Abstract
Background
There are breed differences in several blood variables in healthy dogs.
Objective
Investigate breed variation in plasma endothelin‐1 (ET‐1) concentration, plasma renin activity, and serum cortisol concentration.
Animals
Five‐hundred and thirty‐one healthy dogs of 9 breeds examined at 5 centers (2–4 breeds/center).
Methods
Prospective observational study. Circulating concentrations of ET‐1 and cortisol, and renin activity, were measured using commercially available assays. Absence of organ‐related or systemic disease was ensured by thorough clinical investigations, including blood pressure measurement, echocardiography, ECG, blood and urine analysis.
Results
Median ET‐1 concentration was 1.29 (interquartile range [IQR], 0.97–1.82) pg/mL, median cortisol concentration 46.0 (IQR, 29.0–80.8) nmol/L, and median renin activity 0.73 (IQR, 0.48–1.10) ng/mL/h in all dogs. Overall, breed differences were found in ET‐1 and cortisol concentrations, and renin activity (P < .0001 for all). Pair‐wise comparisons between breeds differed in 67% of comparisons for ET‐1, 22% for cortisol, and 19% for renin activity, respectively. Within centers, breed differences were found at 5/5 centers for ET‐1, 4/5 centers for cortisol, and 2/5 centers for renin activity. Newfoundlands had highest median ET‐1 concentration, 3 times higher than Cavalier King Charles Spaniels, Doberman Pinschers, and Dachshunds. Median renin activity was highest in Dachshunds, twice the median value in Newfoundlands and Boxers. Median cortisol concentration was highest in Finnish Lapphunds, almost 3 times higher than in Boxers.
Conclusions and Clinical Importance
Breed variation might be important to take into consideration when interpreting test results in clinical studies.
Keywords: Biomarker, Breed variation, Canine, Vasoactive
Abbreviations
- ANP
atrial natriuretic peptide
- CKCS
Cavalier King Charles Spaniel
- CVs
coefficients of variation
- ELFA
enzyme‐linked fluorescent assay
- ET‐1
endothelin‐1
- IQR
interquartile range
- NT‐proBNP
N‐terminal pro‐B‐type natriuretic peptide
- RAAS
renin‐angiotensin‐aldosterone system
- RIA
radioimmunoassay
Regulation of blood pressure is complex involving brain, kidneys, cardiovascular system, and vasoactive peptides. Decrease in blood pressure activates the sympathetic nervous system, the renin‐angiotensin‐aldosterone system (RAAS), and vasopressin, leading to vasoconstriction.1 Angiotensin II can stimulate the release of endothelin‐1 (ET‐1), a potent vasoconstrictor, from vascular endothelium, and ET‐1 can in turn stimulate the release of norepinephrine, angiotensin II, and vasopressin.2, 3 The RAAS, together with vasopressin, also stimulates sodium and fluid retention.1 This might be counteracted by the natriuretic peptides, which stimulate natriuresis, diuresis, and vasodilation.1 The systems are also activated in development of cardiac and renal disease and several of these substances have been suggested as biomarkers of disease in dogs.4, 5, 6 However, many physiologic and pathologic factors might influence neuroendocrine concentrations,5, 7, 8 making interpretation of test results difficult. Dog breed is one potential such factor.9
In an European research project, LUPA, over 500 dogs of 9 breeds were examined at five centers looking for genetic determinants of variation in blood pressure, neuroendocrine, and metabolic variables.10 Simultaneously, breed differences in physiological variables were investigated. Results have shown substantial breed differences in plasma concentrations of the natriuretic peptides pro‐ANP 31‐67 and NT‐proBNP,11 prompting cautiousness when using them as biomarkers for heart disease. When investigating a subpopulation of the dogs, we also found breed differences in blood pressure and heart rate in consistency with other studies,12, 13, 14 and in urinary epinephrine and norepinephrine concentrations.15
It has been hypothesized that plasma ET‐1 in horses could be increased in stress conditions and there are breed differences in plasma ET‐1 and serum cortisol concentrations.16 The aim of this study was to investigate breed variation in plasma concentration of ET‐1, plasma renin activity, and serum concentration of cortisol in healthy dogs.
Materials and Methods
Animals
Dogs were examined at 5 centers: University of Liège, Belgium; University of Copenhagen, Denmark; National Veterinary School of Alfort, France; University of Helsinki, Finland; and Swedish University of Agricultural Sciences, Sweden. The study was performed as part of the EU‐funded LUPA‐project,10 and was approved by an ethical committee in each participating country. Dogs were privately owned, and informed owner consent was obtained. To be included, dogs had to be pure‐bred, healthy, and 1–7 years old. Dogs had to have a normal body condition score, and could not be related to each other at parental level. Each center could include 2–5 breeds, and within center, each breed cohort included dogs of 1 sex only, intact males, or females that were spayed or in anestrus, according to LUPA inclusion criteria. Dogs were excluded if any finding indicating systemic or organ‐related disease was observed during the clinical examination described below.
Preparations
To ensure consistent salt intake, owners were instructed to feed their dog only commercial dog food and no treats, 2 weeks before participation in the study. On examination day, all dogs were fasted ≥12 hours and had no access to water for at least 2 hours before examination.
