Abstract
Background
Intervertebral disc herniation is a common cause of spinal cord injury (SCI) causing paralysis and sensory loss. Little quantitative information is available on the loss and recovery of sensation in dogs with SCI.
Objectives
To determine whether quantitative sensory testing (QST) can be used to establish thermal and mechanical sensory thresholds in chrondrodystrophoid dogs and compare thresholds among normal dogs and dogs with different grades of SCI.
Animals
Thirty‐three client‐owned chondrodystrophoid dogs: 15 normal and 18 SCI dogs.
Methods
Thermal testing was performed by placing a hot (49°C) and cold (5°C) probe on the dorsal metatarsus and mechanical thresholds were tested using calibrated forceps to apply force to the lateral digit. Stimuli were applied until acknowledged, and response rate, latency, and force applied to response were recorded. Test‐retest repeatability was determined by calculating intraclass correlation coefficients. Response rates were compared using logistic regression and thresholds were compared using Kaplan–Meier Survival curves.
Results
Testing was feasible with moderate repeatability. Thresholds and response rates were significantly different between normal and SCI dogs for all modalities (P < .001). When dogs were grouped by their clinical grade, each grade was significantly different from normal dogs, and cold stimuli differentiated among all grades.
Conclusion and clinical importance
Sensory thresholds can be measured reliably in chondrodystrophoid dogs and are altered by SCI. The differences in sensation among neurologic grades indicate that these techniques can be used to further characterize recovery of SCI dogs.
Keywords: Allodynia, Mechanical threshold, Nociception, Quantitative sensory testing, Thermal threshold
Abbreviations
- AIS
ASIA Impairment Scale
- ASIA
American Spinal Injury Association
- ICC
intraclass correlation
- QST
quantitative sensory testing
- SCI
spinal cord injury
- TL‐IVDH
thoracolumbar intervertebral disc herniation
Chondrodystrophoid dog breeds such as the Dachshund have a high incidence of spinal cord injury (SCI) as a result of acute thoracolumbar intervertebral disc herniations (TL‐IVDH).1 As injury severity increases, neurologic functions are lost in a specific order, starting with proprioception, then motor function, and lastly, nociception. Nociception is assessed clinically by applying heavy force over a digit with hemostatic forceps and looking for a behavioral response acknowledging the stimulus.2 This categorical assessment of the presence of mechanical nociception serves as a useful prognostic indicator at the time of injury because dogs that maintain nociception have a much higher recovery rate than those that do not.3, 4
Historically, quantification of sensation in humans has been achieved using the American Spinal Injury Association (ASIA) Impairment Scale (AIS) by testing the response to light touch and pinprick at different locations of the body and categorizing the response as normal, abnormal, or absent.5 The AIS motor scores have proven more reliable predictors of outcome than the sensory scores,6, 7 but the response to light touch and pinprick has been used to document recovery of sensation.8 More recently, sensory testing has been refined by quantifying thermal and mechanical thresholds (quantitative sensory testing, QST). The data generated provide important information on the recovery of sensory function and allow quantification of neuropathic pain, a common consequence of SCI in people.9 This type of testing is challenging in pet dogs because of individual differences in behavioral responses, and the difficulty in interpreting whether a response indicates the threshold of sensation or nociception. Additional modifiers of behavioral responses are also difficult to recognize and assess. However, both thermal10, 11 and mechanical12, 13 thresholds can be determined consistently in dogs. Data on dogs with SCI are limited, a modified von Frey device has been used to assess mechanical thresholds in dogs with SCI and indicated that affected dogs had significantly different thresholds from normal dogs, but differences among grades of injury were not detected.14
We hypothesized that QST would provide discriminating data on the sensory deficits associated with different severity of SCI in dogs with acute TL‐IVDH. The aims of this study were to determine the reliability of measuring thermal and mechanical sensory thresholds in normal chondrodystrophoid dogs, to compare these thresholds in dogs with acute TL‐IVDH causing different severity of injury and to describe the temporal recovery of sensory function in dogs after decompressive surgery.
Materials and Methods
Study Subjects
Neurologically normal dogs were recruited from the staff, students, and neurology service clientele of the NC State Veterinary Hospital and the public via Dodgerslist (http://www.dodgerslist.com/). To be included, dogs had to be a chondrodystrophoid breed such as Dachshund or a mixed breed phenotypically similar in skeletal stature to the chondrodystrophoid breeds. Exclusion criteria included prior history of SCI, lameness at the time of participation, current administration of analgesic or behavioral medications, or aggression. Age, breed, sex, and weight were recorded. Dogs with SCI were recruited from the neurology service at NC State Veterinary Hospital. To be included, dogs had to be nonambulatory paraparetic or paraplegic at time of presentation, with neurologic signs localizing to the third thoracic to third lumbar spinal cord segments. If dogs had disc herniations caudal to these segments, but had intact hindlimb spinal reflexes, they were included. The injury had to be caused by an acute TL‐IVDH that was treated surgically. Age, breed, sex, weight, severity of presenting signs (graded using the modified Frankel Scale [Table 1]) and location of disc herniation were recorded. Dogs were excluded if they could not rest calmly in the testing environment after acclimation or behaved aggressively to handlers during the evaluation. Informed consent was obtained from all owners before participation, and all protocols were reviewed and approved by the NC State University Institutional Animal Use and Care Committee (protocol number: 14‐021‐O).
Table 1.
