Abstract
Background
Pleural effusion is a common cause of dyspnea in cats. N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) measurement, using a first‐generation quantitative ELISA, in plasma and pleural fluid differentiates cardiac from noncardiac causes of pleural effusion.
Hypothesis/Objectives
To determine whether NT‐proBNP measurements using second‐generation quantitative ELISA and point‐of‐care (POC) tests in plasma and pleural fluid distinguish cardiac from noncardiac pleural effusions and how results compare to the first‐generation ELISA.
Animals
Thirty‐eight cats (US cohort) and 40 cats (UK cohort) presenting with cardiogenic or noncardiogenic pleural effusion.
Methods
Prospective cohort study. Twenty‐one and 17 cats in the US cohort, and 22 and 18 cats in the UK cohort were classified as having cardiac or noncardiac pleural effusion, respectively. NT‐proBNP concentrations in paired plasma and pleural fluid samples were measured using second‐generation ELISA and POC assays.
Results
The second‐generation ELISA differentiated cardiac from noncardiac pleural effusion with good diagnostic accuracy (plasma: sensitivity, 95.2%, specificity, 82.4%; pleural fluid: sensitivity, 100%, specificity, 76.5%). NT‐proBNP concentrations were greater in pleural fluid (719 pmol/L (134–1500)) than plasma (678 pmol/L (61–1500), P = 0.003), resulting in different cut‐off values depending on the sample type. The POC test had good sensitivity (95.2%) and specificity (87.5%) when using plasma samples. In pleural fluid samples, the POC test had good sensitivity (100%) but low specificity (64.7%). Diagnostic accuracy was similar between first‐ and second‐generation ELISA assays.
Conclusions and clinical importance
Measurement of NT‐proBNP using a quantitative ELISA in plasma and pleural fluid or POC test in plasma, but not pleural fluid, distinguishes cardiac from noncardiac causes of pleural effusion in cats.
Keywords: Biomarker, Blood testing, Cardiomyopathy, Dyspnea, Natriuretic peptide
Abbreviations
- CI
confidence interval
- LA/Ao
left atrial to aortic ratio
- LVFWd
left ventricular wall thickness in diastole
- NT‐proBNP
N‐terminal pro‐B‐type natriuretic peptide
- POC
point‐of‐care
- ROC
receiver operator characteristic
Dyspnea secondary to pleural effusion is a common presenting complaint in cats, and can be the result of a variety of disease processes, including neoplasia, cardiac disease, pyothorax, and feline infectious peritonitis.1, 2 Differentiation of the underlying causes can be challenging in dyspneic cats. These cats might require supplementary oxygen and be too unstable to tolerate extensive diagnostic testing. Measurement of N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) using a first‐generation feline‐specific quantitative ELISA1 in plasma3, 4 and pleural fluid4 samples differentiates cardiac from noncardiac causes of pleural effusion in cats. Measurement in pleural fluid samples is a potentially attractive alternative to measurement in plasma, insofar as therapeutic thoracocentesis is usually performed in cats with significant pleural effusion, whereas venipuncture might cause additional distress to an already dyspneic cat. In human patients, measurement of NT‐proBNP concentrations in pleural fluid has high diagnostic accuracy to distinguish cardiac from noncardiac causes of pleural effusion. Meta‐analysis of 1,120 human patients revealed high sensitivity (94%, 95% confidence interval [CI]: 90–97%) and specificity (94%, 95% CI: 89–97%) with a strong correlation between NT‐proBNP concentrations measured in plasma and pleural fluid samples.5
Second‐generation assays that measure NT‐proBNP in cats are available, including a second‐generation quantitative ELISA1 and a semiquantitative point‐of‐care (POC) test2 that is specifically designed for detection of preclinical or occult forms of cardiomyopathy.6 In contrast to the first‐generation assay, sample collection for the second‐generation assays is performed without the use of a protease inhibitor cocktail and special handling and shipping requirements.3 In emergent cats, one disadvantage of the ELISA assay that is run at a reference laboratory is that results are usually not available until 1–2 working days after sample submission. The POC test offers patient‐side semiquantitative measurement of NT‐proBNP but its utility to distinguish cardiac from noncardiac causes of pleural effusion using either plasma or pleural fluid samples has not been investigated.
