Abstract
Background
Hyperthyroidism, the most common endocrine disorder in cats, has been associated with low serum cobalamin concentrations. Whether this is a functional cobalamin deficiency of clinical importance has not been assessed.
Hypothesis/Objectives
Cats with hyperthyroidism experience a functional cobalamin deficiency which correlates with their clinical catabolic state and is reversible with return of the euthyroid state.
Animals
Thirty‐nine client‐owned hyperthyroid cats.
Methods
Prospective observational study. Serum cobalamin, methylmalonic acid, and clinical scores were determined in each hyperthyroid cat at enrollment and when euthyroid (60 days after radioiodine treatment).
Results
Five of the 39 hyperthyroid cats (13%) had a low serum cobalamin concentration ranging from <150 to 290 ng/L. Serum cobalamin concentrations normalized to >350 ng/L in 2 of the hypocobalaminemic cats once euthyroid. None of the hyperthyroid/hypocobalaminemic cats had increased serum methylmalonic acid concentrations (175–601 nmol/L). In cats with clinical and biochemical hyperthyroidism, there was no correlation between serum cobalamin concentrations with total T4 concentration (P = .12) or clinical scores including body weight (P = .11) and BCS (P = .54).
Conclusions and Clinical Importance
In this population of hyperthyroid cats, the prevalence of hypocobalaminemia was low. Specifically, hyperthyroid cats, in which concurrent gastrointestinal disease is unlikely. Hypocobalaminemia is not a functional deficiency requiring supplementation in hyperthyroid cats without gastrointestinal disease.
Keywords: Feline, Hypocobalaminemia, MMA, Thyroxine, Vitamin B12
Abbreviations
- Hyc
homocysteine
- MMA
methylmalonic acid
- MTHFR
methylene tetrahydrofolate reductase
- FIGLU
formiminoglutamic acid
Cobalamin (vitamin B12) is a water‐soluble vitamin that has an important role in cellular functions involving DNA synthesis and amino acid production.1 It is a cofactor required in many enzymatic reactions, including the conversion of methylmalonyl‐coenzyme A to succinyl‐coenzyme A and the conversion of homocysteine (Hyc) to methionine. Diminished activity of these enzymes leads to increased serum methylmalonic acid (MMA)2 and Hyc3 concentrations. Hypocobalaminemia is defined as a low serum cobalamin concentration; however, it does not accurately reflect a low cellular cobalamin concentration or functional cobalamin deficiency.4 In humans, increased serum concentrations of MMA and Hyc in association with hypocobalaminemia are considered accurate and reliable markers of a cellular cobalamin deficiency.5, 6 A functional cobalamin deficiency is an important metabolic derangement in humans that may lead to substantial morbidities, such as anemia, cognitive impairment, weakness, and peripheral neuropathies.1 Because of its role in DNA synthesis, cobalamin deficiency has a considerable impact on rapidly dividing cells such as the intestinal epithelium, causing atrophy of the intestinal crypts and subsequent malabsorption.7
Cobalamin reserves in cats become depleted within days in part because of the absence of circulating transcobalamin 1 and cobalamin loss via the enterohepatic circulation.8 Cobalamin deficiency in cats is not associated with hyperhomocysteinemia seen in humans,9, 10 suggesting species differences in Hyc metabolism. However, low cobalamin levels in cats are associated with alteration in the metabolism of other sulfur‐containing amino acids, to include methionine, cystathionine, and cysteine.9 In addition, increased serum MMA concentrations are found in 68% of cats with low serum cobalamin, suggesting a functional deficit in this species.11
In a recent study, a significant decrease in serum cobalamin concentrations were reported in hyperthyroid cats, however, MMA concentrations were not measured and the change in cobalamin status after treatment for hyperthyroidism was not assessed.12 Low serum cobalamin concentrations are also associated with hyperthyroidism in humans but are considered functionally unimportant13, 14 as serum cobalamin levels rise significantly once euthyroid.15, 16 Based on known differences in nutritional requirements as well as a much higher cobalamin turnover in cats,8 feline hyperthyroidism may be a risk factor for a functional cobalamin deficiency and a clinical population of cats that may benefit from cobalamin supplementation.5
The purpose of this study was to prospectively determine, in a well‐characterized population of hyperthyroid cats, whether (1) hypocobalaminemia reflects functional cobalamin deficiency, using increased serum MMA as the marker of cellular cobalamin deficiency, (2) determine whether serum cobalamin, and, if abnormal, MMA concentrations normalize when the euthyroid state is achieved after radioiodine treatment, and (3) assess whether the clinical catabolic state associated with feline hyperthyroidism, as defined by body condition score, body weight, and fecal score, correlates with cobalamin status.