Modified Frankel Scale used for clinical grading of affected dogs
| Grade | Description |
|---|---|
| 0 | Normal |
| 1 | Back pain, no neurological deficits |
| 2 | Ambulatory paraparetic/ataxic |
| 3 | Nonambulatory paraparetic |
| 4 | Paraplegic with nociception |
| 5 | Paraplegic with absent nociception |
Quantitative Sensory Testing
All testing was performed in the same quiet environment by 3 investigators, all of whom spent approximately 10 minutes acclimating the dogs before testing. One investigator lightly restrained the dog on a table; each dog was allowed to adopt a comfortable position to avoid misinterpretation of behavioral responses. The second investigator applied the stimuli and noted thresholds, and the third investigator operated the video camera and computer software to capture data. Both hindlimbs were tested, and a subset of normal dogs were retested a minimum of 24 hours after the first testing session to evaluate test‐retest repeatability. Both the same observer and different observers were used to allow comparison of intra‐ and interobserver repeatability. Dogs with SCI were tested starting 48 hours postoperatively and then daily until discharge from the hospital. A subset was evaluated at 2, 4, 6, and 8 weeks postoperatively.
Thermal thresholds were tested using a handheld probe1 set at 49°C (for heat) and 5°C (for cold) (±0.1°C) connected to a Peltier semiconductor heat pump and digital temperature control unit to maintain accurate temperature application. An approximately 2.5cm diameter area of skin was shaved on the dorsal metatarsal region (Fig 1). The probe (at room temperature) was placed on the area to familiarize the animal with the sensation. Three trials of each temperature (49 and 5°C) then were performed on both hindlimbs with a minimum of 1 minute between trials. During each timed trial, the probe was held in place until the dog showed a behavioral response indicating conscious perception of the stimulus or the maximum amount of time had been reached. The maximum times were 30 and 60 seconds for hot and cold, respectively, to ensure no tissue damage occurred. Evidence of conscious perception of the stimulus included orientation towards the stimulus, escape behavior, or vocalization.14 Limb withdrawal in response to a stimulus can occur in dogs as a local reflex.15 Therefore, in evaluating this population of dogs, withdrawal of the limb alone without additional signs was not taken as evidence of conscious perception. The latency to response (time from application of probe to acknowledgment; the maximum latency was recorded if there was no response) and the response rate (number of responses within the maximum time expressed as a percentage of number of tests) were recorded. Feasibility scores were assigned to each testing session to assess the level of difficulty of testing10 (Table 2).
Figure 1.

Dog during a thermal testing session. The 13mm diameter probe is applied to a clipped area of skin on the dorsum of the hind paw (arrowhead).
Table 2.
Number of testing sessions assigned each feasibility score
| Testing Sessions | Feasibility Score | |||||
|---|---|---|---|---|---|---|
| 0 (No Problem) | 1 (Mild Difficulty) | 2 (Moderate Difficulty) | 3 (Significant Difficulty) | 4 (Extreme Difficulty) | 5 (Impossible) | |
| Normal thermal N = 29 | 22 | 3 | 1 | 2 | 0 | 1 |
| Affected thermal N = 76 | 58 | 10 | 4 | 1 | 1 | 2 |
| Normal mechanical N = 23 | 20 | 0 | 3 | 0 | 0 | 0 |
| Affected mechanical N = 74 | 66 | 3 | 3 | 1 | 1 | 0 |
N, number of sessions performed. Testing sessions impossible to complete (Feasibility Score of 5) were not included in data analysis because no data were generated.
The mechanical threshold was tested using purpose‐built instrumented forceps16 (Fig 2). The forceps have a load cell in the handle that measures the force applied to the handle as the jaws are closed. Before testing, dogs were restrained and placed in lateral recumbency. The forceps were placed around the lateral digit perpendicular to the bone, with pressure applied over the second phalanx. Force was applied smoothly until signs of conscious perception of the noxious stimulus or a maximum force of 5 kg was reached. The load cell was connected to a computer program that gave real time data on how much force was being applied. When the dog showed evidence of conscious perception, force application was stopped immediately, and the forceps were removed. Both hindlimbs were tested once because of the potential for skin damage. Threshold was recorded as the force applied at the time of behavioral response; the maximum force was recorded as the threshold if there was no response. Response rate was calculated from testing of both hindlimbs combined.
Figure 2.

The instrumented forceps used for mechanical sensory testing. The operator's thumb is placed over the central button of the load cell when force is applied.
On each day of testing the dogs with SCI, the neurologic status of the dog was assessed and quantified using the modified Frankel Scale (Table 1). Postoperative pain medications being administered were noted daily.
Data Analysis
Summary data (mean ± SD) were generated for response rate, thermal latency and mechanical force threshold for the normal dogs and dogs with SCI grouped together and also grouped according to their neurological grade at time of testing. Repeatability was evaluated in the subset of normal dogs that were tested twice by constructing Bland–Altman plots and calculating intraclass correlation (ICC). Intra‐ and interobserver repeatability also was tested using ICC.
