Abstract
Background
Exenatide extended release (ER) is a glucagon‐like peptide‐1 analogue that increases insulin secretion, inhibits glucagon secretion and induces satiation in humans with type 2 diabetes mellitus. The use of exenatide ER is safe and stimulates insulin secretion in healthy cats.
Objectives
The objective of this study is to assess the safety of exenatide ER and its effect on body weight, remission and metabolic control in newly diagnosed diabetic cats receiving insulin and a low‐carbohydrate diet.
Animals
Thirty client‐owned cats.
Methods
Prospective placebo‐controlled clinical trial. Cats were treated with exenatide ER or 0.9% saline, administered SC, once weekly. Both groups received insulin glargine and a low‐carbohydrate diet. Exenatide ER was administered for 16 weeks, or in cats that achieved remission it was given for 4 weeks after discontinuing insulin treatment. Nonparametric tests were used for statistical analysis.
Results
Cats in the exenatide ER and placebo groups had transient adverse signs including decreased appetite (60% vs. 20%, respectively, P = .06) and vomiting (53% vs. 40%, respectively, P = .715). Body weight increased significantly in the placebo group (P = .002), but not in cats receiving exenatide ER. Cats on exenatide ER achieved remission or good metabolic control in 40% or 89%, respectively, whereas in control cats percentages were 20% or 58% (P = .427 and P = .178, respectively).
Conclusion and clinical importance
Exenatide ER is safe in diabetic cats and does not result in weight gain. Our pilot study suggests that, should there be an additional clinically relevant beneficial effect of exenatide ER in insulin‐treated cats on rate of remission and good metabolic control, it would likely approximate 20% and 30%, respectively.
Keywords: feline, incretin, metabolic control, remission, treatment
Abbreviations
- DM
diabetes mellitus
- exenatide ER
exenatide extended release
- GLP‐1
glucagon‐like peptide‐1
- GIP
glucose‐dependent insulinotropic polypeptide
- IGF‐1
insulin‐like growth factor 1
- T4
thyroxine
- Spec fPL
feline pancreatic lipase
- DGGR
1,2‐o‐dilauryl‐rac‐glycero‐3‐glutaric acid‐(6′‐methylres‐orufin) ester
- BCS
body condition score
Incretins, such as glucagon‐like peptide‐1 (GLP‐1) and glucose‐dependent insulinotropic polypeptide (GIP), have been the focus of recent studies because of their beneficial role in glucose homeostasis. Incretins are gastrointestinal hormones released in response to food intake that increase glucose‐dependent insulin secretion in various species and have been shown to stimulate pancreatic β‐cell proliferation in rodents.1, 2 Additionally, they inhibit glucagon secretion, slow gastric emptying, induce satiation, and promote weight loss.2 However, the half‐lives of GLP‐1 and GIP are only a few minutes because they are rapidly cleaved by dipeptidylpeptidase‐4 (DPP‐4).2, 3 Incretin‐based therapies have been developed for humans with type 2 diabetes mellitus (DM). They include GLP‐1 receptor analogues that are resistant to rapid degradation of DPP‐4, such as liraglutide and exenatide or exenatide extended release (ER), and DPP‐4 inhibitors, such as sitagliptin and vildagliptin, which decrease the degradation of endogenous GLP‐1. Exenatide ER, which requires only once weekly dosing in humans with type 2 DM, was more efficacious at decreasing glycated hemoglobin, and it improved metabolic control and promoted weight loss more often than DPP‐4 inhibitors.4 Exenatide ER resulted in greater improvements in metabolic control, induced nausea and vomiting less frequently than exenatide (which must be given twice daily), but injection site irritation was more common.5, 6 Furthermore, exenatide ER currently is considered an effective second‐line treatment to metformin in humans with type 2 DM because it rarely induces hypoglycemia when used as monotherapy.4, 5, 6
To date, the biology and effects of incretins have been investigated only in healthy cats. In 1 study, GLP‐1 increased after PO glucose administration.7, 8 The DPP‐4 inhibitors were shown to decrease plasma glucagon concentration and to enhance insulin secretion in cats.9, 10 Administration of exenatide, exenatide ER, or liraglutide caused glucose‐dependent insulin secretion, and a decrease in body weight occurred after the administration of exenatide and liraglutide in cats.11, 12, 13, 14 A recent comparison of incretin‐based treatments in cats showed that exenatide and exenatide ER had more pronounced effects on insulin secretion than sitagliptin.15 Furthermore, exenatide ER was considered a better option than exenatide or sitagliptin because it is administered only once weekly and has fewer adverse effects.15
The goal of the present study was to determine whether the administration of exenatide ER is safe in newly diagnosed diabetic cats treated with insulin glargine and a low‐carbohydrate diet. In addition, the effect of exenatide ER on body weight, remission rate and metabolic control was investigated.
Materials and Methods
Animals
This prospective clinical trial used newly diagnosed diabetic cats admitted from January 2013 to January 2015. The diagnosis of DM was based on clinical signs and laboratory test results.16 Cats were excluded if they had received insulin or other antidiabetic medication for >4 weeks before admission, if glucocorticoids or progestagens had been given during the previous 3 months or if a concurrent disease such as renal failure, gastrointestinal disorder, heart disease, another endocrinopathy, or neoplasia was diagnosed. Diabetic cats with ketoacidosis or pancreatitis were included in the study if clinical signs resolved and their general condition improved within 48 hours of treatment. All cats underwent a thorough evaluation including physical examination, blood tests, and urinalysis (Table 1), blood pressure measurement, abdominal and thoracic radiography, and abdominal ultrasonography. A cutoff concentration of >5.3 μg/L was used for Spec fPL concentration.1 For 1,2‐o‐dilauryl‐rac‐glycero‐3‐glutaric acid‐(6′‐methylresorufin) ester (DGGR)‐lipase activity, the cutoff was set at >26 U/L based on the interval previously established in healthy cats (8–26 U/L).17 For insulin‐like growth factor 1 (IGF‐1), a cutoff concentration of >1000 ng/mL was used in accordance with laboratory guidelines.2 The study was approved by authorities in Switzerland (permission: 122/2011, 118/2014) and in Italy (permission: 16/79/2014, CE17/02/14). Informed consent was provided by owners.
Table 1.
Blood and urine examinations at baseline and during all follow‐up examinations
| Blood analysis | Baseline | Follow‐up |
|---|---|---|
| Complete blood cell count | + | + |
| Biochemical profile | + | + |
| Fructosamine | + | + |
| T4 | + | |
| Spec fPL | + | + |
| IGF‐1 | + | |
| Urinalysis with UPC and culture | + |
UPC, urine protein‐to‐creatinine ratio.
