Abstract
Background
Immune‐mediated hemolytic anemia (IMHA) is uncommon in cats, but may result in severe disease. Demographic predispositions for development of the disease and prognostic factors for mortality have not been investigated previously.
Hypothesis/Objectives
To explore possible demographic predispositions for development of primary IMHA in cats and to investigate possible prognostic factors for mortality.
Animals
107 client‐owned cats with IMHA, of which 72 had primary IMHA and 35 had secondary IMHA, and 9,194 control cats.
Methods
Data were collected retrospectively from records of cats with IMHA, defined by the presence of anemia and concurrent autoagglutination, ghost cells without oxidative damage on fresh blood smear, positive titer in a direct antiglobulin test, or evidence of phagocytosis of erythroid precursors in bone marrow. Odds ratios were calculated to assess the risk of development of primary IMHA in different demographic groups and Cox proportional hazards analysis was conducted to evaluate prognostic factors.
Results
No sex or breed predisposition was identified for the development of primary IMHA in comparison to the control cats, but cats in the age range 2.1–5.9 years were predisposed. Higher total bilirubin concentration and age were significant negative prognostic factors and higher lymphocyte numbers and serum globulin concentration were positive prognostic factors in a multivariable model.
Conclusions and Clinical Importance
Young adult cats were more likely to develop primary IMHA than other groups, but no apparent male predisposition was identified in this study, contrary to previous reports. Several prognostic factors were identified, which may be helpful in guiding clinical practice in the future.
Keywords: Autoimmunity, Bone marrow, Direct antiglobulin test, Hemolysis
Abbreviations
- ALT
alanine aminotransferase
- ARC
absolute reticulocyte concentration
- CBC
complete blood cell count
- CI
confidence interval
- CLL
chronic lymphocytic leukemia
- DAT
direct antiglobulin test
- FeLV
feline leukemia virus
- FIV
feline immunodeficiency virus
- IMHA
immune‐mediated hemolytic anemia
- IQR
interquartile range
- PCR
polymerase chain reaction
Immune‐mediated hemolytic anemia (IMHA) occurs when the body mounts an autoimmune response against antigens expressed on the surface of erythrocytes.1 Autoreactive antibodies specific for cytoskeletal components occur naturally in healthy dogs and people, where they may play a role in removal of senescent erythrocytes from the circulation.2, 3 In animals with IMHA, production of antibodies results in destruction of normal erythrocytes by inappropriate activation of the complement cascade, antibody‐dependent cytotoxicity, or facilitated phagocytosis in the liver and spleen, resulting in severe anemia.4
Early reports suggested that IMHA was more likely to be secondary to an underlying infectious, inflammatory, or neoplastic process in cats,5, 6 and there appeared to be a particular association between this disease and concurrent feline leukemia virus (FeLV) infection. More recent studies have suggested that the proportion of cats with primary disease (83% of those with a positive direct antiglobulin test) is higher, and similar to that reported in dogs.7, 8, 9
Diagnosis of IMHA in cats traditionally has relied on detection of antibodies bound to erythrocytes by the direct antiglobulin test (DAT, direct Coombs’ test), but the reported diagnostic accuracy of this test has varied considerably among previous studies,10 with positive results also observed in cats with other diseases, including FeLV infection,6, 11 hemotropic Mycoplasma spp. infection,12 and inflammatory diseases such as pancreatitis,8 cholangitis,8 and pyothorax.13 Persistent agglutination after dilution in saline has been reported in a large proportion of cats with IMHA,5, 6, 7 but this finding also is considered to have low specificity in cats because it also may occur in the diseases listed above. Previous descriptions of IMHA in cats have not discussed the presence of ghost cells, which are partially lysed erythrocytes that retain their shape and basic cytoskeletal structure. These cells indicate intravascular hemolysis, which is most likely to be associated with complement‐mediated lysis,14 particularly if signs of oxidative damage, such as Heinz bodies, are absent. In contrast to dogs, detection of spherocytes on a blood smear is not considered reliable for diagnosis of IMHA in cats because their normal erythrocytes are small and may lack central pallor.1
Little information has been published on the natural history of primary idiopathic IMHA in cats. A case series of 19 cats described several intriguing features of the disease, including a high prevalence of lymphocytosis and hyperglobulinemia, which are not typical of IMHA in dogs and may suggest different underlying immunologic changes in cats.7 A higher proportion of cats with IMHA also had nonregenerative anemia at diagnosis, but reticulocyte numbers were reported to increase in the majority of these cats after commencing treatment.7
More male than female cats were diagnosed with primary IMHA in 3 previous studies,6, 7, 8 but sex and breed frequencies were not compared to control groups. Follow‐up of the cats also suggested that survival may be more favorable in this species with a mortality rate of 23.5% overall, which is lower than the rate of 50–70% that often is cited for IMHA in dogs.15 Prognostic factors for mortality have not been evaluated previously in cats with IMHA.
The aims of this study were to evaluate possible age, breed, and sex predispositions for development of primary idiopathic IMHA in cats, and to evaluate survival times and possible prognostic factors for mortality in a large cohort of cats with this disease.
Materials and Methods
Selection of Cases
The electronic medical record system of a tertiary referral hospital was searched between July 2005 and July 2014 for cats that had a final diagnosis of IMHA, and the full records of selected cases were obtained.
The following data were recorded for each case: signalment; clinical examination findings; results of CBC, serum biochemical profile, reticulocyte count, retroviral and hemotropic Mycoplasma spp. testing, bone marrow, and any other cytologic or histologic examinations; findings from thoracic and abdominal imaging; blood type and records of blood product transfusion; and immunosuppressive drugs administered. The reports of bone marrow analysis were reviewed by 2 of the authors, and, where data were incomplete, the relevant samples were reviewed by a board‐certified clinical pathologist (BS).
Referring veterinarians were contacted by telephone to obtain follow‐up information. The study protocol was approved by an institutional ethical review committee (reference number 2014_1303).
Case Definitions
Cats were considered to have IMHA if they fulfilled all 4 of the following criteria:
Cat was anemic at presentation, with a hematocrit of <24%.
Cat showed signs suggestive of immune‐mediated destruction of erythrocytes by observation of ghost cells (without visible Heinz bodies) on a fresh blood smear, or persistent microscopic or macroscopic autoagglutination, or a titer of at least 1 : 16 in a DAT at 37C with polyvalent antisera, or evidence of phagocytosis of erythroid precursors in bone marrow samples. Agglutination was assessed macroscopically on a glass slide and microscopically after diluting 1 drop of whole blood in a sufficient volume of 0.9% saline to eliminate rouleaux formation. Fresh blood smears were evaluated after staining with modified Wright's stain; new methylene blue stains were requested in cases with a hematocrit <20% or on the request of the examining pathologist.
