Abstract
Background
Noninvasive diagnosis of pulmonary hypertension (PH) primarily relies upon Doppler echocardiography of tricuspid regurgitation (TR). However, TR might be absent or difficult to measure.
Hypothesis/Objectives
To determine the diagnostic value of right pulmonary artery distensibility (RPAD) index for prediction of Doppler‐derived estimates of pulmonary artery (PA) pressure compared to other indices of PH in dogs.
Animals
Sixty‐nine client‐owned dogs with TR.
Methods
Prospective observational study. Dogs were allocated to groups according to TR pressure gradient (TRPG): TRPG <36 mmHg (control, n = 22), TRPG 36–50 (n = 16), TRPG 50–75 (n = 14) and TRPG >75 mmHg (n = 17). Right pulmonary artery distensibility index, acceleration time to peak PA flow (AT), AT: ejection time of PA flow (AT:ET) and main PA size: aorta size (MPA:Ao) were calculated in each dog.
Results
Right pulmonary artery distensibility index demonstrated the strongest correlation (r = −0.90; P < .0001) to TRPG followed by MPA:Ao (r = 0.78; P < .0001), AT (r = −0.69; P < .0001) and AT:ET (r = −0.68; P < .0001). RPAD index possessed the most accurate cutoff (<29.5%; Sensitivity [Sn] 0.84, Specificity [Sp] 0.95) to predict TRPG >50 mmHg compared to AT (<53.9 ms; Sn 0.74, Sp 0.87), AT:ET (<0.30; Sn 0.61, Sp 0.97) and MPA:Ao (>1.04; Sn 0.94, Sp 0.74). All intra‐ and interobserver measurement variabilities exhibited coefficients of variation ≤13%.
Conclusions and Clinical Importance
Right pulmonary artery distensibility index is an accurate predictor of TRPG and should be particularly useful if TR is absent or difficult to measure.
Keywords: Canine, Echocardiography, Prediction, Pulmonary vascular resistance, Right pulmonary artery distensibility
Abbreviations
- 2D
two‐dimensional
- Ao
aorta
- AT
acceleration time to peak pulmonary artery flow
- AUC
area under the ROC curve
- CHF
congestive heart failure
- ET
ejection time of pulmonary artery flow
- LA
left atrial
- MMVD
myxomatous mitral valve disease
- MPA
main pulmonary artery
- PA
pulmonary artery
- PH
pulmonary hypertension
- ROC
receiver operating characteristic
- RPAD
right pulmonary artery distensibility
- RV
right ventricular
- TRPG
peak tricuspid regurgitation systolic pressure gradient
- TR
tricuspid regurgitation
The clinical importance of pulmonary hypertension (PH) in dogs is becoming more apparent. Pulmonary hypertension is commonly encountered in dogs affected with myxomatous mitral valve disease (MMVD), thromboembolic disease, parasitic disease (e.g, dirofilariasis, angiostrongyliasis), congenital cardiac shunts and various respiratory diseases.1, 2, 3, 4, 5, 6, 7 It adversely affects the quality of life and exercise tolerance and can cause syncope,4, 8 frequently influences diagnostic and therapeutic decisions,7 and is associated with a worse outcome in dogs with MMVD.2 Thus, screening for PH is an important part of the diagnostic evaluation in dogs exhibiting clinical signs of PH such as syncope, or affected with diseases commonly associated with PH.
Despite its flaws,9 clinical diagnosis of PH in dogs typically relies on Doppler echocardiographic estimates of pulmonary arterial pressure derived from tricuspid or pulmonary regurgitant jets. However, these regurgitant jets can be absent or difficult to quantify. Clinicians might then be obligated to rely on clinical and indirect echocardiographic findings to help determine if PH is present.7
Several quantitative indirect echocardiographic indices of PH have been evaluated in dogs for predictive/diagnostic value, including main pulmonary artery (MPA) size indexed to the size of the ascending aorta (MPA:Ao),10 pulsed‐wave Doppler‐derived acceleration time to peak PA flow velocity (AT),10, 11 and AT indexed to the ejection time of PA flow (AT:ET).10, 11 Diagnostic value of indices of right ventricular (RV) function (tissue Doppler imaging [TDI] velocities of the RV free wall and RV myocardial performance [Tei] index) has also been evaluated in dogs with PH.10 However, these indices are hindered by relatively low sensitivity and specificity values (MPA:Ao), and technical and alignment limitations (TDI velocities, Tei index, AT, and AT:ET).
Pulmonary hypertension is associated with proximal PA distension and reduced distensibility.12 Indices of proximal PA distensibility or stiffness quantified by echocardiography,13, 14 computed tomography angiography,15 and magnetic resonance imaging12, 16, 17, 18, 19 are attractive noninvasive indices of PH in humans. These indices correlate with “gold standard” invasive measurements of pulmonary vascular resistance and PA pressure,14, 15, 18 are reliable and early noninvasive indicators of PH (even when only exercised‐induced PH is present),16, 18 predict mortality,12, 17, 18 and help predict acute drug responsiveness.19 Recently, a right pulmonary artery distensibility (RPAD) index quantified by echocardiography, essentially a shortening fraction of the right PA, was evaluated in dogs affected with heartworm disease and various degrees of PH.6 The RPAD index showed a strong correlation with invasive PA pressures, demonstrating its potential diagnostic value in dogs. However, diagnostic accuracy for predicting various degrees of PH was not systematically studied and it is unknown how the diagnostic accuracy of the RPAD index compares to other indirect echocardiographic indices of PH in dogs.
The purpose of this study was to determine the diagnostic value of RPAD index for prediction of Doppler‐derived estimates of PA pressure (TRPG) in dogs with various degrees of PH (as estimated by TRPG) compared to other echocardiographic indices of PH. We hypothesized that the RPAD index could predict TRPG pressure in dogs and would be comparable in accuracy to other indirect echocardiographic indices of PH.