Treatment
Enrolled cases were alternately assigned to 1 of 2 treatment groups; the assignment sequence was known to the veterinarian. Both groups received insulin glargine3 and a low‐carbohydrate diet.4 The initial insulin dose was 1.0 IU q12 h in cats weighing <4 kg and 1.5–2.0 IU q12 h in cats ≥4 kg. One group received exenatide ER5 (200 μg/kg) and the other 0.33 mL of 0.9% saline solution (placebo); both treatments were administered SC once weekly. Owners were taught how to prepare and inject the exenatide ER or placebo but, as opposed to the supervising veterinarians, were unaware of the treatment group. If owners found this task problematic, a veterinarian administered the weekly injections.
Exenatide ER was administered for 16 weeks or, in cats that achieved diabetic remission, it was given for 4 weeks after discontinuing insulin treatment.
Follow‐up
Successive follow‐up evaluations were scheduled 1, 3, 6, 10, and 16 weeks after starting exenatide ER or placebo. Each evaluation included assessment of clinical signs, physical examination findings, blood analysis (Table 1) and generation of a glucose curve. Owners were instructed to perform home monitoring of blood glucose concentrations every 1–2 weeks starting 3 weeks after enrollment. To complete all curves, capillary blood glucose concentrations were measured every 2 hours for 8–12 hours using a validated portable blood glucose meter.18 The insulin dose was adjusted based on clinical signs and results of physical examination, glucose curves, and serum fructosamine concentration; the goal was to resolve clinical signs of DM and to obtain curves with blood glucose concentrations ranging between 80 and 270 mg/dL and fructosamine concentrations between 350 and 450 μmol/L.19 In cats that achieved remission, the insulin dose was decreased in increments of 0.5 IU per treatment, once weekly. The last dosage before insulin was discontinued was 0.5 IU once daily, for at least 1 week. The frequency, onset and duration of remission were recorded in both groups. The rate of metabolic control was evaluated at the end of the study. Criteria used to define remission and good metabolic control are presented in Table 2.
Table 2.
| Endpoint | Clinical signs | Fructosamine (μmol/L) | Glucose (mg/dL) | Insulin treatment |
|---|---|---|---|---|
| Remission | Absent | <350 | 72–162 | No (for ≥4 weeks) |
| Good metabolic control | Absent | 350–450 | 80–270 | Yes |
The frequency of hypoglycemia, defined as a blood glucose curve concentration ≤65 mg/dL, was determined. If owners observed clinical signs compatible with hypoglycemia (increased appetite, restlessness, weakness, unsteady gait, loss of consciousness, or twitching), they were instructed to measure capillary blood glucose concentration and to offer food or administer honey.
The median insulin dose per kg per day given during the study period was calculated for each cat. Two calculations were done; in 1 of them, the phases of remission were excluded, in the other 1, the phases of remission were included.
Statistical Analysis
Normality of datasets for continuous and ordered categorical variables was evaluated using the Shapiro‐Wilk normality test. Parameters with or without normal distribution were analyzed with parametric and nonparametric tests, respectively. Accordingly, data are presented as mean and standard deviation or as median and range. Distribution of sex and breed, frequency of cases treated with antidiabetic medications before inclusion, and frequency of ketoacidosis recorded at admission were compared between cats receiving exenatide ER and placebo using Fisher's exact test. The same test was used to compare the frequency of adverse effects and of hypoglycemic episodes, the rate and onset of remission, and the rate of good metabolic control between groups. This test also was used to compare the frequency of remission or good metabolic control between cats that had increased and decreased body weight within each treatment group, if sufficiently represented. Differences between groups for age, body weight, and laboratory results recorded at admission and for daily insulin dosage given over the 16‐week study period were analyzed using the Mann‐Whitney U‐test or t‐test. The same tests were used to compare concentrations of fructosamine, Spec fPL, DGGR‐lipase, and creatinine concentrations between groups at each follow‐up evaluation. Within each group, differences in body weight, body condition score (BCS)6 and fructosamine, Spec fPL, DGGR‐lipase, and creatinine concentrations between baseline and the end of the study were evaluated using the Wilcoxon signed rank test or paired t‐test. A commercial software program7 was used. Significance was set at P < .05.
Results
Animals
Of 52 cats with newly diagnosed DM admitted during the study period, 30 cats fulfilled the inclusion criteria and were enrolled. Twenty‐three were excluded because of acromegaly, hyperadrenocorticism, hyperthyroidism, hypertrophic cardiomyopathy, mesenteric lymph node abscess, neoplasia, pancreatitis, prior corticosteroid administration, relapse of diabetes or because they had received insulin for >4 weeks before admission. Each treatment group consisted of 15 cats. There were no significant differences between the 2 groups with regard to age, body weight, distribution of breeds, sex, or frequency of cats previously treated with antidiabetic medication (Table 3).
Table 3.
Signalment of diabetic cats and pretreatment with antidiabetic medications before inclusion in the study
| Animals | Exenatide ER (n = 15) | Placebo (n = 15) | P‐value |
|---|---|---|---|
| Age in years | |||
| Median (range) | 9.3 (4.3–14.0) | 10 (2.6–15.0) | .468 |
| Mean (SD) | 9.0 (3.2) | 9.8 (3.7) | |
| Body weight in kg | |||
| Median (range) | 5.3 (4.4–7.4) | 4.5 (2.7–8.3) | .110 |
| Mean (SD) | 5.5 (1.0) | 4.9 (1.5) | |
| Domestic short‐ or longhair | 12 (80%) | 14 (93.3%) | .598 |
| Purebred | 3 (20%) | 1 (6.7%) | |
| Neutered male | 9 (60%) | 5 (33.3%) | .143 |
| Intact male | 1 (6.7%) | 0 (0%) | |
| Spayed female | 5 (33.3%) | 10 (66.7%) | |
| Pretreatmenta | 8 (53.3%) | 10 (66.7%) | .710 |
SD, standard deviation.
Antidiabetic medications received before inclusion.
On admission, 9 cats had ketoacidosis, which resolved within 1–2 days of treatment; 3 were allocated to the exenatide ER group and 6 to placebo. The frequency of ketoacidosis did not differ between groups (P = .427). None of the cats died during the study.
Adverse effects
Adverse effects recorded in cats treated with exenatide ER and placebo are documented in Table 4. Most of the gastrointestinal adverse effects were self‐limiting. In 1 cat that vomited 4 weeks after enrollment, the referring veterinarian prescribed cimetidine. Vomiting subsided, but recurred during week 10, at which time exenatide ER treatment was discontinued. This cat was in good metabolic control 12 weeks after admission, but did not achieve remission. The frequency of adverse effects did not differ between groups (Table 4).
Table 4.