Materials and Methods
Cat Selection
Client‐owned hyperthyroid cats that presented to the University of Wisconsin (UW) Veterinary Care for radioiodine treatment between July 2013 and April 2015 were recruited for the study. Cats with clinical signs of hyperthyroidism (weight loss, polyphagia, polyuria, polydipsia, or palpable goiter) and a serum total thyroxine (T4) > 4.8 μg/dL (reference range 1.9–4.8 μg/dL) were eligible for enrollment. Cats previously treated with methimazole were eligible for enrollment as long as they were biochemically hyperthyroid and had not received antithyroid medications for at least 7 days (calculated using a methimazole half‐life of 3 hours in cats) before enrollment.17, 18 Cats previously diagnosed with infiltrative or inflammatory small intestinal disease via surgical or endoscopic biopsies, exocrine pancreatic insufficiency based on a serum trypsin‐like immunoreactivity (TLI) concentration <8 μg/L,19 or supplemented with cobalamin within 65 days of presentation (calculated using a cobalamin half‐life of 13 days in cats) were excluded from the study.20 All study protocols were reviewed, approved, and conducted in accordance with the University of Wisconsin Animal Care and Use Committee. Informed consent was provided by all cat owners before enrollment into the study.
Study Design
A prospective, observational study design was used. At the time of study enrollment, all cats were required to be clinically and biochemically hyperthyroid. After their initial evaluation the cats were treated with subcutaneous radioiodine (ie, I 131) using a standard treatment algorithm, with dosage based on the severity of the cat's clinical signs, size of the goiter, and serum total T4 concentration.21 Cats were reevaluated 60 days after radioiodine treatment, when 95% of cats are euthyroid using this protocol.21 During the 60 days observation period, cats were maintained on the same diet they were eating at the time of study enrollment. Data collected at the time of recruitment and at the 60 day reevaluation included age, breed, sex, spay/neuter status, medical history pertaining to hyperthyroidism and gastrointestinal (GI) disease, current diet, body weight, current drug therapies, and any concurrent conditions. A physical examination and body condition score (scale 1–9) were performed in all cats by a single clinician (BG). In addition, an illustrated fecal scoring chart1 was used by the owner to subjectively score their cat's stool quality. Each cat was assigned a whole number value between 1 (i.e. “very hard”) to 7 (“watery”) based a visual comparison.
Sample Collection and Analysis
Cats were fasted for a minimum of 12 hours before blood collection. Blood and urine samples were collected from all cats at enrollment and 60 days after radioiodine treatment. Five to 6 mL of venous blood (<1% of a cat's body weight) was collected from jugular, cephalic or saphenous vein. Urine (3–6 mL) was collected by cystocentesis or from a voided sample.
All recruited hyperthyroid cats had screening diagnostic procedures before radioiodine treatment including a complete blood count (CBC), biochemical panel, urinalysis (UA), serum total T4, blood pressure, and examination of thoracic radiographs. If screening diagnostics done by referring veterinarians were available for review and performed by a commercial veterinary clinical pathology laboratory within 2 weeks before evaluation at the UW Veterinary Care, additional screening diagnostics were not performed at the time of study enrollment. Screening after radioiodine treatment include a recheck blood pressure, biochemistry panel, serum total T4, and urine specific gravity.