Quantitative sensory testing outcomes were compared between the normal dogs and all dogs with SCI grouped together. Results then were grouped according to neurological grade at time of testing and compared among grades and with the normal dogs. Two different approaches were used. First, a modified Kaplan–Meier strategy was used to evaluate thresholds and latencies by censoring of observations when the maximum duration (30 or 60 seconds for heat or cold, respectively) or force applied (5 kg) was reached. This produced “survival” curves with time replaced by latency (s) or force (kg). Response rates were compared using logistic regression. Second, where data prevented use of logistic regression (eg, response rate was 100% for mechanical testing in normal dogs), Cochrane–Mantel–Haenszel statistics were calculated. P values <.05 were taken as statistically significant, and where indicated, multiple comparisons were adjusted for using the Sidak method. All statistical testing was performed using SAS 9.4.2
Results
Normal Dogs
Fifteen neurologically normal dogs were evaluated. Their mean age was 6.6 ± 3.4 years (range, 1.5–13 years) and mean body weight was 6.3 ± 1.9 kg (range, 4.1–11.2 kg). Breeds represented included 14 Dachshunds and 1 mixed breed (Shih Tzu/Dachshund mix).
Affected Dogs
Eighteen dogs with TL‐IVDH were evaluated including 9 Dachshunds, 4 mixed breeds (1 Maltipoo, 1 Puggle, 1 Corgi/Dachshund mix, 1 Labradoodle), 2 American Cocker Spaniels, 1 Chihuahua, 1 Beagle, and 1 Shih Tzu. The mean age for the group was 5.7 ± 1.4 years (range, 3.7–8.0 years), and their mean body weight was 7.9 ± 2.2 kg (range, 3.9–11.6 kg).
Severity of clinical signs at time of presentation was grade 5 in 9 dogs, grade 4 in 4 dogs and grade 3 in 5 dogs. Hemilaminectomies were performed to remove herniated disc material at T9–10 (1 dog), T11–12 (2 dogs), T11–13 (1 dog), T11–L2 (1 dog), T13–L1 (2 dogs), T13–L2 (1 dog), L1–2 (3 dogs), L1–3 (1 dog), L2–4 (3 dogs), L3–4 (2 dogs), and L3–5 (1 dog). Choice of postoperative pain management was at the discretion of the attending clinician, and medications used are summarized in Table 3.
Table 3.
Drugs used each day of testing
| Day | # Of Dogs Tested | Hydromorphone | Fentanyl (Patch) | Gabapentin | NSAID | Trazadone | Diazepam | Tramadol |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| 2 | 10 | 1 | 10 | 10 | 6 | 4 | 9 | 0 |
| 3 | 11 | 1 | 10 | 11 | 8 | 2 | 9 | 0 |
| 4 | 9 | 0 | 5 | 9 | 8 | 3 | 9 | 0 |
| 5 | 6 | 0 | 3 | 6 | 5 | 5 | 5 | 0 |
| 6 | 7 | 0 | 1 | 7 | 6 | 3 | 5 | 0 |
| 7 | 3 | 0 | 1 | 2 | 2 | 2 | 2 | 0 |
| 8 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 |
| 10 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| 11 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 12 | 2 | 0 | 0 | 2 | 1 | 0 | 1 | 0 |
| 14 | 9 | 0 | 0 | 7 | 3 | 4 | 3 | 1 |
| 15 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 16 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 |
| 27 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 28 | 7 | 0 | 0 | 2 | 0 | 1 | 0 | 0 |
| 32 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 46 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 56 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 73 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 90 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
NSAID, nonsteroidal anti‐inflammatory drug (meloxicam or carprofen). Hydromorphone was administered IV at a dose of 0.05 mg/kg. The fentanyl patch dose was 2–4 μg/kg/h placed for 3–5 days; gabapentin dose was 8–10 mg/kg every 8–12 hours PO; trazadone dose was 2–5 mg/kg every 8–12 hours PO; diazepam was 0.25–0.5 mg/kg every 8 hours PO, and tramadol was 2.75 mg/kg every 8–12 hours PO.
Quantitative Sensory Testing
Normal Dogs
Thermal latencies and mechanical thresholds were evaluated in 15 and 13 normal dogs, respectively, and testing was well tolerated with only 1 dog being impossible to test (Table 2). Two normal dogs underwent thermal testing before finalization of the mechanical testing protocol and were unable to return. Dogs were more responsive to cold (mean response rate, 61.9 ± 38.8%) than heat (mean response rate, 44.1 ± 39.1%). The mean latency for cold trials was 31 ± 25.6 seconds and for heat trials was 23 ± 9.8 seconds. The mean force applied during mechanical testing was 0.5 ± 0.3 kg, and the response rate was 100%. Thermal testing was repeated in 10 dogs, and mechanical testing was repeated in 9 dogs. Test‐retest repeatability in individual dogs was moderate for thermal testing, but using different observers did decrease repeatability (Table 4). For mechanical testing, all dogs responded each time they were tested and response rate was highly repeatable. However, because of the nature of the stimulus, the second test was always associated with a lower force, and test‐retest quantification of the force applied was low (Table 4).
Table 4.
ICC values for test‐retest and observer reliability
| Modality | Test‐Retest | Intra‐Observer | Interobserver |
|---|---|---|---|
| Cold | |||
| Latency | 0.65 | 0.73 | 0.33 |
| Response rate | 0.73 | 0.69 | 0.53 |
| Heat | |||
| Latency | 0.71 | 0.62 | 0.62 |
| Response rate | 0.51 | 0.64 | 0.33 |
| Mechanical | |||
| Max force | −0.14 | −0.17 | −0.05 |
ICC, intraclass correlation; Max, maximum. Coefficients ranging from 0.41 to 0.60 were considered indicators of “fair” repeatability, 0.61–0.80 “moderate,” and 0.81–1.0 “substantial”.26 Response rate was 100% for every episode of mechanical testing, thus had perfect agreement and is not represented in the table.
