Abstract
Background
Diagnosis of pancreatitis in dogs is complicated by extrapancreatic disorders that can alter the results of laboratory tests. Extrapancreatic disorders can also affect the diagnosis of exocrine pancreatic insufficiency (EPI). The effects of acute kidney injury (AKI) on pancreas‐specific lipase activity (Spec cPL ® Test), serum lipase activity and trypsin‐like immunoreactivity (TLI) in dogs have not been evaluated.
Hypothesis/Objectives
Serum Spec cPL, lipase activity, and TLI concentrations will increase secondary to decreased kidney function.
Animals
Five purpose‐bred dogs.
Methods
Experimental prospective study. Gentamicin was used to induce AKI in 5 purpose‐bred dogs. Serum samples were collected for measurement of creatinine, Spec cPL, lipase activity and TLI over 60 days, during both induction of, and recovery from, AKI.
Results
All dogs developed and recovered from AKI. Six of 52 (12%) serum Spec cPL concentrations were increased (2 in the equivocal zone and 4 consistent with pancreatitis) in 2 of 5 (40%) dogs. Two of 51 (4%) serum lipase activity values were increased in 2 of 5 dogs. Serum TLI was increased above the reference range in 17 of 50 (34%) samples in 3 of 5 dogs. For all biomarkers, there was no consistent correlation with increases in serum creatinine concentration.
Conclusions and Clinical Importance
Decreased renal excretion during experimental AKI did not cause consistent and correlated increases in serum Spec cPL, lipase activity, or TLI in this cohort of dogs.
Keywords: Creatinine, Exocrine pancreatic insufficiency, Pancreatitis, Spec cPL
Abbreviations
- AKI
acute kidney injury
- CKD
chronic kidney disease
- EPI
exocrine pancreatic insufficiency
- GFR
glomerular filtration rate
- Spec cPL
Spec cPL® Test; canine pancreas‐specific lipase concentration
- TLI
trypsin‐like immunoreactivity
- VMTH
Veterinary Medical Teaching Hospital
Acute pancreatitis occurs commonly in dogs. Affected dogs can present with a wide spectrum of clinical signs and physical examination findings, including lethargy, anorexia, vomiting, diarrhea, and abdominal pain.1 The definitive antemortem diagnosis of acute pancreatitis remains challenging, despite the availability of improved serologic testing and abdominal imaging modalities.2, 3, 4, 5 Pancreatic histopathology is the gold standard for definitive diagnosis of pancreatitis; however, procurement of pancreatic tissue is invasive, expensive, and impractical in a debilitated dog. Additionally, multifocal disease within the pancreas and the lack of a uniformly accepted classification scheme for pancreatic histopathology in veterinary medicine further limits the utility of histopathology for diagnosis of pancreatitis.6
In the absence of histopathology, the history, physical examination, abdominal ultrasound, and serum biochemical testing remain the current mainstays for diagnosis of pancreatitis in veterinary medicine.1 Historically, serum lipase activity, amylase activity, and trypsin‐like immunoreactivity (TLI) have been used to diagnose pancreatitis, and TLI can be included in a comprehensive gastrointestinal panel with canine pancreas‐specific lipase concentration (Spec cPL® Test) to help evaluate exocrine pancreatic function. These biomarkers can be affected by extrapancreatic diseases, which can lead to decreased specificity.7 One of the speculated causes for this decreased specificity is the effect of renal dysfunction, but this has not been fully evaluated in veterinary medicine. The Spec cPL® Test is reported to be the most sensitive (65–94%) and specific (66–100%) noninvasive biomarker currently available for the diagnosis of pancreatitis in dogs2, 4, 8, 9, 10, 11; 1 study reported a sensitivity of 21% with mild pancreatitis.9
Acute kidney injury (AKI) and pancreatitis can lead to similar clinical signs. In addition, these 2 conditions are common comorbidities, as AKI is a well‐documented cause of pancreatitis via ischemia and hypovolemia, and pancreatitis can lead to AKI via hypovolemia and cytokine‐induced ischemia and inflammation.12, 13 As with other serological biomarkers of pancreatitis, it is plausible that decreased glomerular filtration rate (GFR), as seen with AKI, could result in Spec cPL increase secondary to decreased renal clearance, in the absence of pancreatitis. The influence of altered renal function on the serum concentration of canine pancreatic lipase immunoreactivity (cPLI), a test similar to Spec cPL, was evaluated in 17 dogs with experimentally induced chronic kidney disease (CKD).14 Results showed a positive correlation between serum creatinine and cPLI; however, the clinical utility of the test was not affected by CKD when tested at a single time point in these dogs.14 There has been no critical evaluation of Spec cPL, serum lipase activity or TLI in dogs with AKI, and the sensitivity and specificity of these tests in affected dogs is therefore unknown.