The aims of this study were to determine whether measurement of NT‐proBNP in plasma or pleural fluid samples using either the second‐generation quantitative ELISA or POC assay could distinguish cardiac from noncardiac causes of pleural effusion, and how these results compared to the results of a previous study using the first‐generation quantitative ELISA assay.4
Materials and Methods
The study was approved by the respective institutional animal use and care committees and informed owner consent was obtained. Thirty‐eight cats presenting to one of two US teaching hospitals (Matthew J. Ryan Veterinary Hospital of the University of Pennsylvania, Philadelphia PA and the Foster Hospital for Small Animals, Cummings School of Veterinary Medicine, Tufts University, North Grafton, MA) with pleural effusion undergoing thoracocentesis were prospectively recruited (US cohort). Forty cats presenting to a UK teaching hospital (Queen Mother Hospital for Animals, Royal Veterinary College, North Mymms, Hatfield) with pleural effusion undergoing thoracocentesis were prospectively recruited, as previously described (UK cohort).4 For both cohorts, cats were recruited and the cause of the pleural effusion was determined to be either cardiac or noncardiac by a board‐certified cardiologist on the basis of the echocardiogram, history, physical examination, and results of any additional diagnostic tests performed. The cardiologist was blinded to all quantitative and POC measurements of NT‐proBNP.
Pleural fluid samples were obtained at the time of therapeutic or diagnostic thoracocentesis. Plasma samples were collected when venipuncture for diagnostic sampling was performed during the same hospital visit and as close to the time of therapeutic thoracocentesis as was clinically practicable on the basis of feline stability. One milliliter samples of pleural fluid and 2 milliliter samples of blood were collected in K2‐EDTA treated tubes and were centrifuged at 3000 × g for 5 minutes within 15 minutes of collection. For the US cohort, NT‐proBNP was measured in plasma using the POC assay2 within 30 minutes of centrifugation and the remaining plasma and pleural fluid samples were each transferred to a cryotube and frozen at −80°C for batched analysis. NT‐proBNP was measured at the reference laboratory in plasma and pleural fluid samples using the second‐generation ELISA1 and in pleural fluid samples using the POC assay. 2 For the UK cohort, which was originally recruited for assessment of the first‐generation NT‐proBNP ELISA assay,4 NT‐proBNP was measured at the reference laboratory in plasma and pleural fluid samples using the second‐generation ELISA1 and POC assay2 in samples collected in a protease inhibitor tube3 and frozen at −80°C for batched analysis.
The second‐generation NT‐proBNP in cat assays incorporate a set of antibodies different from the first‐generation assay that attempt to target stable epitopes of NT‐proBNP in cats.4 The lower and upper limits of detection of the reference laboratory assay are 24 pmol/L and 1500 pmol/L, respectively. Values of NT‐proBNP less than the lower limit of detection were assigned values of 24 pmol/L. Values of NT‐proBNP greater than the upper limit of detection were assigned values of 1500 pmol/L. All reference laboratory samples were assayed in duplicate and the mean of the two values used.
The POC test is a bidirectional flow ELISA that uses the same pair of antibodies for the detection of NT‐proBNP in cats as the second‐generation reference laboratory test, and its use in detecting occult cardiomyopathy has been previously described.6 Briefly, to perform the POC assay, 3 drops of plasma or pleural fluid were mixed with 5 drops of assay conjugate and then added to the sample well of the test device. The conjugate‐diluted sample was allowed to flow across the device. Once the sample reached the indicator window, the device was activated by the operator. This initiated the wash and color development steps of the assay. The relative color densities of the sample and reference spots were evaluated visually after 10 minutes of incubation. Positive results were recorded when the density of the sample spot appeared equal to or greater than that of the reference spot. Negative results were recorded when the density of the sample spot appeared less than that of the reference spot. The transition from negative to positive on the POC test occurs in an NT‐proBNP range of 150–200 pmol/L.6
Statistical Analysis
Statistical analysis was performed using commercially available software.5 Data were examined graphically for normality of distribution. Group‐wise comparisons were performed using Mann–Whitney tests, Wilcoxon signed‐rank tests, or Fisher's exact test, as appropriate. Receiver operator characteristic (ROC) curves were constructed to derive cut‐offs for differentiation of cardiac from noncardiac causes of pleural effusion in plasma and pleural fluid samples based on results from the US cohort. Sensitivity, specificity, and positive and negative likelihood ratios were calculated. The positive likelihood ratio is the ratio of true positives to false positives and the negative likelihood ratio is the ratio of false negatives to true negatives. A positive likelihood ratio <5 is considered a reasonable diagnostic test for ruling in a condition and a negative likelihood ratio less than 0.2 is considered a reasonable diagnostic test for ruling out a condition.7 By prespecified design, the cut‐off values generated from the US cohort were subsequently tested in the UK cohort. Spearman's rank order correlation coefficients (rho) and Bland–Altman plots were used to compare quantitative ELISA measurements of NT‐proBNP between plasma and pleural fluid samples and the first‐ and second‐generation assays.8
Results
Thirty‐eight cats with pleural effusion were enrolled in the US cohort between February and November 2014. Twenty‐one cats were assigned to the cardiac group and 17 cats were assigned to the noncardiac group. The population characteristics of the US cohort are presented in Table 1. No differences were detected between groups for age, breed, body weight, heart rate, respiratory rate, or diastolic measurements of interventricular septum and left ventricular free wall thickness (IVSd and LVFWd, respectively). Cats in the cardiac group had significantly greater left atrial to aortic root ratios (LA/Ao), and were significantly more likely to be male, to have a murmur or to have a gallop rhythm on auscultation.