Characterization of Health Status
From each dog, a morning urine sample collected by the owner was brought into clinic. Standard urine analysis was performed by dipstick chemistry test, and refractometer for urine specific gravity. The dog underwent general physical examination including blood pressure measurement by high‐definition oscillometry, according to published guidelines.17 Each dog was assigned a body condition score on a 1–9 points scale.18 Five‐minute ECG recording and echocardiographic examination including 2‐dimensional, M‐mode, and Doppler echocardiography was performed. The echocardiographic examination was performed from right and left sides, using standardized imaging planes19 and continuous ECG monitoring. Blood was collected after health examination by venipuncture into 5‐mL serum and EDTA tubes. In most dogs, blood sampling was performed between 9 am and 2 pm (n = 499), while a few dogs (n = 32, 6% of total number of dogs) were sampled between 2 and 5 pm. Routine hematology and biochemistry analyses including parameters of liver and kidney function, glucose, and serum electrolyte concentrations were performed. All examinations were performed in unsedated dogs.
Analyses of ET‐1, Renin Activity, and Cortisol
EDTA tubes for analysis of ET‐1 concentration and renin activity, and serum tubes for analysis of cortisol concentration, were centrifuged within 30 minutes of sample collection. Plasma and serum were harvested, transferred into plastic cryotubes, frozen and stored at −80°C. For practical reasons, at 1 center samples were stored at −20°C for maximum 2 weeks, after which they were transferred frozen to −80°C and stored for batched analysis. All samples were later transported frozen on dry ice at −80°C to 2 laboratories, 1 laboratory1 for analysis of renin activity and ET‐1, and another laboratory2 for analysis of cortisol.
All analyses were performed using commercially available assays validated for dogs,20, 21, 22 according to manufacturers' instructions. Renin activity was measured by radioimmunoassay (RIA) with solid‐phase‐coated tube separation,3 ET‐1 by ELISA4 and cortisol by enzyme‐linked fluorescent assay (ELFA).5 All samples were analyzed in duplicate by personnel blinded to dog identity, and mean values were used for data analysis. In‐house duplicate coefficients of variation (CVs) were: renin activity 13.8%, ET‐1 7.6%, and cortisol 10.5%. Interassay CVs were: renin activity < 15%, ET‐1 < 10%, and cortisol < 12%. Dynamic ranges were: renin activity 0.17–3.40 ng/mL/h, ET‐1 0.39–50 pg/mL, and cortisol 5.5–2,759 nmol/L; and limits of detection were: renin activity 0.010 ng/mL/h, ET‐1 0.23 pg/mL, and cortisol 5.5 nmol/L.
Statistical Analyses
Commercially available software6 was used for statistical analyses. Data are presented as medians and interquartile ranges (IQR). A value of P < .05 was considered significant, unless otherwise indicated.
The nonparametric Kruskal–Wallis test was used to investigate overall differences among breeds for renin activity, ET‐1, and cortisol, respectively. If a significant difference was detected, pair‐wise breed comparisons were performed by Mann–Whitney U‐test with Bonferroni adjustment; significance level P < .0014.
Due to the uneven breed distribution between centers, breed was highly covariate with center. Kruskal–Wallis test was therefore also used to investigate breed differences within each center for renin activity, ET‐1, and cortisol, respectively. At centers including more than 2 breeds, pair‐wise breed comparisons were performed by Mann–Whitney U‐test with Bonferroni adjustment, if an overall significant difference was detected.
Univariate regression analyses were performed to evaluate potential associations between breed, age, body weight, examination center, and renin activity, ET‐1, and cortisol, respectively. A subanalysis of the same variables by univariate regression analysis was performed in Labrador retrievers, because this breed included the largest number of dogs, was represented at 4 of 5 centers and included both female and male dogs. Therefore, sex also was assessed by univariate regression analysis in the Labrador retriever breed.
To compensate for influence of other confounding factors on renin activity, ET‐1, and cortisol, multiple regression analysis was performed, including variables that reached P < .2 in the univariate regression analysis of all dogs. Analyses were performed in a reverse stepwise manner,23 starting with all included variables and removing the variable with highest P‐value until all remaining variables had a P‐value <.05. All variables were assessed only as main effects; no interaction terms were considered in the model.
Distribution of residuals in the multiple regression analysis was tested for normality using Shapiro–Wilk W test. The adjusted R 2 is defined as the percentage of the total sum of squares that can be explained by the regression and also considers the degrees of freedom for variables added. Univariate and multiple regression analyses were performed on log‐transformed data. No multiple regression analysis was performed in the Labrador retriever cohort due to high covariance between center and sex.
Results
In total, 531 dogs of 9 breeds were included. Twenty‐six examined dogs were excluded due to the following reasons: myxomatous mitral valve disease (n = 10), tricuspid dysplasia (n = 2), aortic stenosis (n = 3), arrhythmia (n = 1), hepatopathy (n = 1), signs of inflammation on blood panel (n = 1), isosthenuria (n = 1), underweight (n = 1), obesity (n = 1), poor condition (n = 1), nonfasted at examination (n = 1), on medication at examination (n = 1), extreme stress during examination (n = 1), character of dog (aggression) (n = 1). Distribution of breeds and dogs included at different centers is shown in Table 1. Each center examined dogs belonging to 2–4 breeds and some breeds were examined at more than 1 center. One breed, the Labrador retriever, was represented at 4 of 5 centers. Sex distribution was uneven with 413 males and 118 female dogs (Table 1). All males were intact, whereas females were spayed or in anestrus. Median age (n = 531) was 3.3 (IQR, 2.6–4.4) years and median body weight (n = 490) was 30.0 (IQR, 23.5–36.0) kg.
Table 1.