Materials and Methods
The Institutional Animal Care and Use Committee at the University of California, Davis, approved all procedures in this study. Owner consent for participation in the study was obtained for each dog.
Animals
Study subjects consisted of privately owned dogs that underwent a complete echocardiographic study as part of a routine clinical work up at the University of California, Davis Veterinary Medical Teaching Hospital, or were referred for the purposes of this study. Over a 14‐month period, dogs were prospectively and consecutively enrolled if they possessed a clearly identifiable systolic TR jet based on color and continuous‐wave Doppler echocardiography (ie, TR jets had to possess a clear Doppler profile). Once enrolled, dogs underwent the specific echocardiographic examination outlined below, which is considered a continuation of the first clinical echocardiographic study. Dogs were allocated to 1 of 4 groups according to the estimated peak tricuspid regurgitation systolic pressure gradient (TRPG) derived from the peak systolic TR jet velocity via the simplified Bernoulli equation (PG = 4 × velocity2): (1) control group, TRPG <36 mmHg, (2) TRPG of 36–50 mmHg, (3) TRPG of 50–75 mmHg and (4) TRPG >75 mmHg.4 None of the dogs in the control group could be taking medications known to affect the cardiovascular or respiratory system and had to have normal RV systolic function based on recently published body weight‐specific reference intervals for fractional area change (FAC).20 In dogs with a TRPG > 36 mmHg (suggestive of at least mild PH), a search for an underlying cause or additional diagnostic testing was performed at the discretion of the attending clinician. Dogs were excluded from the study if right ventricular outflow tract obstruction was detected by 2D and Doppler echocardiography (defined as any visible echocardiographic lesion in the RV, PA or branch PAs possessing a peak systolic velocity great than 2 m/s) or if sildenafil was currently being administered. Cardiogenic ascites was used as an indicator of right‐sided congestive heart failure and was deemed present if ascites was discovered in addition to jugular venous distension, ultrasonographic evidence of right‐sided cardiac disease, hepatomegaly, a subjectively dilated caudal vena cava, or some combination thereof.
Echocardiography
Image Acquisition
All echocardiographic studies1 were performed by a board‐certified veterinary cardiologist (L.C.V or J.A.S.) or a cardiology resident under the direct supervision of a board‐certified veterinary cardiologist and all raw data were captured digitally for offline analysis at a digital workstation.2 Dogs were manually restrained in right and left lateral recumbency. Use of sedation and supplemental oxygen was permitted if deemed necessary by the attending clinician. Conventional imaging planes21 were utilized with continuous ECG monitoring. Care was taken to align the TR jet as parallel as possible to the plane of the ultrasound interrogation cursor. Care was also taken to optimize visualization of the main and right pulmonary arteries via the 2D echocardiographic view from a right parasternal short axis basilar position. Quantification of a pulmonary valve insufficiency jet to estimate mean and diastolic pulmonary artery pressures was not performed for the purposes of this study.
Echocardiographic Measurements
All echocardiographic assessments, measurements and calculations were performed by the same individual (L.C.V.) and in the same order for each dog, with measurement of TR jet velocity obtained last. Therefore, the individual performing echocardiographic measurements was unaware of the TRPG when measuring all other echocardiographic indices. Peak systolic TR jet velocity obtained by 2D/color Doppler‐guided continuous‐wave Doppler was measured from the view that allowed the clearest jet profile and best alignment with direction of the jet. Tricuspid regurgitation jet velocity was only measured when a complete flow profile of the jet was present and peak velocity was clearly visualized. The value for each echocardiographic index consisted of an average of 3 representative but not necessarily consecutive measurements and was always measured while the dog was in sinus rhythm. Heart rate recorded represented the average heart rate of each of the 3 cardiac cycles used to determine each echocardiographic index value.
For determination of the RPAD index, the minimum diastolic (RPAD; usually at the Q wave) and maximum systolic (RPAS; usually coinciding with the largest T wave deflection or early‐to‐midsystole) internal diameter of the right PA was quantified at the same location of the right PA. This was performed using a trailing edge to leading edge technique. Care was taken to clearly visualize the internal borders of right PA throughout the cardiac cycle, and internal diameters of the right PA were measured as perpendicular as possible to the internal borders of the right PA. The RPAD index represents the percent change in diameter of the right PA throughout a single cardiac cycle according to the following formula: RPAD index = ([RPAS−RPAD]/RPAS) × 100 (Fig 1). For each individual dog's measurement, RPAD and RPAS were measured at the same location along the right PA, although this location could have varied slightly from dog to dog. The RPAD index was also determined in 12 randomly selected studies (3 from each of the PH groups) from a short axis 2D imaging view of the right PA (viewed from a right parasternal long axis view optimized for the heart base/right PA in short axis).6
Figure 1.

Representative measurement and calculation of the right pulmonary artery distensibility (RPAD) index in a dog with a peak tricuspid regurgitation systolic pressure gradient (TRPG) <36 mmHg. RPA, right pulmonary artery; RPAD, RPA at its minimum diameter in diastole; RPAS, RPA at its maximum diameter in systole; RA, right atrium; RV, right ventricle; Ao, aorta; PA, pulmonary artery.