For the study, serum was submitted at enrollment and after radioiodine treatment to the GI laboratory at Texas A&M University (College Station, TX) for analysis of folate, cobalamin, feline TLI, and MMA concentrations. Serum samples were stored at <4°C and shipped on ice to the GI laboratory for analysis. Folate was measured as a marker of both duodenal malabsorption and small intestinal bacterial overgrowth (SIBO) which has been correlated with altered MMA absorption in humans.22 MMA was measured using a stable isotope dilution gas chromatography–mass spectrometry assay, which was previously described and validated in cats.11
Hypocobalaminemia was defined as a serum cobalamin concentration <290 ng/L based on the reference interval (290–1499 ng/L) provided by the GI laboratory at Texas A&M University. Serum cobalamin concentrations are reported based on the assay's working range of 150–1000 ng/L; serum samples above or below the assay's working range were either reported as <150 ng/L or >1000 ng/L, respectively.12 The markers used to define a cellular (or functional) cobalamin deficiency were hypocobalaminemia in association with a serum MMA concentration >867 nmol/L.11
Statistical Analysis
An a priori power calculation was used to determine our target enrollment of 30 hyperthyroid cats and was based on the previously reported prevalence of low cobalamin in hyperthyroid cats12 and the anticipated prevalence of high serum MMA concentrations if hypocobalaminemic.11 The enrollment of 30 cats provided >95% power to detect a significant difference between serum cobalamin and MMA concentrations in hyperthyroid cats.
Descriptive statistics were used to define baseline characteristics (age, body weight, and clinicopathological values) of the hyperthyroid cat population and values were compared before and after radioiodine treatment using Wilcoxon signed‐rank test. The prevalence of hyperthyroid cats with a serum cobalamin concentration <290 ng/L and MMA concentration >867 nmol/L before radioiodine treatment was calculated. Serum cobalamin and MMA concentrations were compared in hyperthyroid cats before treatment and after restoration of a euthyroid state using Wilcoxon signed‐rank test. Cats in which the hyperthyroid state persisted at the 60‐day recheck were excluded from the after treatment analysis. In cats with clinical and biochemical hyperthyroidism, clinical scores (body condition score, body weight, and fecal score) were evaluated for correlation with serum cobalamin concentrations using a Spearman rho correlation. Statistical calculations were performed with a commercial software package.2 All values are reported as median and ranges. Statistical significance was set at P < .05.
Results
Study Population
During the active enrollment period, a total of 69 hyperthyroid cats were evaluated and treated with radioiodine treatment with a total of 39 cats enrolled into the study (Fig 1). Demographics, clinical scores (body weight, BCS, fecal score), and results of selected diagnostics of the hyperthyroid cats at enrollment and 60 days after radioiodine treatment are summarized in Tables 1 and 2. After radioiodine treatment and return to the euthyroid state there were significant increases in body weight, BCS, and folate concentrations and a decrease in total T4 concentrations. There was no significant change in the cats' cobalamin concentrations or fecal scores after radioiodine treatment.
Figure 1.

The progression of study events for all cats evaluated and enrolled over the 60 day observation period of the study.
Table 1.
Signalment and clinical scores of the hyperthyroid cats at study enrollment and after radioiodine treatment (60 days). All values reported as median and ranges
| Study Enrollment (n = 39) | After I131 (60 days) (n = 29) | P‐value | |
|---|---|---|---|
| Cats | |||
| Breeds | N/A | ||
| DSH/DLH/DMH | 12/8/5 | 19/6/3 | |
| Other | 1 Siamese | 1 Siamese | |
| Sex (FS/MC) | 19/20 | 16/13 | N/A |
| Age (years) | 13.5 (7.5–18) | 13.5 (7.5–17.5) | 0.77 |
| Clinical Scores | |||
| Body weight (kg) | 3.7 (2.3–5.7) | 4.5 (2.9–7.1) | 0.0058 |
| BCS (1–9) | 4 (2–8) | 5 (3–8) | 0.0087 |
| Fecal score (1–7) | 2 (2–7) | 2 (2–6) | 0.64 |
Abbreviations: I131, iodine 131 or radioiodine; DSH, domestic short hair; DLH, domestic long hair; DMH, domestic medium hair; FS, female spayed; MC, male castrated; BCS, body condition score.
Table 2.