Distribution of dogs by center of examination, breed, and sex
| Belgium | Denmark | Finland | France | Sweden | Total | |
|---|---|---|---|---|---|---|
| Box | 15M | 15 | ||||
| BS | 95M | 25M | 121 | |||
| CKCS | 34M | 34 | ||||
| Dach | 26M | 16M | 42 | |||
| Dob | 24M | 23 | ||||
| FinL | 50M | 50 | ||||
| GS | 16M | 60M | 76 | |||
| Lab | 6M | 45F | 29F | 46M | 126 | |
| NF | 44F | 44 | ||||
| Total | 117 | 89 | 136 | 78 | 111 | 531 |
Box, Boxer; BS, Belgian Shepherd; CKCS, Cavalier King Charles Spaniel; Dach, Dachshund; Dob, Doberman pinscher; FinL, Finnish Lapphund; GS, German Shepherd; Lab, Labrador retriever; NF, Newfoundland; M, male; F, female.
ET‐1, Renin Activity, and Cortisol
Median ET‐1 concentration (n = 506) was 1.29 (IQR, 0.97–1.82) pg/mL, median cortisol concentration (n = 528) was 46.0 (IQR, 29.0–80.8) nmol/L, and median renin activity (n = 528) was 0.73 (IQR, 0.48–1.10) ng/mL/h. The number of missing samples was n = 25 for ET‐1, n = 3 for cortisol, and n = 3 for renin activity. Concentrations of ET‐1 and cortisol, and renin activity by breed are shown in Fig 1 and Table 2.
Figure 1.
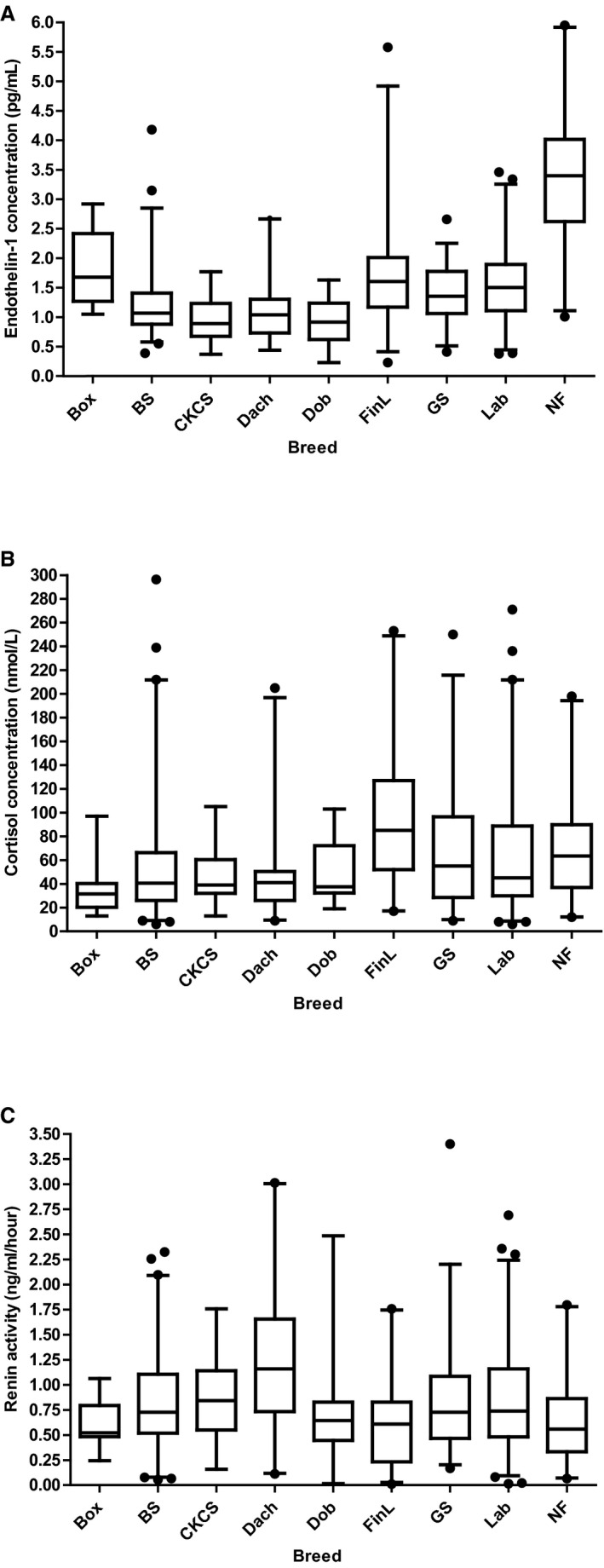
Boxplots showing distribution of endothelin‐1 (A), cortisol (B), and renin activity (C) by breed. The top, bottom, and line within each box correspond to the 75th percentile (top quartile), the 25th percentile (bottom quartile), and the 50th percentile (median), respectively. The whiskers extend from the bottom 2.5th percentile to the top 97.5th percentile. Outliers, represented by black dots, were included in statistical analyses. There was an overall significant breed difference for all 3 substances (P < .0001). For information on which breeds that differed significantly, see Table 3. Box, Boxer; BS, Belgian Shepherd; CKCS, Cavalier King Charles Spaniel; Dach, Dachshund; Dob, Doberman pinscher; FinL, Finnish Lapphund; GS, German Shepherd; Lab, Labrador retriever; NF, Newfoundland.
Table 2.