From the same view used to quantify the RPAD index, diastolic measurements of the internal dimensions of the main pulmonary artery (MPA) in long axis and the ascending aorta (Ao) in short axis were obtained, and the MPA:Ao ratio was calculated.10 The MPA measurement was obtained approximately midway between the pulmonary valve and origin of the right and left PAs.22
Pulmonary artery flow was measured and recorded with pulsed‐wave Doppler imaging from the standard right parasternal short axis view only, using color Doppler imaging to guide placement of the sample volume (1–3 mm) centrally between the opened pulmonary valve leaflets. AT was measured from the onset of the pulsed‐wave Doppler flow to peak flow velocity. ET was measured from the onset to the end of the pulsed‐wave Doppler PA flow signal, and an AT:ET ratio was calculated.11 The presence of systolic “notching” during deceleration of the PA flow profile was noted.4
Right ventricular (RV) size and function was assessed from a standard left apical four‐chamber view. Right ventricular function was quantified by FAC, where measurements of RV area were obtained by tracing the RV endocardial border at end‐diastole (RVAD) and end‐systole (RVAS).20 Right ventricular percent FAC was calculated using the formula: FAC = ([RVAD−RVAS]/RVAD) × 100. Fractional area change values were compared to body weight‐specific reference intervals.20 Right ventricular size was assessed from the same view as FAC and, for the purposes of this study, the presence of (severe) RV enlargement was deemed present if the RV chamber and/or wall thickness was subjectively greater than that of the left ventricle.4
The presence of interventricular septal flattening was subjectively assessed throughout the cardiac cycle from standard short‐ and long‐axis views.
Left atrial (LA) size was assessed from a standard right parasternal long axis four‐chamber view and was determined by indexing the maximum systolic dimension23 to the aortic valve annulus, which was measured at the hinge points of opened leaflets in an early systolic frame from a separate long axis view optimized for the aortic valve annulus. A ratio greater than 2.6 indicated enlargement.24
Echocardiographic Indices Measurement Variability
Intraobserver measurement variability was determined by comparison of echocardiographic indices of PH (RPAD index, AT, AT:ET, and MPA:Ao) on these studies by a masked investigator (L.C.V.) on 3 separate occasions. The same studies and echocardiographic indices of PH were analyzed by once by a 2nd trained and masked investigator (M.K.I.) to determine interobserver measurement variability.
Statistical Analysis
Statistical analyses were performed using a commercial software package.3 Descriptive statistics were generated and normality testing with the D'Agostino–Pearson test was performed for all continuous data. Data are reported as median and range (minimum‐maximum) unless otherwise stated. A value of P < .05 was considered statistically significant.
Differences in continuous data among groups was determined by one‐way analysis of variance with subsequent pair‐wise comparisons determined using Tukey's multiple comparisons test (for normally distributed data) or by Kruskal–Wallis test with subsequent pair‐wise comparisons determined using the Dunn test (for non‐normally distributed data). Proportions were compared using the Chi‐square test with subsequent pair‐wise comparisons determined using multiple z‐tests for comparing 2 proportions, while adjusting the P‐value based on the Holm–Bonferroni (sequentially rejective) method for the number of comparisons.
The Pearson product‐moment correlation was used to determine the strength of association between TRPG and quantitative echocardiographic indices of PH (RPAD index, AT, AT:ET, and MPA:Ao). Simple linear regression analyses were used to predict TRPG from the same indices of PH. Standard assumptions for linear regression (linearity, homoscedasticity, and normality and independence of the residuals) was examined and verified before all analyses. The presence of outliers and influential values were assessed with standardized residual plots and Cook's distances.
Receiver operating characteristic (ROC) analyses were performed to determine optimal cutoff values for RPAD index, AT, AT:ET, and MPA:Ao in the prediction of TRPG >36 mmHg (suggestive of at least mild PH) and TRPG >50 mmHg (suggestive of at least moderate PH) and to evaluate the relationships among various sensitivity and specificity values. A value of TRPG >50 mmHg was chosen as an additional cutoff because moderate PH has implications for therapeutic intervention,1 suggesting the presence of irreversible PH,25 and has been demonstrated to be associated with a worse outcome in dogs with myxomatous mitral valve disease (MMVD).2 Optimal cutoff values were chosen for each indirect echocardiographic PH index from all combinations of sensitivity and specificity based on the highest Youden index (Youden index = [sensitivity−specificity] + 1). Cutoff values with the highest Youden index (optimal combination of sensitivity and specificity) represent a clinically relevant cutoff, with the least amount of overlap between groups. The area under the ROC curve was used to represent diagnostic accuracy and to quantify the predictive value of the indirect echocardiographic indices of PH.
Right pulmonary artery distensibility index measurements with the right PA in long versus short axis were evaluated for bias and for limits of agreement by the Bland–Altman method. The average percent coefficient of variation (CV) was used to quantify echocardiographic measurement variability studies, where percent CV = (standard deviation of the measurements/average of the measurements) × 100.
Results
Clinical and Echocardiographic Data
A summary of clinical and echocardiographic data for all dogs is presented in Table 1. The study sample consisted of 69 dogs designated into control (TRPG <36 mmHg; n = 22), TRPG 36–50 mmHg (n = 16), TRPG 50–75 mmHg (n = 14) and TRPG >75 mmHg (n = 17) groups and was comprised of primarily aged, small breed dogs. Nine each were mixed breeds and Chihuahuas, 7 were shih tzus, 4 each were Cavalier King Charles Spaniels and miniature poodles, 3 were Yorkshire terriers, 2 each were papillons, miniature schnauzers, miniature dachshunds, Pomeranians, pugs, West Highland white terriers, boxers, and pit bull terriers. The other breeds (Bichon Frise, Boston terrier, Jack Russell terrier, Shetland sheepdog, Affenpinscher, Siberian husky, Pekingese, Toy fox terrier, Havanese, Maltese, Whippet, English setter, beagle, border collie, Australian shepherd, Labrador retriever, and Schipperke) were each represented once. Median body weight of dogs in the control group (8.9, range 3.7–39.0) was slightly but significantly (P < .05) more than dogs in the TRPG >75 mmHg group (5.6, range 1.4–39.0). Heart rate of all dogs with TRPG >36 mmHg was significantly (all P < .05) higher than dogs in the control group. No statistically significant (P > .05) differences in age or sex were encountered among groups. Significantly (P < .05) more dogs in the TRPG >75 mmHg group (n = 6 [35%]) experienced right‐sided congestive heart failure compared to control dogs (n = 0 [0%]) and dogs in the TRPG 36–50 mmHg group (n = 0 [0%]). None of the dogs included in this study had left‐sided CHF. Median FAC was significantly (P < .05) lower in dogs in the TRPG >75 mmHg group (37.7%, range 19.9–56.0%) compared to dogs in the TRPG 36–50 mmHg group (50.0%, range 23.3–65.0%), and significantly (P < .05) more dogs in the TRPG >75 mmHg group (n = 11 [65%]) had a FAC value below the reference interval as compared to dogs in the TRPG 36–50 mmHg group (n = 1 [6.0%]). Characteristic qualitative changes associated with severe and chronic PH (notched PA flow signal, interventricular septal flattening, and RV enlargement) were documented in significantly (all P < .05) more dogs in the TRPG >75 mmHg group compared to the other groups (Table 1).