Cat did not have historical evidence or clinical signs of hemorrhage or exposure to oxidative toxins as the cause of anemia.
Cat was treated with immunosuppressive medications.
Cats were considered to have primary idiopathic IMHA if no underlying cause of disease was detected. All cats underwent imaging of the thorax and abdomen, CBC with examination of a fresh blood smear, serum biochemical profile, and serologic tests for FeLV and feline immunodeficiency virus (FIV). At the discretion of the attending clinician, blood samples also were submitted from some cats for polymerase chain reaction (PCR) for hemotropic Mycoplasma spp. and bone marrow samples were collected for cytologic examination, histologic examination, or both. Cats were considered to have nonregenerative IMHA (a clinical descriptive term) if the absolute reticulocyte count remained <50,000/μL for at least 5 days after presentation.
Breed and sex were recorded for all other cats that were presented to the same institution during the same time period as the cats with IMHA to act as a control population. For this group, the age at presentation also was calculated for a random sample of 504 cats. The results of all blood typing procedures in cats at the same institution also were obtained over the same time period, together with the breeds of the typed cats.
Statistical Analysis
All analyses were conducted using statistical software packages.1 , 2 Variables were assessed for normality by visual assessment of histograms and use of the Shapiro–Wilks test. Continuous variables were compared using Student's t‐test if normally distributed or the Mann–Whitney U‐test if not normally distributed. Categorical variables were compared using Chi‐squared test or Fisher's exact test, depending on the number of cases per cell.
Breed, sex, or age predispositions for the development of primary IMHA were assessed by the calculation of odds ratios, comparing the proportion of cats with primary IMHA with any particular signalment feature to the proportion of control cats with the same feature. Only breeds represented by at least 3 cats with primary IMHA were considered. Cases and controls were stratified into 4 balanced age groups based on calculation of the quartiles for age distribution from the entire sample. Bonferroni corrections were applied to P values to account for the effect of multiple comparisons.
Survival was assessed by Kaplan–Meier product limit estimates, and differences between groups were assessed using the log rank test. Cox proportional hazards analysis was used to assess possible prognostic factors for mortality. Fifteen variables were selected based on their a priori importance and to decrease redundancy and correlation between variables. Individual variables were first visualized as 3 groups using Kaplan–Meier product limit estimates. Where there was an evident difference between 1 group and the others, these variables were entered as categorical variables. Where there was an ordinal change in survival, these variables were entered as continuous variables. Variables first were entered individually for univariable analysis and all those with a P < .1 were entered into the multivariable model. The model was constructed using manual backward selection based on calculation of likelihood ratios, and the validity of the proportional hazards assumption was determined by visual assessment of the Schoenfeld residuals for each variable in the final model.
Results
Diagnosis of IMHA
One hundred and seven cats were included in this study, and the results of tests used to make the diagnosis of IMHA are shown in Table 1. A DAT was performed in 11 cases: in 3 cats DAT produced a titer ≥1 : 16, which was supportive of a diagnosis of IMHA. Of the 8 cats with a titer of <1 : 16, ghost cells were observed on a fresh blood smear in 5, persistent agglutination after saline dilution was detected in 7 and findings supportive of IMHA were observed on examination of bone marrow samples in 1 cat.
Table 1.
Criteria used to make the diagnosis of IMHA. Percentages add up to more than 100% because some cats were included in >1 group
| Diagnostic Criterion | Number of Cats | % | Number of Cats in which this was the only Diagnostic Criterion Fulfilled |
|---|---|---|---|
| Significant titer in direct antiglobulin test | 3 | 2.8 | 1 |
| Persistent agglutination (confirmed by saline dilution) | 70 | 65.4 | 10 |
| Ghost cells observed on fresh blood smear | 62 | 57.9 | 5 |
| Phagocytosis of erythroid precursors observed on bone marrow sample | 23 | 21.5 | 7 |
Of the cats diagnosed with IMHA, 35 (32.7%) were found to have an underlying disease that could have caused secondary immune‐mediated disease. These diseases included neoplasia (n = 16), including lymphoma (5), erythroleukemia (2), histiocytic sarcoma (1), multiple myeloma (1), anaplastic sarcoma with giant cells (1), and uncharacterized masses (6); infection with FeLV (n = 1), Mycoplasma hemofelis (1), Mycoplasma hemominutum (1), concurrent M. hemofelis and M. hemominutum (1), or feline infectious peritonitis (n = 3); cholangitis, pancreatitis or both (n = 6), urinary tract infection and suspected pyelonephritis (n = 1), and other inflammatory or infectious diseases (n = 7).
Of the cats considered to have primary IMHA (n = 72), immunochromatographic point‐of‐care tests yielded a positive result for FeLV p27 antigen in 2 cases, but PCR for proviral DNA on EDTA‐anticoagulated blood subsequently were negative in both cats. Point‐of‐care tests for antibodies to FIV were negative in all cases. Quantitative polymerase chain reactions for hemotropic Mycoplasma spp. were submitted in 62 cats and negative results were obtained in all cases.
Demographic Data
Data describing age, breed, and sex in cats with primary IMHA (n = 72), cats with secondary IMHA (n = 35) and all other cats presented to the institution (n = 9,194) are shown in Table 2.
Table 2.
Demographic data from cats with primary and secondary IMHA and control cats
| Variable | Cats with IMHA | ||
|---|---|---|---|
| Primary Disease | Secondary Disease | Control Cats | |
| Age (years) | |||
| Median | 4.2 | 6.8 | 6.1 |
| Interquartile range | 1.9–8.8 | 4.1–10.0 | 2.0–11.6 |
| Range | 0.2–17.9 | 0.5–18.0 | 0.1–23.0 |
| N | 72 | 35 | 504 |
| Sex (N, %) | |||
| Intact male | 2 (2.8) | 0 | 525 (5.7) |
| Neutered male | 30 (41.7) | 22 (62.9) | 4,744 (51.8) |
| Intact female | 4 (5.6) | 0 | 532 (5.8) |
| Neutered female | 36 (50.0) | 13 (37.1) | 3,351 (36.6) |
| N | 72 | 35 | 9,152 |
| Breed (N, %) | |||
| DSH | 48 (66.7) | 23 (65.7) | 6,147 (66.9) |
| DLH | 8 (11.1) | 4 (11.4) | 849 (9.2) |
| Siamese | 4 (5.6) | 3 (8.6) | 204 (2.2) |
| Other | 12 (16.7) | 5 (14.3) | 1,994 (21.7) |
| N | 72 | 35 | 9,194 |
DSH, domestic short hair; DLH, domestic long hair.
Cats with primary IMHA were significantly younger than those with secondary IMHA (P = .031), but there was no difference in the proportion of cats in these groups that were male and female (P = .074).