Type, time of occurrence, and duration of adverse effects in diabetic cats treated with exenatide ER or placebo
| Adverse effect | Exenatide ER | Placebo | P‐value | ||||
|---|---|---|---|---|---|---|---|
| Cats (n = 15) | Dpi | Between week | Cats (n = 15) | Dpi | Between week | ||
| Decreased appetite | 9 (60%) | 1–3 | 1–10 | 3 (20%) | 0–7 | 2–6 | .060 |
| Vomiting | 8 (53.3%) | 1–4 | 0–15 | 6 (40%) | 1–6 | 0–16 | .715 |
| Diarrhea | 1 (6.7%) | 6 | 1–14 | 3 (20%) | 1–3 | 1–14 | .598 |
| Increased sleeping | 5 (33.3%) | 1–3 | 1–6 | 1 (6.7%) | 1–3 | 0–6 | .169 |
| Hiding in dark areas | 3 (20%) | 0–3 | 0–3 | 1 (6.7%) | 1 | 9 | .598 |
Dpi, days postinjection.
Skin lesions were not observed at the injection site of exenatide ER or placebo‐treated cats. One cat treated with exenatide ER developed nonpruritic histiocytic panniculitis, which was deemed unrelated to drug administration because it was 5–10 cm away from the injection site.
Hypoglycemia
Fourteen of 15 cats (93.3%) treated with exenatide ER and 12 of 15 cats (80%) treated with placebo had episodes of hypoglycemia based on blood glucose curves; the frequency of hypoglycemic episodes did not differ between groups (P = .598). Median glucose concentration during hypoglycemia was 47 mg/dL (range, 25–65) in the exenatide ER group and 50 mg/dL (range, 29–63) in the placebo group. Most of the cats did not have clinical signs of hypoglycemia. However, 2 cats with hypoglycemia in the exenatide ER group showed clinical signs including decreased appetite, vomiting, and lethargy which disappeared after PO administration of honey. Episodes of hypoglycemia were observed more often in weeks 6 and 7 in the exenatide ER group and in weeks 8 to 12 in the placebo group (Fig 1), although significant differences were not identified.
Figure 1.
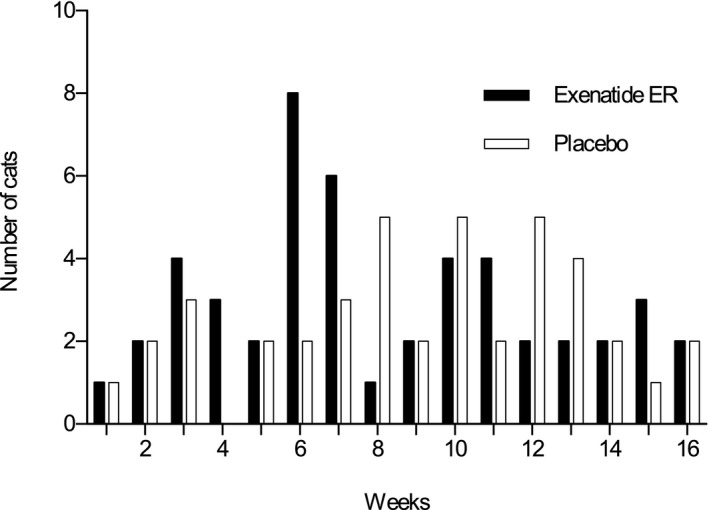
Number of diabetic cats with episodes of hypoglycemia during the 16‐week study period.
Laboratory Results and Insulin Dosage
Baseline results of a CBC, biochemical profile, urinalysis, and blood pressure measurement did not differ between groups (Table 5); positive urine culture was obtained in 1 (6.7%) cat in the exenatide ER group and in 2 (13.3%) in the placebo group.
Table 5.
Laboratory results and blood pressure measurements at baseline in diabetic cats treated with exenatide ER vs. placebo
| Variable | Exenatide ER | Placebo | Reference interval | P‐value | ||
|---|---|---|---|---|---|---|
| Median (range) | Mean (SD) | Median (range) | Mean (SD) | |||
| Haematocrit (%) | 37 (29–47) | 38 (4.9) | 34 (25–47) | 35 (6.7) | 33–45 | .268 |
| Leukocytes (103/μL) | 8.9 (3.2–20.5) | 9.3 (4.0) | 10.8 (4.8–22.1) | 11.6 (5.1) | 4.6–12.8 | .165 |
| Platelets (103/μL) | 340 (207–525) | 372 (108.5) | 347 (156–672) | 386 (143.2) | 180–680 | .923 |
| Glucose (mg/dL) | 329 (101–810) | 383 (174.0) | 428 (70–536) | 384 (135.6) | 72–162 | .543 |
| Fructosamine (μmol/L) | 620 (402–883) | 657 (162.4) | 582 (438–878) | 625 (134.0) | 202–340 | .604 |
| Cholesterol (mg/dL) | 256 (132–565) | 279 (113.6) | 267 (112–662) | 300 (150.0) | 101–263 | .000 |
| Triglycerides (mg/dL) | 79 (26–350) | 108 (96.8) | 70 (35–3478) | 578 (1104.2) | 26–114 | .537 |
| Total protein (g/dL) | 7.4 (6.4–11.4) | 7.7 (1.2) | 7.4 (6.3–8.3) | 7.3 (0.6) | 6.4–8.0 | .369 |
| Albumin (g/dL) | 3.6 (3.1–4.1) | 3.6 (0.3) | 3.5 (2.7–4.4) | 3.6 (0.4) | 3.0–4.0 | .760 |
| Urea (mg/dL) | 30.0 (17.6–52.1) | 30.3 (10.0) | 29.1 (21.6–37.2) | 28.9 (5.8) | 20.7–35.3 | .494 |
| Creatinine (mg/dL) | 1.1 (0.9–1.9) | 1.2 (0.3) | 1.2 (0.6–1.7) | 1.1 (0.3) | 1.1–1.8 | .460 |
| Sodium (mEq/L) | 157 (145–165) | 157 (5.5) | 158 (145–165) | 157 (4.9) | 158–165 | .848 |
| Chloride (mEq/L) | 118 (99–126) | 116 (7.4) | 116 (105–121) | 114 (5.1) | 121–131 | .280 |
| Potassium (mEq/L) | 4.7 (4.3–5.4) | 4.7 (0.3) | 4.8 (3.4–5.9) | 4.8 (0.8) | 3.8–5.4 | .690 |
| Phosphorus (mg/dL) | 4.5 (2.7–6.5) | 4.5 (0.9) | 4.2 (2.5–5.0) | 4.1 (0.7) | 2.8–5.6 | .130 |
| Calcium (mg/dL) | 10.3 (1.1–11.9) | 9.8 (2.5) | 10.4 (9.3–11.9) | 10.4 (0.7) | 9.6–11.2 | .810 |
| Bilirubin (mg/dL) | 0.09 (0.02–0.43) | 0.13 (0.1) | 0.08 (0.01–0.43) | 0.11 (0.1) | 0–0.2 | .747 |
| ALP (U/L) | 50 (30–112) | 54 (21.9) | 49 (32–92) | 54 (19.0) | 16–43 | .975 |
| ALAT (U/L) | 76 (34–424) | 122 (119.3 | 87 (33–379) | 110 (91.0) | 34–98 | .709 |
| ASAT (U/L) | 29 (19–215) | 45 (49.2) | 41 (17–133) | 48 (29.9) | 19–44 | .184 |
| DGGR‐Lipase (U/L) | 25 (11–92) | 29 (22.8) | 23 (13–48) | 25 (11.7) | 8–26 | .734 |
| Spec fPL (μg/L) | 2.2 (0.6–41.8) | 6.6 (11.0) | 3.0 (0.6–16.8) | 4.6 (4.4) | >5.3 | .878 |
| T4 (μg/L) | 1.5 (0.7–2.4) | 1.5 (0.5) | 1.4 (0.5–1.8) | 1.3 (0.5) | <3.5 | .434 |
| IGF‐1 (ng/mL)a | 372 (227–1163) | 524 (303.5) | 405 (167–1041) | 419 (223.6) | <1000 | .408 |
| UPCa | 0.22 (0.08–1.04) | 0.31 (0.3) | 0.2 (0.1–1.2) | 0.3 (0.3) | ≤0.40 | .913 |
| SAP (mm Hg)a | 144 (96–224) | 153 (42.2) | 145 (85–189) | 141 (30.1) | 80–160 | .573 |
SD, standard deviation; ALP, alkaline phosphatase; ALAT, alanine aminotransferase; ASAT, aspartate aminotransferase; UPC, urine protein‐to‐creatinine ratio; SAP, systolic arterial pressure.