Results of the selected diagnostics of the hyperthyroid cats at study enrollment and after radioiodine treatment (60 days). All values reported as median and ranges
| Selected Diagnostics | Reference Interval | Study Enrollment (n = 39) | After I131 (60 days) (n = 29) | P‐value |
|---|---|---|---|---|
| TT4 (μg/dL) | 0.6–3.5 | 9 (4–26.8) | 1.4 (0.7–3.2) | <0.0001 |
| Blood pressure (mmHg) | <150 | 150 (96–200) | 144a (121–223) | 0.30 |
| HCT (%) | 31–48 | 39 (30–48) | N/A | N/A |
| BUN (mg/dL) | 15–35 | 23 (11–36) | 28 (20–46) | 0.0004 |
| Creatinine (mg/dL) | 0.9–2.3 | 1.0 (0.40–2.2) | 1.7 (0.90–3.3) | <0.0001 |
| Urine SG | >1.035 | 1.026 (1.009–1.057) | 1.025b (1.012–1.060) | 0.18 |
| ALT (U/L) | 20–108 | 136 (47–683) | 60 (31–120) | <0.0001 |
| ALP (U/L) | 23–107 | 68 (30–262) | 35 (22–63) | <0.0001 |
| Albumin (g/dL) | 2.7–3.9 | 3.2 (2.3–3.9) | 3.4 (2.6–3.9) | 0.012 |
| Globulin (g/dL) | 2.3–3.8 | 3.5 (3–4.5) | 3.9 (3.2–4.5) | 0.0002 |
| TLI (μg/L) | 12.1–81.9 | 40 (12.6–145.1) | N/A | N/A |
| Folate (μg/L) | 9.7–21.6 | 12.5 (5.2–34) | 18.25 (9.2–30.7) | <0.0001 |
| Cobalamin (ng/L) | 290–1000 | 774 (<150–>1000) | 866 (<150–>1000) | 0.24 |
| MMA (nmol/L) | 139–898 | 292 (129–1215) | 347 (129–1555) | 0.16 |
As a result of cat compliance blood pressure measurements were only available in 27 cats.
As a result of cat compliance urine specific gravity measurements were only available in 20 cats.
Abbreviations: I131, iodine 131 or radioiodine; TT4, total thyroxine; RI, reference interval; HCT, hematocrit; BUN, blood urea nitrogen; SG, specific gravity; ALT, alanine aminotransferase; ALP, alkaline phosphatase; TLI, trypsin‐like immunoreactivity; MMA, methylmalonic acid.
Cobalamin and MMA concentrations
Five of the 39 hyperthyroid cats (13%) had a low serum cobalamin concentration ranging from <150 to 290 ng/L (Fig 2). In 4 of these 5 cats with 60 day follow‐up data, 2/4 hypocobalaminemic cats had serum cobalamin concentrations increased to >350 ng/L once euthyroid. In the remaining 2 cats, serum cobalamin concentrations remained <290 ng/mL. None of the hyperthyroid/hypocobalaminemic cats had increased serum MMA concentrations (175–601 nmol/L). In cats with clinical and biochemical hyperthyroidism, there was no correlation between serum cobalamin concentrations with total T4 concentrations (P = .12) or clinical scores including body weight (P = .11) and BCS (P = .54). There was a weak negative correlation between serum cobalamin concentrations with fecal score in cats with clinical and biochemical hyperthyroidism, Spearman rho correlation coefficient of −0.3677 (95% confidence interval −0.62 to −0.049. P = .021).
Figure 2.
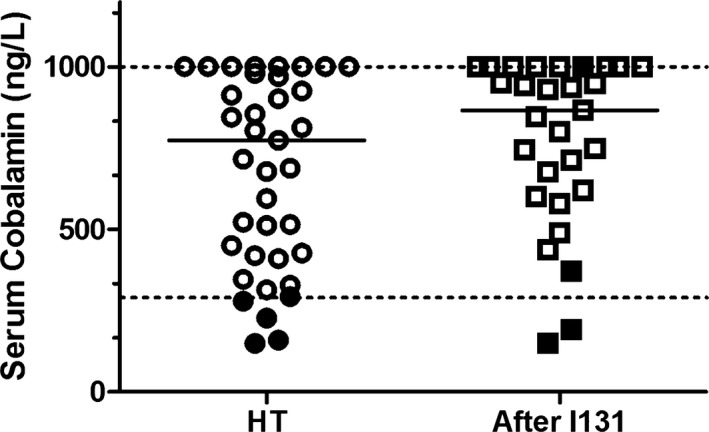
Serum cobalamin (vitamin B12) concentrations plotted for the hyperthyroid cats before and after radioiodine treatment. Five hyperthyroid cats (●) had cobalamin concentrations <290 ng/L. Four of these 5 cats with 60 day follow‐up data (■), 2/4 had cobalamin concentrations increase to >350 ng/L once euthyroid and for the remaining 2 cats cobalamin concentrations remained <290 ng/L.
