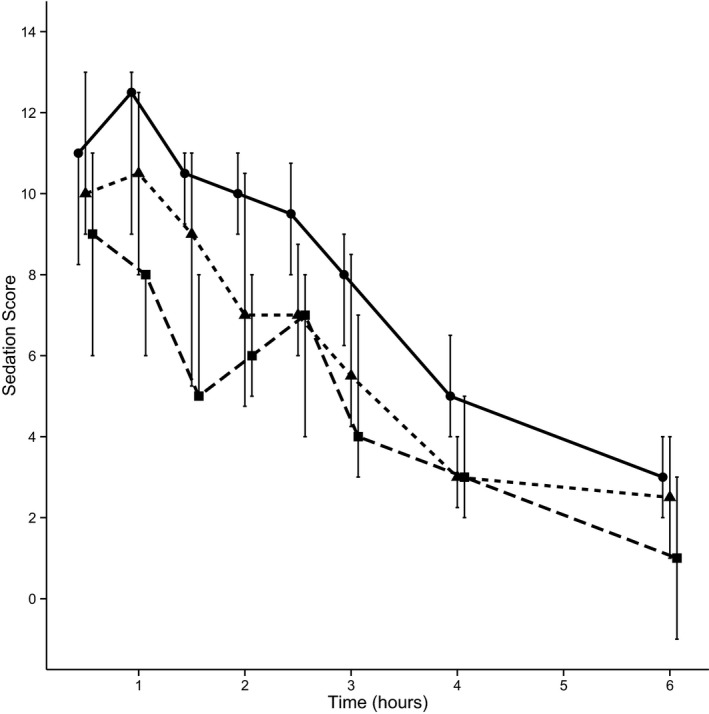
Median dog sedation scores normalized to baseline (± the interquartile ranges), for the ABCB1‐1Δ genotypes mut/mut (solid line), mut/nor (short dashes), and nor/nor (long dashes) measured over 6 hours after IV administration of acepromazine.
To compare the sedation scores for each genotype over the total 6 hours after acepromazine sedation the AUC for each dogs normalized sedation score was calculated and the median, ranges and interquartile ranges compared (Fig 2). Compared to the nor/nor dogs, the AUCs of the ABCB1 mut/mut dogs were found to be significantly higher (P = .028) while the AUCs of the ABCB1 nor/mut dogs were not (P = .447).
Figure 2.
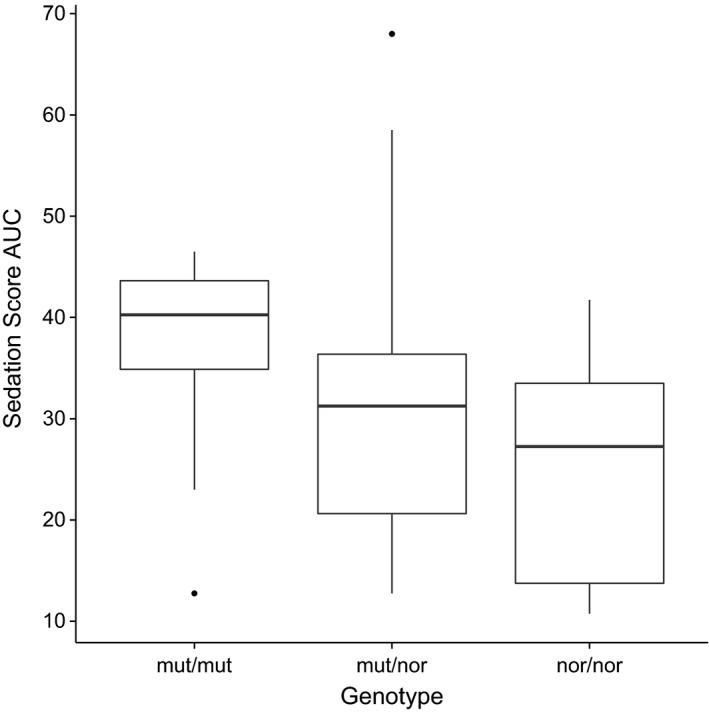
Box and whisker plot of the sedation score AUCs for each genotype. The box shows the 1st and 3rd quartiles with the median plotted as the band inside the box. Whiskers show the upper and lower ranges with outliers plotted as circles.