Cats in the age range 2.1 to 5.9 years were 2.0 times (95% confidence interval [CI], 1.19–3.41) more likely to develop primary IMHA than all other age groups combined (Table 3). Siamese cats also appeared to be predisposed to development of primary IMHA compared to all other breeds, but this association did not reach significance after Bonferroni correction. No sex group was predisposed to development of primary IMHA.
Table 3.
Age, sex, and breed predispositions for cats with primary IMHA compared to control cats
| Variable | Number of Cats with Primary IMHA (%) | Number of Control Cats (%) | Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|---|---|
| Agea | |||||
| 0–2.05 | 20 (27.8) | 127 (25.2) | 1.14 | 0.65–1.98 | .652 |
| 2.06–5.90 | 26 (36.1) | 110 (21.8) | 2.01 | 1.19–3.41 | .008 |
| 5.91–11.01 | 14 (19.4) | 135 (26.8) | 0.66 | 0.35–1.22 | .178 |
| 11.02–23.50 | 12 (16.7) | 130 (25.8) | 0.57 | 0.29–1.10 | .090 |
| N | 72 | 504 | |||
| Sexa | |||||
| Intact male | 2 (2.8) | 525 (5.7) | 0.47 | 0.12–1.93 | .440 |
| Neutered male | 30 (41.7) | 4,744 (51.8) | 0.67 | 0.42–1.07 | .093 |
| Intact female | 4 (5.6) | 532 (5.8) | 0.96 | 0.35–2.64 | 1.000 |
| Neutered female | 36 (50.0) | 3,351 (37.1) | 1.74 | 1.10–2.77 | .017 |
| N | 72 | 9,192 | |||
| Breedb | |||||
| DSH | 48 (80.0) | 6,147 (85.4) | 0.99 | 0.61–1.62 | .972 |
| DLH | 8 (13.3) | 849 (11.8) | 1.23 | 0.59–2.57 | .584 |
| Siamese | 4 (6.7) | 204 (2.8) | 2.59 | 0.94–7.17 | .078 |
| N | 60 | 7,200 | |||
| Blood typeb | |||||
| A | 43 (75.4) | 277 (75.1) | 0.98 | 0.51–1.87 | .952 |
| B | 9 (15.8) | 70 (19.0) | 1.25 | 0.59–2.67 | .565 |
| AB | 5 (8.8) | 22 (6.0) | 0.66 | 0.24–1.82 | .418 |
| N | 57 | 369 | |||
DSH, domestic short hair; DLH, domestic long hair.
P value ≤.0125 indicates significance at .05 level after Bonferroni correction.
P value ≤.0167 indicates significance at .05 level after Bonferroni correction.
Clinical Data
Results of CBC and serum biochemical profiles for cats with primary or secondary IMHA are shown in Table 4. The lymphocyte count was significantly higher in cats with primary IMHA (median: 2,400/μL; interquartile range [IQR], 1,300–4,300; range: 420–20,280) compared to those with secondary IMHA (1,000/μL; IQR: 600–2,200; range: 90–17,440; P < .001; Fig 1), whereas the serum albumin concentration was significantly lower in cats with secondary IMHA (mean: 2.65 ± 1.05 g/dL) compared to those with primary IMHA (3.18 ± 0.66 g/dL; P < .001).
Table 4.
Hematologic and serum biochemical data from cats with primary and secondary IMHA
| Parameter | Cats with Primary IMHA | Cats with Secondary IMHA | P Value |
|---|---|---|---|
| Red blood cell concentration (×106/μL) | 2.07 (1.63–2.42) | 2.41 (1.96–3.38) | .016 |
| Hematocrit (%, mean and standard error) | 11.5 (±0.45) | 13.0 (±0.70) | .057a |
| Hemoglobin concentration (g/dL, mean and standard error) | 4.0 (±0.14) | 4.5 (±0.23) | .063a |
| Mean cell volume (fL) | 54.0 (46.4–65.9) | 48.8 (45.2–56.1) | .086 |
| Mean cell hemoglobin concentration | 34.3 (32.1–37.0) | 34.5 (31.9–36.6) | .722 |
| Red cell distribution width (%) | 21.3 (18.6–24.8) | 19.9 (17.7–23.6) | .205 |
| Nucleated red blood cells (per 100 leukocytes) | 3.0 (0–11.0) | 2.0 (0–6.0) | .412 |
| Absolute reticulocyte count (/μL) | 32,480 (12,320–101,200) | 38,600 (7,340–106,640) | .861 |
| Blood type | |||
| A | 45 | 18 | .336 |
| B | 9 | 7 | |
| AB | 3 | 3 | |
| Total white blood cell count (×103/μL) | 9.0 (6.1–14.2) | 9.6 (5.6–19.1) | .705 |
| Neutrophil concentration | 4.5 (3.2–8.0) | 7.8 (3.8–15.3) | .031 |
| Band neutrophil concentration | 0 (0–0.1) | 0 (0–0.1) | .595 |
| Lymphocyte concentration | 2.4 (1.3–4.3) | 1.0 (0.6–2.2) | <.001 |
| Monocyte concentration | 0.5 (0.2–0.9) | 0.6 (0.2–1.0) | .698 |
| Eosinophil concentration | 0 (0–0.2) | 0 (0–0.09) | 0.089 |
| Platelet count (×103/μL) | 150 (100–250) | 150 (91–252) | .832 |
| Serum total protein concentration (g/dL) | 7.0 (6.1–8.0) | 6.4 (5.6–7.3) | 0.049 |
| Serum albumin concentration (g/dL) | 3.2 (±0.66) | 2.7 (±1.05) | <.001 a |
| Serum globulin concentration (g/dL) | 3.8 (2.8–4.8) | 3.5 (2.8–4.9) | .844 |
| Serum total bilirubin concentration (mg/dL) | 0.4 (0.1–1.0) | 0.5 (0.2–1.5) | .327 |
| Serum urea nitrogen concentration (mg/dL) | 26 (19–34) | 26 (20–36) | .945 |
| Serum creatinine concentration (mg/dL) | 1.2 (1.1–1.4) | 1.4 (1.0–1.7) | .429 |
| Serum ALT activity (U/L) | 78 (44–158) | 68 (37–127) | .533 |
| Serum ALP activity (U/L) | 14 (6–28) | 16 (10–32) | .167 |
ALT, alanine aminotransferase; ALP, alkaline phosphatase.
Values are median and IQR and comparisons are by Mann–Whitney U‐test unless otherwise stated. P values ≤.002 denote significance at the .05 level following Bonferroni correction.
Student's t‐test.
Figure 1.