Table 1.
Clinical and echocardiographic characteristics of all study dogs (n = 69)
| Clinical and Echocardiographic Data | Control (n = 22) | TRPG 36–50 mmHg (n = 16) | TRPG 50–75 mmHg (n = 14) | TRPG >75 mmHg (n = 17) |
|---|---|---|---|---|
| Body weight (kg) | 8.9 (3.7–39.0) | 7.4 (3.4–15.1) | 6.0 (3.3–12.9) | 5.6 (1.4–39.0)a |
| Heart rate (bpm) | 103 (72–158) | 133 (77–186)a | 133 (90–162)a | 130 (60–190)a |
| Age (years) | 9.5 (3.0–14.0) | 10.5 (6.0–15.0) | 13.0 (4.0–15.0) | 9.0 (2.0–15.0) |
| Female: number (%) | 10 (45%) | 8 (50%) | 8 (57%) | 9 (53%) |
| Right‐sided CHF: number (%) | 0 (0%) | 0 (0%) | 2 (14%) | 6 (35%)a , b |
| RV FAC (%) | 46.0 (33.6–63.8) | 50.0 (23.3–65.0) | 42.3 (34.0–55.6) | 37.7 (19.9–56.0)b |
| RV FAC below RI: number (%) | 0 (0%) | 1 (6.0%) | 5 (36%) | 11 (65%)b |
| TRPG (mmHg) | 25.2 (13.7–29.8) | 39.2 (36.5–46.8)a | 59.0 (53.0–70.9)a | 93.7 (77.8–141.6)a , b |
| RPAD index (%) | 40.5 (29.1–46.8) | 37.2 (22.6–43.8) | 27.2 (16.9–36.0)a | 9.4 (4.0–20.1)a , b |
| AT (ms) | 79.2 (51.0–119.7) | 63.4 (40.6–101.2)a | 52.7 (39.5–71.2)a | 37.8 (20.5–67.8)a , b , c |
| AT:ET | 0.39 (0.31–0.52) | 0.37 (0.28–0.59) | 0.33 (0.22–0.44)a | 0.21 (0.14–0.38)a , b , c |
| MPA:Ao | 0.93 (0.80–1.08) | 1.04 (0.86–1.33) | 1.10 (1.00–1.27)a | 1.25 (0.96–1.53)a , b |
| LA:Ao long axis | 2.5 (2.0–2.6) | 2.8 (2.0–5.1) | 2.4 (2.1–4.6) | 2.3 (1.7–2.6)b |
| LA enlargement: number (%) | 0 (0%) | 7 (44%)a | 5 (36%)a | 0 (0%)b , c |
| Notched PA flow: number (%) | 0 (0%) | 0 (0%) | 0 (0%) | 13 (76%)a , b , c |
| Septal flattening: number (%) | 0 (0%) | 0 (0%) | 4 (29%)a | 16 (94%)a , b , c |
| RV enlargement: number (%) | 0 (0%) | 0 (0%) | 3 (21%) | 17 (100%)a , b , c |
Data represent median (min–max) unless otherwise stated. CHF, congestive heart failure; RV, right ventricle; FAC, fractional area change; RI, reference interval; TRPG, peak tricuspid regurgitation systolic pressure gradient; RPAD, right pulmonary artery distensibility; AT, acceleration time to peak pulmonary artery flow; ET, ejection time of pulmonary artery flow; MPA, main pulmonary artery; Ao, aorta; PA, pulmonary artery; LA, left atrial.
P < .05 as compared to Control group.
P < .05 as compared to TRPG 36–50 mmHg group.
P < .05 as compared to TRPG 50–75 mmHg group.
In the control group, 8 of 22 dogs (36%) were echocardiographically normal and the remaining 14 dogs (64%) were diagnosed with MMVD but without an elevated TRPG. All dogs in this study that had LA enlargement were diagnosed with MMVD as the primary cause. In dogs with normal or reduced LA size, a cause for PH was not definitively diagnosed in the vast majority of dogs (unless stated). Conversely, in the remainder of the dogs that were diagnosed with LA enlargement, the cause of the PH was presumed to be at least partially secondary to left‐sided cardiac disease (ie, postcapillary PH). In the TRPG 36–50 mmHg group, all dogs had MMVD and 2 dogs were receiving enalapril. In the TRPG 50–75 mmHg group, 9 dogs had MMVD. Two dogs were receiving theophylline, 1 dog was receiving enalapril, and 1 dog was receiving furosemide. In the TRPG >75 mmHg group, there were significantly less dogs with LA enlargement (0 of 17 [0%]) compared to the other groups. In this group, 3 had MMVD, 3 dogs had a patent ductus arteriosus (with 2 demonstrating right‐to‐left shunting via agitated saline contrast echocardiography) and 2 dogs had severe heartworm disease. Dogs in the TRPG >75 mmHg group were receiving a variety of medications at the time of echocardiography, including furosemide (7 dogs), enalapril (4 dogs), and pimobendan (2 dogs), and 1 dog each was receiving theophylline, prednisone, or atenolol.