Analysis of the heart rate, respiratory rate and mean arterial blood pressure over 6 hours after IV dosing of acepromazine showed no statistical correlation with any of the 3 ABCB1 genotypes and remained within clinical acceptable limits.
Discussion
The results from this study suggest that dogs that are homozygous for the ABCB1‐1Δ mutation are likely to show increased and prolonged sedation after IV acepromazine compared to ABCB1 heterozygous or normal dogs. We selected a dose rate of 0.04 mg/kg of acepromazine because we considered that this dose would be sufficient to see moderate levels of sedation in all dogs but should not result in life threatening adverse effects even if sedation was dramatically pronounced and extended in ABCB1 mut/mut dogs. It is unknown what level of sedation would result with higher or lower doses rate of acepromazine.
We have not determined what effect giving acepromazine intramuscularly would have on sedation in ABCB1 mut/mut dogs. However, the ABCB1‐1Δ mutation results in increased drug levels in the central nervous system because of a non‐functional P‐gp.16 This is likely to be the mechanism responsible for increased acepromazine sedation seen in this study. It is reasonable to assume that this will still be the case in dogs dosed intramuscularly. This suggests that for ABCB1 mut/mut dogs the doses of acepromazine should be reduced and dogs should be monitored carefully, regardless of the route of administration.
We found that the ABCB1 mut/nor dogs did not show statistically significant increased sedation compared to the ABCB1 nor/nor animals. This is in agreement with sensitivity to macrocyclic lactones such as ivermectin where heterozygous carriers of the ABCB1 mutation do not show an increased sensitivity compared to homozygous normal dogs at normal doses.3 However, it is known that ABCB1 mut/nor dogs do show some increased toxicity to ivermectin for doses greater than 120 μg/kg especially if they are administered over multiple days.17 Furthermore the ABCB1‐1Δ mutation can predict the hematologic toxicity of vincristine in both mut/mut and mut/nor dogs.18 It is unknown if higher doses of acepromazine would have resulted in increased sedation in the ABCB1 mut/nor dogs and our study does not rule this out. Increased sedation may have been found to be statistical significant in the mut/nor groups if more dogs were included in the study. Therefore, caution with acepromazine doses would be prudent, even in dogs that are heterozygous for the ABCB1 mutation.
Acepromazine is considered a relatively “safe” sedative, whereas the level of sedation seen in an individual ABCB1 mut/mut dog is likely to be unpredictable because of differences in drug disposition between individuals. We were careful to ensure that the dogs included in this study were apparently healthy with no detected abnormalities in liver or kidney function. Any metabolic disturbance of these organs in an ABCB1 mut/mut dog could result in prolonged metabolism and delayed excretion with the potential for an even greater sedation level. There is no published literature on acepromazine metabolism in dogs, but in horses acepromazine is metabolized by the liver and is excreted as conjugated and unconjugated metabolites in the urine.19 It would be advisable to perform a CBC, serum chemistry, and urinary analysis on dogs that carry ABCB1‐1Δ mutation before sedation or anesthesia. Dogs that are very old or very young may also require particular care caused by geriatric‐associated diseases and organ immaturity, respectively.
Acepromazine is not the only drug used for sedation in dogs that could potentially be transported by P‐gp. Various opioids such as morphine, methadone, butorphanol, pethidine, and fentanyl are thought to be substrates for P‐gp as shown by a variety of in vitro and in vivo assays.20, 21, 22 These opiates are known to cause sedation in dogs and are frequently used in combinations with acepromazine.23 These drugs have not been tested for the effects on sedation in ABCB1 mut/mut dogs but there is anecdotal evidence that butorphanol may result in increased sedation.11 Combinations of these drugs may result in an increased depth and duration of sedation that exceeds what was seen in this study. Without thoughtful dose rate selection and careful monitoring during all stages of sedation or anesthesia these combinations are very likely to pose an increased safety risk in dogs that are ABCB1 mut/mut.
There are several limitations to this study. Firstly the number of dogs enrolled in the study was small, although each group was similar in their age and sex distributions. Obtaining client‐owned dogs for studies such as these is challenging, as owners are naturally cautious to commit their animals to unnecessary sedation. However, we did find owners and breeders were interested in working with us to increase the knowledge and improve the safety of sedation and analgesia in their dogs. This work is important to improve the safety of drug treatment in dogs carrying the ABCB1‐1Δ mutation, whereas in the long term it would be possible for breeders to selectively breed out, or at least reduce the frequency, of the mutation by careful selection of sires for mating.