Table 1.
Characteristics of the cardiac and noncardiac groups in the US cohort. The median and ranges are shown for continuous variables. NB sex was not recorded for one cat in the cardiac group
| Variable | Noncardiac (n = 17) | Cardiac (n = 21) | P value |
|---|---|---|---|
| Age (years) | 12.0 (0.5–16.0) | 12.0 (1.5–18.0) | 0.86 |
| Pedigree breed (yes/no) | 2/15 | 1/20 | 0.58 |
| Sex (male/female) (n = 37) | 6/11 | 16/4 | 0.008 |
| Weight (kg) | 4.1 (2.1–8.8) | 4.9 (2.7–6.9) | 0.74 |
| Heart rate (beats per minute) | 200 (160–260) | 180 (130–300) | 0.27 |
| Respiratory rate (breaths per minute) | 68 (44–92) | 60 (30–88) | 0.17 |
| Murmur (yes/no) | 5/12 | 15/6 | 0.021 |
| Gallop (yes/no) | 1/16 | 13/8 | 0.001 |
| LA/Ao | 1.3 (1.0–1.9) | 2.6 (1.6–3.2) | <0.001 |
| IVSd (mm) (n = 35) | 5.5 (3.5–7.5) | 6.2 (3.7–10.0) | 0.37 |
| LVFWd (mm) (n = 35) | 5.8 (2.9–11.0) | 7.2 (3.8–18.6) | 0.16 |
Forty cats with pleural effusion were enrolled in the UK cohort between February 2011 and June 2012. The study population and utility of NT‐proBNP concentration using the first‐generation assay have been described previously.4 Twenty‐two cats were assigned to the cardiac group and 18 cats were assigned to the noncardiac group. A cut‐off of 213.3 pmol/L in plasma samples had a sensitivity of 86.4% (95% confidence interval (CI) 66.7–95.3%) and specificity of 88.9% (95% CI 67.2–96.9%), and a cut‐off of 322.3 pmol/L in pleural fluid samples had a sensitivity of 100% (95% CI 85.1–100.0%) and a specificity of 94.4% (74.2–99%) to distinguish cardiac from noncardiac causes of pleural effusion. Samples were stored at −80°C for 30–46 months before analysis.
Results of NT‐proBNP measurements in the US cohort using the second‐generation reference laboratory ELISA and the POC test are presented in Table 2. The result of the POC test using a plasma sample was not available for one cat in the noncardiac group. NT‐proBNP concentrations in plasma and pleural fluid samples were significantly higher in the cardiac group than in the noncardiac group (P < 0.0001). The proportion of positive POC results from both plasma and pleural fluid samples was significantly higher in the cardiac group than in the noncardiac group (P < 0.0001). ROC curve analysis in the US cohort revealed an optimal plasma NT‐proBNP cut‐off of ≥199 pmol/L with a sensitivity of 95.2% (95% CI 77.3–99.2%), specificity of 82.4% (95% CI 59.0–93.8%), positive likelihood ratio of 5.40 (95% CI 1.92–15.14), and negative likelihood ratio of 0.06 (95% CI 0.01–0.40). The area under the ROC curve was 0.952 (95% CI 0.891–1.00). A pleural fluid NT‐proBNP cut‐off of ≥240 pmol/L yielded a sensitivity of 100% (95% CI 84.5–100%), specificity of 76.5% (95% CI 52.7–90.4), and positive likelihood ratio of 4.25 (95% CI 1.80–10.01). The negative likelihood ratio could not be calculated because all 21 cats with pleural effusion of cardiac origin had pleural fluid NT‐proBNP ≥240 pmol/L. The area under the pleural fluid ROC curve was 0.923 (95% CI 0.832–1.00). There was no significant difference between the area under the pleural fluid ROC curve and the area under the plasma ROC curve (P = 0.26).
