Abstract
Background
Obese dogs risk poor life quality, creating a need for increased knowledge of metabolism in overweight dogs.
Objectives
Investigate postprandial metabolic and hormonal responses to a high‐fat mixed‐meal in dogs and responses of lean versus overweight dogs.
Animals
Twenty‐eight healthy intact male Labrador Retrievers were included.
Methods
Prospective observational study. Twelve dogs were grouped as lean (body condition score (BCS 4–5), 10 as slightly overweight (BCS 6), and 6 as overweight (BCS 6.5–8) on a 9‐point scale. After an overnight fast, urine and blood samples were collected. Dogs were then fed a high‐fat mixed‐meal, and blood was collected hourly for 4 hours and urine after 3 hours.
Results
Postprandial concentrations of insulin and glucagon were increased at 1 hour (both P < 0.0001), triglycerides at 2 hours (P < 0.0001), and glucose at 3 hours (P = 0.004); and all remained increased throughout the feed‐challenge in all dogs. Postprandial urine cortisol/creatinine ratio was higher than fasting values (P = 0.001). Comparing between groups, there was an overall higher triglyceride response in overweight compared to lean (P = 0.001) and slightly overweight (P = 0.015) dogs. Overweight dogs also had higher fasting cortisol/creatinine ratio compared to lean dogs (P = 0.024).
Conclusions and Clinical Importance
Postprandial responses of dogs to a high‐fat mixed‐meal were similar to those previously reported in people. The higher postprandial triglyceride response and fasting cortisol/creatinine ratio in the overweight dogs could be early signs of metabolic imbalance. Thus, although overweight dogs often appear healthy, metabolic alterations might be present.
Keywords: Canine, Glucagon, Obesity, Triglycerides, Urine cortisol
Abbreviations
- BCS
body condition score
- BW
body weight
- CV
coefficient of variation
- DER
daily energy requirements
- DEXA
dual‐energy X‐ray absorptiometry
- FFA
free fatty acids
- HOMAIS
basal insulin sensitivity homeostasis model assessment
- ME
metabolizable energy
- SD
standard deviation
Obese dogs have decreased quality of life,1 increased risk of developing chronic diseases at a young age, and shortened life‐span.2 Dogs might become overweight for many reasons, and obesity constitutes a complex health problem that cannot easily be solved only by eating less and exercising more.3, 4 Increasing obesity in the dog population5, 6 is related to metabolic dysfunctions and hormonal imbalances.7, 8, 9
Meal composition influences postprandial increases in glucose and insulin concentrations in lean dogs, with time to peak glucose concentration delayed and peak insulin concentration lowered by the food with the highest fat content.10 In contrast to lean dogs, obese dogs react differently to a standardized dog meal with higher postprandial serum concentrations of glucose, insulin, and triglycerides.11
The role of cortisol in human obesity has been extensively studied.12 Potentially, chronic elevations of cortisol in the body could be connected to fat deposition.13, 14 Accumulation of abdominal body fat is associated with increased serum as well as urinary cortisol concentrations in people,15 but basal plasma cortisol concentration of obese dogs was not increased.16 To assess the role of cortisol in obesity, measurement of urinary free cortisol during night‐time has been suggested as well as challenging the system by different meals.13 In this study, urine cortisol/creatinine ratio was therefore measured in morning urine taken at home as well as after the feed‐challenge.
The aim was to investigate postprandial metabolic and hormonal responses to a high‐fat mixed‐meal in healthy dogs and to study if responses differed between lean and overweight groups.
Materials and Methods
Animals
This prospective observational study was performed at the Swedish University of Agricultural Sciences, Uppsala, Sweden. The study population consisted of privately owned intact male show‐type Labrador Retrievers and to be included each dog had to be considered healthy by its owner and pass the health examination outlined below. Exclusion criteria consisted of historic or present systemic or organ‐related diseases and treatment with antibiotics, nonsteroid anti‐inflammatory drugs, steroids, deworming drugs, and proton pump inhibitors within 3 months of the examination day. Dogs were recruited by personal letter to owners of male Labrador Retrievers, mostly located within 100 km of Uppsala. Recruitment and data collection was performed during 1 year and all dogs were sampled once at the same time of day according to a predesigned protocol. Study invitation letters were sent to owners of 715 potentially eligible dogs. Sixty owners replied and their dogs were examined for eligibility by an online survey of the dogs' health status and feeding and exercise routines. Thirty‐two dogs were not invited to data collection after the survey due to the exclusion criteria. The remaining 28 dogs were invited to the study, were all confirmed eligible, included in the study, and underwent data collection in the feed‐challenge test.
General Study Design
The study was approved by the Ethical Committee for Animal Experiments, Uppsala, Sweden (C180/12), and owner consent in writing was obtained. Dietary history was acquired for each dog and is presented in the Supporting Information. No adjustments were made in the dogs' regular home diets of dry or wet complete dog foods and treats before participation in the study. After an overnight fast of 14–17 hours, a morning spot urine sample was taken from each dog before they left home on the examination day. On arrival at the clinic, dogs were physically examined and fasting blood samples were taken, followed by intake of a test‐meal. Postprandially, blood was collected hourly for 4 hours and urine was collected after 3 hours. The study followed guidelines for reporting observational studies in epidemiology.17
Assessment of Health Status
The physical examination included general condition, skin condition, rectal temperature, mucus membranes, lymph nodes, heart‐ and lung auscultation, abdominal palpation, and gait. The dogs were weighed and photographed. Routine analyses of hematology1 and serum biochemistry2 of liver‐ and kidney function, fructosamine, thyroid, and thyroid stimulating hormone, total protein, albumin, C‐reactive protein, and electrolytes were performed. Urine was analyzed with standard dipstick chemistry test3 and refractometer for urine specific gravity.4
Body Condition and Grouping
Dogs were assigned a body condition score (BCS) according to the 1‐ to 9‐point scale18 by the same veterinarian (JS). Dogs with BCS 4–5 were considered lean, dogs with BCS 6 slightly overweight, and dogs with BCS 6.5–8 overweight. Dogs were classified as slightly overweight (BCS 6) when no palpable fat deposits were present. Dogs with palpable fat deposits were classified as overweight. A BCS of 6.5 was specified as a dog fulfilling all criteria of BCS 7 but with less defined fat deposits.