Median, interquartile range (IQR), minimum (min) and maximum (max) values for endothelin‐1 and cortisol concentrations, and renin activity displayed by breed
| Breed | Endothelin‐1 (pg/mL) | Cortisol (nmol/L) | Renin Activity (ng/mL/h) | |||
|---|---|---|---|---|---|---|
| Median (IQR) | Min–Max | Median (IQR) | Min–Max | Median (IQR) | Min–Max | |
| Box | 1.68 (1.27–2.42) | 1.05–2.92 | 31.5 (20.3–40.3) | 13.0–97.0 | 0.52 (0.48–0.79) | 0.24–1.06 |
| BS | 1.07 (0.88–1.41) | 0.39–4.18 | 39.0 (26.0–65.0) | 6.0–321 | 0.73 (0.52–1.11) | 0.05–2.33 |
| CKCS | 0.89 (0.68–1.24) | 0.37–1.77 | 39.0 (32.0–60.5) | 13.0–105 | 0.84 (0.55–1.14) | 0.16–1.76 |
| Dach | 1.04 (0.73–1.30) | 0.44–2.68 | 41.0 (26.0–50.5) | 9.0–205 | 1.16 (0.73–1.66) | 0.11–3.01 |
| Dob | 0.92 (0.63–1.24) | 0.23–1.63 | 37.5 (32.3–72.3) | 19.0–103 | 0.64 (0.45–0.83) | 0.01–2.49 |
| FinL | 1.61 (1.17–2.01) | 0.23–5.58 | 85.0 (52.0–127) | 17.0–253 | 0.61 (0.23–0.83) | 0.01–1.76 |
| GS | 1.36 (1.06–1.78) | 0.41–2.66 | 55.0 (28.5–96.5) | 9.0–250 | 0.73 (0.47–1.08) | 0.17–3.40 |
| Lab | 1.51 (1.11–1.90) | 0.38–3.46 | 45.0 (30.0–88.8) | 6.0–271 | 0.74 (0.48–1.16) | 0.01–2.69 |
| NF | 3.40 (2.62–4.02) | 1.01–5.95 | 63.5 (37.0–89.8) | 12.0–198 | 0.56 (0.33–0.86) | 0.07–1.80 |
Box, Boxer; BS, Belgian Shepherd; CKCS, Cavalier King Charles Spaniel; Dach, Dachshund; Dob, Doberman pinscher; FinL, Finnish Lapphund; GS, German Shepherd; Lab, Labrador retriever; NF, Newfoundland.
Group‐wise Comparisons, All Dogs
Overall significant breed differences were found for ET‐1 and cortisol concentrations, and renin activity (P for all < .0001). Pair‐wise breed comparisons showed significant differences for 24 of 36 comparisons (67%) for ET‐1, 8 of 36 comparisons (22%) for cortisol, and 7 of 36 comparisons (19%) for renin activity (Table 3). Concentration of ET‐1 was highest in Newfoundlands with a median concentration more than 3 times higher than median values in Cavalier King Charles Spaniels (CKCS), Doberman Pinschers and Dachshunds, which had lowest concentrations. Renin activity was highest in Dachshunds with a median value twice the values in Boxers and Newfoundlands, which had the lowest activities. Cortisol concentration was highest in Finnish Lapphunds with a median almost 3 times higher than in Boxers, which had the lowest concentration (Fig 1).
Table 3.
Pair‐wise comparisons between breeds in endothelin‐1 and cortisol concentrations, and renin activity
| Box | CKCS | Dach | Dob | FinL | GS | Lab | NF | ||
|---|---|---|---|---|---|---|---|---|---|
| BS | * | * | * | * | * | Endothelin‐1 | |||
| * | Cortisol | ||||||||
| * | Renin activity | ||||||||
| Box | * | * | * | * | Endothelin‐1 | ||||
| * | * | Cortisol | |||||||
| * | Renin activity | ||||||||
| CKCS | * | * | * | * | Endothelin‐1 | ||||
| * | Cortisol | ||||||||
| Renin activity | |||||||||
| Dach | * | * | * | * | Endothelin‐1 | ||||
| * | * | Cortisol | |||||||
| * | * | * | * | * | Renin activity | ||||
| Dob | * | * | * | * | Endothelin‐1 | ||||
| * | Cortisol | ||||||||
| Renin activity | |||||||||
| FinL | * | Endothelin‐1 | |||||||
| * | Cortisol | ||||||||
| Renin activity | |||||||||
| GS | * | Endothelin‐1 | |||||||
| Cortisol | |||||||||
| Renin activity | |||||||||
| Lab | * | Endothelin‐1 | |||||||
| Cortisol | |||||||||
| Renin activity | |||||||||
Box, Boxer; BS, Belgian Shepherd; CKCS, Cavalier King Charles Spaniel; Dach, Dachshund; Dob, Doberman pinscher; FinL, Finnish Lapphund; GS, German Shepherd; Lab, Labrador retriever; NF, Newfoundland.
The asterisks denote significant differences using a Bonferroni corrected P‐value of <.0014.
Group‐wise Comparisons, Within Center
Overall significant breed differences were found at 5 of the 5 centers for ET‐1 (P ≤ .030), at 4 centers for cortisol (P ≤ .043), and at 2 centers for renin activity (P ≤ .0002). Pair‐wise comparisons showed significant differences in 8 of 16 comparisons of ET‐1, 5 of 15 comparisons of cortisol, and 4 of 4 comparisons of renin activity.
Univariate Regression Analysis, All Dogs
The univariate regression analysis of all dogs showed association between ET‐1 and breed (R 2 = 0.39, P < .0001), as well as center of examination (R 2 = 0.24, P < .0001). Cortisol was associated with breed (R 2 = 0.08, P < .0001) and center of examination (R 2 = 0.09, P < .0001), and renin activity with breed (R 2 = 0.07, P < .0001) and center of examination (R 2 = 0.02, P = .013). ET‐1 increased with increasing body weight (R 2 = 0.14, P < .0001) and renin activity decreased with increasing body weight (R 2 = 0.02, P = .0009). Age was not associated with any of the 3 examined substances.