Table 2.
Results of NT‐proBNP measurements for the cardiac and noncardiac groups using 2 assay methods for the US cohort. The median and interquartile ranges are shown for continuous variables
| Cardiac (n = 21) | Noncardiac (n = 17) | P value | |
|---|---|---|---|
| Second‐generation ELISA (pmol/L) | |||
| Plasma | 1500 (790–1500) | 58 (31–174.5) | <0.0001 |
| Pleural fluid | 1500 (918–1500) | 108 (56–324.5) | <0.0001 |
| Point‐of‐care test (no. positive/no. negative) | |||
| Plasma (n = 37) | 20/1 | 2/14 | <0.0001 |
| Pleural fluid | 21/0 | 6/11 | <0.0001 |
In the US cohort, the POC test using plasma samples differentiated the cardiac and noncardiac groups with a sensitivity of 95.2% (95% CI 77.3–99.2%), specificity of 87.5% (95% CI 64.0–96.5%), positive likelihood ratio of 7.62 (95% CI 2.08–27.95), and negative likelihood ratio of 0.05 (95% CI 0.01–0.37). The area under the ROC curve for the POC assay using plasma samples was 0.914 (95% CI 0.818–1.00). The POC test using pleural fluid samples differentiated the cardiac and noncardiac groups with a sensitivity of 100% (95% CI 84.5–100%), specificity of 64.7% (95% CI 41.3–82.7%), and positive likelihood ratio of 2.83 (95% CI 1.49–5.39) in the US cohort. The negative likelihood ratio could not be calculated because all 21 cats with pleural effusion of cardiac origin had a positive POC assay. The area under the ROC curve for the POC assay using pleural fluid samples was 0.824 (95% CI 0.706–0.941) and was not statistically different from the area under the ROC curve for plasma samples (P = 0.096).
Results of NT‐proBNP measurements in the UK cohort using the second‐generation reference laboratory ELISA and the POC test are presented in Table 3. Insufficient plasma sample volume was available to measure NT‐proBNP using the second‐generation version of the ELISA in 3 cats and using the POC test in 6 cats. When the cut‐off values derived from the US cohort were applied to plasma and pleural fluid measurements of NT‐proBNP using the second‐generation assay from the UK cohort, plasma NT‐proBNP ≥199 pmol/L yielded a sensitivity of 95.0% (95% CI 76.4–99.1%), specificity of 82.4% (95% CI 59.0–93.8%), positive likelihood ratio of 5.40 (95% CI 1.92–15.11), and negative likelihood ratio of 0.06 (95% CI 0.01–0.42). Pleural fluid NT‐proBNP ≥240 pmol/L yielded a sensitivity of 100% (95% CI 85.1–100%), specificity of 66.7% (95% CI 43.8–83.7%), and positive likelihood ratio of 3.00 (95% CI 1.56–5.77). The negative likelihood ratio could not be calculated because all 22 cats with pleural effusion of cardiac origin had pleural fluid NT‐proBNP ≥240 pmol/L. For both plasma and pleural fluid samples, the 95% CI of the sensitivity and specificity of the second‐generation ELISA assay overlapped with reported confidence intervals using the first‐generation ELISA assay.4 In the UK cohort, the POC test using plasma samples differentiated cardiac from noncardiac causes of pleural effusion with a sensitivity of 88.2% (95% CI 65.7–96.7%), specificity of 88.2% (95% CI 65.7–96.7%), positive likelihood ratio of 7.50 (95% CI 2.02–27.89), and negative likelihood ratio of 0.13 (95% CI 0.04–0.50). The POC test using pleural fluid samples differentiated cardiac from noncardiac causes of pleural effusion with a sensitivity of 100% (95% CI 85.1–100%), specificity of 77.8% (95% CI 54.8–91.0%), and positive likelihood ratio of 4.50 (95% CI 1.89–10.68). The negative likelihood ratio could not be calculated because all 22 cats with pleural effusion of cardiac origin had a positive POC assay.