Discussion
In this well‐characterized population of hyperthyroid cats the prevalence of hypocobalaminemia was low (13%), and none of the cats with low cobalamin had a concurrent increase in MMA concentrations. Half of the hyperthyroid/hypocobalaminemic cats had a significant increase in their cobalamin concentrations once euthyroid. The results of this study suggest that hyperthyroid cats with a concurrent hypocobalaminemia do not experience a functional cobalamin deficiency or altered cobalamin concentration at the cellular level. In addition, there was no correlation between serum cobalamin and T4 concentrations or clinical score further supporting that a low serum cobalamin in some hyperthyroid cats is not clinically important. Two cats had persistently low cobalamin concentrations (<290 ng/L) after radioiodine treatment, suggesting the possibility of concurrent subclinical malabsorptive gastrointestinal disease. Neither cat had clinical evidence of diarrhea (fecal scores before I131, 2 and 4 versus after I131, 2 and 2, respectively) or significant decrease in body condition (BCS before I131, 3 and 3 versus after I131, 3 and 4, respectively) or body weight (before I131, 3.8 kg and 2.7 kg versus after I131, 3.7 kg and 3.5 kg, respectively). In addition, neither of these cats required subsequent reevaluation for clinical signs suggestive of gastrointestinal disease as of the writing of this manuscript.
Similar to hyperthyroidism in women, the results of this study suggest feline hyperthyroidism is not associated with a clinically important functional deficiency in cobalamin.13, 14 However, the prevalence of hyperthyroid/hypocobalaminemia in this study is in contrast with the a recent retrospective study which reported a 40% prevalence in their population of hyperthyroid cats.12 Both studies used the same validated analytical methodology (an automated chemiluminescence assay) via the GI laboratory at Texas A&M to quantify serum cobalamin concentrations. A possible reason for the discrepancy in the prevalence of hypocobalaminemia between studies is the difference in the feline populations studied. The feline population was prospectively enrolled in this study and was a well‐characterized group of clinically hyperthyroid cats. None of the enrolled cats had clinical evidence of concurrent illness, specifically EPI or gastrointestinal disease. In contrast to the cat population presented here, the cat population studied previously was acquired retrospectively through convenience sampling of available serum samples from cats reported to be hyperthyroid but in the absence of any clinical history. No information was provided at the time of blood collection about the cats including clinical signs or presenting complaint, previous medical history, diagnostics to rule out concurrent illnesses including gastrointestinal disease, current medications (ie, antithyroid treatment or cobalamin supplementation), or dietary information. In the absence of any clinical history, the prevalence of hypocobalaminemia may have been confounded by common diseases affecting an older (median age 13 years, range 6–22) population of cats (ie, malabsorptive gastrointestinal disease including inflammatory bowel disease or lymphosarcoma and chronic kidney disease), which impact serum cobalamin concentrations. However, other plausible explanations for the contrasting results between studies should not be discounted including sample size. The lower‐than‐expected prevalence of hypocobalaminemia in this population of hyperthyroid cats resulted in an underpowered study and our negative findings. Our a priori power calculation determined a statistical power of >95% to detect hypocobalaminemia in this population of cats.12
An unexpected but interesting outcome of this study is that when cats are hyperthyroid, serum folate concentrations were significantly lower than when euthyroid. Serum folate concentrations significantly increased once the cats were euthyroid without a change in diet or supplementation. The altered serum folate concentrations experienced by hyperthyroid cats is likely relative based on serum levels not falling outside the reference interval and serum folate concentrations spontaneously increasing once cats were euthyroid. The clinical importance of a lower serum folate concentrations in hyperthyroid cats is unknown. Low folate in the absence of hypocobalaminemia has been reported in other ill cat populations including cats with aortic thromboembolism (mean folate concentration 13.8 μg/L)23 and a heterogeneous group of systemically ill cats (n = 103). Forty of the 103 ill cats were reported to have a serum folate lower than the reference interval.24 Additional research is needed to assess if a lower serum folate concentration in cats is associated with a functional folate deficiency. A concurrent increase in the urinary excretion formiminoglutamic acid (or FIGLU)25 is one proposed marker of a functional folate deficiency. For example, increased urinary excretion of FIGLU was described in kittens fed a diet deficient in folate.26 A deficiency in folate results in the inability to metabolize FIGLU to glutamic acid leading to an increase in FIGLU concentrations in the urine.