Univariate Regression Analysis, Labrador Retrievers
The univariate regression analysis of Labrador retrievers showed association between center of examination and cortisol (R 2 = 0.19, P < .0001) as well as renin activity (R 2 = 0.14, P = .0003), whereas ET‐1 was not associated with center of examination. Sex, age, and bodyweight were not associated with renin activity, ET‐1, or cortisol.
Multiple Regression Analysis, All Dogs
In the multiple regression analysis for ET‐1, only breed (P < .0001) remained significant in the final model with an adjusted model R 2 of 0.39. For cortisol, multiple regression analysis confirmed an effect of breed (P < .0001) and center of examination (P < .0001) with an adjusted model R 2 of 0.14. For renin activity, breed (P < .0001) and center of examination (P = .0002) remained significant in the final model, which had an adjusted model R 2 of 0.11.
Discussion
Results of this study demonstrate that ET‐1 can be included among vasoactive substances that differ between breeds, whereas breed difference was smaller for renin activity. There are breed differences in concentrations of natriuretic peptides and catecholamines in the same healthy dogs.11, 15 Several of these substances are altered in development of cardiac or renal disease and have been investigated as potential biomarkers for disease in dogs.4, 5, 6, 8, 24 A clinically useful test requires an upper reference limit for healthy dogs, and cut‐off values for dogs with subclinical disease or clinical signs of disease.
Endothelin‐1 is mainly produced in vascular endothelial cells and acts locally as a potent vasoconstrictor, but it is also produced in organs such as the heart and kidney.2, 25 In dogs, increased concentrations of ET‐1 have been found in chronic kidney disease and ET‐1 has been suggested a biomarker of hypertension.6 In one study, plasma concentrations of ET‐1 were twice as high in dogs with congestive heart failure compared to controls,26 and studies indicate that ET‐1 could aid in distinguishing between cardiac and noncardiac causes of dyspnea in dogs.5, 8 ET‐1 has also been suggested an useful predictor of poor prognosis in dogs with dilated cardiomyopathy.4 In that study, ET‐1 concentrations were 3 times higher in dogs with overt dilated cardiomyopathy compared to controls. Among included breeds in this study, Newfoundlands had the highest median ET‐1 concentration with values more than 3 times higher than median values in CKCS, Doberman Pinschers, and Dachshunds. In our previous study of natriuretic peptide concentrations in the same population of dogs, interestingly, Newfoundlands were found to have the highest and Dachshunds the lowest NT‐proBNP concentration.11 ET‐1 has been shown to directly induce BNP transcription in cultured ventricular myocytes27, and ET‐1 has also been suggested to be involved in increased synthesis of natriuretic peptides in dogs with congestive heart failure.28
In this study, RAAS activation was evaluated by measurement of renin activity. Renin release is stimulated by decrease in arterial blood pressure, decrease in sodium chloride concentration and flow in the nephron, and increase in sympathetic nervous activity.29 The RAAS is activated in cardiac and renal disease and blockers of different components of RAAS are used therapeutically for both cardiac and renal disease in dogs.20, 30, 31 Despite an overall significant breed difference, significant differences were found in less than a fifth of pair‐wise comparisons, and within only 2 of the centers. It was primarily one breed which differed from the others in renin activity, namely the Dachshunds which had the highest activity. The same breed had the lowest NT‐proBNP concentration of all breeds,11 and a low median ET‐1 concentration. Newfoundlands, on the other hand, had low renin activity and high ET‐1 as well as NT‐proBNP concentration.11 Natriuretic peptides are known to suppress RAAS activation in dogs as well as people,32, 33 and ET‐1 has been suggested to inhibit renin production, but the interaction between hormonal systems is complex.34 Taken together, the present results indicate that if ET‐1 and natriuretic peptides are high, the renin activity is low and vice versa. Why Dachshund and Newfoundlands had opposite response patterns is currently unknown. Further study into the interplay between these substances in healthy dogs and in dogs with cardiac and renal disease is warranted to optimize their use as biomarkers of disease.
Dogs in the LUPA project all underwent a thorough standardized health examination. Although attempts were made to stress dogs as little as possible, in a subpopulation of dogs examined at one center, we found substantial increases in urine epinephrine and norepinephrine postexamination compared to pre‐examination in home environment, indicating that dogs experienced stress or excitement during travel and examination at the clinic.15 We also found breed differences in the 2 catecholamines, with lower concentrations in Labrador retrievers compared to CKCS and Dachshunds.15 In this study, we analyzed cortisol concentration in serum samples taken from all dogs after the clinical examination. The observation that breed differences were found within 4 of the 5 centers indicates that differences in handling of the dogs between centers could not be the sole explanation. In contrast to the catecholamines, no breed differences were shown between CKCS, Dachshunds, and Labrador retrievers. Instead, cortisol concentration was highest in Finnish Lapphunds with median concentration almost 3 times higher than median concentration in Boxers, which had the lowest concentration. However, although statistically significant, the actual breed difference in cortisol concentration was comparatively small and variation within breeds substantial. The individual stress response might vary and other factors than breed might be more relevant for an individual dog in the clinical situation.