IGF‐1, UPC, and SAP were increased in 3, 3, and 4 cats of the exenatide ER group, respectively, and in 1, 4, and 3 cats of the placebo group, respectively.
In cats treated with exenatide ER, the median fructosamine concentration was 620 μmol/L (range, 402–883) at baseline and 361 μmol/L (range, 267–483) after 16 weeks. In the placebo group, the median fructosamine concentration was 582 μmol/L (range, 438–878) at baseline and 377 μmol/L (range, 256–718) after 16 weeks. A significant decrease in serum fructosamine concentration was observed in both groups (P = .001, each). Differences between groups were not identified at any time point.
In both groups at the end of the study, concentrations of Spec fPL (exenatide ER, 1.8 μg/L; range, 0.6–25.4; placebo, 2.15 μg/L; range, 1.0–14.3; P = .115 and P = .650, respectively) and DGGR‐lipase (exenatide ER, 19 U/L; range, 10–89; placebo, 18.5 U/L; range, 12–56; P = .372 and P = .479, respectively) did not differ from baseline concentrations (Table 5). There was no difference in the concentration of either enzyme between groups at any time point (Fig 2). In the exenatide ER group, baseline Spec fPL concentration was higher than normal in 3 (20%) cats and remained increased throughout the study. The Spec fPL was higher than normal at 16 weeks in 1 of the 12 (8.3%) cats with a baseline Spec fPL concentration ≤5.3 μg/L. In the placebo group, the baseline Spec fPL concentration was higher than normal in 5 (33.3%) cats, and it remained increased throughout the study in 2 of them. Two of 10 (20%) cats with initial Spec fPL concentrations ≤5.3 μg/L had higher than normal Spec fPL concentrations after 16 weeks (Fig 2A). The concentration of serum DGGR‐lipase was measured in 23 of the 30 cats. In the exenatide ER group, baseline serum DGGR‐lipase concentration was higher than normal in 4 of 11 (36.4%) and remained increased throughout the study in 3 of them. In the placebo group, baseline serum DGGR‐lipase concentration was higher than normal in 5 of 12 (41.7%) cats and remained increased throughout the study in 4 of them (Fig 2B). The concentration of serum DGGR‐lipase did not increase by the end of the study in any of the cats that had normal baseline concentrations. Cats with increased Spec fPL or DGGR‐lipase concentrations did not have obvious clinical signs or ultrasonographic evidence of pancreatitis.
Figure 2.
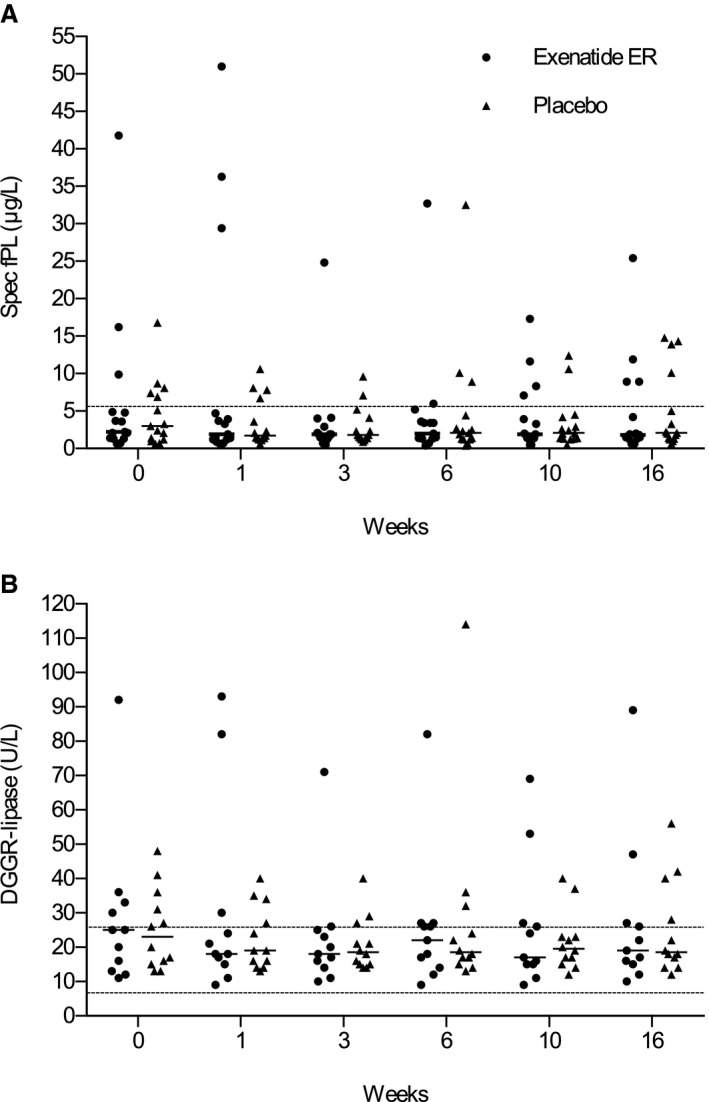
Serum concentration of Spec fPL (A) and DGGR‐lipase (B) in exenatide ER‐ and placebo‐treated cats during the 16‐week study period. Medians are shown. Dashed lines identify the reference interval. There were no differences between the groups.