The aim of this study was to evaluate serum Spec cPL concentrations, lipase activity, and TLI in dogs during induction of, and recovery from, AKI. It was hypothesized that experimentally induced AKI would lead to increases in serum Spec cPL concentrations, lipase activity, and TLI as a consequence of decreased renal excretion.
Materials and Methods
Dogs
Six 2‐year‐old female intact, purpose‐bred hound dogs were screened for inclusion by physical examination, complete blood count, serum chemistry panel, urinalysis, and bacterial urine culture and sensitivity. This prospective study was approved by the University of California‐Davis Institutional Animal Care and Use Committee (IACUC).
Induction of AKI and Monitoring
Acute kidney injury was induced by gentamicin administration at 8 mg/kg subcutaneously q8 hours. If AKI was not demonstrated after 7 days, the gentamicin dose was increased to 10 mg/kg subcutaneously q8 hours.15, 16 This dose was maintained until induction of AKI, which was defined by a 50% increase in creatinine above the baseline (day 0) value.17, 18, 19
During the course of the study, dogs were monitored for development of any abnormal clinical signs, including hypodipsia and hyporexia. For any dog that developed hypodipsia, based on the subjective assessment of the attending clinician, supplemental subcutaneous fluid administration was instituted to prevent dehydration, hypovolemia, or both. This was done in an attempt to maintain adequate renal perfusion and facilitate recovery from AKI.
Blood and Urine Collection
Blood was collected via jugular or lateral saphenous venipuncture from each dog for evaluation of serum creatinine and BUN concentrations, and urine was collected by cystocentesis or free catch for measurement of USG at the following time points: day 0 (defined as the day before gentamicin administration), twice daily (at 8 am and 4 pm) during induction of AKI, once daily for 4–6 days after AKI was documented, and then on days 26, 28, 31, 35, 39, 46, and 60. After collection, blood and urine samples were immediately placed on ice before centrifugation in a refrigerated centrifuge. Serum creatinine, BUN, and USG were analyzed the day of collection at the VMTH laboratory.1 Remaining serum and urine samples were stored at −80°C and select serum samples were subsequently submitted for batched biomarker analysis.
Pancreatic Biomarker Analysis
In order to analyze biomarkers during both AKI induction and recovery, creatinine was used to estimate changes in GFR, and subsequently, renal excretion. Time points for biomarker analysis were selected based on changes in serum creatinine concentration compared to baseline and compared to maximum serum creatinine concentration.20 Stored serum samples from each dog were submitted frozen on dry ice to a commercial laboratory2 for batched measurements of serum Spec cPL, lipase activity, and TLI at the following time points: (1) baseline serum creatinine concentration before gentamicin administration (day 0), (2) the first documented increase in serum creatinine concentration above 25%, 50%, 75%, and 100% of the baseline creatinine concentration, (3) the maximum creatinine concentration, (4) during the recovery phase of AKI, as reflected by the first documented decrease in serum creatinine of 25%, 50%, and 75% below the maximum creatinine concentration, (5) at the end of the study (day 60). After preliminary Spec cPL results were analyzed, additional serum samples were then submitted for Spec cPL, lipase activity, and TLI analysis in dogs with Spec cPL increases, in order to further elucidate the relationship between these biomarkers and creatinine.