Correlation Analyses & Prediction of TRPG
Results of the correlation and simple linear regression analyses are shown in Figure 2 and Table 2. The RPAD index exhibited the strongest correlation (r = −0.90; P < .0001) to TRPG, followed by MPA:Ao (r = 0.78; P < .0001), AT (r = −0.69; P < .0001), and AT:ET (r = −0.68; P < .0001). Predictive equations for TRPG derived from each of the 4 echocardiographic indices of PH are presented in Table 2.
Figure 2.
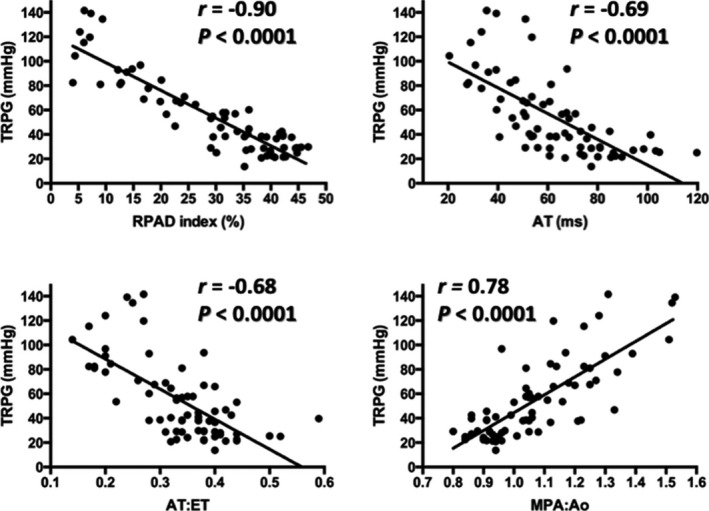
Scatter plots illustrating significant (all P < .0001) correlations (r) between peak tricuspid regurgitation systolic pressure gradient (TRPG) and the 4 indirect echocardiographic indices of pulmonary hypertension: right pulmonary artery distensibility (RPAD) index (upper left), acceleration time to peak pulmonary artery flow (AT; upper right), acceleration time to peak pulmonary artery flow to ejection time of pulmonary artery flow ratio (AT:ET; lower left), and main pulmonary artery to aorta internal diameter ratio (MPA:Ao; lower right). The solid line within each scatter plot represents the line of best fit.
Table 2.
Results of correlation and simple linear regression analyses for the prediction of TRPG from 4 echocardiographic indices of PH in 69 dogs where TRPG could be reliably quantified
| Variable | r | 95% CI of r | R 2 | P | Regression Equation |
|---|---|---|---|---|---|
| RPAD index | −0.90 | −0.94 to −0.85 | 0.81 | <.0001 | TRPG = (−2.27 × RPAD index) + 121.5 |
| MPA:Ao | 0.78 | 0.67–0.86 | 0.61 | <.0001 | TRPG = (146.0 × MPA:Ao)−101.5 |
| AT | −0.69 | −0.80 to −0.54 | 0.48 | <.0001 | TRPG = (−1.06 × AT) + 120.5 |
| AT:ET | −0.68 | −0.79 to −0.53 | 0.46 | <.0001 | TRPG = (−246.5 × AT:ET) + 137.8 |
r, correlation coefficient; CI, confidence interval; R 2, coefficient of determination; PH, pulmonary hypertension; See Table 1 for remainder for the remainder of the key.
Diagnostic Accuracy and Optimal Cutoffs for Predicting Pulmonary Hypertension
The ROC curves for each echocardiographic index for the prediction of TRPG >50 mmHg (suggestive of at least moderate PH) is presented in Figure 3.
Figure 3.

Receiver operating characteristic (ROC) curves of the 4 indirect echocardiographic indices of PH: right pulmonary artery distensibility (RPAD) index (upper left), acceleration time to peak pulmonary artery flow (AT; upper right), acceleration time to peak pulmonary artery flow to ejection time of pulmonary artery flow ratio (AT:ET; lower left), and main pulmonary artery to aorta internal diameter ratio (MPA:Ao; lower right) for the prediction of peak tricuspid regurgitation systolic pressure gradient (TRPG) >50 mmHg in 69 dogs. AUC, area under the ROC curve; CI, confidence interval.
Three cutoff values were determined from the ROC analyses for each indirect echocardiographic PH index for predicting TRPG >36 mmHg (Table 3) and TRPG >50 mmHg (Table 4); 1 for optimal test efficiency (defined by the highest Youden index), 1 for maximum sensitivity (least number of false negatives), and 1 for maximal specificity (least number of false positives).
Table 3.
Sensitivity, specificity, and Youden index of different cutoff points of 4 echocardiographic indices of PH for the prediction of peak tricuspid regurgitation systolic pressure gradient (TRPG) >36 mmHg in 69 dogs
| Variable | AUC | 95% CI | P | Cutoff | Sensitivity | Specificity | Youden Index |
|---|---|---|---|---|---|---|---|
| MPA:Ao | 0.89 | 0.81–0.97 | <.0001 | >0.85 | 1.00 | 0.14 | 0.14 |
| >0.98 | 0.85 | 0.86 | 0.71 | ||||
| >1.10 | 0.55 | 1.00 | 0.55 | ||||
| RPAD index (%) | 0.88 | 0.80–0.96 | <.0001 | <44.2 | 1.00 | 0.23 | 0.23 |
| <34.6 | 0.74 | 0.91 | 0.65 | ||||
| <27.7 | 0.53 | 1.00 | 0.53 | ||||
| AT (ms) | 0.88 | 0.80–0.97 | <.0001 | <102.3 | 1.00 | 0.14 | 0.14 |
| <72.1 | 0.89 | 0.77 | 0.67 | ||||
| <50.7 | 0.43 | 1.00 | 0.43 | ||||
| AT:ET | 0.80 | 0.69–0.90 | <.0001 | <0.56 | 1.00 | 0.05 | 0.05 |
| <0.38 | 0.79 | 0.68 | 0.47 | ||||
| <0.31 | 0.45 | 1.00 | 0.45 |
AUC, area under receiver operating characteristic curve; PH, pulmonary hypertension; See Table 1 for remainder for the remainder of the key. Bolded values represent a clinically relevant cutoff, with the least amount of overlap between groups (optimal combination of sensitivity and specificity and highest Youden index).