Urine and Blood Sample Collections
Urine was collected by the dog owners with a free catch sampling device.a Before the examination day, dogs had experienced the urine sampling procedure at least 3 times. On examination day, spot morning urine was collected at home and kept cold on ice during transport. At the clinic, urine samples were transferred to polypropylene tubes5 and immediately frozen at −70°C. Three hours after the test meal, postprandial urine was collected and frozen as above.
After the physical examination, a catheterc was placed into the distal cephalic vein and blood samples were collected 15 minutes before (fasting), and then hourly for 4 hours after the test‐meal (postprandial period). The catheter was flushed with 2 mL physiologic saline solution after each collection. Serum tubes6 were left to clot at room temperature for 30 minutes and then centrifuged at 1500 × g for 10 minutes. Serum was transferred into polypropylene tubesb and immediately frozen at −70°C. The catheter was removed after the final blood sampling.
Feed‐challenge Test
All dogs were exposed to the same feed‐challenge test. Dogs were given half their daily energy requirement (DER) of a high‐fat mixed‐meal. The DER was based on actual body weight (BW) in lean dogs and on the calculated ideal body weight11, 18 in slightly overweight and overweight dogs. The DER formula used (137 kcal*BWkg 0.75) has previously been developed for adult intact Labrador Retrievers.19 The test‐meal was weighed and served with water added to the meal (same amount in grams as the individual test meals). The test‐feed7 provided 4230 kcal/kg with 51% of the metabolizable energy (ME) as fat, 26% as carbohydrate, and 23% as protein, according to the manufacturer. Nutrient composition and energy content of the test‐feed was confirmed by an independent authorized laboratory.8 The postprandial period started at the first bite and all 28 dogs voluntarily consumed all food and water within 10 minutes of being served. The dogs had nothing further to eat or drink and were kept indoors until completion of postprandial sampling.
Analysis of Metabolic and Hormonal Response
Assays
The ELISA assays were performed according to manufacturers' instructions by trained personnel blinded to dog identity. Leptin serum concentration was analyzed in fasting samples, whereas all other serum and urine variables were analyzed in fasting and postprandial samples. Commercially available canine ELISA assays were used to analyze serum leptin9 and insulin10 concentrations, with mean intra‐assay CVs of 5.4% and 5.1%, respectively. Analysis of urine cortisol11 (in‐house validation available in Supporting Information) and creatinine12 concentrations gave mean intra‐assay CVs of 2.8% and 2.2%, respectively. All samples were analyzed in duplicate, and the mean of the two results used for data analyses. Serum glucose, triglycerides, free fatty acids,13 and total cholesterol were analyzed by routine automatic methodsa at the Clinical Pathology Laboratory, University Animal Hospital, Swedish University of Agricultural Sciences, Uppsala, Sweden.
Validation of a Glucagon ELISA for Canine Serum
A human glucagon ELISA (25 μL)14 was validated for canine serum at the Mercodia laboratory, Uppsala, Sweden. The process included evaluation of precision of 4 replicates in 7–8 runs, and of recovery in spiked and diluted samples (5 and 7 samples, respectively).
The precision study (at 5.41–9.42 pmol/L) gave a mean inter‐assay CV of 8.6% (range 8.2–8.9%) and a mean intra‐assay CV of 3.9% (range 2.5–4.8%). Mean recovery upon dilution was 87% (range 74–98%) and in spiked samples 107% (range 93–126%). The serum samples in the feed‐challenge test were analyzed in duplicate for glucagon concentration with mean intra‐assay CV of 3.5% and mean inter‐assay CV for the low and high control of 10% and 4.3% respectively.
Statistical Analyses
Commercially available software15 was used for statistical analyses and P < 0.05 was considered significant. Results were expressed as mean ± standard deviation (SD). Serum responses to the feed‐challenge were evaluated by the Mixed Procedure for Repeated Measures20 in SAS.21, 22 In the statistical model, body condition group was defined as an independent variable and the fasting value was included as a time point. The model analyzes the response over time (from fasting to four hours after feeding) in all dogs, as well as differences between groups (lean, slightly overweight and overweight). Thus, the model is capable of overall as well as pair‐wise comparisons, but pair‐wise comparisons were only interpreted when the overall effect was significant. The model corrects for multiple comparisons by Tukey–Kramer adjustment. The correction factor for this adjustment depends on the data set as a whole and cannot be separately stated.22 Logarithmic transformation of raw data was performed to correct non‐normality for insulin and glucagon concentrations.
Pearson correlation (r) was used to evaluate the relation between fasting leptin concentration and body condition score. One‐way analysis of variance and Kruskal–Wallis tests were used for normally and non‐normally distributed comparisons between the three groups for leptin and cortisol. Paired t‐tests and Wilcoxon signed‐rank tests were used for normally and non‐normally distributed paired comparisons of urine cortisol between time points. Correction for multiple comparisons was made with Bonferroni (correction factor 3).
Basal insulin sensitivity was estimated using the homeostasis model assessment (HOMAIS) 23 for fasting glucose (mmol/L) and insulin (μU/mL) concentrations. The calculations were made using the non‐linear HOMA Calculator.16 Fasting serum insulin concentrations <2.9 μU/mL were entered as 2.9 (the minimum concentration accepted in the calculation). Kruskal–Wallis tests were used for the non‐normally distributed comparisons between the body condition groups.