Spec cPL was assayed by a monoclonal antibody sandwich ELISA as previously described.5 According to the manufacturer, the assay has 3 ranges of interpretation: 0–200 μg/L is within the normal reference range; 201–399 μg/L is an equivocal zone; and ≥400 μg/L is consistent with pancreatitis. Serum lipase activity (reference range 138–755 U/L) was analyzed using a 1,2‐diglyceride enzymatic/colorimetric assay,3 and TLI (reference range 5–35 μg/L) was analyzed using a chemiluminescence immunoassay.4
Statistical Analysis
Linear regressions between creatinine concentration and biomarkers (ie, Spec cPL, lipase activity and TLI) were evaluated both individually for each dog, and as a group analysis (all dogs) with Student's t‐tests to determine if the regression and correlation coefficients (r) were significantly different from 0. For group analysis, linear regressions with robust variance estimation to account for the replicate measurements within individual dogs were similarly used to test if the correlation coefficients were significantly different from 0. P values <.05 were considered statistically significant. Data were analyzed using Stata/IC 13.1 software.5
Results
Baseline Renal Function
One dog was excluded from the study because of the presence of an E. coli bacterial cystitis, and therefore, 5 dogs were enrolled. Before gentamicin administration, all 5 dogs had unremarkable biochemistry analyses. Median baseline creatinine concentration was 1.0 mg/dL (range 0.7–1.0 mg/dL; reference range 0.8–1.5 mg/dL1) and median baseline USG was 1.046 (range 1.024–1.050).
Induction of Acute Kidney Injury
All dogs developed AKI within 14–20 days (median 14 days) after gentamicin commencement, and all dogs required the increased gentamicin dose (10 mg/kg subcutaneously q 8 hours). The median serum creatinine concentration at the peak of the AKI was 4.2 mg/dL (range 1.4–5.1 mg/dL; reference range 0.8–1.5 mg/dL) and the mean peak serum creatinine concentration was 3.5 ± 1.67 mg/dL. One dog did not become azotemic, but did develop AKI with a 100% increase above baseline creatinine, from 0.7 to 1.4 mg/dL. Serum creatinine concentrations were higher than the upper limit of the reference range in 27 of 52 (52%) serum samples. Additionally, 35 of 52 (67%) serum creatinine concentrations documented AKI. Median duration to development of isosthenuria (USG ≤ 1.012) was 12 days (range 7–13 days).
Dogs weighed a median of 22.3 kg (range 20.0–22.7 kg) and were administered lactated ringers solution at approximately 50–100 mL/kg/day (1 L subcutaneously q 12–24 hours per dog) when hypodipsia was noted. Mild hyporexia also occurred when hypodipsia was present. Fluid administration was indicated only during the recovery phase of AKI, with a median of 5 (range 1–9) fluid administrations per dog. The start of the recovery phase was defined when serum creatinine first decreased after its peak value and occurred at a median of 23 days (range 21–24 days).
Pancreatic Biomarker Analysis
Based on the above mentioned criteria for sample selection for biomarker analysis, it was anticipated to have 10 samples measured per dog; however, sample overlap (eg, 100% above baseline creatinine also being the maximum creatinine value) eliminated a total of 7 samples. Therefore, in 3 dogs, 9 samples were initially evaluated, and in 2 dogs, 8 samples were initially evaluated. In the 2 dogs with increased Spec cPL concentrations noted after initial evaluation, additional serum samples were submitted, for a total of 12 samples in 1 dog and 14 samples in the other dog. These additional serum samples were from days 19, 21, 24, and 39 for 1 dog and days 18, 19, 22, 28, and 35 for the other dog.