Table 3.
Results of NT‐proBNP measurements for the cardiac and noncardiac groups using 2 assay methods for the UK cohort. The median and interquartile ranges are shown for continuous variables
| Cardiac (n = 22) | Noncardiac (n = 18) | P value | |
|---|---|---|---|
| Second‐generation ELISA (pmol/L) | |||
| Plasma | 849 (590–1500) (n = 20) | 73 (33–138) (n = 17) | <0.0001 |
| Pleural fluid | 1001 (640–1500) (n = 22) | 98 (45–273) (n = 18) | <0.0001 |
| Point‐of‐care test (no. positive/no. negative) | |||
| Plasma (n = 34) | 15/2 | 2/15 | <0.0001 |
| Pleural fluid (n = 40) | 22/0 | 4/14 | <0.0001 |
NT‐proBNP concentrations in paired plasma and pleural fluid samples from the US cohort were measured using the second‐generation ELISA assay, and were significantly correlated (Spearman rho = 0.969; P < 0.0001); however, the limits of agreement between the two sample types were wide, and NT‐proBNP concentrations in pleural fluid were systematically greater than those in plasma samples (pleural fluid, 719 pmol/L [134–1500] versus plasma, 678 pmol/L [61–1500]; P = 0.003) with a mean bias of +67.9 pmol/L (Fig 1). NT‐proBNP concentrations in plasma samples from the UK cohort using the first‐ and second‐generation ELISA assay were significantly correlated (Spearman rho = 0.864; P < 0.0001); however, the limits of agreement between the two assay generations were wide, and NT‐proBNP concentrations in plasma assayed using the second‐generation assay were systematically greater than those in plasma samples assayed using the first‐generation assay (plasma second‐generation, 531 pmol/L (84–874) versus plasma first‐generation 254 pmol/L (50–620); P = 0.002), with a mean bias of +156.1 pmol/L (Fig 2). NT‐proBNP concentrations in pleural fluid samples from the UK cohort using the first‐ and second‐generation ELISA assay were significantly correlated (Spearman rho = 0.966; P < 0.0001). Pleural fluid NT‐proBNP concentrations were not significantly different between the two different generations of NT‐proBNP ELISA assay (pleural second generation, 603 pmol/L (121–1135) versus pleural first generation 542 pmol/L (142–1374); P = 0.95).
Figure 1.
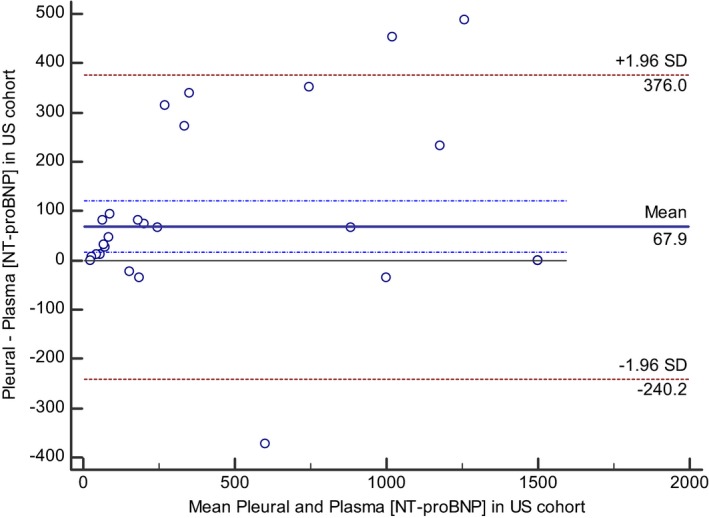
Bland–Altman plot illustrating agreement between measurements of NT‐proBNP concentrations in pleural fluid and plasma samples in cats using the second‐generation assay in samples from the US cohort. The mean bias and the dash‐dot lines representing the 95% confidence intervals for the bias indicate that the NT‐proBNP concentration of pleural fluid samples was on average 67.9 pmol/L greater than NT‐proBNP concentration from paired plasma sample. The 95% limits of agreement between the two sample types are displayed as ±1.96 times the standard deviation of the difference.