In the exenatide ER group, serum creatinine concentration was slightly above the reference range in 1 (6.7%) cat at baseline but it normalized by the end of the study. Serum creatinine concentration increased significantly from baseline (Table 5) to the end of the study in both groups (exenatide ER, 1.4 mg/dL; range, 1.1–1.9; placebo, 1.4 mg/dL; range, 1.0–1.9; P = .012 and P = .005, respectively). In only 1 cat per group however did it slightly exceed the reference range. Differences between groups were not identified at any time point.
The median baseline concentration of IGF‐1 did not differ significantly between the exenatide ER and placebo groups (Table 5). One cat of each group had an IGF‐1 concentration >1000 ng/mL at week 0 (exenatide ER, 1163 ng/mL; placebo, 1041 ng/mL). At week 16, increased IGF‐1 concentrations decreased to normal in 1 cat (exenatide ER, 660 ng/mL) and remained increased in the other (placebo, 1136 ng/mL). None of the cats developed clinical signs of acromegaly; these cats had no organ enlargement on abdominal ultrasonography and the cat in the exenatide ER group had a normal pituitary gland on computed tomography of the head. These 2 cats achieved remission at weeks 8 and 10, respectively, and still are in remission at the time of writing (after approximately 1 year).
The median insulin dose administered to cats receiving exenatide ER or placebo throughout the entire study did not differ if phases of remission were excluded (exenatide ER, 0.41 IU/kg/day; range, 0.11–0.88; placebo, 0.38 IU/kg/day; range: 0.22–1.5; P = .663; Fig 3). The median insulin dose administered throughout the entire study in cats also did not differ if phases of remission were not excluded from the calculation (exenatide ER, 0.36 IU/kg/day; range, 0.07–0.88; placebo, 0.33 IU/kg/day; range, 0.13–1.5; P = 0.494).
Figure 3.
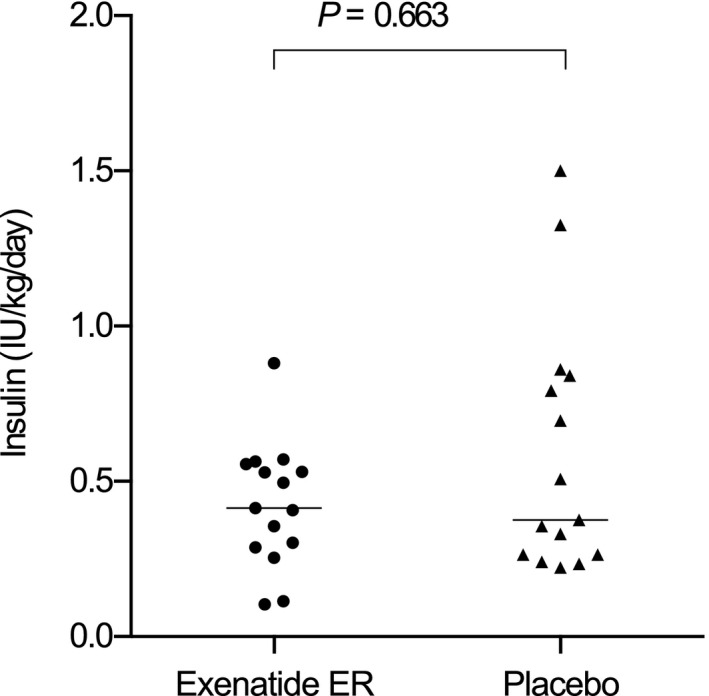
Dot plots of median insulin dose administered per kg body weight per day in cats receiving exenatide ER or placebo. Horizontal lines mark the group medians.
Remission and Metabolic Control
Remission of DM was achieved in 6 (40%) cats treated with exenatide ER and in 3 (20%) cats treated with placebo (P = .427). All cats that achieved remission had no relapse of DM at the end of the study. In the exenatide ER group, remission occurred 10–14 weeks (median, 11 weeks) after initiation of treatment, and in the placebo group, 8–10 weeks (median, 10) after initiation of treatment. The onset of remission did not differ between groups (Table 6). Good metabolic control was obtained in 8 of 9 (88.9%) nonremission cats in the exenatide ER group and in 7 of 12 (58.3%) nonremission cats in the placebo group (P = .178). If cats in remission were grouped together with those achieving good metabolic control, 14 of 15 (93.3%) diabetic cats treated with exenatide ER had remission or good metabolic control compared with 10 of 15 (66.7%) diabetic cats treated with placebo (P = .169). In the exenatide ER group, 2 additional cats only required insulin for 2 weeks (1 cat between weeks 10 and 12 and the other between weeks 14 and 16).
Table 6.
Onset of remission in diabetic cats treated with exenatide ER or placebo (P = .167)
| Onset of remission | Exenatide ER (number of cats) | Placebo (number of cats) |
|---|---|---|
| 0–6 weeks after discharge | 0 | 0 |
| 7–10 weeks after discharge | 2 | 3 |
| 11–16 weeks after discharge | 4 | 0 |
Body Weight
In the exenatide ER group, body weight decreased in 6 cats (40%; median, −0.22 kg [−3.3%]; range, −0.14 to −0.64 kg [−2.6 to −14.5%]), increased in 8 (53.3%; median, +0.89 kg [+18.4%]; range, +0.20 to +1.50 kg [+3.4 to +21.1%]), and did not change in 1 (6.7%) during the study period. In the placebo group, body weight decreased in 2 cats (13.3%; (median, −0.28 kg [−5.9%]; range, −0.2 to −0.35 kg [−4.4 to −7.3%]) and increased in 13 (86.7%; (median, +0.76 kg [+21.6%]; range, +0.18 to +2.00 kg [+4.1 to +34.2%]). Overall, median body weight increased in both groups during the study; the increase was significant in the placebo group (P = .002), but not in the exenatide ER‐treated cats (P = .084; Fig 4). Body condition score increased significantly in the placebo group (P = .002), but not in the exenatide ER group (P = .058; Table 7).
Figure 4.