Results
Health Status
Twenty‐eight Labrador Retrievers (mean ± SD age 5.2 ± 1.5 years) were included. No clinically relevant abnormalities were detected by hematology, serum biochemistry, or urine analysis. Vital variables at physical examination were within reference ranges, but small health problems were found, e.g. slightly stiff gait and mild lameness, signs of periodontitis, palpable periarticular osteophyte formation, and skin furunculosis. None of the dogs exhibiting the small health problems were excluded as vital variables were normal. For all collected data of included dogs, there were only two missing values (in cortisol concentration).
Classification and Grouping
Twelve dogs were classified and grouped as lean, 10 as slightly overweight, and 6 as overweight according to BCS (Table 1). Fasting serum leptin concentrations were 3.4 ± 1.9 in lean, 3.7 ± 2.1 in slightly overweight, and 9.0 ± 2.7 ng/mL in overweight dogs (both lean and slightly overweight dogs, P < 0.05 versus overweight dogs). A strong positive correlation between leptin concentration and BCS was confirmed (Pearson's r = 0.69, P < 0.0001). No significant differences were found in dietary history between lean, slightly overweight, and overweight dogs (data presented in Supporting Information).
Table 1.
Descriptive statistics of the 28 male show‐type Labrador Retriever dogs included in the study and the amount of test food given in the feed‐challenge
Metabolic and Hormonal Responses, All Dogs
Metabolic and hormonal serum responses to the high‐fat mixed‐meal of all dogs are summarized in Table 2. In all dogs, there was an overall significant difference between time points in triglyceride, free fatty acid, glucose, insulin, and glucagon concentrations (P < 0.0001 for all) but not in cholesterol concentration. Postprandial concentrations of insulin and glucagon were increased at 1 hour (both P < 0.0001), triglycerides at 2 hours (P < 0.0001), and glucose at 3 hours (P = 0.004); and all remained increased throughout the feed‐challenge. Free fatty acids were decreased 1 hour postprandially (P < 0.0001) and then remained unchanged. Fasting urine cortisol/creatinine ratio in all dogs was 9.1 ± 2.8 and increased to 11.8 ± 4.6 in postprandial urine (P = 0.001).
Table 2.
Fasting and postprandial concentrations of metabolic and hormonal serum variables in 28 Labrador Retriever dogs subjected to a feed‐–challenge test with a high‐fat mixed‐meal. Fasting blood samples were taken 15 minutes before the meal and postprandially samples were taken hourly for 4 hours.c
| Fasting | Postprandial | SEc | |||
|---|---|---|---|---|---|
| Mean ± SDa | Hours | Mean ± SDa | P‐valueb | ||
| Triglycerides (mmol/L)[mg/dL] | 0.53 ± 0.17 [46.9 ± 15.1] | 1 | 0.64 ± 0.23 [56.7 ± 20.4] | 0.31 | 0.054 |
| 2 | 1.13 ± 0.38 [100 ± 33.7] | <0.001 | 0.070 | ||
| 3 | 1.36 ± 0.58 [121 ± 51.4] | <0.001 | 0.079 | ||
| 4 | 1.18 ± 0.51 [105 ± 45.2] | <0.001 | 0.085 | ||
| Free fatty acids (mmol/L) | 0.90 ± 0.23 | 1 | 0.39 ± 0.13 | <0.001 | 0.027 |
| 2 | 0.37 ± 0.10 | <0.001 | 0.035 | ||
| 3 | 0.39 ± 0.09 | <0.001 | 0.039 | ||
| 4 | 0.44 ± 0.11 | <0.001 | 0.041 | ||
| Total cholesterol (mmol/L) | 6.05 ± 0.94 | 1 | 6.11 ± 0.96 | 0.90 | 0.075 |
| 2 | 6.18 ± 0.89 | 0.74 | 0.104 | ||
| 3 | 6.06 ± 1.12 | 1.0 | 0.126 | ||
| 4 | 6.11 ± 1.04 | 0.98 | 0.143 | ||
| Glucose (mmol/L) [mg/dL] | 5.21 ± 0.38 [93.8 ± 6.9] | 1 | 5.36 ± 0.32 [96.5 ± 5.8] | 0.31 | 0.076 |
| 2 | 5.44 ± 0.37 [97.9 ± 6.7] | 0.13 | 0.096 | ||
| 3 | 5.60 ± 0.47 [101 ± 8.5] | 0.004 | 0.107 | ||
| 4 | 5.91 ± 0.51 [106 ± 9.2] | <0.001 | 0.113 | ||
| Insulin (ng/L) | 87.4 ± 40.0 | 1 | 170 ± 65.7 | <0.001 | 0.036 |
| 2 | 200 ± 82.2 | <0.001 | 0.044 | ||
| 3 | 269 ± 109 | <0.001 | 0.047 | ||
| 4 | 321 ± 119 | <0.001 | 0.049 | ||
| Glucagon (pmol/L) | 5.60 ± 3.06 | 1 | 10.6 ± 5.08 | <0.001 | 0.029 |
| 2 | 11.4 ± 4.66 | <0.001 | 0.038 | ||
| 3 | 9.83 ± 3.59 | <0.001 | 0.043 | ||
| 4 | 9.14 ± 5.03 | <0.001 | 0.046 | ||
Calculations were performed by a mixed procedure model with significance level P < 0.05.
Results shown as means ± standard deviations (SD).
P‐values for comparisons between fasting and postprandial time points.
Standard Errors (SE) for comparisons between fasting and postprandial time points.