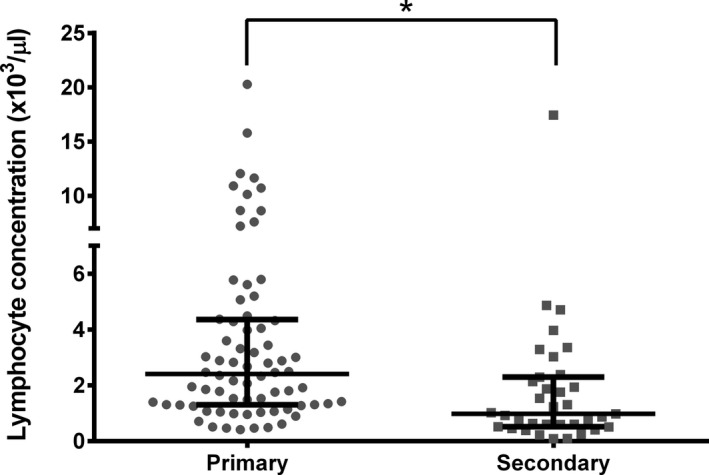
Lymphocyte concentrations in cats with primary or secondary IMHA. Lines represent median and interquartile range. *p < 0.001.
Fifty‐seven cats with primary IMHA were blood typed, and the majority were type A (n = 45, 78.9%) and a smaller number were type B (n = 9, 15.8%) or AB (n = 3, 5.3%). Three hundred and sixty‐nine cats without IMHA were typed over the same time period as this study, of which 277 (75.1%) were type A, 70 (19.0%) were type B, and 22 (6.0%) were type AB. Cats of 1 particular blood type were not at increased risk of development of primary IMHA (Table 3), and there was no difference in the proportion of cats belonging to each blood type among groups.
The median absolute reticulocyte concentration (ARC) for all cats with IMHA on presentation was 32,850/μL (IQR: 9,160–106,640; range: 0–795,400), and did not differ between cats with primary or secondary IMHA (Table 4, P = .861). Forty (55.6%) cats with primary IMHA had an ARC < 50,000/μL on presentation, and, of these, 16 failed to show evidence of appropriate regeneration within the next 5 days after starting to receive immunosuppressive treatment, 18 did become regenerative, 3 died within the first 5 days, and 3 did not have a repeat blood sample at an appropriate time to determine whether the reticulocyte count had changed. The proportion of cats with nonregenerative anemia 5 days after presentation did not differ between those with primary IMHA (16/63, 25.4%) and those with secondary disease (8/27, 29.6%, P = .677).
Comparisons of age, hematologic and serum biochemical variables between cats with primary IMHA with either regenerative anemia or persistently nonregenerative anemia did not identify any significant differences between groups after Bonferroni correction (Table S1) aside from the expected difference in ARC. Cats with nonregenerative IMHA had lower serum bilirubin concentrations, higher hematocrits and lower mean corpuscular volume, and red cell distribution width, but these differences were not significant.
Of the cats with primary IMHA, 55 had concurrent deficiency of ≥1 other blood cell types, including 44 with thrombocytopenia (platelet concentration <200,000/μL), 12 with neutropenia (<2,500/μL), and 24 with lymphopenia (<1,500/μL).
Bone Marrow Findings
Bone marrow aspirates were obtained from 60 cats with IMHA, of which 13 (21.7%) samples were considered to be poorly diagnostic. Core biopsies were obtained from 46 cats, with 16 (34.8%) of samples being poorly diagnostic. Major findings from bone marrow examination are shown in Table 5. In cases where a bone marrow aspirate provided little information, a diagnostic core biopsy was obtained in 7 (53.8%) cases. Stainable iron and phagocytosis of erythroid precursors were noted in samples from cats with primary and secondary IMHA (Fig 2A,B).
Table 5.
Results of cytologic and histopathologic examination of samples of bone marrow from cats with primary or secondary IMHA
| Parameter | Cat with primary IMHA (n = 47) | Cats with secondary IMHA (n = 18) | P value |
|---|---|---|---|
| Myeloid: erythroid ratio (IQR) | 0.30 (0.20–0.70) | 0.55 (0.37–1.35) | .130a |
| Proportion of lymphocytes observed (IQR) | 8 (2–25) | 6 (3.5–12.5) | .429a |
| Stainable iron deposits observed (n, %) | 5 (11.4%) | 8 (44.4%) | .004b |
| Erythrophagocytosis observed (n, %) | 16 (34.0%) | 10 (55.6%) | .128b |
| Major features observed (n) | |||
| Erythroid hyperplasia | 36 | 15 | |
| Erythroid hypoplasia or aplasia | 6 | 2 | |
| Myeloid hyperplasia | 7 | 9 | |
| Megakaryocytic hyperplasia | 9 | 5 | |
| Lymphoid hyperplasia | 14 | 1 | |
| Dyserythropoiesis, including maturation arrest | 6 | 0 | |
| Neoplastic infiltration, including erythroleukemia | 0 | 3 |
Values are presented as median and IQR, unless stated otherwise.
Mann–Whitney U‐test.
Chi‐squared test.
Figure 2.
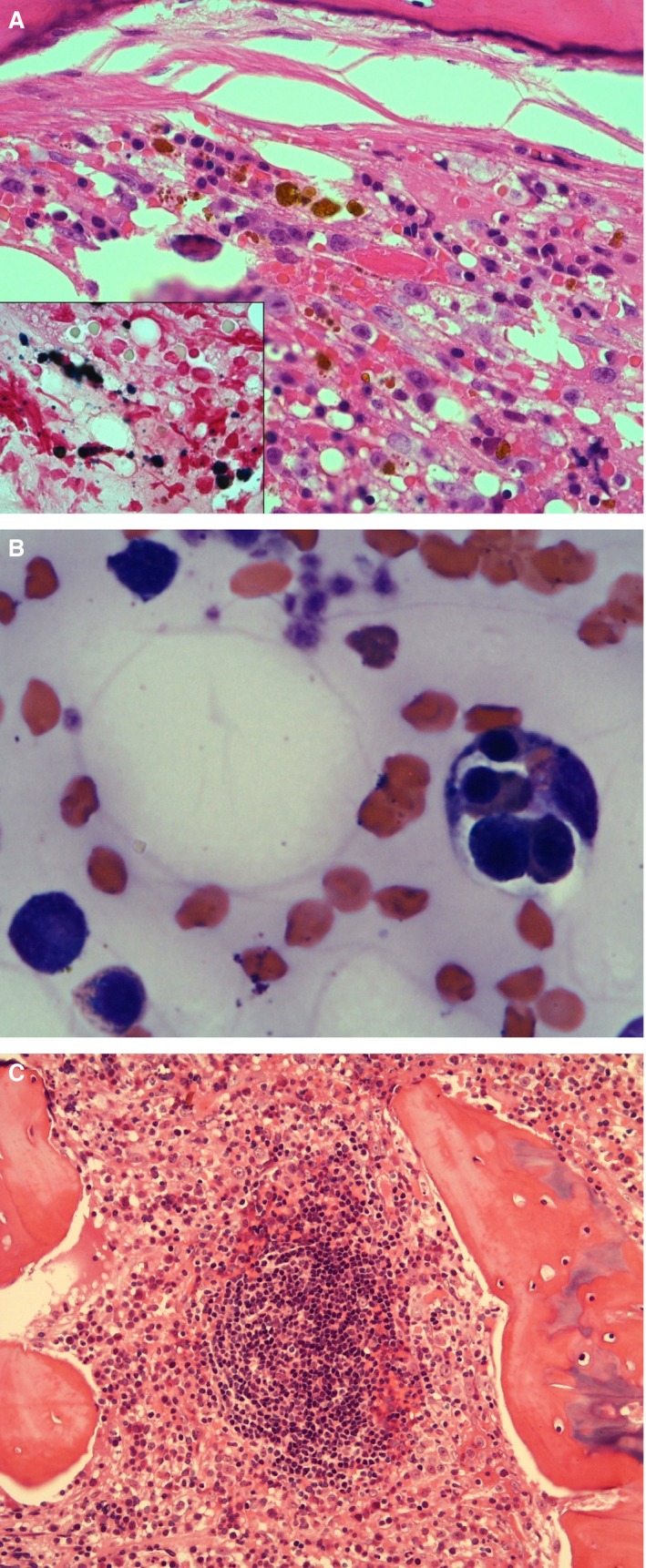
(A) Bone marrow core biopsy section of a cat with numerous foci of iron (H&E stain, 400x). Inset: stainable iron in the same sample (Perl's Prussian Blue stain, 1000x). (B) Bone marrow aspirate from a cat. Macrophage on the right with four intact erythroid precursors phagocytosed, including a basophilic rubricyte, a polychromatophilic rubricyte, and two metarubricytes (Modified Wright's stain, 1000x). (C) Prominent lymphoid follicle in a core biopsy section of feline bone marrow (H&E stain, 200x).