Other physiologic factors, which might influence concentration of vasoactive peptides, include sex and age.35, 36 Due to the uneven sex distribution among centers as well as breeds, sex was not included in the univariate or multiple regression analyses of all dogs, but was instead assessed in the univariate regression analysis of the Labrador retriever cohort. No association was found between sex and ET‐1 concentration, cortisol concentration, or renin activity. This study was, however, not designed to evaluate sex differences and no multiple regression analysis was performed in the Labrador retriever cohort, due to the high covariance between center and sex. Age was not associated with any of the 3 investigated substances, neither including all dogs nor in the Labrador retriever cohort. This was not surprising, as the study only included young adult to middle‐aged dogs and was not designed to evaluate age differences. Age and sex differences have been shown for cortisol and components of RAAS in dogs.37, 38, 39 Age and sex differences have been shown for ET‐1 in people,40, 41 whereas scarce information is available for dogs. Additional study into the effect of sex and age on investigated variables in dogs is warranted.
Study Limitations
Breed differences in ET‐1 concentration were highly significant within all 5 centers. However, the study only included 9 breeds and cannot be considered representative of the entire dog population. Because of the uneven sex representation and narrow age span of included dogs, results should not be interpreted as reference values. To establish breed‐specific reference values, studies evaluating additional breeds with an even sex and age representation would have to be performed. Breed differences were found at 2 centers for renin activity and at 4 centers for cortisol concentration. For the latter 2 substances, the different number of individuals of each breed and the uneven breed distribution among centers could have played a role because the variation was small compared to ET‐1. Furthermore, within‐dog variation in the substances have not been tested, and could have affected the results.
Sample handling was standardized, but samples were kept frozen at −20°C for a short time at 1 of the 5 centers. Stability has been shown at the freezing temperatures used for cortisol in dogs and for plasma renin activity in people.42, 43 Stability of ET‐1 has not been investigated in dogs, but in one human study, ET‐1 was stable at −80°C, but showed degradation when stored at −30°C for 3 weeks.44 Center of examination did not affect ET‐1 concentration in the multiple regression analysis of all dogs or in the univariate regression analysis of the Labrador retriever cohort, where this center was included. Hence, it is unlikely that sample handling had any major effect on the results.
The interassay variabilities in this study were good (<10%) to acceptable (<15%), and should therefore not have had any major effect on the results. Another factor which could affect results is time‐to‐time variation. In dogs, circadian variation has been shown for serum cortisol45 and plasma renin activity,46 but has not been investigated for plasma ET‐1. In a small human study, time‐to‐time variation in ET‐1 was found.47 To account for circadian variation, blood sampling was performed between 0900 and 1400 hours in this study. However, for practical reasons 6% of the dogs were instead sampled between 1400 and 1700 hours, which could have had a minor effect on the results. Further study into time‐to‐time variation in ET‐1 in dogs is warranted.
In order to avoid excessive salt intake, dogs were fed only commercial dog foods and no treats for 2 weeks preceding examination. However, as salt content might vary between food brands, salt intake between dogs could not be standardized which might have affected plasma renin activity. Dogs had no access to water for at least 2 hours before examination in order not to affect urine density measurements and assessment of renal function. According to newly published human recommendations,48 water should be allowed during the fasting period for most plasma/serum blood tests and the ingested volume of water should mirror the usual daily ingested water volume of each individual. However, urine density as well as hematocrit was within reference range for all participating dogs and water restriction should therefore not have had any major effect on the results.
Conclusion
Breed variation was considerable in circulating ET‐1, but less prominent for circulating cortisol and renin activity in healthy dogs. Evidence for breed variation in yet another vasoactive peptide highlights the need for deeper understanding of their role in health and disease. Furthermore, breed variation might be important to take into consideration when interpreting test results in clinical studies.
Acknowledgments
We thank the Universities involved for allowing completion of the study, and Michel Georges and Yukihide Momozawa for their contributions. We thank the laboratory personnel for their excellent work and the dog owners for their willingness to enroll their dogs.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Planning of the study started in 2007. The study protocol was finalized in 2008. There was a follow‐up meeting in 2010. The current article was finalized and approved by the investigators in August 2015. The publication committee consisted of Katja Höglund, Anne‐Sophie Lequarré, Ingrid Ljungvall, and Jens Häggström.
Presented in part at the 24th European College of Veterinary Internal Medicine—Companion Animal Congress, Mainz, Germany, September 2014.
The study was funded by the European Commission (FP7‐LUPA, GA‐201370).