Possible explanations for the lower serum folate concentrations in association with hyperthyroidism in cats are likely multifactorial including decreased absorption, increased loss, or increased utilization (or altered metabolism). Hyperthyroidism has been associated with faster gastrointestinal transit in cats,27, 28 suggesting the possibility of either increased gastrointestinal loss or decreased gastrointestinal absorption of folate in cats. The altered metabolic state associated with hyperthyroidism includes increased renal blood flow (or GFR) and altered active tubular transport29 suggesting the possible role of increased urinary loss of folate when cats are hyperthyroid that subsequently decreases once euthyroid. In this group of hyperthyroid cats, the significant increase in creatinine and folate once euthyroid (60 days after radioiodine treatment) may suggest that the urinary excretion of folate is altered in hyperthyroid cats.30 Finally, the increased catabolic state associated with hyperthyroidism may contribute to an increase in the demand for folate by the remethylation pathway of Hyc to methionine. Folate is essential in the remethylation pathway to produce S‐adenosylmethionine (the universal methyl donor needed in nucleic acid synthesis) and regenerate methionine as well as central to cellular metabolism, specifically its role in one‐carbon oxidation/reduction reactions.31 In humans, folate deficiency is often associated with hyperhomocysteinemia.32
Cats have several unique metabolic and nutritional requirements making comparisons across species difficult. For example, cats relative to humans have differences in Hyc metabolism9, 10 and dietary amino acids (ie, taurine, arginine, and methionine) requirements.33 The findings of this study further support species differences (ie, human versus cat) in B vitamin concentrations during illness.34, 35 Hyperthyroid cats do not have abnormally low cobalamin and higher folate concentrations as do hyperthyroid women14 but rather have no significant change in serum cobalamin and significantly decreased levels of serum folate. Experimental studies in hyperthyroid humans and rodent models support increased activity of methylene tetrahydrofolate reductase (MTHFR) leading to decreased folate concentrations.14, 36 A similar mechanism seems unlikely in hyperthyroid cats. Further assessment as to the mechanism and/or clinical importance of decreased folate concentrations in hyperthyroid cats may include an assessment of MTHFR activity, Hyc concentrations, and/or urinary FIGLU levels, which were not evaluated in this study.
The strengths of this study are the prospective study design and the use of a well‐characterized population of clinically hyperthyroid cats before and post definitive treatment with radioiodine. As previously discussed, the limitations of this study include the relatively small population of hyperthyroid cat studied and the inability to more definitively rule out concurrent gastrointestinal disease in this cat population. However, the study's inclusion/exclusion criteria used not only history and clinical signs but also biochemical testing and previous gastrointestinal biopsies to exclude any cat suspected or diagnosed with underlying gastrointestinal disease from study enrolment, minimizing the confounding variables in the interpretation of the serum cobalamin concentrations. Additional diagnostics including a noninvasive abdominal ultrasound of the cats with low cobalamin may have helped rule out concurrent malabsorptive disease, however, ultrasonographic changes generally correlate poorly with small intestinal histological findings.37
In conclusion, the prevalence of hypocobalaminemia is low in this group of hyperthyroid cats. In some hyperthyroid cats in which underlying gastrointestinal disease is unlikely, concurrent hypocobalaminemia is not a functional deficiency in need of supplementation. However, further investigation for subclinical gastrointestinal disease is recommended in any cat with persistent hypocobalaminemia once euthyroid after radioiodine treatment. Significantly lower serum folate concentrations occur in cats when hyperthyroid, but levels increase once euthyroid, suggesting a clinically unimportant observation.
Acknowledgments
The authors thank Small Animal Internal Medicine technicians for their assistance in patient recruitment and care as well as sample collection and handling.
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Grant support: University of Wisconsin‐Madison, School of Veterinary Medicine, Companion Animal Grant.
Meetings: Portions of the data herein were first presented as an oral research abstract at the 2014 American College Veterinary Internal Medicine Forum, Nashville, TN, June 5, 2014.