Figure 2.
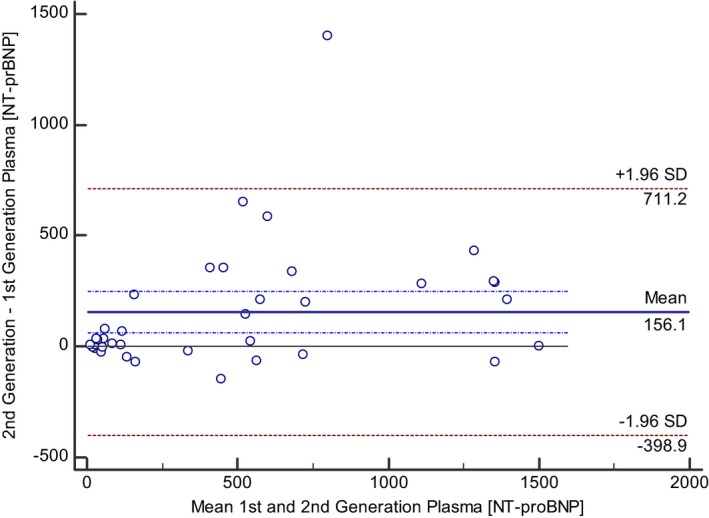
Bland–Altman plot illustrating agreement between measurements of NT‐proBNP concentrations in plasma samples from cats using the first‐ and second‐generation assay in samples from the UK cohort. The mean bias and the dash‐dot lines representing the 95% confidence intervals for the bias indicate that the NT‐proBNP concentration of plasma samples using the second‐generation assay was on average 156.1 pmol/L greater than NT‐proBNP concentration from paired plasma sample using the first generation assay. The 95% limits of agreement between the two sample types are displayed as ±1.96 times the standard deviation of the difference.
Discussion
This study utilized data from two separate cohorts to investigate the diagnostic characteristics of a quantitative ELISA and semiquantitative POC test to differentiate cardiac and noncardiac causes of pleural effusion in cats. Our results demonstrate that measurement of NT‐proBNP in plasma samples using either a quantitative ELISA or POC test differentiates these etiologies of pleural effusion with good diagnostic accuracy. Measurement of NT‐proBNP concentration in pleural fluid samples using the quantitative ELISA also had good diagnostic accuracy as long as a higher cut‐off value is used for pleural fluid samples (≥240 pmol/L) compared to the cut‐off value used for plasma samples (≥199 pmol/L). In the emergency setting, POC testing of either plasma or pleural fluid is attractive because of the immediacy of assay results. Testing of pleural effusion, specifically, is attractive in instances in which cats with significant pleural effusion would undergo therapeutic thoracocentesis but might not be stable enough for venipuncture. However, the results of this study suggest that the diagnostic value of POC testing of pleural effusion samples is limited. Using pleural fluid samples, a negative POC assay result helped to rule out cardiac causes of pleural effusion, but a positive result was associated with a relatively low positive predictive value, and the likelihood ratio associated with a positive pleural fluid POC test from either the US or UK cohort (i.e., 2.83 and 4.5) was lower than the level (i.e. 5.0) generally considered to indicate a useful diagnostic test.7 Thus, a positive POC result using pleural fluid samples frequently represents a false positive result, and this limits its diagnostic value. The discrepancy in performance between testing plasma and pleural fluid samples using the POC test probably reflects the NT‐proBNP concentration at which the POC device transitions from a negative to positive result. A previous study has shown this to occur at a NT‐proBNP concentration between 150 and 200 pmol/L,6 which approximates the cut‐off value in plasma (199 pmol/L) but is lower than the optimal cut‐off for pleural fluid samples (240 pmol/L) found in this study.
Likelihood ratios can be used to estimate post‐test probability of pleural effusion of cardiac cause. In this study, plasma POC test results were associated with approximate positive and negative likelihood ratios of 7.50 and 0.10, respectively. These values roughly translate into an absolute increase or decrease in the post‐test probability for a cardiac cause of pleural effusion of 40% compared to the pretest probability.9 For example, in a population of cats whose pretest probability of pleural effusion because of a cardiac cause is 60%, a positive plasma POC test would increase this probability to nearly 100% while a negative plasma POC test result would reduce the probability to 20%. Likewise, in a population of cats where a cardiac cause of pleural effusion is 10%, and hence unlikely, a positive POC test increases this probability to only 50%. It is therefore important, as with any clinical test, that measurements of NT‐proBNP be interpreted in combination with historical and physical examination findings and the results of other diagnostic tests such as imaging and not be relied upon in isolation for clinical decision‐making.