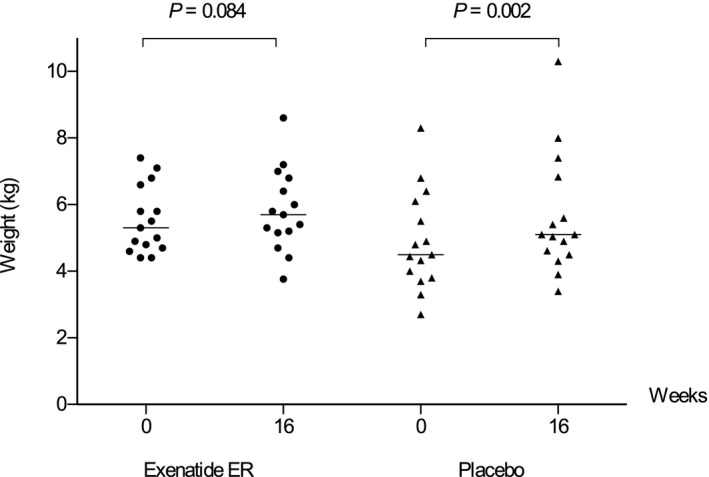
Body weight of cats of the exenatide ER and placebo groups at baseline and week 16. Horizontal lines indicate the median body weight.
Table 7.
Body condition score (BCS) in diabetic cats treated with exenatide ER or placebo at baseline (week 0) and at week 16. Within each group, cats are divided according to their body weight at week 16, which is compared to week 0 and reported as increased, decreased, or no change
| Group | Body weight after 16 weeks | Number of cats | BCS (1–9) | |
|---|---|---|---|---|
| Week 0 | Week 16 | |||
| Exenatide ER | Increased | 2 | 4 | 5 |
| 1 | 4 | 6 | ||
| 2 | 5 | 5 | ||
| 2 | 5 | 6 | ||
| 1 | 7 | 8 | ||
| Decreased | 2 | 4 | 4 | |
| 2 | 5 | 5 | ||
| 1 | 6 | 6 | ||
| 1 | 6 | 5 | ||
| No change | 1 | 5 | 5 | |
| Placebo | Increased | 1 | 3 | 4 |
| 1 | 3 | 5 | ||
| 3 | 4 | 5 | ||
| 3 | 5 | 6 | ||
| 2 | 6 | 6 | ||
| 1 | 6 | 8 | ||
| 1 | 7 | 8 | ||
| 1 | 7 | 9 | ||
| Decreased | 2 | 5 | 5 | |
In the exenatide ER group, among the 8 cats that gained body weight, 3 went into remission, 4 achieved good metabolic control and 1 did not achieve remission or good metabolic control. In the same group, among the 6 cats that lost body weight, 2 went into remission and 4 achieved good metabolic control. One additional cat had stable body weight and achieved remission. In the placebo group, among the 13 cats that gained body weight, 1 cat went into remission, 7 achieved good metabolic control and 5 did not achieve remission or good metabolic control. In the same group, the 2 cats that lost body weight achieved remission. In the exenatide ER group, remission or metabolic control was not associated with changes in body weight (P = 1.000). The analysis was not performed in the placebo group because of the small number of cats that lost body weight.
Discussion
In humans with type 2 DM, exenatide ER administration generally is well tolerated, but transient mild gastrointestinal adverse effects, which may in part be due to decreased gastric emptying, frequently are reported.5, 6, 21, 22 Gastrointestinal adverse effects of exenatide ER have been described recently in healthy cats15 and were commonly observed in these diabetic cats treated with the GLP‐1 analogue, but the frequency of adverse effects was not significantly higher than in the placebo group. In these diabetic cats treated with exenatide ER, the adverse effects usually were mild and self‐limiting, which is in agreement with findings in other species.3 Increased duration of sleeping and hiding in dark areas frequently were reported by owners of diabetic cats treated with exenatide ER, but these behaviors were observed in the placebo group as well. Because adverse effects were not associated with exenatide ER in the present study and none was moderate to severe or long‐lasting, we believe the GLP‐1 analogue can be safely used in diabetic cats. Of note, injection site irritation as well as nodular, eosinophil‐rich, granulomatous panniculitis at the injection site of exenatide has been described in humans.23, 24 To date, dermatologic irritation has not been reported in cats.11, 13, 15 Skin nodules that were identified in 1 of our diabetic cats were likely unrelated to exenatide ER injections. The lesions were nonpruritic, 5 to 10 cm from the injection site, and diagnosed histologically as histiocytic panniculitis.
There have been concerns about incretin‐based therapies and pancreatitis in humans, but studies did not find a significant association.25, 26 The Food and Drug Administration and the European Medicines Agency recently stated that it is currently unclear whether there is a causal relationship between incretin‐based drugs and pancreatitis.27 To our knowledge, a possible association between incretin‐based treatment and pancreatitis in cats has not been investigated. Previous investigations showed that many cats with DM may have subclinical pancreatitis.28, 29 In our study, some cats treated with exenatide ER had clinical signs compatible with pancreatitis that included decreased appetite and vomiting, but only 1 (8.3%) cat with normal baseline Spec fPL and DGGR‐lipase concentrations had an increase in Spec fPL concentration during treatment. In our study, to identify a significant difference for pancreatitis based on increased Spec fPL and assuming a 10% prevalence caused by exenatide ER, sample size should have been approximately 50 cats per group according to a statistical power calculation.
An increase in serum creatinine concentration has been reported in some humans treated with GLP‐1 analogues, which suggests that renal failure may occur or worsen during treatment. Based on this information, exenatide treatment is not recommended, particularly in human patients with severe renal impairment.8 In the present study, the increase in serum creatinine concentration did not differ between the 2 groups. In most cats, serum creatinine concentrations stayed within the normal range. However, diabetic cats with relevant kidney disease were excluded; it is therefore possible that an increase in serum creatinine concentration would have occurred if diabetic cats with renal failure had been included. The significant increase of creatinine observed in either group, although not above the reference range, might be explained by the fact that body weight, and possibly muscle mass, increased in most cats during insulin treatment.
An investigation of type 2 diabetic humans receiving insulin glargine and short‐acting exenatide or placebo showed that the incidence of hypoglycemic episodes was similar in both groups.30 In healthy cats treated with exenatide ER, hypoglycemia was observed, whereas clinical evidence of hypoglycemia did not occur.13 Similarly, in the present study hypoglycemia was a common finding, but there was no difference between the 2 groups. Clinical signs of hypoglycemia were observed in 2 cats of the exenatide ER group. Because these were 2 single episodes that were short‐lived and there was no difference compared with placebo, we hypothesize that exenatide ER is safe when used in conjunction with insulin to treat diabetic cats.