Footnotes
Nestle Purina, Vevey, Switzerland
Graphpad Prism 5, La Jolla, CA
References
- 1. Langan RC, Zawistoski KJ. Update on vitamin B12 deficiency. Am Fam Physician 2011;83:1425–1430. [PubMed] [Google Scholar]
- 2. Allen RH, Stabler SP, Savage DG, et al. Metabolic abnormalities in cobalamin (vitamin B12) and folate deficiency. FASEB J 1993;7:1344–1353. [DOI] [PubMed] [Google Scholar]
- 3. Chadefaux B, Cooper BA, Gilfix BM, et al. Homocysteine: Relationship to serum cobalamin, serum folate, erythrocyte folate, and lobation of neutrophils. Clin Invest Med 1994;17:540–550. [PubMed] [Google Scholar]
- 4. Banerjee R. B12 trafficking in mammals: A for coenzyme escort service. ACS Chem Biol 2006;1:149–159. [DOI] [PubMed] [Google Scholar]
- 5. Allen RH, Stabler SP, Savage DG, et al. Diagnosis of cobalamin deficiency I: Usefulness of serum methylmalonic acid and total homocysteine concentrations. Am J Hematol 1990;34:90–98. [DOI] [PubMed] [Google Scholar]
- 6. Lindenbaum J, Savage DG, Stabler SP, et al. Diagnosis of cobalamin deficiency: II. Relative sensitivities of serum cobalamin, methylmalonic acid, and total homocysteine concentrations. Am J Hematol 1990;34:99–107. [DOI] [PubMed] [Google Scholar]
- 7. Whitehead R. The interpretation and significance of morphological abnormalities in jejunal biopsies. J Clin Path Suppl (Roy Coll Path) 1971;5:108–124. [Google Scholar]
- 8. Hall EJ, German AJ. Diseases of the small intestine, 7th ed St. Louis: Saunders; 2010. [Google Scholar]
- 9. Ruaux CG, Steiner JM, Williams DA. Metabolism of amino acids in cats with severe cobalamin deficiency. Am J Vet Res 2001;62:1852–1858. [DOI] [PubMed] [Google Scholar]
- 10. Ruaux CG, Steiner JM, Williams DA. Early biochemical and clinical responses to cobalamin supplementation in cats with signs of gastrointestinal disease and severe hypocobalaminemia. J Vet Intern Med 2005;19:155–160. [DOI] [PubMed] [Google Scholar]
- 11. Ruaux CG, Steiner JM, Williams DA. Relationships between low serum cobalamin concentrations and methlymalonic acidemia in cats. J Vet Intern Med 2009;23:472–475. [DOI] [PubMed] [Google Scholar]
- 12. Cook AK, Suchodolski JS, Steiner JM, et al. The prevalence of hypocobalaminaemia in cats with spontaneous hyperthyroidism. J Small Anim Pract 2011;52:101–106. [DOI] [PubMed] [Google Scholar]
- 13. Demirbas B, Ozkaya M, Cakal E, et al. Plasma homocysteine levels in hyperthyroid patients. Endocr J 2004;51:121–125. [DOI] [PubMed] [Google Scholar]
- 14. Orzechowska‐Pawilojc A, Siekierska‐Hellmann M, Syrenicz A, et al. Homocysteine, folate, and cobalamin levels in hyperthyroid women before and after treatment. Endokrynol Pol 2009;60:443–448. [PubMed] [Google Scholar]
- 15. Alperin JB, Haggard ME, Haynie TP. A study of vitamin B 12 requirements in a patient with pernicious anemia and thyrotoxicosis: Evidence of an increased need for vitamin B 12 in the presence of hyperthyroidism. Blood 1970;36:632–641. [PubMed] [Google Scholar]
- 16. Gyftaki H, Kesse‐Elias M, Koutras D, et al. Serum vitamin B12 and folic acid levels in hyperthyroidism. Nuklearmedizin 1979;18:278–282. [PubMed] [Google Scholar]
- 17. Trepanier LA, Peterson ME, Aucoin DP. Pharmacokinetics of intravenous and oral methimazole following single‐ and multiple‐dose administration in normal cats. J Vet Pharmacol Ther 1991;14:367–373. [DOI] [PubMed] [Google Scholar]
- 18. Trepanier LA, Peterson ME, Aucoin DP. Pharmacokinetics of methimazole in normal cats and cats with hyperthyroidism. Res Vet Sci 1991;50:69–74. [DOI] [PubMed] [Google Scholar]
- 19. Steiner JM, Williams DA. Serum feline trypsin‐like immunoreactivity in cats with exocrine pancreatic insufficiency. J Vet Intern Med 2000;14:627–629. [DOI] [PubMed] [Google Scholar]
- 20. Simpson KW, Fyfe J, Cornetta A, et al. Subnormal concentrations of serum cobalamin (vitamin B12) in cats with gastrointestinal disease. J Vet Intern Med 2001;15:26–32. [DOI] [PubMed] [Google Scholar]
- 21. Peterson ME, Becker DV. Radioiodine treatment of 524 cats with hyperthyroidism. J Am Vet Med Assoc 1995;207:1422–1428. [PubMed] [Google Scholar]
- 22. Hvas AM, Juul S, Gerdes LU, et al. The marker of cobalamin deficiency, plasma methylmalonic acid, correlates to plasma creatinine. J Intern Med 2000;247:507–512. [DOI] [PubMed] [Google Scholar]
- 23. Hohenhaus A, Simantov R, Fox P, et al. Evaluation of plama homocysteine and B vitamin concentrations in cardiomyopathic cats with congestive heart failure and aterial thromboembolism. Proceedings of the 1999 Purina Nutrition Forum, St Louis, MO 1999;170.