The optimal cut‐offs determined in this study are somewhat lower than those previously reported. Cut‐offs for differentiation of cardiac from noncardiac causes of dyspnea using plasma samples have been reported as 220 pmol/L,10 265 pmol/L,11 214 pmol/L4 and 258 pmol/L.3 The former two studies did not restrict inclusion to cats with pleural effusion, whereas the latter two did; all of these studies used the first‐generation quantitative ELISA. A cut‐off for pleural fluid samples of 322 pmol/L has been reported to distinguish cardiac and noncardiac causes of dyspnea using the first‐generation ELISA.4 Given that plasma measurements of NT‐proBNP were found to be higher using the second‐ compared with the first‐generation ELISA and that no difference was found in pleural fluid measurements between assay generations, it seems surprising that the optimal cut‐offs were lower in this study than those previously reported. However, this probably reflects the relatively small sample sizes in each study, population differences (United states versus United Kingdom versus Germany) and differences in the methods used to select the optimal cut‐off values.
This study revealed that NT‐proBNP concentrations were significantly higher in pleural fluid samples than in plasma samples when measured using the quantitative second‐generation ELISA, resulting in the need for two different cut‐off values depending on sample type. These findings agree with a previous study that compared pleural fluid and plasma samples in cats using the first‐generation assay,4 but are in contrast to human patients, in whom pleural fluid and plasma NT‐proBNP concentrations are reported to be almost identical.12 The potential reasons for this discrepancy between species are not immediately clear. In this study, plasma NT‐proBNP concentrations were significantly higher using the second‐generation versus the first‐generation assay, whereas in contrast NT‐proBNP concentrations in pleural fluid samples were not significantly different between assay generations. One possible explanation is that the rate of postsample degradation is lower in pleural fluid than in plasma samples, perhaps because of lower concentrations of protease enzymes. Further studies are necessary to investigate this possibility.
This study has a number of important limitations. While attempts were made to collect plasma and pleural fluid samples at the same time, feline instability might have caused pleural fluid and blood samples to be obtained hours apart from each other. Cats were assigned to the cardiac or noncardiac groups based on the opinion of the blinded cardiologist, and misclassification of cases could have occurred. This study involved use of different sample collection, handling and storage protocols; however, samples collected for ELISA from the US cohort were handled according to current manufacturer recommendations and results between the US and UK cohorts were comparable. The pleural fluid POC tests from both the US and UK cohorts were performed on batched samples which had been stored at −80°C, and further studies that utilize the POC test in pleural fluid samples in a truly pet‐side manner, including comparison of assay performance when used immediately, compared with on previously frozen samples, should be performed. Pleural fluid sample POC testing was not performed immediately in this study because the POC device had not been validated for use with pleural fluid samples at the time of sample collection. One disadvantage of using the POC device is that the result is either positive or negative; this is in contrast to the quantitative ELISA, which allows for interpretation of the results throughout a range of possible values. The POC device is not specifically designed to differentiate causes of pleural effusion in cats, but rather to detect occult cardiomyopathy in cats without obvious respiratory signs, and the device's cut‐off value between a negative and positive result is relatively low for differentiation of cardiac versus noncardiac etiologies of pleural effusion. Nevertheless, the POC assay when used with plasma was able to differentiate cause of pleural effusion with good diagnostic accuracy.
In conclusion, the results of this study suggest that quantitative measurements of NT‐proBNP in plasma and pleural fluid samples using a second‐generation assay allow differentiation of cardiac from noncardiac causes of pleural effusion with good diagnostic accuracy. Cut‐off values specific for sample type should be used. Measurement of NT‐proBNP in plasma samples using a POC test also allows differentiation of cardiac from noncardiac causes of pleural effusion with good diagnostic accuracy; however, although sensitivity is excellent and a negative POC result in pleural fluid samples helps rule out a cardiac cause of pleural effusion, the specificity of the POC test is low, resulting in a low positive predictive value. Measurements of NT‐proBNP in plasma fluid samples obtained using the second‐generation assay are systematically greater than those obtained using the first‐generation assay, and cut‐offs specific to the second‐generation assay should be used.