Table 4.
Sensitivity, specificity, and Youden index of different cutoff points of 4 echocardiographic indices of PH for prediction of peak tricuspid regurgitation systolic pressure gradient (TRPG) >50 mmHg in 69 dogs
| Variable | AUC | 95% CI | P | Cutoff | Sensitivity | Specificity | Youden Index |
|---|---|---|---|---|---|---|---|
| RPAD index (%) | 0.97 | 0.93–1.00 | <.0001 | <36.2 | 1.00 | 0.68 | 0.68 |
| <29.5 | 0.84 | 0.95 | 0.79 | ||||
| <21.8 | 0.65 | 1.00 | 0.65 | ||||
| MPA:Ao | 0.90 | 0.83–0.97 | <.0001 | >0.96 | 1.00 | 0.58 | 0.58 |
| >1.04 | 0.94 | 0.74 | 0.67 | ||||
| >1.33 | 0.16 | 1.00 | 0.16 | ||||
| AT (ms) | 0.89 | 0.82–0.96 | <.0001 | <72.1 | 1.00 | 0.58 | 0.58 |
| <53.9 | 0.74 | 0.87 | 0.61 | ||||
| <40.1 | 0.39 | 1.00 | 0.39 | ||||
| AT:ET | 0.87 | 0.78–0.96 | <.0001 | <0.47 | 1.00 | 0.08 | 0.08 |
| <0.30 | 0.61 | 0.97 | 0.59 | ||||
| <0.28 | 0.52 | 1.00 | 0.52 |
Bland–Altman Analysis and Echocardiographic Measurement Variability
Bland–Altman analysis of long versus short axis RPAD index showed minimal bias (0.25 ± 1.3%) with narrow limits of agreement (95% limits of agreement, −2.3 to 2.8%). Coefficients of variation for intraobserver measurement variability was 9.5% for RPAD index, 7.7% for AT, 9.4% for AT:ET, and 6.2% for MPA:Ao. Coefficients of variation for interobserver measurement variability was 13.0% for RPAD index, 6.4% for AT, 6.7% for AT:ET, and 8.4% for MPA:Ao.
Discussion
Results obtained in this study confirmed our hypothesis that the RPAD index is a predictor of TRPG measured by Doppler echocardiography and is comparable in accuracy to other indices of PH for predicting TRPG >36 mmHg (suggestive of a least mild PH). Further, our results suggest the RPAD index might be more accurate than other studied indices for predicting TRPG >50 mmHg (suggestive of at least moderate PH), which is potentially more clinically relevant. We found that acquisition and measurement of the RPAD index in dogs with varying levels of TRPG (suggestive of varying levels of PH) was repeatable, with good agreement when measured from long or short axis. Our results support the use of the RPAD index when screening dogs for PH via echocardiography, particularly when TR is absent or difficult to measure.
Other investigators have recently evaluated the RPAD index in dogs affected with heartworm disease and PH.6 Despite differences in acquiring the RPAD index (M‐mode imaging with the right PA in short axis compared to 2D imaging with the right PA in long axis performed in this study), the authors also found the index to be repeatable and documented a similar strong correlation (r = −0.88) to Doppler‐derived estimates of systolic PA pressure. In contrast to a previous study,6 our study consisted of a more heterogeneous sample of dogs that were affected with a variety of diseases suspected to be contributing to precapillary, postcapillary, or mixed causes of PH. This is important to highlight because most clinicians are screening dogs for PH secondary to a variety of diseases, making this study more broadly applicable.
Although there are more advanced noninvasive methods of quantifying PA distensibility in humans, echocardiography is the most practical imaging modality for clinical use in dogs. Others have introduced quantifying PA distensibility echocardiographically in dogs using 2D imaging of the MPA22 and M‐mode imaging of the right PA in short6 and long axis.26 We felt quantifying PA distensibility using the relative diameter change in the right PA when viewed from a long axis plane using 2D echocardiography would optimize practicality and accuracy compared to other methods. We also felt measuring the diameter of the right PA from long axis plane would have less potential to falsely lengthen or foreshorten the right PA. We specifically chose quantifying right PA distensibility versus MPA distensibility because a study demonstrated that right PA distensibility (utilizing computed tomography angiography) yielded the best diagnostic value for PH in people.15 Further, the RPAD index described herein can be efficiently acquired using a digital caliper from a standard imaging plane21 routinely acquired by most veterinary echocardiographers that permits clear visualization of the internal borders of the right PA in most dogs. Ultimately, however, additional studies would be necessary to determine which method of quantifying PA distensibility is most accurate and reliable in dogs.