Metabolic Responses, Comparisons Between Body Condition Groups
The overall triglyceride response (from fasting to 4 hours postprandially) differed significantly between overweight and lean dogs (P = 0.001), and between overweight and slightly overweight dogs (P = 0.015), whereas slightly overweight and lean dogs did not differ. Pair‐wise comparisons between groups at different time‐points demonstrated that postprandial triglyceride concentrations were almost two‐fold higher in overweight compared with lean dogs at 3 and 4 hours (P < 0.0001 and P = 0.0005, respectively), and 1.6‐fold higher in overweight compared with slightly overweight dogs at 4 hours (P = 0.018). Triglyceride concentrations at 3 hours were: lean 1.07 ± 0.36, slightly overweight 1.35 ± 0.45, and overweight dogs 1.95 ± 0.74 mmol/L, which corresponded to 95 ± 32, 120 ± 40, and 173 ± 66 mg/dL, respectively, and at 4 hours; lean 0.96 ± 0.28, slightly overweight 1.10 ± 0.42, and overweight dogs 1.77 ± 0.61 mmol/L, which corresponded to 85 ± 25, 97 ± 37, and 157 ± 54 mg/dL, respectively. There were no significant differences between groups in fasting triglyceride concentrations; lean 0.44 ± 0.10, slightly overweight 0.52 ± 0.11, and overweight dogs 0.72 ± 0.21 mmol/L, which corresponded to 39 ± 9, 46 ± 10, and 64 ± 19 mg/dL, respectively. Similarly, there were no significant group differences in postprandial triglyceride concentrations at 1 and 2 hours postprandially (Fig 1A).
Figure 1.
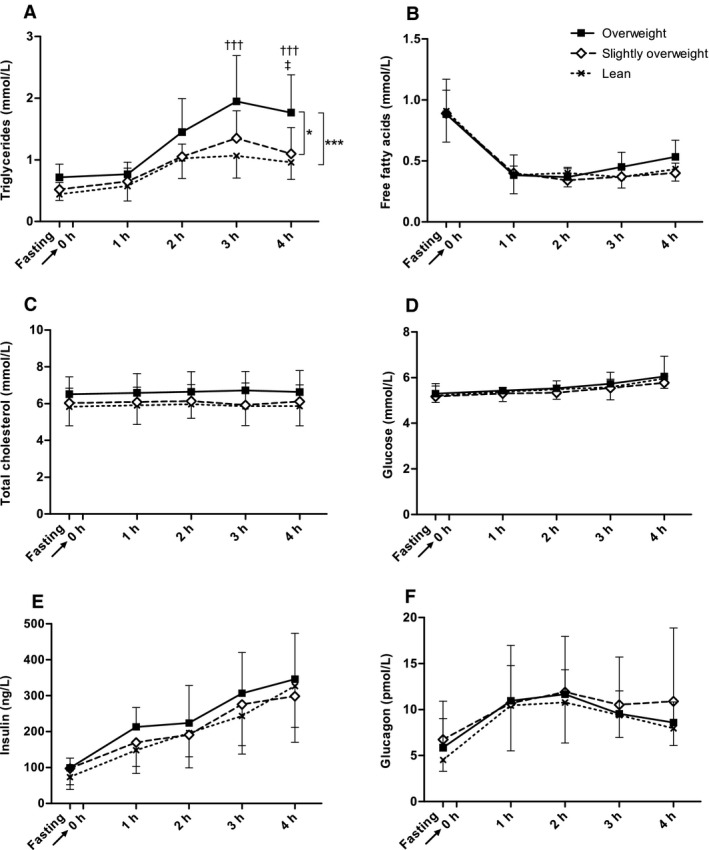
Serum responses to the feed‐challenge test. Triglycerides (A), free fatty acids (B), total cholesterol (C), glucose (D), insulin (E), and glucagon (F) are shown as response curves from fasting to 4 hours after feeding. Fasting blood sample was taken 15 minutes before serving of a test‐meal at time 0 (arrow). Dogs were divided into body condition groups; lean (BCS 4–5, n = 12), slightly overweight (BCS 6, n = 10) and overweight (BCS 6.5–8, n = 6). Values are given as mean ± SD, and were logarithmically transformed for insulin and glucagon before statistical analyses. Calculations were performed by a mixed procedure model with significance level P < 0.05. Significant differences in overall responses between body condition groups are indicated by asterisks (*P < 0.05, ***P < 0.001). Differences in triglyceride concentrations between overweight and lean dogs (†††P < 0.001) and between overweight and slightly overweight dogs (‡P < 0.05) at specific time points are indicated. Note the gradual postprandial increase in glucose, insulin, and triglyceride concentrations.
Comparing between groups, there were no overall significant differences in free fatty acid, cholesterol, and glucose responses (Fig 1B–D).
Hormonal Responses, Comparisons Between Body Condition Groups
Comparing between body condition groups, there were no significant differences in insulin and glucagon responses (Fig 1E–F). Similarly, there were no differences between groups in basal insulin sensitivity assessed by HOMAIS; lean dogs 261 ± 3.6%, slightly overweight dogs 240 ± 48%, and overweight dogs 251 ± 24%. Fasting mean urine cortisol/creatinine ratio was significantly higher in overweight compared with lean dogs (P = 0.024), whereas no significant differences between groups were seen at 3 hours postprandially. In lean dogs, there was a significant increase in cortisol/creatinine ratio between fasting and the 3 hour postprandial sample (P = 0.007), whereas no significant differences were found in slightly overweight and overweight dogs (Fig 2).
Figure 2.