Compared with the results of other studies of diabetic cats, the remission rates in the present investigation were relatively low in both groups. Other studies have followed diabetic cats for longer periods of time (eg 6 months) than our study (4 months),31, 32 and it therefore is possible that more cats would have achieved remission if the study had been longer. In addition, it is known that the rate of remission is higher if diabetic cats have received corticosteroids before diagnosis of DM.19 In the present study, cats with prior corticosteroid administration were not included, whereas in 1 study with high remission rate such cats were included.33 This difference in selection criteria may explain at least in part the different remission rates. Furthermore, our definition of remission was the maintenance of euglycemia without insulin treatment for 4 weeks, whereas another study defined remission as 2 weeks of euglycemia without insulin.33 If we had used this latter definition, there would have been 2 additional cats in remission in the exenatide ER group (but none in the placebo group) and thus, the rate of remission would have increased from 40% to 53.3% (8 of 15 cats). A recent literature review on feline diabetes suggested adopting the definition of diabetic remission used in the present study.34
In humans with type 2 DM, exenatide ER once weekly was superior to other GLP‐1 analogues in controlling blood glucose concentrations.5, 6 Exenatide ER, in addition to PO administered antidiabetic agents, also was able to provide better glycemic control than insulin detemir.35 The present study shows that the percentage of cats that achieved good metabolic control was higher in the exenatide ER group than in the placebo group (93.3% vs. 66.7%, respectively) when cats in remission were grouped together with those with good metabolic control. The difference between groups was not significant because of the relatively low number of cats. According to the calculation of statistical power and sample size and assuming the same percentages of remission and good metabolic control, 28 instead of 14 cats per group would have been necessary to achieve statistical significance.7
In humans with type 2 DM, treatment with GLP‐1 analogues is associated with weight loss, whereas insulin glargine treatment is associated with weight gain.35 Weight loss was described in healthy cats during treatment with exenatide or liraglutide.12, 14 In our study, a significant weight gain and increase in BCS were observed in cats of the placebo group, likely due to the anabolic effect of insulin treatment, but the increase was not significant in the exenatide ER group. Prevention of weight gain is advised in diabetic cats with a high BCS, whereas weight gain to normalize body condition is preferable for diabetic cats with low BCS. In general, exenatide ER administration was linked to increased BCS in cats with low baseline BCS, but not in cats with high baseline BCS. In humans treated with GLP‐1 analogues, weight control can be attributed to a reduction in appetite because of increased satiation and a delay in gastric emptying.21, 36 In cats of our study treated with exenatide ER weight control was not associated with remission or metabolic control.
Our study had some limitations including the lack of veterinarian blinding and the relatively low number of cats. Significant differences for rates of remission and good metabolic control may have been observed with a larger number of diabetic cats. Furthermore, the follow‐up period was set at 4 months and it is possible that more cats would have achieved remission or good metabolic control if a longer study period had been chosen. In addition, the 2 cats with increased IGF‐1 might have had acromegaly. However, compatible clinical signs, abdominal organ enlargement, or pituitary gland abnormalities were not detected and both cats achieved long‐lasting remission. The IGF‐1 concentration was measured again at the end of the study and was normal in 1 cat and remained increased in the other. An IGF‐1 concentration above the cutoff (>1000 ng/ml) currently is considered highly suspicious for acromegaly.37 Therefore, although compatible clinical findings were not documented, the hypothesis of acromegaly could not be entirely excluded in both cats. Finally, it is also worth noting that costs of exenatide ER might limit its use, and a month of treatment using the present protocol costs approximately $150 USD.
In summary, exenatide ER was not associated with local or systemic adverse effects and can be safely used in diabetic cats. Exenatide ER treatment does not result in a significant increase in body weight. Our study also suggests that, should there be an additional significant beneficial effect of exenatide ER in insulin‐treated cats on rate of remission and good metabolic control, it would likely be of a magnitude of approximately 20% and 30%, respectively. These results enable calculation of statistical power and sample size for a definitive study.
Acknowledgments
Conflict of Interest Declaration
Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
The study was performed at the Clinic for Small Animal Internal Medicine, Vetsuisse Faculty, University of Zurich, Switzerland, and at the Department of Veterinary Medical Sciences, University of Bologna, Italy.
The abstract has been presented at the 24th Congress of the European College of Veterinary Internal Medicine – Companion Animals 2014 in Mainz, Germany.
Footnotes
NationWide, Specialist Laboratories, Cambridge, UK
Lantus, Sanofi Aventis, Meyrin, Switzerland
DM Purina Veterinary Diets; Medical Solution, Steinhausen, Switzerland
Bydureon; Amylin Pharmaceuticals, San Diego, CA
Body condition chart (cat); Nestlé Purina, St. Louis, MO
SPSS 18.0; SPSS, Chicago, IL
References
- 1. Buteau J, Foisy S, Joly E, et al. Glucagon‐like peptide 1 induces pancreatic β‐cell proliferation via transactivation of the epidermal growth factor receptor. Diabetes 2003;52:124–132. [DOI] [PubMed] [Google Scholar]
- 2. Baggio LL, Drucker DJ. Biology of incretins: GLP‐1 and GIP. Gastroenterology 2007;132:2131–2157. [DOI] [PubMed] [Google Scholar]
- 3. Nauck MA. Incretin‐based therapies for type 2 diabetes mellitus: Properties, functions, and clinical implications. Am J Med 2011;124:3–18. [DOI] [PubMed] [Google Scholar]
- 4. Bergenstal RM, Wysham C, MacConell L, et al. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION‐2): a randomised trial. Lancet 2010;376:431–439. [DOI] [PubMed] [Google Scholar]
- 5. Blevins T, Pullman J, Malloy J, et al. DURATION‐5: exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes. J Clin Endocrinol Metab 2011;96:1301–1310. [DOI] [PubMed] [Google Scholar]