Footnotes
Laboratoire de Physiologie, Faculté de Médecine, Bruxelles, Belgium
Laboratoire Vebio, Arcueil, France
Plasma renin activity, RIA, Diasorin, Stillwater, MN
ET‐1, ELISA, IBL‐27165, Aramachi, Takasaki‐Shi, Gunma, Japan
Cortisol, ELFA, VIDAS, Biomérieux SA, Lyon, France
JMP Pro, version 11.0.0, SAS Institute Inc, Cary, NC
References
- 1. Laragh JH. Atrial natriuretic hormone, the renin‐aldosterone axis, and blood pressure‐electrolyte homeostasis. N Engl J Med 1985;313:1330–1340. [DOI] [PubMed] [Google Scholar]
- 2. Yanagisawa M, Kurihara H, Kimura S, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988;332:411–415. [DOI] [PubMed] [Google Scholar]
- 3. Goetz KL, Wang BC, Madwed JB, et al. Cardiovascular, renal, and endocrine responses to intravenous endothelin in conscious dogs. Am J Physiol 1988;255:R1064–R1068. [DOI] [PubMed] [Google Scholar]
- 4. O'Sullivan ML, O'Grady MR, Minors SL. Plasma big endothelin‐1, atrial natriuretic peptide, aldosterone, and norepinephrine concentrations in normal Doberman Pinschers and Doberman Pinschers with dilated cardiomyopathy. J Vet Intern Med 2007;21:92–99. [DOI] [PubMed] [Google Scholar]
- 5. Tessier‐Vetzel D, Tissier R, Chetboul V, et al. Diagnostic and prognostic value of endothelin‐1 plasma concentrations in dogs with heart and respiratory disorders. Vet Rec 2006;158:783–788. [DOI] [PubMed] [Google Scholar]
- 6. Rossi G, Giordano A, Breda S, et al. Big‐endothelin 1 (big ET‐1) and homocysteine in the serum of dogs with chronic kidney disease. Vet J 2013;198:109–115. [DOI] [PubMed] [Google Scholar]
- 7. Balion CM, Santaguida P, McKelvie R, et al. Physiological, pathological, pharmacological, biochemical and hematological factors affecting BNP and NT‐proBNP. Clin Biochem 2008;41:231–239. [DOI] [PubMed] [Google Scholar]
- 8. Prosek R, Sisson DD, Oyama MA, et al. Distinguishing cardiac and noncardiac dyspnea in 48 dogs using plasma atrial natriuretic factor, B‐type natriuretic factor, endothelin, and cardiac troponin‐I. J Vet Intern Med 2007;21:238–242. [DOI] [PubMed] [Google Scholar]
- 9. Misbach C, Chetboul V, Concordet D, et al. Basal plasma concentrations of routine variables and packed cell volume in clinically healthy adult small‐sized dogs: Effect of breed, body weight, age, and gender, and establishment of reference intervals. Vet Clin Pathol 2014;43:371–380. [DOI] [PubMed] [Google Scholar]
- 10. Lequarre AS, Andersson L, Andre C, et al. LUPA: A European initiative taking advantage of the canine genome architecture for unravelling complex disorders in both human and dogs. Vet J 2011;189:155–159. [DOI] [PubMed] [Google Scholar]
- 11. Sjöstrand K, Wess G, Ljungvall I, et al. Breed differences in natriuretic peptides in healthy dogs. J Vet Intern Med 2014;28:451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bodey AR, Michell AR. Epidemiological study of blood pressure in domestic dogs. J Small Anim Pract 1996;37:116–125. [DOI] [PubMed] [Google Scholar]
- 13. Rasmussen CE, Vesterholm S, Ludvigsen TP, et al. Holter monitoring in clinically healthy Cavalier King Charles Spaniels, Wire‐haired Dachshunds, and Cairn Terriers. J Vet Intern Med 2011;25:460–468. [DOI] [PubMed] [Google Scholar]
- 14. Hezzell MJ, Dennis SG, Humm K, et al. Relationships between heart rate and age, bodyweight and breed in 10,849 dogs. J Small Anim Pract 2013;54:318–324. [DOI] [PubMed] [Google Scholar]
- 15. Höglund K, Hanås S, Carnabuci C, et al. Blood pressure, heart rate, and urinary catecholamines in healthy dogs subjected to different clinical settings. J Vet Intern Med 2012;26:1300–1308. [DOI] [PubMed] [Google Scholar]
- 16. Söder J, Bröjer JT, Nostell KE. Interday variation and effect of transportation on indirect blood pressure measurements, plasma endothelin‐1 and serum cortisol in Standardbred and Icelandic horses. Acta Vet Scand 2012;54:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brown S, Atkins C, Bagley R, et al. Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med 2007;21:542–558. [DOI] [PubMed] [Google Scholar]
- 18. Laflamme DP. Development and validation of a body condition score system for dogs: A clinical tool. Canine Pract 1997;22:10–15. [Google Scholar]
- 19. Thomas WP, Gaber CE, Jacobs GJ, et al. Recommendations for standards in transthoracic two‐dimensional echocardiography in the dog and cat. Echocardiography Committee of the Specialty of Cardiology, American College of Veterinary Internal Medicine. J Vet Intern Med 1993;7:247–252. [DOI] [PubMed] [Google Scholar]
- 20. Tidholm A, Häggström J, Hansson K. Effects of dilated cardiomyopathy on the renin‐angiotensin‐aldosterone system, atrial natriuretic peptide activity, and thyroid hormone concentrations in dogs. Am J Vet Res 2001;62:961–967. [DOI] [PubMed] [Google Scholar]
- 21. Schellenberg S, Grenacher B, Kaufmann K, et al. Analytical validation of commercial immunoassays for the measurement of cardiovascular peptides in the dog. Vet J 2008;178:85–90. [DOI] [PubMed] [Google Scholar]
- 22. Proverbio D, Groppetti D, Spada E, et al. Comparison of the VIDAS and IMMULITE‐2000 methods for cortisol measurement in canine serum. Vet Clin Pathol 2009;38:332–336. [DOI] [PubMed] [Google Scholar]
- 23. Bland M. Multifactorial methods In: Bland M, ed. An Introduction to Medical Statistics, 2nd ed Oxford: Oxford University Press; 1995:322–323. [Google Scholar]