Affected Dogs
Thermal testing was performed in 16 of 18 dogs. One dog would not remain still for testing, and no test data could be generated; 1 dog was tested using a different protocol, and thus its data were excluded. Mechanical testing was performed in 16 of 18 dogs; 2 dogs underwent thermal testing before the final mechanical testing protocol was implemented. Testing of affected dogs was tolerated well for both modalities; the biggest challenge for the dogs was remaining calm and still for the full duration of thermal testing (Table 2). The number of testing sessions and individual trials performed at each clinical grade are summarized in Table 5. In 4 dogs, it was not possible to perform thermal testing in 1 hind limb for 1–2 testing sessions each because of obstruction of the testing site by placement of a fentanyl patch. In 1 dog, 1 mechanical testing session was not performed because of superficial damage to the skin of the toes.
Table 5.
Summary of the number of testing sessions and individual trials performed at each clinical grade
| Modality | Normal Dogs | All Affected Dogs | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|---|---|
| Cold | ||||||
| Dogs | 15 | 16 | 10 | 11 | 9 | 4 |
| Sessions | 28 | 74 | 16 | 22 | 20 | 16 |
| Trials | 168 | 389 | 93 | 113 | 102 | 81 |
| Heat | ||||||
| Dogs | 15 | 16 | 10 | 11 | 9 | 4 |
| Sessions | 28 | 74 | 16 | 22 | 20 | 16 |
| Trials | 168 | 391 | 93 | 115 | 102 | 81 |
| Mechanical | ||||||
| Dogs | 13 | 16 | 11 | 10 | 9 | 4 |
| Sessions | 23 | 74 | 18 | 20 | 20 | 16 |
| Trials | 46 | 148 | 36 | 40 | 40 | 32 |
Number of dogs tested, subdivided according to their clinical grade at time of testing. The number of testing sessions performed is provided as well as the number of trials completed in the testing sessions. Note, 7 of the normal dogs that underwent thermal testing had 2 testing sessions, and 3 had 3 sessions. Eight of the normal dogs that underwent mechanical testing had 2 testing sessions, and 1 had 3 sessions. The number of trials includes repetitions in both hindlimbs.
The response rates, latencies and threshold forces for all testing sessions in the whole group and all testing sessions subdivided by neurologic grade at time of testing are provided in Table 6. Using Kaplan–Meier analysis, a significant difference was identified between control dogs and all neurologic dogs for cold latency (P < .001), heat latency (P < .001) and mechanical threshold (P < .001); (Fig 3). Similarly, a significant difference in response rates was identified between normal and affected dogs for cold (P < .001), heat (P < .001) and mechanical (P < .001) stimuli. When considering neurologic grade at the time of testing, important differences were identified among sensory modalities. For each modality, dogs with each clinical grade had significantly lower response rates and longer latencies or higher force thresholds than did normal dogs (Table 7). When testing cold latency, dogs with grade 4 and 5 signs were very similar with low response rates and significantly longer latencies than for all other grades (Fig 4 and Table 7). Dogs with grade 2 and 3 signs also were very similar and were significantly slower to respond than were normal dogs and faster than dogs with grade 4 and 5 signs. Heat testing discriminated less because of the low response rate overall (Fig 4). However, the response rate was significantly lower in grade 4 and 5 dogs when compared to grade 2 and 3 dogs. Mechanical sensation was absent in grade 5 dogs, and this group was significantly different from all other clinical grades, whereas dogs with clinical grades 2, 3, and 4 did not differ significantly from each other (Fig 4 and Table 7).
Table 6.
Results of QST in affected dogs
| Modality | Normal Dogs Mean (SD) | Affected Dogs Mean (SD) | Grade 2 Mean (SD) | Grade 3 Mean (SD) | Grade 4 Mean (SD) | Grade 5 Mean (SD) |
|---|---|---|---|---|---|---|
| Cold | ||||||
| Latency (s) | 31.5 (25.6) | 53.1 (16.9) | 47.3 (21.1) | 47.1 (21.6) | 60.0 (0.0) | 59.4 (5.2) |
| Response rate % | 61.9 (38.8) | 13.7 (26.1) | 29.2 (26.9) | 24.2 (35.2) | 0.0 | 1.0 (4.2) |
| Heat | ||||||
| Latency (s) | 22.7 (9.8) | 28.6 (4.6) | 27.2 (6.9) | 28.3 (4.3) | 29.9 (0.7) | 29.2 (4.1) |
| Response rate % | 44.1 (39.1) | 9.7 (19.1) | 17.7 (24.7) | 15.9 (23.8) | 0.8 (3.7) | 4.2 (9.6) |
| Mechanical | ||||||
| Max Force (kg) | 0.5 (0.3) | 2.2 (1.9) | 1.2 (1.5) | 1.3 (1.1) | 1.8 (1.7) | 5.0 (0.0) |
| Response rate % | 100.0 | 71.0 (45.3) | 88.9 (32.3) | 95.0 (22.4) | 87.5 (31.9) | 0.0 |
QST, quantitative sensory testing. Grade represents the clinical grade on the day of testing.
Figure 3.