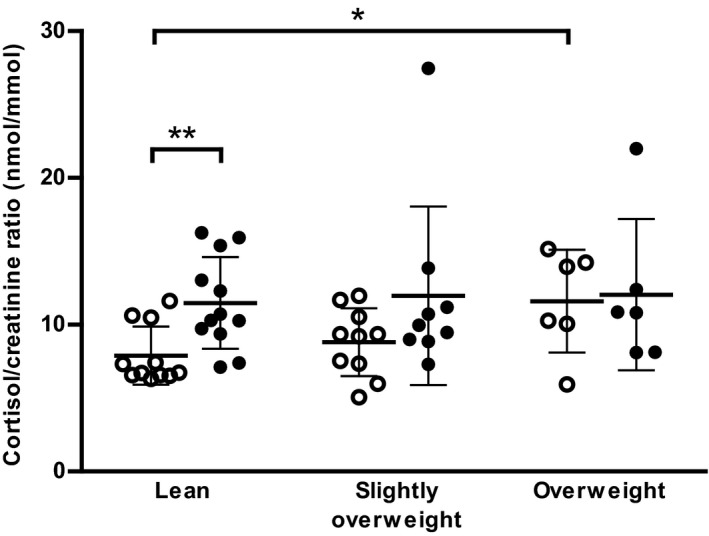
Urine cortisol/creatinine ratios in fasting and postprandial samples. Dogs were divided into body condition groups; lean (BCS 4–5, n = 12), slightly overweight (BCS 6, n = 10), and overweight (BCS 6.5–8, n = 6). Values are given as scatter plots with mean ± SD. Significant differences between groups or time points are indicated by asterisks (*P < 0.05, **P < 0.01). ○ fasting, ● postprandial
Discussion
In the fasted state, serum metabolic and hormonal values did not differ between lean, slightly overweight, and overweight healthy dogs, but after the high‐fat meal overweight dogs had higher overall triglyceride response than both lean and slightly overweight dogs, which could be an early sign of metabolic imbalance. Both obesity and overweight are increasing in the dog population.5, 6 Although overweight dogs often appear healthy, the results of this feed‐challenge test show that metabolic alterations might be present.
Dog breeds vary considerably in body composition and studies reveal important differences in breed morphology;24 so, only healthy intact male Labrador Retrievers of show‐type and of approximately the same age were studied. This breed was chosen since there might be a genetic basis of obesity in Labrador Retrievers.25
Postprandial hypertriglyceridemia was considered normal in dogs,26 but recent attempts have been made to define reference ranges for triglyceride concentrations.17 In this study, triglyceride concentrations increased after 2 hours and remained increased 4 hours after the meal. Concentrations in lean and slightly overweight dogs were in the range of results in healthy lean dogs of mixed breeds.27 The overweight dogs had the highest peak triglyceride concentration, but their values were approximately half of that reported in an earlier study of obese dogs.11 The results give some support to the existence of a close relationship between triglyceride concentrations and body fat, which is reported both in people28 and dogs.29
The magnitude and time of the peak glucose concentration depend on a variety of factors, including the timing, quantity, and composition of the meal. Dogs on a traditional diet (55% carbohydrate and 23% fat of ME, respectively) had a higher postprandial glucose peak after 1 hour compared with the same dogs fed a low carbohydrate (25%) – moderate fat (32%) diet,27 whereas a meal consisting of 40% fat and 37% carbohydrates of ME, respectively, resulted in peak glucose concentrations after 2 to 3 hours.11 In this study, postprandial glucose concentration was not significantly higher than fasting values until 3 hours after the meal. The high amount of fat in proportion to carbohydrates in the test meal (51% and 26%, of ME, respectively) could explain the slow glucose response in our study in support of earlier observations.30
Adding fat to a carbohydrate‐rich meal lowers the postprandial glucose response in people, without reducing the insulin response.30 In this study, postprandial insulin was significantly increased after 1 hour and continued to increase throughout the study period. Similarly, healthy men ingesting a fat‐enriched meal show increased postprandial concentrations of insulin and triglycerides, without significant changes in glucose.31 In this study, no dog displayed hyperinsulinemia or hyperglycemia in the fasting state and no differences were found in postprandial insulin response between groups. All groups had HOMAIS percent similar to values earlier measured in lean dogs.32 The HOMA method for estimation of basal insulin sensitivity was originally developed for use in people. It is validated for use in dogs but is recommended only for group comparisons in this species.32 The dogs in this study had naturally acquired overweight, but none of them was markedly obese, which could explain why no differences between groups could be detected by the HOMAIS.
Validation of the canine glucagon assay showed good results, with inter‐ and intra‐assay CVs ≤10%. The sandwich‐ELISA uses N‐terminal for capture and C‐terminal for detection, making cross reactivity to pro‐glucagon derived peptides, a common problem with previous assays, less likely.33, 34 , 18 In response to the high‐fat mixed‐meal, glucagon concentration increased significantly from 1 hour postprandially, and the overall response was similar to that observed in people after a mixed‐meal.35, 36 In people, the rapid increase in glucagon concentration after ingestion of pure fat diminishes when carbohydrates are added to the food,37 whereas ingestion of pure glucose leads to decreasing glucagon concentrations.36 Adding large amounts of fat to a diet increases postprandial glucagon concentrations in people, but can be balanced by carbohydrates.37, 38 The high‐fat content in the test meal could therefore be the cause of the rise in glucagon concentration in the present dogs.
In this study, morning urine cortisol/creatinine ratio was higher in overweight than in lean dogs. Similarly, night‐time urine cortisol was higher in obese human patients than in normal weight controls.13 Although a hypothetical role of glucocorticoids in human obesity has been suggested,13 the role is debated with inconclusive results regarding the assumed positive relationship between cortisol secretion and body fat content.39 Increased sensitivity along the hypothalamic‐pituitary‐adrenal axis as well as peripheral alterations of cortisol metabolism could play a role in the pathophysiology of abdominal obesity.13 A chronically higher cortisol level in the body could potentially increase insulin resistance, and hyperinsulinemia and overproduction of glucocorticoids might result in fat deposition.13, 14 In this study, the urine‐cortisol ratio was at the same level before and after the meal in overweight dogs, whereas that increased level was reached first after the meal in lean dogs. All dogs followed the same protocol and were sampled at the same time of day by the same persons.40 Apart from metabolic functions, cortisol is one of the hormones involved in stress. Excitement caused by the clinical environment and the meal cannot be excluded. We have previously found higher concentrations of stress hormones in a clinical situation compared to morning samples taken in home environment.41 Furthermore, Labrador Retriever is a breed known to enjoy food. The potential interrelationship between cortisol secretion, metabolism, and stress is interesting and warrants further investigation in overweight and obese dogs.