Further Characterization of Cats with Lymphocytosis
Eleven (15.3%) of the 72 cats with primary IMHA had a lymphocyte count higher than the upper reference limit (7,000/μL), and the distribution of lymphocyte counts is shown in Figure 1. The median age of these cats was 2.6 years (IQR: 1.8–4.8; range: 1.6–6.4). Bone marrow samples were obtained in 7 cats, and lymphoid cells constituted a median of 20% of the bone marrow cells counted (IQR: 6–30) in these cases. The appearance and organization of these lymphocytes varied, with some arranged in distinct follicles or aggregates (Fig 2C) and some spread diffusely throughout the bone marrow cells.
Treatment of Cats with Primary IMHA
Sixty cats were discharged with PO medications, of which 29 received only a glucocorticoid (either prednisolone, n = 28 or dexamethasone, n = 1),3 , 4 , 5 22 received prednisolone and chlorambucil,6 6 received prednisolone and cyclosporine,7 1 received only cyclosporine, and 2 received prednisolone, chlorambucil, and cyclosporine. The average starting dosages of each drug are shown in Table S2. Twenty‐five cats received courses of doxycycline8 after samples were submitted for PCR for hemotropic Mycoplasma spp.
Temporal Changes in Hematocrit
Of the 65 cats with primary IMHA that survived for >5 days after presentation, 25 (38.5%) recovered a normal hematocrit or packed cell volume of at least 25% within 1 month of discharge from the hospital, of which 3 died before the month had elapsed. The normal hematocrit or packed cell volume was not achieved within 1 month in 23 cats (35.4%), of which 11 died or were euthanized before the month had elapsed. Results of follow‐up blood samples were unavailable in 17 cats (26.2%) during this period.
Survival Data
Not including those cats that died or were euthanized while hospitalized, the median follow‐up time for all cats with IMHA was 239 days (IQR: 41–781), with 3 cats lost to follow‐up after discharge. The median survival time for cats with primary IMHA (n = 71) was 516 days (first quartile 22 days, third quartile not reached) and 14 days (IQR: 4–504) for cats with secondary IMHA (n = 33). Of the cats with complete follow‐up for at least 6 months after diagnosis, 35 of 59 (59.3%) with primary IMHA were still alive at 6 months, compared to 8 of 31 (25.8%) cats with secondary IMHA. Survival curves for both groups are shown in Figure 3, and there was a significant difference in survival time between the 2 groups (P < .001) and in the proportion of cats alive at 6 months (P = .002).
Figure 3.
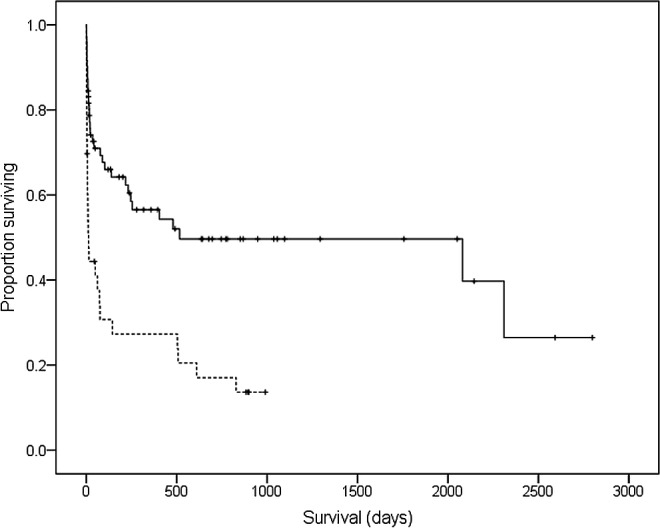
Kaplan–Meier survival curve to compare survival times in cats with primary IMHA (solid line) and those with secondary IMHA (dashed line). Tick marks indicate censored cases.
Prognostic Factors
Fifteen variables were evaluated as possible prognostic factors for mortality in cats with primary IMHA, and results of univariable Cox proportional hazards analysis are shown in Table 6. Seven variables (lymphocyte count, serum globulin concentration, serum total bilirubin concentration, age, heart rate on presentation, neutrophil count and serum alanine aminotransferase [ALT] activity) were taken forward to multivariable analysis, of which the first 4 were retained in the final model, which is shown in Figure 4. There were no significant interactions among variables included in the final model, and violations of the proportional hazards assumption were not detected on assessment of the Schoenfeld residuals.
Table 6.