Other investigators have evaluated the relationship between Doppler echocardiographic estimates of systolic PA pressure in dogs and AT,10, 11 AT:ET,10, 11 and MPA:Ao10 and very similar correlations were encountered despite slightly different methods of estimating systolic PA pressure in each study. We elected not to add estimates of right atrial pressure to the TRPG in this study, as consensus is lacking on how best to do so in dogs and adding right atrial pressure to TRPG only minimally improved accuracy of estimating invasive systolic PA pressure in a recent experimental study of acute PH in dogs.9 There is also evidence in humans that adding estimations of right atrial pressure to estimate systolic PA pressure can result in overestimation of PH severity.27 Also, compared to 2 previous studies in dogs that evaluated diagnostic accuracy of AT, AT:ET, and MPA:Ao in predicting PH (ie, echocardiographic evidence of at least mild PH was present),10, 11 similar to slightly improved sensitivity, specificity, and AUC values were obtained. Our cutoff values are slightly different and can likely be explained by the use of different values to define a TRPG cutoff suggestive of PH in each study and the more homogenous patient sample (West Highland white terriers) of a previous study.11
The RPAD index is an appealing indirect and complementary index of estimating PA pressures in dogs. It recently demonstrated strong correlation (r = −0.81) with invasive “gold standard” systolic PA pressures in a relatively large number of dogs6 and appears to be a repeatable and relatively easy/practical index to acquire via 2D imaging. Conversely, Doppler‐derived echocardiographic indices of PH (AT, AT:ET, Tei index, and RV TDI velocities) can be hampered by technical and alignment limitations. Clinical experience indicates that meticulous placement of the Doppler sample volume is vital for accurate AT/AT:ET values and “clean” Doppler signals can be difficult to acquire in some dogs, particularly those with respiratory difficulty.7, 11 This could explain why 2D‐based indices in this study (RPAD index, MPA:Ao) were often more accurate in predictors of PH. Furthermore, predicting a TRPG >50 mmHg (suggestive of at least moderate PH) is an important and clinically relevant finding, as the presence of at least moderate PH often influences therapeutic decisions,1 provides important prognostic information,2 and suggests that PH could become refractory to therapy in dogs with MMVD.25
This study has several limitations. It utilized Doppler echocardiography‐derived estimates of PA pressure (TRPG without adding estimated right atrial pressure) as a surrogate measure of PA pressure. It is well known that TRPG is an imperfect estimate of invasive PA pressure and relevant differences might exist between echocardiographically estimated and invasively measured PA pressure.9 Therefore, clinicians should be mindful of this limitation when diagnosing and classifying dogs with PH, and utilizing the regression equations and cutoffs values presented herein. However, a strong correlation of the RPAD index with invasive PA pressures was recently reported in a relatively large number of dogs,6 thus strengthening the results of our study. There is unfortunately a lack of consensus throughout the veterinary literature on the cutoff of PA pressure that constitutes PH. We elected to use a relatively conservative cutoff of 36 mmHg (equates to a peak TR jet velocity of 3 m/s), excluding adding estimates of right atrial pressure. We acknowledge that this cutoff could have misclassified or falsely excluded some dogs with a diagnosis of PH as estimated echocardiographically or, less likely, falsely diagnosed some dogs with PH (eg, dogs with heightened adrenergic tone and increased cardiac output). An additional limitation was that, despite being masked to the TRPG while recording other echocardiographic indices (TRPG measured last in each dog), this study lacked true blinding to the degree of PH during the time the indices of PH were measured, thus potentially biasing our results. However, we contend that true blinding would be very difficult, as typical morphologic changes associated with PH can be observed during measurement of indirect echocardiographic indices of PH. Also, we acknowledge potential confounding effects of the various medications that relatively few dogs were taking at the time of the echocardiographic examination. It would be difficult to standardize medications given the heterogeneous study sample; yet, many of these medications possess hemodynamic effects relevant to this study. Lastly, our study sample consisted of dogs with clinical and echocardiographic signs suggestive of chronic PH. Caution is advised when using these indices to predict PH suspected to be secondary to acute events (eg, acute pulmonary thromboemboli). Human studies have demonstrated that PA distensibility is still abnormal, albeit affected differently by diseases causing acute (versus chronic) PH.28
We conclude by advocating for the use of the RPAD index when utilizing echocardiography to screen dogs for PH. This index will likely provide beneficial complementary information in dogs with PH as estimated echocardiographically, especially when TR is absent or (more commonly) inconsistent. Further study is warranted to determine if there is additional clinical value of this index.
Acknowledgments
The authors gratefully acknowledge the contributions of Mikaela Mueller, Catherine Gunther‐Harrington, Catherine Bélanger, Denise Berger, Judy Schettler, Chrissy Kinkade, and Eric Ontiveros. This study was supported in part by the American Kennel Club Canine Health Foundation and the Center for Companion Animal Health at the University of California, Davis.
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This work was performed at the William R. Pritchard Veterinary Medical Teaching Hospital, University of California, Davis. Presented in abstract form as an oral presentation at the 2015 ACVIM Forum, Indianapolis, IN.