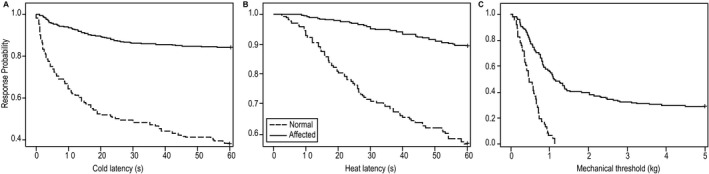
Kaplan–Meier curves comparing normal and affected dogs for cold (A) and heat (B) latencies (s) and mechanical (C) thresholds (kg). Data from every trial of every dog were included. Responses were censored when there was no response by 60 (cold) or 30 (heat) seconds. Affected and normal dogs are significantly different for all 3 modalities (P < .0001).
Table 7.
P values when QST outcomes were compared among clinical grades of affected dogs and normal dogs
| Modality | Grade | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|---|
| Cold (latency) | Normal | <.0001 | <.0001 | <.0001 | <.0001 |
| 2 | 1.0000 | <.0001 | .003 | ||
| 3 | .0006 | .015 | |||
| 4 | .97 | ||||
| Cold (response rate) | Normal | .0003 | .0003 | .0003 | .0003 |
| 2 | .78 | .0003 | .0003 | ||
| 3 | .0003 | .0003 | |||
| 4 | .53 | ||||
| Heat (latency) | Normal | <.0001 | <.0001 | <.0001 | <.0001 |
| 2 | 1.0000 | .03 | .56 | ||
| 3 | .11 | .83 | |||
| 4 | .89 | ||||
| Heat (response rate) | Normal | .0007 | .0007 | .0007 | .0007 |
| 2 | 1.00 | .01 | .03 | ||
| 3 | .02 | .04 | |||
| 4 | .26 | ||||
| Mechanical (force) | Normal | <.0001 | <.0001 | <.0001 | <.0001 |
| 2 | 1.00 | .95 | <.0001 | ||
| 3 | 1.00 | <.0001 | |||
| 4 | <.0001 | ||||
| Mechanical (response rate) | Normal | .16 | .47 | .10 | .0007 |
| 2 | .55 | .85 | .0007 | ||
| 3 | .55 | .0007 | |||
| 4 | .0007 |
QST, quantitative sensory testing. P values were corrected for multiple comparisons using the Sidak method.
Figure 4.
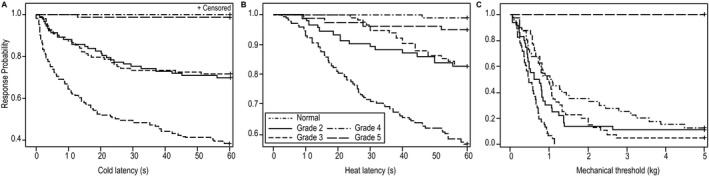
Kaplan–Meier curves comparing normal and affected dogs for cold (A) and heat (B) latencies (s) and mechanical (C) thresholds (kg) subdivided according to clinical grade at time of testing. Latencies of each of the clinical grades for each testing modality were significantly different to the normal dogs (Table 7). (A) Dogs with grades 4 and 5 clinical signs had similar cold latencies, as did grades 2 and 3. Significance was reached between all grades except for grades 4 and 5 (Table 7). (B) While the latency to heat clearly stratified according to grade, only grades 2 and 4 were significantly different (P = .0343). (C) Dogs with grade 5 signs had significantly higher mechanical thresholds than all other groups.
The recovery of a subset of 8 dogs that presented with grade 5 clinical signs was followed during the first postoperative week and then at 2, 4, 6, and 8 weeks. These data were used to describe the sequence of recovery of each sensory modality. Of the 8 dogs, 1 failed to recover motor or sensory function over the 8‐week period. Two additional dogs failed to recover consistent mechanical nociception as tested clinically, but they did recover some motor function by the 8‐week evaluation and were classified as nonambulatory paraparetic, nociception‐negative at this time point. One of these nonambulatory paraparetic, nociception‐negative dogs went on to recover independent walking ability 4 months after injury while remaining nociception‐negative as tested clinically. This dog had a low positive response rate (1 of 3 testing trials in 1 foot only on each of these sessions) to heat on days 2 and 3. At 2 weeks the dog had a positive response to heat and cold but not the mechanical stimulus. This dog's responses continued to vary, with no responses at 4 weeks, a response to the mechanical stimulus at 6 weeks and no response to any stimuli at 8 weeks. In the remaining 5 dogs, all of which recovered motor and sensory function, 1 would not remain still long enough for thermal testing to be performed reliably, but mechanical testing was possible. In the 4 dogs with thermal and mechanical testing, the first modality recovered was mechanical nociception, followed by motor function. Recovery of cold and heat sensation occurred in 2 dogs when nonambulatory paraparetic and 2 dogs when they became ambulatory paraparetic. One of the dogs that recovered consistent mechanical nociception and motor function did respond to cold and heat on days 5 and 6 of recovery but then lost this response. This deterioration was not accompanied by deterioration in motor recovery (Fig 5). This pattern of decreased and then increased thresholds was repeated for mechanical nociception in 2 other dogs.
Figure 5.