Free fatty acids decreased significantly 1 hour after the meal and no difference in response was seen between body condition groups. Free fatty acids can inhibit insulin signaling and, in people, incraesed FFA are associated with obesity and insulin resistance.42 In an experimental set up, plasma FFA were higher in diabetic dogs than in control dogs.43 The lack of difference between groups in this study is in accordance with the normal insulin and glucose concentrations measured, and the normal insulin sensitivity according to the HOMA calculation. In this study, neither fasting nor postprandial total cholesterol was related to body condition group, and the concentration was not influenced by intake of the high‐fat mixed‐meal. Fasting total cholesterol concentrations can be increased in obese dogs7, and this discrepancy between studies might be explained by differences in body fat content.
The DER obtained with the calculation used (137 kcal* BWkg 0.75) corresponded well to a previously recommended formula (DER = 1.8*(70*BWkg 0.75)) for intact canine adults, but a lower multiplying factor is suggested for obesity prone or inactive dogs.44 Instead of lowering the multiplying factor in the overweight group of dogs, we chose to feed all dogs according to the calculated ideal body weight.11, 18 The advantage of this adjustment for food intake is that it could be used both in the slightly overweight and overweight group as it is based on assigned body condition score. The calculated ideal bodyweight and corresponding amount of test feed offered to the overweight group differed significantly from the other two groups (Table 1). This difference could be due to the ideal‐BW calculation‐formula that might overestimate lean bodyweight in dogs with BCS ≥7, or could simply be because the overweight dogs were taller and longer than the other dogs in the study (which they visually were). The 1‐ to 9‐point scale for clinically estimating BCS and body fat content correlates well with dual‐energy X‐ray absorptiometry (DEXA) for dogs with BCS 4–8.45 All dogs investigated in this study fell within this BCS‐range; therefore, the risk of underestimating body fat content was low.
Study Limitations
Despite great efforts, a relatively low number of dogs could finally be included, especially in the overweight group. This was a result of overweight dogs being more difficult to enroll, mainly due to the inclusion criteria of the study, but also to owners' commitment. Hence, negative findings cannot be absolutely trusted in this study. To find relevant differences, power calculations should be made to assess the sample sizes needed for similar future studies. A greater number of dogs and a more equal distribution of lean and overweight dogs would have been preferred, but this proved impossible to achieve. Hence, it was decided before data collection was completed, to divide the dogs based on a clinical perspective. In our clinical experience, a BCS 6 is generally considered only slightly overweight, and as this group of dogs represents a great part of the canine population they were grouped separately. Dogs with palpable fat deposits (≥BCS 6.5) would clinically be considered overweight and were therefore grouped together. The strict inclusion/exclusion criteria otherwise strengthen the study, as previous studies of privately owned dogs with naturally occurring obesity have often included a more heterogeneous dog population that has not been as thoroughly screened as in this study. The sampling time in the postprandial period could have been extended for more than 4 hours, but this was not possible for practical reasons. The diet in home environment was not controlled and differences in feeding regimen could possibly have influenced the results, even though all dogs were fed mainly complete diets, underwent the same fasting period, and had the same test‐feed. Furthermore, none of the dogs were fed a high‐fat high‐energy diet in the home environment (such as the test‐meal) or were under extreme training (e.g. hunting dogs, sled‐dogs, service dogs) during the period preceding the data collection.
The classification of dogs was made by the same veterinarian according to clinical body condition scoring, which was also confirmed by leptin concentrations. Although this scoring system has proven highly comparable with DEXA within this range of body condition, it is possible that a DEXA‐scan could have provided more information. However, DEXA‐scan evaluation was not possible in this set up. The dogs in the lean group were mainly in the upper range of normal body composition (BCS 5), and dogs in the slightly overweight group were defined as BCS 6. These two groups of dogs were subsequently quite similar, and the chances of finding significant differences between them therefore reduced.
Conclusions
Overall, postprandial responses to the high‐fat mixed‐meal were similar to previous findings in people with rapid increase in insulin and glucagon, whereas glucose was not significantly increased until 3 hours postprandially. All dogs responded to the high‐fat meal by increase in triglyceride concentrations, but the response was higher in overweight dogs. The other metabolic and hormonal variables did not differ between groups except fasting cortisol/creatinine ratio which was higher in overweight dogs. Although overweight dogs often appear healthy, the results of this feed‐challenge test show that metabolic alterations might be present. This emphasizes the need for further studies of this large group of dogs.
Supporting information
Table S1. Summary of dietary history of the 28 male show‐type Labrador Retriever dogs included in the study.
Data S1. In‐House validation of a cortisol ELISA assay for canine urine.
Acknowledgments
We thank all dog owners for enrolling their dogs in the study, Sanna Truelsen Lindåse for help with sample collection, prof Ulf Olsson for statistical consultations, prof Kerstin Olsson for involvement in manuscript writing, and the personnel at Clinical Pathology Laboratory, Swedish University of Agricultural Sciences (SLU), Uppsala, Sweden, for help with cortisol validation. The study was financially supported by Future Animal Health and Welfare Initiative, the Foundation of Thure F. and Karin Forsberg, the Foundation of Michael Forsgren, Sweden, and the Mercodia Laboratory, Uppsala, Sweden.
Conflict of Interest Declaration: Validation of the glucagon ELISA for canine serum and analyses of glucagon concentrations in experimental samples were performed and partly financed by the Mercodia laboratory, Uppsala, Sweden.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
The study was performed at Swedish University of Agricultural Sciences, Uppsala, Sweden.
Presented in part at the 24th European College of Veterinary Internal Medicine, Companion Animal Congress, Mainz, Germany, September 2014 and at the Swedish Veterinary Congress Uppsala, Sweden, November 2014.