Baseline Biomarker Results
Before induction of AKI, all dogs had baseline Spec cPL concentrations within the reference range (0–200 μg/L) with a median concentration of 51 μg/L (range 31–108 μg/L) and a mean of 64.8 ± 33.4 μg/L. Median serum lipase activity at baseline was 338 U/L (range 220–779 U/L; reference range 138–755) with a mean of 457 ± 252 U/L. One dog had an increased serum lipase activity at baseline (779 U/L); however, the Spec cPL (42 μg/L) and TLI (27.8 μg/L) concentrations were within their respective reference ranges. The median serum TLI concentration at baseline was 27.4 μg/L (range 22.7–48.5 μg/L; reference range 5–35) with a mean of 30.3 ± 10.3 μg/L. One dog had an increased baseline serum TLI concentration (48.5 μg/L); however, baseline Spec cPL (108 μg/L) and serum lipase activity (220 U/L) were within the reference ranges for this dog.
Biomarkers During AKI Induction and Recovery
A total of 52 serum samples from the 5 dogs were analyzed for Spec cPL concentrations (Fig 1). Spec cPL was in the equivocal range (201–399 μg/L) in 2 of 52 (4%) samples and was ≥400 μg/L in 4 of 52 (8%) samples. These increases were documented in 2 of 5 dogs. For each of these 2 dogs, the increases in Spec cPL concentration occurred sequentially, and values before and after these increases were within the normal reference range. Only 1 of these 2 dogs had a significant positive linear correlation between Spec cPL and serum creatinine concentration (r = 0.704, P = .005) (Table 1). The highest documented serum creatinine concentration for all dogs was 5.1 mg/dL, which was correspondent with a normal Spec cPL of 178 μg/L. Spec cPL remained <200 μg/L throughout the study in the other 3 dogs and there was no significant linear correlation between Spec cPL and serum creatinine in these 3 dogs (Table 1, Fig 2). When analyzing the data as a group, there was minimal and nonsignificant correlation between serum creatinine concentration and Spec cPL in the 5 dogs (r = 0.235, P = .095) (Fig 1).
Figure 1.
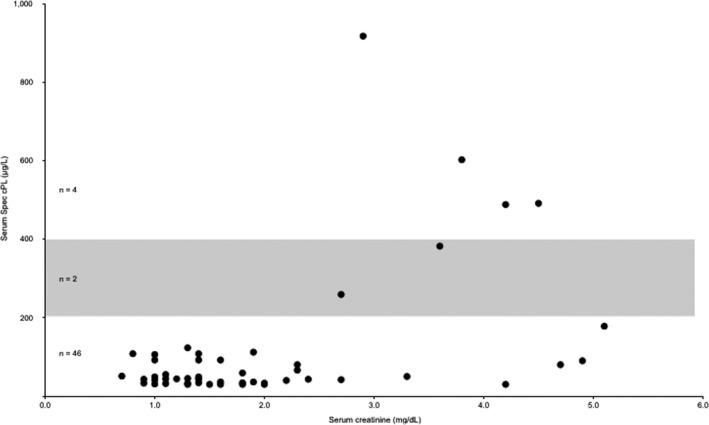
Scatter plot representing creatinine concentrations and Spec cPL concentrations for all 5 dogs. Creatinine reference range (0.8–1.5 mg/dL). Spec cPL reference range (0–200 μg/L). Spec cPL equivocal range (201–399 μg/L) denoted by shaded box.
Table 1.
Correlation coefficients (r) and P‐values (Ho: r=0) for serum creatinine with each biomarker
| Dog(s) | Creat:Spec cPL | Creat:Lipase | Creat:TLI | |||
|---|---|---|---|---|---|---|
| r | P Value | r | P Value | r | P Value | |
| 1 | 0.224 | .56 | −0.761 | .02 | −0.266 | .53 |
| 2 | 0.562 | .06 | 0.211 | .53 | 0.887 | <.001 |
| 3 | −0.393 | .30 | −0.652 | .06 | −0.706 | .03 |
| 4 | 0.704 | .01 | 0.600 | .02 | 0.115 | .70 |
| 5 | 0.161 | .70 | 0.528 | .18 | −0.906 | .00 |
| All dogs | 0.235 | .10 | 0.190 | .51 | 0.458 | .06 |
Creat, serum creatinine concentration; Spec cPL, serum canine pancreas‐specific lipase activity; Lipase, serum lipase activity; TLI, serum trypsin‐like immunoreactivity.