The second limitation of this study is the lack of a saline control group along with the acepromazine treatment. A control group was not included for practical reasons as owners were reluctant to commit their dogs to 2 treatments on 2 separate days. It is clear that a saline control is important for sedation studies to help avoid a type II error, but is frequently not included in many studies.24, 25 It is also clear that the sedation score of a dog will increase with saline treatment as a dog adapts and relaxes to a new environment.26, 27 To minimize the effects of the absence of a saline control we acclimated the dogs to the room used for sedation scoring for 2 hours before administration of acepromazine. The sedation score at 2 hours was then used as a baseline to measure any subsequent changes in sedation after acepromazine injection. Also, dogs that were ABCB1 nor/nor acted as suitable control to the ABCB1 mut/mut group.
We are also limited in drawing conclusions from these studies to rough‐coated collies only. Although it is reasonable to assume that other breeds that are ABCB1 mut/mut would sedate to a greater extent than homozygous normal dogs, it is not certain. Other genotypes specific to other breeds may play a role attributable to unknown interactions.
More studies in dogs carrying the ABCB1‐1Δ mutation are required to evaluate commonly used sedatives and analgesics, both individually and in combination. However, these studies are challenging to perform, require large numbers of dogs, and rely on the goodwill of owners to contribute their animals. Until more studies are completed care with drug doses and limiting sedative–analgesic combinations when possible are sensible steps to reduce the risk complications in these dogs during procedures requiring sedation or anesthesia.
Acknowledgments
This work was supported by a CAS/CAHF Project Grant, a grant from the Post‐graduate Research Fund and the McGeorge Research Fund. We acknowledge the invaluable work of Raewyn Mullen for helping us find collie owners and encouraging them to participate in our study.
Conflict of Interest Declaration: Dr. Kate Hill is the Associate Editor of the Journal of Veterinary Internal Medicine.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
The work was presented in part as an oral research abstract at the 2014 American College of Veterinary Internal Medicine Forum in Nashville, TN.
References
- 1. Martinez M, Modric S, Sharkey M, et al. The pharmacogenomics of P‐glycoprotein and its role in veterinary medicine. J Vet Pharmacol Ther 2008;31:285–300. [DOI] [PubMed] [Google Scholar]
- 2. Schinkel AH, Wagenaar E, Mol CA, et al. P‐glycoprotein in the blood‐brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J Clin Invest 1996;97:2517–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mealey KL, Bentjen SA, Gay JM, et al. Ivermectin sensitivity in collies is associated with a deletion mutation of the mdr1 gene. Pharmacogenetics 2001;11:727–733. [DOI] [PubMed] [Google Scholar]
- 4. Roulet A, Puel O, Gesta S, et al. MDR1‐deficient genotype in Collie dogs hypersensitive to the P‐glycoprotein substrate ivermectin. Eur J Pharmacol 2003;460:85–91. [DOI] [PubMed] [Google Scholar]
- 5. Nelson OL, Carsten E, Bentjen SA, et al. Ivermectin toxicity in an Australian Shepherd dog with the MDR1 mutation associated with ivermectin sensitivity in Collies. J Vet Intern Med 2003;17:354–356. [PubMed] [Google Scholar]
- 6. Krugman L, Bryan JN, Mealey KL, et al. Vincristine‐induced central neurotoxicity in a collie homozygous for the ABCB1Δ mutation. J Small Anim Pract 2012;53:185–187. [DOI] [PubMed] [Google Scholar]
- 7. Mealey KL, Northrup NC, Bentjen SA. Increased toxicity of P‐glycoprotein‐substrate chemotherapeutic agents in a dog with the MDR1 deletion mutation associated with ivermectin sensitivity. J Am Vet Med Assoc 2003;223:1453–1455, 1434. [DOI] [PubMed] [Google Scholar]