Acknowledgments
We acknowledge Kristen Antoon and Drs. Suzanne Cunningham, Vicky Yang, and Kursten Roderick.
Conflict of Interest Declaration:Dr. Hezzell's residency is partly funded by IDEXX Laboratories.
Off‐label Antimicrobial Declaration:Authors declare no off‐label use of antimicrobials.
The work was performed at the University of Pennsylvania, Tufts University and the Royal Veterinary College, University of London.
The study was supported by a PetSavers grant and by IDEXX Laboratories, Inc.
An abstract based on some of these data was presented at the 2015 ACVIM Forum, Indianapolis, Indiana.
Footnotes
Cardiopet® Feline proBNP, IDEXX Laboratories Inc., Westbrook, ME
SNAP® Feline proBNP, IDEXX Laboratories Inc., Westbrook, ME
Cardiopet® proBNP specimen tubes, IDEXX Laboratories Inc., Westbrook, ME
Mainville et al. Analytical validation of an immunoassay for the quantification of N‐terminal pro‐B‐type natriuretic peptide in feline blood. Journal of Veterinary Diagnostic Investigation (In Press).
SPSS 22; IBM, Armonk NY; MedCalc 15.2.2; MedCalc Software, Ostend, Belguim
References
- 1. Rozanski E, Chan DL. Approach to the patient with respiratory distress. Vet Clin North Am Small Anim Pract 2005;35:307–317. [DOI] [PubMed] [Google Scholar]
- 2. Dempsey SM, Ewing PJ. A review of the pathophysiology, classification, and analysis of canine and feline cavitary effusions. J Am Anim Hosp Assoc 2011;47:1–11. [DOI] [PubMed] [Google Scholar]
- 3. Hassdenteufel E, Henrich E, Hildebrandt N, et al. Assessment of circulating N‐terminal pro B‐type natriuretic peptide concentration to differentiate between cardiac from noncardiac causes of pleural effusion in cats. J Vet Emerg Crit Care (San Antonio) 2013;23:416–422. [DOI] [PubMed] [Google Scholar]
- 4. Humm K, Hezzell M, Sargent J, et al. Differentiating between feline pleural effusions of cardiac and non‐cardiac origin using pleural fluid NT‐proBNP concentrations. J Small Animal Practice 2013;54:656–661. [DOI] [PubMed] [Google Scholar]
- 5. Janda S, Swiston J. Diagnostic accuracy of pleural fluid NT‐pro‐BNP for pleural effusions of cardiac origin: a systematic review and meta‐analysis. BMC Pulm Med 2010;10:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Machen MC, Oyama MA, Gordon SG, et al. Multi‐centered investigation of a point‐of‐care NT‐proBNP ELISA assay to detect moderate to severe occult (pre‐clinical) feline heart disease in cats referred for cardiac evaluation. J Vet Cardiol 2014;16:245–255. [DOI] [PubMed] [Google Scholar]
- 7. Jaeschke R, Guyatt GH, Sackett DL. Users' guides to the medical literature. III. How to use an article about a diagnostic test. B. What are the results and will they help me in caring for my patients? The Evidence‐Based Medicine Working Group. JAMA 1994;271:703–707. [DOI] [PubMed] [Google Scholar]
- 8. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–310. [PubMed] [Google Scholar]
- 9. McGee S. Simplifying likelihood ratios. J Gen Intern Med 2002;17:646–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Connolly DJ, Soares Magalhaes RJ, Fuentes VL, et al. Assessment of the diagnostic accuracy of circulating natriuretic peptide concentrations to distinguish between cats with cardiac and non‐cardiac causes of respiratory distress. J Vet Cardiol 2009;11(Suppl 1):S41–S50. [DOI] [PubMed] [Google Scholar]
- 11. Fox PR, Oyama MA, Reynolds C, et al. Utility of plasma N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) to distinguish between congestive heart failure and non‐cardiac causes of acute dyspnea in cats. J Vet Cardiol 2009;11(Suppl 1):S51–S61. [DOI] [PubMed] [Google Scholar]
- 12. Kolditz M, Halank M, Schiemanck CS, et al. High diagnostic accuracy of NT‐proBNP for cardiac origin of pleural effusions. Eur Respir J 2006;28:144–150. [DOI] [PubMed] [Google Scholar]