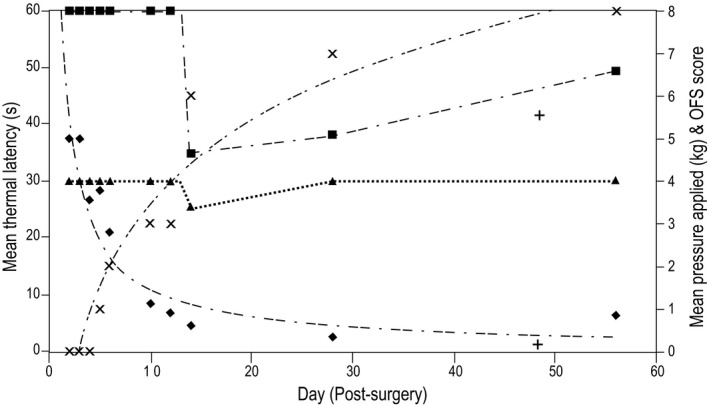
Graph showing the recovery of each sensory modality and motor function over time (days) in a dog that presented with complete sensorimotor loss and then recovered both sensation and motor function. For the visual purposes of this figure, motor function has been graded using an expanded open field scale (OFS) that ranges from 0 (paraplegic) to 14 (normal).27 The dog transitioned from nonambulatory paraparetic to ambulatory paraparetic at OFS grade 6. Note the decrease in thermal thresholds between days 10 and 14 that subsequently increased potentially representing hyperesthesia. ■: Cold latency (s); ▲: Heat latency (s); ♦: Mechanical threshold (kg); X: Motor open field score.
Discussion
Our study indicated that thermal and mechanical QST can be performed reliably in chondrodystrophoid pet dogs and that test‐retest repeatability is enhanced by using the same observer. Using these testing protocols, we distinguished between normal dogs and dogs recovering from acute SCI and among different grades of injury, and we also identified differences in temporal recovery of different sensory modalities during recovery from injury.
Our study was performed to address a lack of data on sensory loss and recovery and the development of neuropathic pain in dogs with SCI. The clinical importance of the presence of nociception in response to a noxious mechanical stimulus is well‐recognized as an important prognostic indicator,3, 4, 17 but little information is available on the quantification of this response.14 Clinically in veterinary patients, nociception may be classified into “superficial pain” (a behavioral response to force applied with forceps to skin) versus “deep pain” (a behavioral response to force applied with forceps over the digit, compressing bone). The reliability of this categorization has not been tested, and the pathways involved are unclear.18 No information is available on thermal sensation in dogs with SCI. Describing and quantifying these sensory modalities may allow better documentation of recovery and also may identify pain syndromes. Neuropathic pain occurs in >80% of people after SCI,9 but little is known about this problem in dogs with naturally occurring SCI beyond a recognition that post‐injury self mutilation may occur as a consequence of neuropathic pain.17
Our testing protocols were adapted from QST protocols described previously to assess sensory thresholds in dogs with chronic pain (eg, osteoarthritis)11 and to assess efficacy of analgesics or nerve blocks.16 Protocols were adapted with a focus on decreased sensation rather than increased sensitivity and to be practical in animals that are unable to move their hindlimbs voluntarily. For example, protocols that required the animal to stand were not possible, and distinguishing reflex limb withdrawal (which can occur in animals with complete spinal cord transections as a local reflex rather than a conscious response to stimulation) was critical. Because of the frequency of acute SCI in chondrodystrophoid breeds, in particular Dachshunds, and the potential influence of body size on QST results,11, 19 a normal population of chrondrodystrophoid dogs was used to establish normal ranges. Behavioral responses were defined before testing, and testing sessions were videotaped to allow group consensus to be established in problematic cases. Animals were familiarized to the investigators and allowed to adopt a comfortable position that would give access to the limb being tested to ensure they would not struggle. The effects of an unfamiliar environment and anxiety on rodent QST are recognized.20 The approach adopted here resulted in feasibility scores of 0 in the majority of cases, even those that had undergone recent surgery, and indicated that this type of behavioral testing, although challenging, can be performed reliably in client‐owned pets.
It is not possible to reliably distinguish between threshold of sensation and threshold of nociception as is commonly performed in people because of the need to interpret a behavioral response in dogs. Rather than try to categorize the responses as a sensory versus a nociceptive threshold, we simply reported the thresholds and response rates and ensured that a consistent protocol was followed. Another challenge to quantifying nociception in dogs that have just undergone surgery is the administration of analgesics. All dogs, regardless of neurologic grade, received very similar analgesic protocols in the first 2 postoperative weeks. Thus, comparisons among groups of different clinical grade were valid, but it could be argued that the difference between the normal dogs and dogs with SCI was due in part to analgesics and that recovery of sensation over time also was influenced by the tapering of analgesic drugs being administered.
Mechanical thresholds have been evaluated using the von Frey needle in rodents, humans, and dogs.12, 20, 21, 22 This technique classically requires the animal to stand while the stimulus is applied to the underside of the foot. It was adapted recently to evaluate ambulatory paraparetic dogs placed in lateral recumbency with the stimulus applied to the dorsal metatarsus.14 Positioning of the dogs was facilitated by habituation to the environment and use of the dog appeasement pheromone. Using this technique, it was determined that dogs with SCI had different thresholds when compared to normal dogs.14 There was no correlation with injury severity, but only 6 dogs of similar injury severity and a wide range of body size were tested. In this study, we chose to investigate the use of calibrated forceps to apply force in a fashion similar to the clinical testing performed routinely in evaluation of paralyzed dogs with the intent of being able to apply a strong stimulus to dogs with blunted sensory function. These forceps were developed to assess cutaneous sensation after nerve blocks.16 Because of the potential to cause tissue damage, the maximum force to be applied was defined ahead of time, and only one testing session was used per limb. Using this protocol, there was only 1 occasion in which mechanical testing was not attempted because of pre‐existing tissue damage despite affected dogs undergoing standard clinical testing for nociception every morning by the clinician caring for the dog. This limitation to a single test point, however, did prevent us from following the usual protocol of testing repeatedly to generate an average at each testing session. The fact that the stimulus is controlled by the operator introduces variability in the rate of force application. To minimize this variability, the operator practiced applying force smoothly while watching the force curve develop using the associated software.16 In addition, different morphology among animals also introduced variability. The repeatability of this technique in terms of response rate was perfect, but the quantification of the mechanical threshold repeatability was low with dogs consistently reacting extremely quickly to a lower force when retesting on another date was attempted. This reflects the noxious nature of this stimulus and implies it would not be useful when testing dogs with normal sensation or hyperesthesia. However, in our patient population with absent to markedly decreased sensation, the calibrated forceps were useful to quantify initial recovery of mechanical nociception. The von Frey technique described previously14 may better differentiate levels of mechanical sensation once it has been recovered and identify allodynia.