- 6. Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open‐label, non‐inferiority study. Lancet 2008;372:1240–1250. [DOI] [PubMed] [Google Scholar]
- 7. Hoenig M, Jordan ET, Ferguson DC, et al. Oral glucose leads to a differential response in glucose, insulin, and GLP‐1 in lean versus obese cats. Domest Anim Endocrinol 2010;38:95–102. [DOI] [PubMed] [Google Scholar]
- 8. Gilor C, Graves TK, Gilor S, et al. The incretin effect in cats: Comparison between oral glucose, lipids, and amino acids. Domest Anim Endocrinol 2011;40:205–212. [DOI] [PubMed] [Google Scholar]
- 9. Furrer D, Kaufmann K, Tschuor F, et al. The dipeptidyl peptidase IV inhibitor NVP‐DPP728 reduces plasma glucagon concentration in cats. Vet J 2010;183:355–357. [DOI] [PubMed] [Google Scholar]
- 10. Nishii N, Takashima S, Iguchi A, et al. Effects of sitagliptin on plasma incretin concentrations after glucose administration through an esophagostomy tube or feeding in healthy cats. Domest Anim Endocrinol 2014;49:14–19. [DOI] [PubMed] [Google Scholar]
- 11. Gilor C, Graves TK, Gilor S, et al. The GLP‐1 mimetic exenatide potentiates insulin secretion in healthy cats. Domest Anim Endocrinol 2011;41:42–49. [DOI] [PubMed] [Google Scholar]
- 12. Seyfert TM, Brunker JD, Maxwell LK, et al. Effects of a glucagon‐like peptide‐1 mimetic (exenatide) in healthy cats. Intern J Appl Res Vet Med 2012;10:147–156. [Google Scholar]
- 13. Rudinsky AJ, Adin CA, Borin‐Crivellenti S, et al. Pharmacology of the glucagon‐like peptide‐1 analog exenatide extended‐release in healthy cats. Domest Anim Endocrinol 2015;51:78–85. [DOI] [PubMed] [Google Scholar]
- 14. Hall MJ, Adin CA, Borin‐Crivellenti S, et al. Pharmacokinetics and pharmacodynamics of the glucagon‐like peptide‐1 analog liraglutide in healthy cats. Domest Anim Endocrinol 2015;51:114–121. [DOI] [PubMed] [Google Scholar]
- 15. Padrutt I, Lutz TA, Reusch CE, et al. Effects of the glucagon‐like peptide‐1 (GLP‐1) analogues exenatide, exenatide extended‐release, and of the dipeptidylpeptidase‐4 (DPP‐4) inhibitor sitagliptin on glucose metabolism in healthy cats. Res Vet Sci 2015;99:23–29. [DOI] [PubMed] [Google Scholar]
- 16. Tschuor F, Zini E, Schellenberg S, et al. Remission of diabetes mellitus in cats cannot be predicted by the arginine stimulation test. J Vet Intern Med 2011;25:83–89. [DOI] [PubMed] [Google Scholar]
- 17. Oppliger S, Hartnack S, Riond B, et al. Agreement of the serum Spec fPLTM and 1,2‐o‐dilauryl‐rac‐glycero‐3‐glutaric acid‐(6'‐methylresorufin) ester lipase assay for the determination of serum lipase in cats with suspicion of pancreatitis. J Vet Intern Med 2013;27:1077–1082. [DOI] [PubMed] [Google Scholar]
- 18. Zini E, Moretti S, Tschuor F, et al. Evaluation of a new portable glucose meter designed for the use in cats. Schweizer Archiv für Tierheilkunde 2009;15:448–451. [DOI] [PubMed] [Google Scholar]
- 19. Reusch CE. Feline diabetes mellitus In: Feldman EC, Nelson RW, Reusch CE, Scott‐Moncrieff JC, eds. Textbook of Canine and Feline Endocrinology, 4th ed St. Louis, MO: Saunders; 2015:258–314. [Google Scholar]
- 20. Sieber‐Ruckstuhl NS, Kley S, Tschuor F, et al. Remission of diabetes mellitus in cats with diabetic ketoacidosis. J Vet Intern Med 2008;22:1326–1332. [DOI] [PubMed] [Google Scholar]
- 21. Campbell RK. Clarifying the role of incretin‐based therapies in the treatment of type 2 diabetes mellitus. Clin Ther 2011;33:511–527. [DOI] [PubMed] [Google Scholar]
- 22. Näslund E, Gutniak M, Skogar S, et al. Glucagon‐like peptide 1 increases the period of postprandial satiety and slows gastric emptying in obese men. Am J Clin Nutr 1998;68:525–530. [DOI] [PubMed] [Google Scholar]
- 23. Boysen NC, Stone MS. Eosinophil‐rich granulomatous panniculitis caused by exenatide injection. J Cutan Pathol 2014;41:63–65. [DOI] [PubMed] [Google Scholar]
- 24. Shan SJ, Guo Y. Exenatide‐induced eosinophilic sclerosing lipogranuloma at the injection site. Am J Dermatopathol 2014;36:510–512. [DOI] [PubMed] [Google Scholar]
- 25. Romley JA, Goldman DP, Solomon M, et al. Exenatide therapy and the risk of pancreatitis and pancreatic cancer in a privately insured population. Diabetes Technol Ther 2012;14:904–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alves C, Batel‐Marques F, Macedo AF. A meta‐analysis of serious adverse events reported with exenatide and liraglutide: acute pancreatitis and cancer. Diabetes Res Clin Pract 2012;98:271–284. [DOI] [PubMed] [Google Scholar]
- 27. Egan AG, Blind E, Dunder K, et al. Pancreatic safety of incretin‐based drugs – FDA and EMA assessment. N Engl J Med 2014;370:794–797. [DOI] [PubMed] [Google Scholar]
- 28. Zini E, Hafner M, Kook P, et al. Longitudinal evaluation of serum pancreatic enzymes and ultrasonographic findings in diabetic cats without clinically relevant pancreatitis at diagnosis. J Vet Intern Med 2015;29:589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Forcada Y, German AJ, Noble PJM, et al. Determination of serum fPLI concentrations in cats with diabetes mellitus. J Feline Med Surg 2008;10:480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Buse JB, Bergenstal RM, Glass LC, et al. Use of twice‐daily exenatide in basal insulin‐treated patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med 2011;154:103–112. [DOI] [PubMed] [Google Scholar]
- 31. Zini E, Hafner M, Osto M, et al. Predictors of clinical remission in cats with diabetes mellitus. J Vet Intern Med 2010;24:1314–1321. [DOI] [PubMed] [Google Scholar]
- 32. Hafner M, Dietiker‐Moretti S, Kaufmann K, et al. Intensive intravenous infusion of insulin in diabetic cats. J Vet Intern Med 2014;28:1753–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Roomp K, Rand J. Intensive blood glucose control is safe and effective in diabetic cats using home monitoring and treatment with glargine. J Feline Med Surg 2009;11:668–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gostelow R, Forcada Y, Graves T, et al. Systemic review of feline diabetic remission: separating fact from opinion. Vet J 2014;202:208–221. [DOI] [PubMed] [Google Scholar]
- 35. Davies M, Heller S, Sreenan S, et al. Once‐weekly exenatide versus once‐ or twice‐daily insulin detemir: randomized, open‐label, clinical trial of efficacy and safety in patients with type 2 diabetes treated with metformin alone or in combination with sulfonylureas. Diabetes Care 2013;36:1368–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Drucker DJ, Nauck MA. The incretin system: glucagon‐like peptide‐1 receptor agonists and dipeptidyl peptidase‐4 inhibitors in type 2 diabetes. Lancet 2006;368:1696–1705. [DOI] [PubMed] [Google Scholar]
- 37. Niessen SJ, Forcada Y, Mantis P, et al. Studying cat (Felis catus) diabetes: beware of the acromegalic imposter. PLoS ONE 2015;10:e0127794. [DOI] [PMC free article] [PubMed] [Google Scholar]