- 24. Reed N, Gunn‐Moore D, Simpson K. Cobalamin, folate and inorganic phosphate abnormalities in ill cats. J Feline Med Surg 2007;9:278–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Luhby AL, Cooperman JM, Teller DN. Urinary excretion of formiminoglutamic acid: Application in diagnosis of clinical folic acid deficiency. Am J Clin Nutr 1959;7:397–406. [DOI] [PubMed] [Google Scholar]
- 26. Yu S, Morris JG. Folate requirement of growing kittens to prevent elevated formiminoglutamic acid excretion following histidine loading. J Nutr 1998;128:2606S–2608S. [DOI] [PubMed] [Google Scholar]
- 27. Papasouliotis K, Muir P, Gruffydd‐Jones T, et al. Decreased orocaecal transit time, as measured by the exhalation of hydrogen, in hyperthyroid cats. Res Vet Sci 1993;55:115–118. [DOI] [PubMed] [Google Scholar]
- 28. Peachey SE, Dawson JM, Harper EJ. Gastrointestinal transit times in young and old cats. Comp Biochem Physiol A Mol Integr Physiol 2000;126:85–90. [DOI] [PubMed] [Google Scholar]
- 29. Adams WH, Daniel GB, Legendre AM. Investigation of the effects of hyperthyroidism on renal function in the cat. Can J Vet Res 1997;61:53–56. [PMC free article] [PubMed] [Google Scholar]
- 30. Adams WH, Daniel GB, Legendre AM, et al. Changes in renal function in cats following treatment of hyperthyroidism using 131I. Vet Radiol Ultrasound 1997;38:231–238. [DOI] [PubMed] [Google Scholar]
- 31. Bailey LB, Gregory JF 3rd. Folate metabolism and requirements. J Nutr 1999;129:779–782. [DOI] [PubMed] [Google Scholar]
- 32. Selhub J, Jacques PF, Wilson PW, et al. Vitamin status and intake as primary determinants of homocysteinemia in an elderly population. J Am Med Assoc 1993;270:2693–2698. [DOI] [PubMed] [Google Scholar]
- 33. Zoran DL. The carnivore connection to nutrition in cats. J Am Vet Med Assoc 2002;221:1559–1567. [DOI] [PubMed] [Google Scholar]
- 34. Fulmer AK, Mauldin GE, Mauldin GN. Evaluation of plasma folate and homocysteine concentrations in cats with and without oral squamous cell carcinoma. Vet Comp Oncol 2008;6:248–256. [DOI] [PubMed] [Google Scholar]
- 35. McMichael MA, Freeman LM, Selhub J, et al. Plasma homocysteine, B vitamins, and amino acid concentrations in cats with cardiomyopathy and arterial thromboembolism. J Vet Intern Med 2000;14:507–512. [DOI] [PubMed] [Google Scholar]
- 36. Selhub J. Homocysteine metabolism. Annu Rev Nutr 1999;19:217–246. [DOI] [PubMed] [Google Scholar]
- 37. Gaschen L. Ultrasonography of small intestinal inflammatory and neoplastic diseases in dogs and cats. Vet Clin North Am Small Anim Pract 2011;41:329–344. [DOI] [PubMed] [Google Scholar]