Thermal thresholds have not been evaluated in dogs with SCI but play an important role in quantifying neuropathic pain.21 Different techniques have been described in dogs including use of a light box to apply heat to the underside of a standing dog's paw (the canine nociceptive thermal escape model)10 and use of a cold probe.11 Given the inability of many of our patients to stand, both heat and cold were tested using a probe applied to the dorsum of the paw, a location shown to be a reliable testing point in rodents with SCI and in dogs.14, 20 One of the challenges with this testing is applying a large enough stimulus to get a response without causing tissue damage. The maximum duration of probe application was established in preliminary work (unpublished work by BDXL). Using the protocol described here, testing in normal dogs had acceptable intersession and intra‐observer variability.
In normal chondrodystrophoid dogs, the response rate was higher to cold when compared to heat. Indeed, the low response rate to heat was such that there was little differentiation between SCI dogs and normal dogs. Extending the duration of the stimulus might have produced a higher response rate but was avoided because of the increased risk of burns. Cold testing was more useful in our population although the response rate still was only 62%. Two strategies were employed to address the issue of lack of response. The first was to compare response rate, and the second was to use a Kaplan–Meier survival curve strategy that allowed plotting of probability of response against latency or force with censoring once the maximum stimulus had been reached. In this instance, each data point was considered independent. This approach provided clear discrimination among paraplegic dogs (grades 4 and 5), nonambulatory and ambulatory paraparetic dogs (grades 2 and 3) and normal dogs.
The presence of apparently decreased thresholds to thermal (1 dog) and mechanical (2 dogs) stimuli in the first week after recovery of mechanical sensation is of interest. The numbers of animals followed this closely was too low to determine whether these findings are reliable, but this finding may represent a transient period of hyperalgesia. In humans, hyperalgesia detected by QST occurs acutely after surgical procedures.23, 24 This transient change is likely distinct from the development of chronic neuropathic pain as documented in people and rats after SCI.21, 22 Another explanation is simply that the repeated testing (these dogs were also evaluated for the presence of mechanical nociception once in the morning by their clinician) produced anticipation.25 The recording of intermittent presence of a positive response to thermal and mechanical stimuli in 1 dog that was clinically nociception‐negative by the 8‐week study end point was surprising. This dog did go on to recover effective ambulation despite a persistent lack of clinically tested mechanical nociception. Thus, the daily QST may have uncovered minimal sensation indicating an incomplete lesion and may have predicted the ultimate recovery of motor function.
Conclusions
Quantitative sensory testing can be used to quantify pet dogs’ responses to mechanical, heat, and cold stimuli. Dogs with SCI caused by acute TL‐IVDH have significantly different thresholds and response rates to these sensory stimuli when compared to normal dogs and when different grades of injury are compared. Further exploration of transient postoperative hyperalgesia and more chronic neuropathic pain associated with SCI is warranted.
Acknowledgment
The authors thank the owners of all dogs that took part in this study.
Funding: One of the authors was funded by the NC State Veterinary Scholars Program and the NIH/T35 Interdisciplinary Biomedical Research Program: T35OD011070. Research support was provided by the Department of Defense Congressionally Directed Medical Research Program grant ID: W81XWH‐11‐1‐0772 and the Morris Animal Foundation grant ID: D10CA‐022.
Conflict of Interest Declaration: The authors confirm that there are no known conflicts of interest associated with this publication.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Presentation of Data: Preliminary results were presented as a poster at the Merial‐NIH Veterinary Scholars Program Symposium, Ithaca, NY August July 31st–August 3rd, 2014 and as an oral abstract at the North Carolina State University College of Veterinary Medicine Research Forum, Raleigh, NC September 18th, 2015.