- 8. Sartor LL, Bentjen SA, Trepanier L, et al. Loperamide toxicity in a collie with the MDR1 mutation associated with ivermectin sensitivity. J Vet Intern Med 2004;18:117–118. [DOI] [PubMed] [Google Scholar]
- 9. Mealey KL, Gay JM, Martin LG, et al. Comparison of the hypothalamic‐pituitary‐adrenal axis in MDR1‐1delta and MDR1 wildtype dogs. J Vet Emerg Crit Care 2007;17:61–66. [Google Scholar]
- 10. Henik RA, Kellum HB, Bentjen SA, et al. Digoxin and mexiletine sensitivity in a Collie with the MDR1 mutation. J Vet Intern Med 2006;20:415–417. [DOI] [PubMed] [Google Scholar]
- 11. Mealey KL. Adverse drug reactions in herding‐breed dogs: the role of P‐gp. Compendium 2006;28:23–33. [Google Scholar]
- 12. Neff MW. Breed distribution and history of canine mdr1‐1, a pharmacogenetic mutation that marks the emergence of breeds from the collie lineage. Proc Natl Acad Sci USA 2004;101:11725–11730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sarkadi B, Homolya L, Szakacs G, et al. Human multidrug resistance ABCB and ABCG transporters: participation in a chemoimmunity defense system. Physiol Rev 2006;86:1179–1236. [DOI] [PubMed] [Google Scholar]
- 14. Smith LJ, Yu JK, Bjorling DE, et al. Effects of hydromorphone or oxymorphone, with or without acepromazine, on preanesthetic sedation, physiologic values, and histamine release in dogs. J Am Vet Med Assoc 2001;218:1101–1105. [DOI] [PubMed] [Google Scholar]
- 15. R: A language and environment for statistical computing . R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org/
- 16. Mealey KL, Greene S, Bagley R, et al. P‐glycoprotein contributes to the blood‐brain, but not blood‐cerebrospinal fluid, barrier in a spontaneous canine P‐glycoprotein knockout model. Drug Metab Dispos 2008;36:1073–1079. [DOI] [PubMed] [Google Scholar]
- 17. Mealey KL. Canine ABCB1 and macrocyclic lactones: heartworm prevention and pharmacogenetics. Vet Parasitol 2008;158:215–222. [DOI] [PubMed] [Google Scholar]
- 18. Mealey KL, Fidel J, Gay JM, et al. ABCB1‐1Delta polymorphism can predict hematologic toxicity in dogs treated with vincristine. J Vet Intern Med 2008;22:996–1000. [DOI] [PubMed] [Google Scholar]
- 19. Dewey EA, Maylin GA, Ebel JG, et al. The metabolism of promazine and acetylpromazine in the horse. Drug Metab Dispos 1981;9:30–36. [PubMed] [Google Scholar]
- 20. Thompson SJ, Koszdin K, Bernards CM. Opiate‐induced analgesia is increased and prolonged in mice lacking P‐glycoprotein. Anesthesiology 2000;92:1392–1399. [DOI] [PubMed] [Google Scholar]
- 21. Wandel C, Kim R, Wood M, et al. Interaction of morphine, fentanyl, sufentanil, alfentanil, and loperamide with the efflux drug transporter P‐glycoprotein. Anesthesiology 2002;96:913–920. [DOI] [PubMed] [Google Scholar]
- 22. Callaghan R, Riordan JR. Synthetic and natural opiates interact with P‐glycoprotein in multidrug‐resistant cells. J Biol Chem 1993;268:16059–16064. [PubMed] [Google Scholar]
- 23. Tranquilli WJ, Thurmon JC, Grimm KA, et al. Lumb & Jones’ Veterinary Anesthesia and Analgesia, 4th ed Ames, IA: Blackwell Publisher; 2007:xv, 1096 p. [Google Scholar]
- 24. Hofmeister EH, Chandler MJ, Read MR. Effects of acepromazine, hydromorphone, or an acepromazine‐hydromorphone combination on the degree of sedation in clinically normal dogs. J Am Vet Med Assoc 2010;237:1155–1159. [DOI] [PubMed] [Google Scholar]
- 25. Giuffrida MA. Type II error and statistical power in reports of small animal clinical trials. J Am Vet Med Assoc 2014;244:1075–1080. [DOI] [PubMed] [Google Scholar]
- 26. Hofmeister EH, Egger CM. Evaluation of diphenhydramine as a sedative for dogs. J Am Vet Med Assoc 2005;226:1092–1094. [DOI] [PubMed] [Google Scholar]
- 27. Hofmeister EH, King J, Read MR, et al. Sample size and statistical power in the small‐animal analgesia literature. J Small Anim Pract 2007;48:76–79. [DOI] [PubMed] [Google Scholar]