Results of univariable Cox proportional hazards analysis for assessment of potential prognostic factors for mortality in feline primary IMHA
| Variable | Univariable Analysis | ||
|---|---|---|---|
| Hazard Ratio | 95% Confidence Interval | P Value | |
| Agea | 1.159 | 1.074–1.251 | <.001 |
| Rectal temperature | 0.800 | 0.535–1.196 | .277 |
| Heart rate | 0.990 | 0.975–1.005 | .179 |
| Respiratory rate | 1.003 | 0.985–1.022 | .742 |
| Neutrophil count | 1.033 | 0.987–1.080 | .163 |
| Lymphocyte counta (>2,500/μL) | 0.436 | 0.211–0.902 | .025 |
| Hematocrit | 0.999 | 0.918–1.088 | .990 |
| Type of anemia (nonregenerative) | 0.765 | 0.311–1.882 | .560 |
| Platelet count | 1.000 | 0.998–1.002 | .664 |
| Serum albumin concentration | 0.734 | 0.375–1.435 | .365 |
| Serum globulin concentrationa | 0.642 | 0.452–0.912 | .013 |
| Serum creatinine concentration | 1.010 | 0.523–1.952 | .976 |
| Serum ALT activitya (≥85 U/L) | 0.517 | 0.239–1.116 | .093 |
| Serum total bilirubin concentrationa | 1.255 | 1.005–1.567 | .046 |
| Autoagglutination (confirmed by saline dilution) | 1.050 | 0.505–2.181 | .897 |
ALT, alanine aminotransferase.
Variables considered in multivariable regression.
Figure 4.
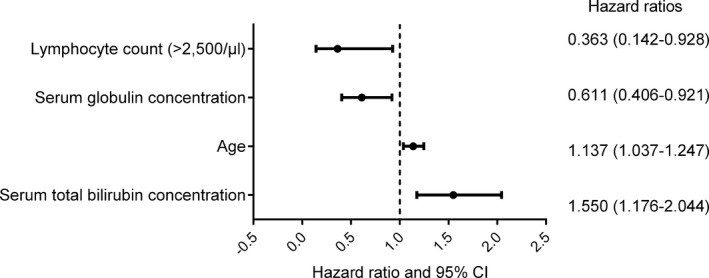
Forest plot to show the variables included in the final multivariable model constructed using Cox proportional hazards analysis.
Discussion
This study describes the largest reported cohort of cats with primary idiopathic IMHA and shows that cats in the age group from 2 to 6 years were more likely to develop the disease than other age groups, but that neither sex and no individual breed was predisposed. The majority of cats with IMHA did not have any underlying cause for the disease and therefore were considered to have idiopathic IMHA. Survival time was significantly longer in cats with primary IMHA compared to those with secondary IMHA, and Cox proportional hazards analysis identified higher serum bilirubin concentration and older age as significant negative prognostic factors for mortality and higher lymphocyte concentration and higher serum globulin concentration as positive prognostic factors.
In this study, IMHA was most commonly diagnosed in cats with persistent agglutination and in cases where ghost cells were observed on fresh blood smears. These findings have been reported in cats with other diseases,4, 16 but we believe that these abnormalities are suggestive of primary immune‐mediated hemolysis in anemic cats with no other detectable diseases or signs of oxidative hemolysis.
Direct antiglobulin tests were requested in a minority of cases that were ultimately diagnosed with primary IMHA because the test was considered unnecessary when autoagglutination or ghost cells were detected in the absence of any other cause of moderate or severe anemia. Traditionally, there also were concerns that the DAT had an unacceptably low specificity for diagnosis of IMHA because clinically relevant titers were obtained from nonanemic cats in a previous study.11 A more recent study using a more complete panel of monovalent antisera directed against IgM and IgG isotypes indicated that a clinically relevant positive titer with either monovalent or polyvalent antisera at 37C had a sensitivity of 81% and specificity of 92% for diagnosis of IMHA,8 suggesting that this DAT should be considered as a diagnostic test more frequently, particularly in those cases without convincing autoagglutination or ghost cells. The relatively low sensitivity of the DAT may create difficulties if used as a sole diagnostic test because cases of presumed primary IMHA have been reported in the absence of a clinically relevant titer, both in a previous study6 and in the current investigation.
This study has a number of limitations, chiefly related to its retrospective design. There was some variation in the diagnostic imaging modalities and types of infectious disease testing among different cats. It is therefore difficult to be sure that all cases were equivalent or classified into the correct groups. Some cats were still alive or were lost to follow‐up at the time that survival times were calculated, which means that the Cox proportional hazards model was based partly on censored data, as indicated in Figure 3. Although it was possible to describe dosages and types of medications used at commencement of treatment in this study, it was difficult to obtain complete treatment records for all cats after discharge. We were therefore unable to consider the effect of different treatment or tapering regimens as prognostic factors in this analysis.
In this study, no underlying causes were detected in the majority of cats diagnosed with IMHA, which is in agreement with a recent study of the disease in Germany.7 In previous studies in the 1970s and 1980s, a substantial proportion of cases were found to be associated with FeLV infection, and we suggest that the difference could be related to the decreasing incidence of FeLV infection in the face of widespread testing and vaccination.17, 18 The proportion of cats in this study with IMHA and no underlying cause is similar to that reported in dogs with IMHA,9, 19, 20 but the relative incidences of the disease in the 2 species have not been reported or compared to date.
In the majority of small descriptive case series, male cats were diagnosed with IMHA more frequently than females,5, 7, 8 but this association was not detected in our study when sex distribution was compared to a large group of cats presented to the same institution over the same period of time. These findings are likely to be related to the relative numbers of male and female cats that were examined at our institution during the period of the study, which was not taken into account in previous studies of IMHA in cats. The predominance of male cats presented for veterinary care has been reported previously in the emergency referral setting,21 but not in first opinion practices in the United Kingdom,22 suggesting that the caseload of individual institutions should be assessed when investigating possible demographic associations for particular diseases.
Most reported cases of primary IMHA have been diagnosed in young adult cats, with medians of 2 years,7 2.5 years,8 and 3.5 years5 stated in 3 previous studies. We report a similar median age for cats with primary IMHA in this study (4.2 years), and also show that cats within the age range from 2.1 to 5.9 years were significantly more likely to develop primary IMHA than any other age group. This age of onset differs from those reported in dogs and people, in whom the diagnosis is made more frequently in middle or older age groups.23, 24, 25
Siamese cats appeared to be overrepresented among the cases with primary IMHA, but this association did not reach significance and the odds ratio had a large CI because of the small number of Siamese cats with IMHA. We cannot therefore conclude that any particular breed is predisposed to development of primary IMHA, but the results of our investigation are in agreement with previous studies that showed that the majority of cases were diagnosed in genetically outbred (domestic short‐haired) cats.5, 7, 8
Clinical, hematologic, and biochemical data obtained from cats with IMHA showed several similarities when compared to previous studies. Severe anemia has been reported in the majority of cats diagnosed with primary IMHA, and the mean hematocrit of 11.5% in this study is similar to results of recent investigations.7 Cats with primary IMHA had a significantly higher lymphocyte count than those with secondary IMHA, and lymphocytosis was observed in 32% of cats with primary IMHA in a previous case series.7 Lymphoid hyperplasia also was observed on bone marrow samples in a large proportion of cats in our study, and this feature has been described previously in cats with IMHA.26
Immune‐mediated hemolytic anemia commonly is associated with chronic lymphocytic leukemia (CLL) in people,27 and this possibility could not be excluded completely in the cats with lymphocytosis and increased numbers of bone marrow lymphocytes in this study. There is no single reliable test to differentiate between the 2 diseases in cats, and a final diagnosis usually is based on consideration of the signalment, magnitude, and chronicity of the lymphocytosis and phenotypic and genotypic characteristics of the lymphocyte population.26, 28 According to these criteria, we consider it unlikely that the cats with peripheral lymphocytosis in this study had CLL because all were younger than 7 years at presentation and because the lymphocytosis resolved rapidly with treatment in all cases. Severe anemia is not reported to be a common feature of cats with CLL, whereas lymphocytosis and increased numbers of bone marrow lymphocytes have been reported consistently in cats with IMHA and pure red cell aplasia.7, 26, 29 Immunophenotyping of peripheral lymphocytes was requested in 1 cat in this study, and indicated a predominance of B cells, which also was more suggestive of reactive proliferation28 , 9 Although the phenotype and functional characteristics of lymphocytes in cats with IMHA have yet to be investigated, we hypothesize that these may represent B cells specific for autoantigens, and some of these B cells ultimately may differentiate into plasma cells and produce autoantibodies.