Footnotes
NTE‐2A, Physitemp Instruments, Clifton, NJ
SAS Institute, Cary, NC
References
- 1. Brisson BA. Intervertebral disc disease in dogs. Vet Clin North Am Small Anim Pract 2010;40:829–858. [DOI] [PubMed] [Google Scholar]
- 2. Garosi L, Lowrie M. The neurological examination In: Platt SR, Olby N, eds. BSAVA Manual of Canine and Feline Neurology. Vol 4th ed Quedgeley: British Small Animal Veterinary Association; 2013:1–24. [Google Scholar]
- 3. Olby N, Harris T, Burr J, et al. Recovery of pelvic limb function in dogs following acute intervertebral disc herniations. J Neurotrauma 2004;21:49–59. [DOI] [PubMed] [Google Scholar]
- 4. Ferreira AJA, Correia JHD, Jaggy A. Thoracolumbar disc disease in 71 paraplegic dogs: Influence of rate of onset and duration of clinical signs on treatment results. J Small Anim Pract 2002;43:158–163. [DOI] [PubMed] [Google Scholar]
- 5. Kirshblum SC, Burns SP, Biering‐Sørensen F, et al. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med 2011;34:535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Steeves JD, Lammertse D, Curt A, et al. Guidelines for the conduct of clinical trials for spinal cord injury (SCI) as developed by the ICCP panel: Clinical trial outcome measures. Spinal Cord 2007;45:206–221. [DOI] [PubMed] [Google Scholar]
- 7. Yavuz N, Tezyürek M, Akyüz M. A comparison of two functional tests in quadriplegia: The quadriplegia index of function and the functional independence measure. Spinal Cord 1998;36:832–837. [DOI] [PubMed] [Google Scholar]
- 8. Betz RR, Chafetz RS, Vogel LC, et al. Description of sensory preservation in children and adolescents with incomplete spinal cord injury. J Spinal Cord Med 2011;34:297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Siddall PJ, McClelland JM, Rutkowski SB, Cousins MJ. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain 2003;103:249–257. [DOI] [PubMed] [Google Scholar]
- 10. Williams MD, Kirkpatrick AE, Griffith E, et al. Feasibility and repeatability of thermal quantitative sensory testing in normal dogs and dogs with hind limb osteoarthritis‐associated pain. Vet J 2014;199:63–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Briley JD, Williams MD, Freire M, et al. Feasibility and repeatability of cold and mechanical quantitative sensory testing in normal dogs. Vet J 2014;199:245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. KuKanich B, Lascelles B. Assessment of a von Frey device for evaluation of the antinociceptive effects of morphine and its application in pharmacodynamic modeling of morphine in dogs. Am J Vet Res 2005;66:1616–1622. [DOI] [PubMed] [Google Scholar]
- 13. KuKanich B, Lascelles B. Use of a von Frey device for evaluation of pharmacokinetics and pharmacodynamics of morphine after intravenous administration as an infusion or multiple doses in dogs. Am J Vet Res 2005;66:1968–1974. [DOI] [PubMed] [Google Scholar]
- 14. Moore SA, Hettlich BF, Waln A. The use of an electronic von Frey device for evaluation of sensory threshold in neurologically normal dogs and those with acute spinal cord injury. Vet J 2013;197:216–219. [DOI] [PubMed] [Google Scholar]
- 15. Fulton JF, Sherrington CS. State of the flexor reflex in paraplegic dog and monkey respectively. J Physiol (Lond) 1932;75:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Trumpatori BJ, Carter JE, Hash J, et al. Evaluation of a midhumeral block of the radial, ulnar, musculocutaneous and median (RUMM block) nerves for analgesia of the distal aspect of the thoracic limb in dogs. Vet Surg 2010;39:785–796. [DOI] [PubMed] [Google Scholar]
- 17. Aikawa T, Fujita H, Kanazono S, et al. Long‐term neurologic outcome of hemilaminectomy and disk fenestration for treatment of dogs with thoracolumbar intervertebral disk herniation: 831 cases (2000–2007). J Am Vet Med Assoc 2012;241:1617–1626. [DOI] [PubMed] [Google Scholar]
- 18. de Lahunta A. General somatic afferent system – GSA In: de Lahunta, ed. Veterinary Neuroanatomy and Clinical Neurology. Philadelphia, PA: WB Saunders Co; 1983:166–169. doi: 10.1089/neu.2015.4040. [Google Scholar]
- 19. Basso DM. Behavioral testing after spinal cord injury: Congruities, complexities and controversies. J Neurotrauma 2004;21:395–404. [DOI] [PubMed] [Google Scholar]
- 20. Detloff MR, Fisher LC, Deibert RJ, Basso DM. Acute and chronic tactile sensory testing after spinal cord injury in rats. J Vis Exp 2012;62:e3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Widerstrom‐Noga E, Felix ER, Adcock JP, et al. Multidimensional neuropathic pain phenotypes after spinal cord injury. J Neurotrauma 2015; doi: 10.1089/neu.2015.4040 [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22. Lindsey AE, LoVerso RL, Tovar CA, et al. An analysis of changes in sensory thresholds to mild tactile and cold stimuli after experimental spinal cord injury in the rat. Neurorehabil Neural Repair 2000;14:287–300. [DOI] [PubMed] [Google Scholar]
- 23. Wilder‐Smith OHG, Tassonyi E, Crul BJP, Arendt‐Nielsen L. Quantitative sensory testing and human surgery: Effects of analgesic management on postoperative neuroplasticity. Anesthesiology 2003;98:1214–1222. [DOI] [PubMed] [Google Scholar]
- 24. Martinez VR, Fletcher D, Bouhassira D, et al. The evolution of primary hyperalgesia in orthopedic surgery: Quantitative sensory testing and clinical evaluation before and after total knee arthroplasty. Anesth Analg 2007;105:815–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Coleman KD, Schmiedt CW, Kirkby KA, et al. Learning confounds algometric assessment of mechanical thresholds in normal dogs. Vet Surg 2014;43:361–367. [DOI] [PubMed] [Google Scholar]
- 26. Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psychol Bull 1979;86:420–428. [DOI] [PubMed] [Google Scholar]
- 27. Olby NJ, De Risio L, Muñana KR, et al. Development of a functional scoring system in dogs with acute spinal cord injuries. Am J Vet Res 2001;62:1624–1628. [DOI] [PubMed] [Google Scholar]