Footnotes
Philips IE33, Philips Healthcare, Andover, MA
Syngo® Dynamic Workplace, Version 10.0.01_HF04_Rev5 (Build 2884), Siemens Medical Solutions, USA, Inc., Malvern, PA
Prism 6 for Mac OS X, Version 6.0f, GraphPad Software, Inc., La Jolla, CA
References
- 1. Kellihan HB, Stepien RL. Pulmonary hypertension in canine degenerative mitral valve disease. J Vet Cardiol 2012;14:149–164. [DOI] [PubMed] [Google Scholar]
- 2. Borgarelli M, Abbott J, Braz‐Ruivo L, et al. Prevalence and prognostic importance of pulmonary hypertension in dogs with myxomatous mitral valve disease. J Vet Intern Med 2015;29:569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stepien RL. Pulmonary arterial hypertension secondary to chronic left‐sided cardiac dysfunction in dogs. J Small Anim Pract 2009;50(Suppl 1):34–43. [DOI] [PubMed] [Google Scholar]
- 4. Johnson L, Boon J, Orton EC. Clinical characteristics of 53 dogs with Doppler‐derived evidence of pulmonary hypertension: 1992–1996. J Vet Intern Med 1999;13:440–447. [DOI] [PubMed] [Google Scholar]
- 5. Borgeat K, Sudunagunta S, Kaye B, et al. Retrospective evaluation of moderate‐to‐severe pulmonary hypertension in dogs naturally infected with Angiostrongylus vasorum . J Small Anim Pract 2015;56:196–202. [DOI] [PubMed] [Google Scholar]
- 6. Venco L, Mihaylova L, Boon JA. Right Pulmonary Artery Distensibility Index (RPAD Index). A field study of an echocardiographic method to detect early development of pulmonary hypertension and its severity even in the absence of regurgitant jets for Doppler evaluation in heartworm‐infected dogs. Vet Parasitol 2014;206:60–66. [DOI] [PubMed] [Google Scholar]
- 7. Kellihan HB, Stepien RL. Pulmonary hypertension in dogs: diagnosis and therapy. Vet Clin North Am Small Anim Pract 2010;40:623–641. [DOI] [PubMed] [Google Scholar]
- 8. Brown AJ, Davison E, Sleeper MM. Clinical efficacy of sildenafil in treatment of pulmonary arterial hypertension in dogs. J Vet Intern Med 2010;24:850–854. [DOI] [PubMed] [Google Scholar]
- 9. Soydan LC, Kellihan HB, Bates ML, et al. Accuracy of Doppler echocardiographic estimates of pulmonary artery pressures in a canine model of pulmonary hypertension. J Vet Cardiol 2015;17:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Serres F, Chetboul V, Gouni V, et al. Diagnostic value of echo‐Doppler and tissue Doppler imaging in dogs with pulmonary arterial hypertension. J Vet Intern Med 2007;21:1280–1289. [DOI] [PubMed] [Google Scholar]
- 11. Schober KE, Baade H. Doppler echocardiographic prediction of pulmonary hypertension in West Highland white terriers with chronic pulmonary disease. J Vet Intern Med 2006;20:912–920. [DOI] [PubMed] [Google Scholar]
- 12. Gan CT, Lankhaar JW, Westerhof N, et al. Noninvasively assessed pulmonary artery stiffness predicts mortality in pulmonary arterial hypertension. Chest 2007;132:1906–1912. [DOI] [PubMed] [Google Scholar]
- 13. Ertan C, Tarakci N, Ozeke O, et al. Pulmonary artery distensibility in chronic obstructive pulmonary disease. Echocardiography 2013;30:940–944. [DOI] [PubMed] [Google Scholar]
- 14. Pasierski TJ, Starling RC, Binkley PF, et al. Echocardiographic evaluation of pulmonary artery distensibility. Chest 1993;103:1080–1083. [DOI] [PubMed] [Google Scholar]
- 15. Revel MP, Faivre JB, Remy‐Jardin M, et al. Pulmonary hypertension: ECG‐gated 64‐section CT angiographic evaluation of new functional parameters as diagnostic criteria. Radiology 2009;250:558–566. [DOI] [PubMed] [Google Scholar]
- 16. Sanz J, Kariisa M, Dellegrottaglie S, et al. Evaluation of pulmonary artery stiffness in pulmonary hypertension with cardiac magnetic resonance. JACC Cardiovasc Imaging 2009;2:286–295. [DOI] [PubMed] [Google Scholar]
- 17. Stevens GR, Garcia‐Alvarez A, Sahni S, et al. RV dysfunction in pulmonary hypertension is independently related to pulmonary artery stiffness. JACC Cardiovasc Imaging 2012;5:378–387. [DOI] [PubMed] [Google Scholar]
- 18. Swift AJ, Rajaram S, Condliffe R, et al. Pulmonary artery relative area change detects mild elevations in pulmonary vascular resistance and predicts adverse outcome in pulmonary hypertension. Invest Radiol 2012;47:571–577. [DOI] [PubMed] [Google Scholar]
- 19. Jardim C, Rochitte CE, Humbert M, et al. Pulmonary artery distensibility in pulmonary arterial hypertension: an MRI pilot study. Eur Respir J 2007;29:476–481. [DOI] [PubMed] [Google Scholar]
- 20. Visser LC, Scansen BA, Schober KE, et al. Echocardiographic assessment of right ventricular systolic function in conscious healthy dogs: repeatability and reference intervals. J Vet Cardiol 2015;17:83–96. [DOI] [PubMed] [Google Scholar]
- 21. Thomas WP, Gaber CE, Jacobs GJ, et al. Recommendations for standards in transthoracic two‐dimensional echocardiography in the dog and cat. Echocardiography Committee of the Specialty of Cardiology, American College of Veterinary Internal Medicine. J Vet Intern Med 1993;7:247–252. [DOI] [PubMed] [Google Scholar]
- 22. Boon JA. Hypertensive heart disease In: Boon JA, ed. Veterinary Echocardiography, 2nd ed Ames, IA: Wiley‐Blackwell; 2011:335–358. [Google Scholar]
- 23. Rishniw M, Erb HN. Evaluation of four 2‐dimensional echocardiographic methods of assessing left atrial size in dogs. J Vet Intern Med 2000;14:429–435. [DOI] [PubMed] [Google Scholar]
- 24. Smith DN, Bonagura JD, Culwell NM, et al. Left ventricular function quantified by myocardial strain imaging in small‐breed dogs with chronic mitral regurgitation. J Vet Cardiol 2012;14:231–242. [DOI] [PubMed] [Google Scholar]
- 25. Chiavegato D, Borgarelli M, D'Agnolo G, et al. Pulmonary hypertension in dogs with mitral regurgitation attributable to myxomatous valve disease. Vet Radiol Ultrasound 2009;50:253–258. [DOI] [PubMed] [Google Scholar]
- 26. Tian L, Kellihan HB, Henningsen J, et al. Pulmonary artery relative area change is inversely related to ex vivo measured arterial elastic modulus in the canine model of acute pulmonary embolization. J Biomech 2014;47:2904–2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fisher MR, Forfia PR, Chamera E, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med 2009;179:615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu DK, Hsiao SH, Lin SK, et al. Main pulmonary arterial distensibility: different presentation between chronic pulmonary hypertension and acute pulmonary embolism. Circ J 2008;72:1454–1459. [DOI] [PubMed] [Google Scholar]