Using Cox proportional hazards analysis, several prognostic factors were identified for mortality in cats with primary IMHA. Age of the patient often is considered to have an impact on survival, but this variable was retained when the majority of cases were diagnosed in cats <9 years of age. Serum bilirubin concentration and clinically detectable icterus have been identified as negative prognostic factors for mortality in several studies of IMHA in dogs,9, 30, 31, 32 and this result probably is related to the severity of hemolysis. Increased serum bilirubin concentration also could be associated with hepatic dysfunction caused by hypoxia and to formation of hepatic or portal thomboemboli, although the latter complication was only detected in 1 cat in this study by ultrasonography. Higher serum globulin concentration and lymphocyte count were associated with improved survival times, but the cause of this relationship was not apparent. As discussed above, increased serum globulin concentration and lymphocyte count traditionally have raised concerns that hemolysis could be related to underlying disease, but they also could be features of a form of IMHA that results in more marked lymphocyte (possibly B cell) proliferation, but that is more responsive to treatment.
As reported previously,6, 7 a large proportion of cats with primary IMHA had an ARC of <50,000/μL at diagnosis, but many then developed a robust regenerative response within the next 5 days. Lack of regeneration by 5 days after diagnosis did not have an independent effect on survival time, which suggests that this group of cats may still respond to immunosuppressive treatment and may have a similar long‐term prognosis compared to cats that have a regenerative response at diagnosis.
Approximately 35% of cats with primary IMHA that survived the first 5 days after presentation to the hospital went on to recover a normal packed cell volume or hematocrit within 1 month, and the mortality rate in this group was lower than among those cats that did not attain a normal value within this time period. These results should be interpreted with caution because data were unavailable for a large proportion of cats and because there was no systematic program of follow‐up testing after discharge, so that recovery of a normal value may have been detected sooner in cats that were being tested more frequently. Similarly, although the speed of recovery of normal erythroid parameters is likely to be an important prognostic factor, we chose not to include this variable in our prognostic model because we considered that the data were of insufficient quality to draw conclusions.
In summary, we showed that primary IMHA occurred more commonly than secondary IMHA, that male cats were not predisposed to the disease, and that several prognostic factors had a significant association with mortality. The findings of this study ideally would be confirmed by prospective investigations, which also would permit assessment of the efficacy of different therapeutic protocols and tapering regimens. Because the disease is not diagnosed commonly, representing just 1.2% of cats presented to our specialist veterinary hospital, the feasibility of such studies remains questionable.
Supporting information
Table S1. Comparisons of hematologic and serum biochemical data from cats with primary IMHA with either regenerative anemia or persistently nonregenerative anemia. Values are median and interquartile range and comparisons are by Mann–Whitney U‐test unless otherwise stated. P < .0025 denote significance at the .05 level after Bonferroni correction.
Table S2. Starting doses of drugs used in the treatment of primary IMHA in cats. Numbers of cats differ from those stated in text as bodyweight was not recorded for all cases to enable calculation of dose rates.
Acknowledgments
This manuscript was assessed according to the Royal Veterinary College's code of good research practice (manuscript number CSS_00939).
Conflict of Interest Declaration : Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration : Doxycycline was used for presumptive treatment of hemotropic Mycoplasma spp., for which it is not licensed for use in cats in the United Kingdom.
This study was conducted at the Royal Veterinary College, University of London.
Footnotes
IBM SPSS Statistics for Windows, Version 20.0, IBM Corp, Armonk, New York
GraphPad Prism version 6.00 for Windows, GraphPad Software, La Jolla, California
Prednicare (5 mg prednisolone tablets), Animalcare Ltd, York, UK
Prednidale (25 mg prednisolone tablets), Dechra Ltd, Stoke‐on‐Trent, Staffordshire, UK
Dexamethasone (2 mg tablets), Auden Mckenzie (Pharma Division) Ltd, Ruislip, Middlesex, UK
Chlorambucil (2 mg tablets), Aspen Pharma Trading Ltd, Dublin, Ireland
Atopica (cyclosporine, 100 mg/mL oral solution), Novartis Animal Health UK Ltd, Camberley, Surrey, UK
Ronaxan (20 mg doxycycline tablets), Merial Animal Health Ltd, Harlow, Essex, UK
Workman HC, Vernau W, Schmidt PS et al. Chronic lymphocytic leukemia in cats is primarily a T helper cell disease. Presented at the 55th Annual Meeting of the American College of Veterinary Pathologists, Orlando, November 13–17, 2004
References
- 1. Mitchell K, Kruth S. Immune‐mediated hemolytic anemia and other regenerative anemias In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine, 7th ed St Louis: Saunders Elsevier; 2010:768–769. [Google Scholar]
- 2. Lutz HU, Wipf G. Naturally occurring autoantibodies to skeletal proteins from human red blood cells. J Immunol 1982;128:1695–1699. [PubMed] [Google Scholar]
- 3. Barker RN, Elson CJ. Red cell‐reactive non‐specific immunoglobulins and autoantibodies in the sera of normal and anaemic dogs. Vet Immunol Immunopathol 1993;39:339–354. [DOI] [PubMed] [Google Scholar]
- 4. Naik R. Warm autoimmune hemolytic anemia. Hematol Oncol Clin North Am 2015;29:445–453. [DOI] [PubMed] [Google Scholar]
- 5. Scott DW, Schultz RD, Post JE, et al. Autoimmune hemolytic anemia in the cat. J Am Anim Hosp Assoc 1973;9:530–539. [Google Scholar]