- 24. Ettinger SJ, Farace G, Forney SD, et al. Evaluation of plasma N‐terminal pro‐B‐type natriuretic peptide concentrations in dogs with and without cardiac disease. J Am Vet Med Assoc 2012;240:171–180. [DOI] [PubMed] [Google Scholar]
- 25. Sakurai T, Yanagisawa M, Inoue A, et al. cDNA cloning, sequence analysis and tissue distribution of rat preproendothelin‐1 mRNA. Biochem Biophys Res Commun 1991;175:44–47. [DOI] [PubMed] [Google Scholar]
- 26. Prosek R, Sisson DD, Oyama MA, et al. Plasma endothelin‐1 immunoreactivity in normal dogs and dogs with acquired heart disease. J Vet Intern Med 2004;18:840–844. [DOI] [PubMed] [Google Scholar]
- 27. Nakagawa O, Ogawa Y, Itoh H, et al. Rapid transcriptional activation and early mRNA turnover of brain natriuretic peptide in cardiocyte hypertrophy. J Clin Invest 1995;96:1280–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Luchner A, Stevens TL, Borgeson DD, et al. Differential atrial and ventricular expression of myocardial BNP during evolution of heart failure. Am J Physiol 1998;274:H1684–H1689. [DOI] [PubMed] [Google Scholar]
- 29. Persson PB. Renin: Origin, secretion and synthesis. J Physiol 2003;552:667–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lefebvre HP, Toutain PL. Angiotensin‐converting enzyme inhibitors in the therapy of renal diseases. J Vet Pharmacol Ther 2004;27:265–281. [DOI] [PubMed] [Google Scholar]
- 31. Sisson DD. Neuroendocrine evaluation of cardiac disease. Vet Clin North Am Small Anim Pract 2004;34:1105–1126. [DOI] [PubMed] [Google Scholar]
- 32. Stevens TL, Burnett JC Jr, Kinoshita M, et al. A functional role for endogenous atrial natriuretic peptide in a canine model of early left ventricular dysfunction. J Clin Invest 1995;95:1101–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yoshimura M, Yasue H, Morita E, et al. Hemodynamic, renal, and hormonal responses to brain natriuretic peptide infusion in patients with congestive‐heart‐failure. Circulation 1991;84:1581–1588. [DOI] [PubMed] [Google Scholar]
- 34. Rossi GP, Sacchetto A, Cesari M, et al. Interactions between endothelin‐1 and the renin‐angiotensin‐aldosterone system. Cardiovasc Res 1999;43:300–307. [DOI] [PubMed] [Google Scholar]
- 35. DeFrancesco TC, Rush JE, Rozanski EA, et al. Prospective clinical evaluation of an ELISA B‐type natriuretic peptide assay in the diagnosis of congestive heart failure in dogs presenting with cough or dyspnea. J Vet Intern Med 2007;21:243–250. [DOI] [PubMed] [Google Scholar]
- 36. Eriksson AS, Jarvinen AK, Eklund KK, et al. Effect of age and body weight on neurohumoral variables in healthy Cavalier King Charles spaniels. Am J Vet Res 2001;62:1818–1824. [DOI] [PubMed] [Google Scholar]
- 37. Mongillo P, Prana E, Gabai G, et al. Effect of age and sex on plasma cortisol and dehydroepiandrosterone concentrations in the dog (Canis familiaris). Res Vet Sci 2014;96:33–38. [DOI] [PubMed] [Google Scholar]
- 38. Chang YJ, Chan IP, Cheng FP, et al. Relationship between age, plasma renin activity, and renal resistive index in dogs. Vet Radiol Ultrasound 2010;51:335–337. [DOI] [PubMed] [Google Scholar]
- 39. Doursout MF, Chelly JE, Wouters P, et al. Effect of gender in centrally induced angiotensin II hypertension in dogs. Hypertension 1990;15:I117–I120. [DOI] [PubMed] [Google Scholar]
- 40. Polderman KH, Stehouwer CD, van Kamp GJ, et al. Influence of sex hormones on plasma endothelin levels. Ann Intern Med 1993;118:429–432. [DOI] [PubMed] [Google Scholar]
- 41. Van Guilder GP, Westby CM, Greiner JJ, et al. Endothelin‐1 vasoconstrictor tone increases with age in healthy men but can be reduced by regular aerobic exercise. Hypertension 2007;50:403–409. [DOI] [PubMed] [Google Scholar]
- 42. Reimers TJ, McCann JP, Cowan RG, et al. Effects of storage, hemolysis, and freezing and thawing on concentrations of thyroxine, cortisol, and insulin in blood samples. Proc Soc Exp Biol Med 1982;170:509–516. [DOI] [PubMed] [Google Scholar]
- 43. Locsei Z, Racz K, Patocs A, et al. Influence of sampling and storage conditions on plasma renin activity and plasma renin concentration. Clin Chim Acta 2009;402:203–205. [DOI] [PubMed] [Google Scholar]
- 44. Walczak M, Fedorowicz A, Chlopicki S, et al. Determination of endothelin‐1 in rats using a high‐performance liquid chromatography coupled to electrospray tandem mass spectrometry. Talanta 2010;82:710–718. [DOI] [PubMed] [Google Scholar]
- 45. Palazzolo DL, Quadri SK. The effects of aging on the circadian rhythm of serum cortisol in the dog. Exp Gerontol 1987;22:379–387. [DOI] [PubMed] [Google Scholar]
- 46. Mochel JP, Fink M, Peyrou M, et al. Chronobiology of the renin‐angiotensin‐aldosterone system in dogs: Relation to blood pressure and renal physiology. Chronobiol Int 2013;30:1144–1159. [DOI] [PubMed] [Google Scholar]
- 47. Herold M, Cornelissen G, Loeckinger A, et al. About 8‐hour variation of circulating human endothelin‐1. Peptides 1998;19:821–825. [DOI] [PubMed] [Google Scholar]
- 48. Simundic AM, Cornes M, Grankvist K, et al. Standardization of collection requirements for fasting samples: For the Working Group on Preanalytical Phase (WG‐PA) of the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM). Clin Chim Acta 2014;432:33–37. [DOI] [PubMed] [Google Scholar]