Footnotes
LaserCyte hematology system; IDEXX laboratories inc, Westbrook, ME
Architect c4000, Abbott Park, IL
Test strips for urine, Krulab, Langeskov, Denmark
Refractometer Master‐URC/NOx, Atago, Bellevue, WA
Uripet, Rocketmedical, Washington, UK
SC Micro Tube PCR‐PT, Sarstedt AG & Co, Nümbrecht, Germany
Venflon ™ Pro 1.1*32 mm; Becton Dickinson, Singapore City, Singapore
Hettich Vacuette Z Serum Clot Activator, Greiner bio‐one, Kremsmünster, Austria
Science Plan ™ Canine Adult Performance, Hills, Etten Leur, The Netherlands
Food & Agro Testing Sweden AB, Eurofins, Lidköping, Sweden
Canine Leptin ELISA, Millipore, MO
Canine Insulin ELISA, Mercodia, Uppsala, Sweden
Cortisol Urine ELISA, IBL, Hamburg, Germany
Canine Urinary Creatinine ELISA, Arbor, MI
Free fatty acid reagent, Wako NEFA‐HR(2), Neuss, Germany
Human Glucagon ELISA(25 μL), Mercodia, Uppsala, Sweden
SAS 9.4 Institute Inc, Cary, NC; GraphPad Prism 5.0 San Diego, CA
HOMA Calculator version 2.2.3, Diabetes Trial Unit, University of Oxford, UK
Elliott, KF, Fleeman, LM, Rand, JS, et al. Triglyceride reference values for a meal challenge test to assist diagnosis and management of canine hyperlipidemia. Abstract presented at ACVIM 2008
Schwanbeck M, Karamihos E, Ritzén H, et al. Development and validation of a high sensitivity enzyme‐linked immunosorbent assay for specific measurement of glucagon. Abstract presented at Insulin Club 2013.
References
- 1. German AJ, Holden SL, Wiseman‐Orr ML, et al. Quality of life is reduced in obese dogs but improves after successful weight loss. Vet J 2012;192:428–434. [DOI] [PubMed] [Google Scholar]
- 2. Kealy RD, Lawler DF, Ballam JM, et al. Effects of diet restriction on life span and age‐related changes in dogs. J Am Vet Med Assoc 2002;220:1315–1320. [DOI] [PubMed] [Google Scholar]
- 3. Zoran DL. Obesity in dogs and cats: a metabolic and endocrine disorder. Vet Clin North Am Small Anim Pract 2010;40:221–239. [DOI] [PubMed] [Google Scholar]
- 4. Handl S, Iben C. The current situation of obesity in the dog and cat I: Risk factors, associated diseases and pathophysiological background. Kleintierpraxis 2012;57:196–210. [Google Scholar]
- 5. Becker N, Dillitzer N, Sauter‐Louis C, et al. Feeding of dogs and cats in Germany. Tierärztliche Praxis Kleintiere 2012;40:391–397. [PubMed] [Google Scholar]
- 6. Witzel AL, Kirk CA, Henry GA, et al. Use of a novel morphometric method and body fat index system for estimation of body composition in overweight and obese dogs. J Am Vet Med Assoc 2014;244:1279–1284. [DOI] [PubMed] [Google Scholar]
- 7. Jeusette IC, Lhoest ET, Istasse LP, et al. Influence of obesity on plasma lipid and lipoprotein concentrations in dogs. Am J Vet Res 2005;66:81–86. [DOI] [PubMed] [Google Scholar]
- 8. Tvarijonaviciute A, Ceron JJ, Holden SL, et al. Obesity‐related metabolic dysfunction in dogs: a comparison with human metabolic syndrome. BMC Vet Res 2012;8:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Verkest KR, Fleeman LM, Rand JS, et al. Evaluation of beta‐cell sensitivity to glucose and first‐phase insulin secretion in obese dogs. Am J Vet Res 2011;72:357–366. [DOI] [PubMed] [Google Scholar]
- 10. Nguyen P, Dumon H, Buttin P, et al. Composition of meal influences changes in postprandial incremental glucose and insulin in healthy dogs. J Nutr 1994;124:2707S–2711S. [DOI] [PubMed] [Google Scholar]
- 11. Verkest KR, Rand JS, Fleeman LM, et al. Spontaneously obese dogs exhibit greater postprandial glucose, triglyceride, and insulin concentrations than lean dogs. Domest Anim Endocrinol 2012;42:103–112. [DOI] [PubMed] [Google Scholar]
- 12. Björntorp P, Rosmond R. Obesity and cortisol. Nutrition 2000;16:924–936. [DOI] [PubMed] [Google Scholar]
- 13. Pasquali R, Vicennati V, Cacciari M, et al. The hypothalamic‐pituitary‐adrenal axis activity in obesity and the metabolic syndrome. Ann NY Acad Sci 2006;1083:111–128. [DOI] [PubMed] [Google Scholar]
- 14. Arnaldi G, Angeli A, Atkinson AB, et al. Diagnosis and complications of Cushing's syndrome: a consensus statement. J Clin Endocrinol Metab 2003;88:5593–5602. [DOI] [PubMed] [Google Scholar]
- 15. Mårin P, Darin N, Amemiya T, et al. Cortisol secretion in relation to body fat distribution in obese premenopausal women. Metabolism 1992;41:882–886. [DOI] [PubMed] [Google Scholar]
- 16. Martin L, Siliart B, Dumon H, et al. Hormonal disturbances associated with obesity in dogs. J Anim Physiol An N 2006;90:355–360. [DOI] [PubMed] [Google Scholar]
- 17. Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Prev Med 2007;45:247–251. [DOI] [PubMed] [Google Scholar]
- 18. Laflamme DP. Development and validation of a body condition score system for dogs: a clinical tool. Canine Pract 1997;22:10–15. [Google Scholar]
- 19. Kienzle E, Rainbird A. Maintenance energy requirement of dogs: what is the correct value for the calculation of metabolic body weight in dogs? J Nutr 1991;121:S39–S40. [DOI] [PubMed] [Google Scholar]
- 20. Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Hoboken, New Jersey: John Wiley & Sons; 2012. [Google Scholar]