- 6. Werner LL, Gorman NT. Immune‐mediated disorders of cats. Vet Clin North Am Small Anim Pract 1984;14:1039–1064. [DOI] [PubMed] [Google Scholar]
- 7. Kohn B, Weingart C, Eckmann V, et al. Primary immune‐mediated hemolytic anemia in 19 cats: Diagnosis, therapy, and outcome (1998–2004). J Vet Intern Med 2006;20:159–166. [DOI] [PubMed] [Google Scholar]
- 8. Tasker S, Murray JK, Knowles TG, et al. Coombs’, haemoplasma and retrovirus testing in feline anaemia. J Small Anim Pract 2010;51:192–199. [DOI] [PubMed] [Google Scholar]
- 9. Piek CJ, Junius G, Dekker A, et al. Idiopathic immune‐mediated hemolytic anemia: Treatment outcome and prognostic factors in 149 dogs. J Vet Intern Med 2008;22:366–373. [DOI] [PubMed] [Google Scholar]
- 10. Wardrop KJ. Coombs’ testing and its diagnostic significance in dogs and cats. Vet Clin North Am Small Anim Pract 2012;42:43–51. [DOI] [PubMed] [Google Scholar]
- 11. Dunn JK, Searcy GP, Hirsch VM. The diagnostic significance of a positive direct antiglobulin test in anemic cats. Can J Comp Med 1984;48:349–353. [PMC free article] [PubMed] [Google Scholar]
- 12. Tasker S, Peters IR, Papasouliotis K, et al. Description of outcomes of experimental infection with feline haemoplasmas: Copy numbers, haematology, Coombs’ testing and blood glucose concentrations. Vet Microbiol 2009;139:323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ottenjann M, Weingart C, Arndt G, et al. Characterization of the anemia of inflammatory disease in cats with abscesses, pyothorax, or fat necrosis. J Vet Intern Med 2006;20:1143–1150. [DOI] [PubMed] [Google Scholar]
- 14. Stockham SL, Scott MA. Erythrocytes In: Stockham SL, Scott MA, eds. Fundamentals of Veterinary Clinical Pathology, 2nd ed Ames: Blackwell Publishing; 2008:137. [Google Scholar]
- 15. Piek CJ. Canine idiopathic immune‐mediated haemolytic anaemia: A review with recommendations for future research. Vet Q 2011;31:129–141. [DOI] [PubMed] [Google Scholar]
- 16. Christopher MM, White JG, Eaton JW. Erythrocyte pathology and mechanisms of Heinz body‐mediated hemolysis in cats. Vet Pathol 1990;27:299–310. [DOI] [PubMed] [Google Scholar]
- 17. Gleich SE, Krieger S, Hartmann K. Prevalence of feline immunodeficiency virus and feline leukaemia virus among client‐owned cats and risk factors for infection in Germany. J Feline Med Surg 2009;11:985–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meichner K, Kruse DB, Hirschberger J, et al. Changes in prevalence of progressive feline leukaemia virus infection in cats with lymphoma in Germany. Vet Rec 2012;171:348. [DOI] [PubMed] [Google Scholar]
- 19. Cotter S. Autoimmune hemolytic anemia. Compend Contin Educ Small Anim 1992;14:E93–E100. [Google Scholar]
- 20. Jackson ML, Kruth SA. Immune‐mediated hemolytic anemia and thrombocytopenia in the dog: A retrospective study of 55 cases diagnosed from 1979 through 1983 at the Western College of Veterinary Medicine. Can Vet J 1985;26:245–250. [PMC free article] [PubMed] [Google Scholar]
- 21. Ruple‐Czerniak A, Aceto HW, Bender JB, et al. Using syndromic surveillance to estimate baseline rates for healthcare‐associated infections in critical care units of small animal referral hospitals. J Vet Intern Med 2013;27:1392–1399. [DOI] [PubMed] [Google Scholar]
- 22. O'Neill DG, Church DB, McGreevy PD, et al. Prevalence of disorders recorded in cats attending primary‐care veterinary practices in England. Vet J 2014;202:286–291. [DOI] [PubMed] [Google Scholar]
- 23. Roumier M, Loustau V, Guillaud C, et al. Characteristics and outcome of warm autoimmune hemolytic anemia in adults: New insights based on a single‐center experience with 60 patients. Am J Hematol 2014;89:E150–E155. [DOI] [PubMed] [Google Scholar]
- 24. Piek CJ, van Spil WE, Junius G, et al. Lack of evidence of a beneficial effect of azathioprine in dogs treated with prednisolone for idiopathic immune‐mediated hemolytic anemia: A retrospective cohort study. BMC Vet Res 2011;7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vagace JM, Bajo R, Gervasini G. Diagnostic and therapeutic challenges of primary autoimmune haemolytic anaemia in children. Arch Dis Child 2014;99:668–673. [DOI] [PubMed] [Google Scholar]
- 26. Visco C, Barcellini W, Maura F, et al. Autoimmune cytopenias in chronic lymphocytic leukemia. Am J Hematol 2014;89:1055–1062. [DOI] [PubMed] [Google Scholar]
- 27. Weiss DJ. Differentiating benign and malignant causes of lymphocytosis in feline bone marrow. J Vet Intern Med 2005;19:855–859. [DOI] [PubMed] [Google Scholar]
- 28. Campbell MW, Hess PR, Williams LE. Chronic lymphocytic leukaemia in the cat: 18 cases (2000–2010). Vet Comp Oncol 2013;11:156–264. [DOI] [PubMed] [Google Scholar]
- 29. Weiss DJ. Bone marrow pathology in dogs and cats with non‐regenerative immune‐mediated haemolytic anaemia and pure red cell aplasia. J Comp Pathol 2008;138:46–53. [DOI] [PubMed] [Google Scholar]
- 30. Swann JW, Skelly BJ. Systematic review of prognostic factors for mortality in dogs with immune‐mediated hemolytic anemia. J Vet Intern Med 2015;29:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reimer ME, Troy GC, Warnick LD. Immune‐mediated hemolytic anemia: 70 cases (1988–1996). J Am Anim Hosp Assoc 1999;35:384–391. [DOI] [PubMed] [Google Scholar]
- 32. Weinkle TK, Center SA, Randolph JF, et al. Evaluation of prognostic factors, survival rates, and treatment protocols for immune‐mediated hemolytic anemia in dogs: 151 cases (1993–2002). J Vet Am Vet Med Assoc 2005;226:1869–1880. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparisons of hematologic and serum biochemical data from cats with primary IMHA with either regenerative anemia or persistently nonregenerative anemia. Values are median and interquartile range and comparisons are by Mann–Whitney U‐test unless otherwise stated. P < .0025 denote significance at the .05 level after Bonferroni correction.
Table S2. Starting doses of drugs used in the treatment of primary IMHA in cats. Numbers of cats differ from those stated in text as bodyweight was not recorded for all cases to enable calculation of dose rates.