- 21. SAS Institute Inc . SAS 9.4 Reference Guide Statistics, 4th ed Cary, NC: SAS Institute Inc; 2015. [Google Scholar]
- 22. Litell R, Milliken G, Stroup W, et al. SAS for Mixed Models, 2nd ed Cary, NC: SAS Institute Inc; 2006. [Google Scholar]
- 23. Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 1998;21:2191. [DOI] [PubMed] [Google Scholar]
- 24. Parker HG, Ostrander EA. Canine genomics and genetics: running with the pack. PLoS Genet 2005;1:e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Raffan E, Becker J, Yeo G, et al. The coding sequence of POMC and obesity and appetite in Labrador retriever dogs. The Lancet 2014;383:S86. [Google Scholar]
- 26. Downs L, Crispin S, Legrande‐Defretin V, et al. The effect of dietary changes on plasma lipids and lipoproteins of six Labrador Retrievers. Res Vet Sci 1997;63:175–181. [DOI] [PubMed] [Google Scholar]
- 27. Elliott KF, Rand JS, Fleeman LM, et al. Use of a meal challenge test to estimate peak postprandial triglyceride concentrations in dogs. Am J Vet Res 2011;72:161–168. [DOI] [PubMed] [Google Scholar]
- 28. Miller M, Stone NJ, Ballantyne C, et al. Triglycerides and cardiovascular disease a scientific statement from the American Heart Association. Circulation 2011;123:2292–2333. [DOI] [PubMed] [Google Scholar]
- 29. Peña C, Suárez L, Bautista I, et al. Relationship between analytic values and canine obesity. J Anim Physiol An N 2008;92:324–325. [DOI] [PubMed] [Google Scholar]
- 30. Collier G, O'Dea K. The effect of coingestion of fat on the glucose, insulin, and gastric inhibitory polypeptide responses to carbohydrate and protein. Am J Clin Nutr 1983;37:941–944. [DOI] [PubMed] [Google Scholar]
- 31. López S, Bermúdez B, Pacheco YM, et al. Distinctive postprandial modulation of β cell function and insulin sensitivity by dietary fats: monounsaturated compared with saturated fatty acids. Am J Clin Nutr 2008;88:638–644. [DOI] [PubMed] [Google Scholar]
- 32. Verkest K, Fleeman L, Rand J, et al. Basal measures of insulin sensitivity and insulin secretion and simplified glucose tolerance tests in dogs. Domest Anim Endocrin 2010;39:194–204. [DOI] [PubMed] [Google Scholar]
- 33. Holst JJ. Enteroglucagon. Annu Rev Physiol 1997;59:257–271. [DOI] [PubMed] [Google Scholar]
- 34. Albrechtsen NJW, Hartmann B, Veedfald S, et al. Hyperglucagonaemia analysed by glucagon sandwich ELISA: nonspecific interference or truly elevated levels? Diabetologia 2014;57:1919–1926. [DOI] [PubMed] [Google Scholar]
- 35. Toft‐Nielsen M‐B, Damholt MB, Madsbad S, et al. Determinants of the impaired secretion of glucagon‐like peptide‐1 in type 2 diabetic patients. J Clin Endocrinol Metab 2001;86:3717–3723. [DOI] [PubMed] [Google Scholar]
- 36. Carr RD, Larsen MO, Jelic K, et al. Secretion and dipeptidyl peptidase‐4‐mediated metabolism of incretin hormones after a mixed meal or glucose ingestion in obese compared to lean, nondiabetic men. J Clin Endocrinol Metab 2010;95:872–878. [DOI] [PubMed] [Google Scholar]
- 37. Radulescu A, Gannon MC, Nuttall FQ. The effect on glucagon, glucagon‐like peptide‐1, total and acyl‐ghrelin of dietary fats ingested with and without potato. J Clin Endocrinol Metab 2010;95:3385–3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gutniak M, Grill V, Efendlć S. Effect of composition of mixed meals—low‐versus high‐carbohydrate content—on insulin, glucagon, and somatostatin release in healthy humans and in patients with NIDDM. Diabetes Care 1986;9:244–249. [DOI] [PubMed] [Google Scholar]
- 39. Abraham S, Rubino D, Sinaii N, et al. Cortisol, obesity, and the metabolic syndrome: A cross‐sectional study of obese subjects and review of the literature. Obesity 2013;21:E105–E117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vonderen IK, Kooistra HS, Rijnberk A. Influence of veterinary care on the urinary corticoid: creatinine ratio in dogs. J Vet Int Med 1998;12:431–435. [DOI] [PubMed] [Google Scholar]
- 41. Höglund K, Hanås S, Carnabuci C, et al. Blood pressure, heart rate, and urinary catecholamines in healthy dogs subjected to different clinical settings. J Vet Int Med 2012;26:1300–1308. [DOI] [PubMed] [Google Scholar]
- 42. Boden G, Shulman G. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and β‐cell dysfunction. Eur J Clin Invest 2002;32:14–23. [DOI] [PubMed] [Google Scholar]
- 43. Basso LV, Havel RJ. Hepatic metabolism of free fatty acids in normal and diabetic dogs. J Clin Invest 1970;49:537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mark Morris Institute . Small Animal Clinical Nutrition, 5th ed Topeka, Kanas: Mark Morris Institute; 2010:1314. [Google Scholar]
- 45. Mawby DI, Bartges JW, d'Avignon A, et al. Comparison of various methods for estimating body fat in dogs. J Am Anim Hosp Assoc 2004;40:109–114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Summary of dietary history of the 28 male show‐type Labrador Retriever dogs included in the study.
Data S1. In‐House validation of a cortisol ELISA assay for canine urine.