Figure 2.
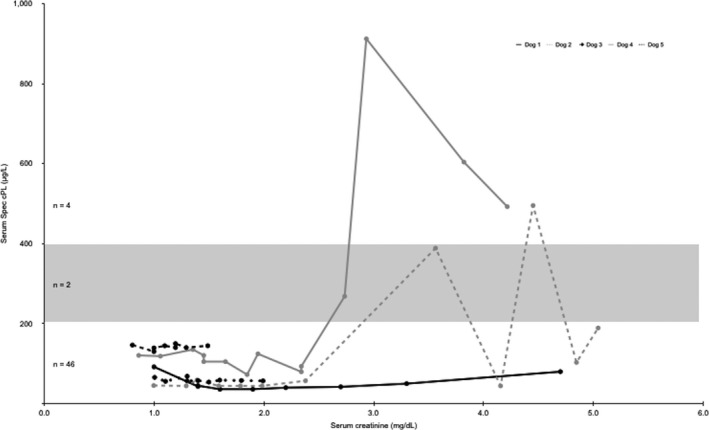
Serum creatinine concentrations and Spec cPL concentrations for each dog (N = 5). Creatinine reference range (0.8–1.5 mg/dL). Spec cPL reference range (0–200 μg/L). Spec cPL equivocal range (201–399 μg/L) denoted by shaded box.
Serum lipase activity was increased in 2 of 51 (4%) samples evaluated (Fig 3). These 2 increases occurred in 2 dogs. In 1 dog, the increased lipase activity was documented at the baseline measurement when creatinine concentration was 1.0 mg/dL, and concurrent Spec cPL (42 μg/L) and TLI (27.8 μg/L) were within the reference range. There was a negative linear correlation between lipase activity and creatinine in this dog (r = −0.652, P = .057). In the second dog with an increased lipase activity, there was a positive moderate linear correlation between serum lipase activity and creatinine (r = 0.600, P = .023). When analyzing the data as a group, there was minimal and nonsignificant correlation between serum creatinine concentration and lipase activity in the 5 dogs (r = 0.190, P = .51).
Figure 3.
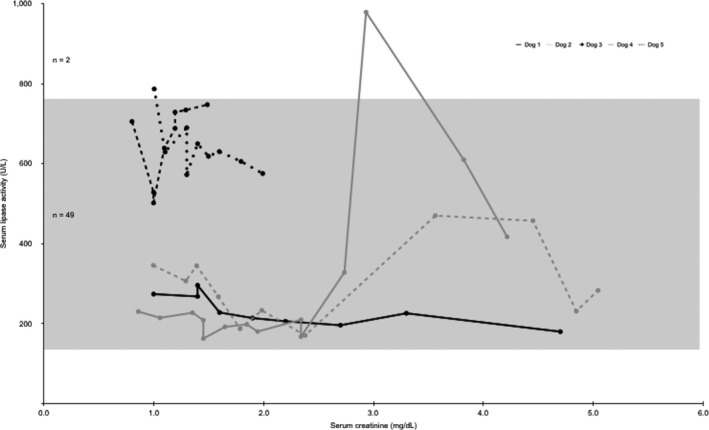
Serum creatinine concentrations and lipase activities for each dog (N = 5). Creatinine reference range (0.8–1.5 mg/dL). Lipase activity reference range (138–755 U/L) denoted by the shaded box.
Trypsin‐like immunoreactivity was increased in 17 of 50 (34%) samples evaluated (Fig 4). These increases occurred in 3 dogs. One dog had 1 of 8 TLI values increased (35.7 μg/L), which occurred when concurrent serum creatinine concentration was 1.4 mg/dL. A second dog had 4 of 11 TLI samples increased; this was the only dog to show a positive linear correlation between TLI and serum creatinine concentration (r = 0.887, P < .001). A third dog had 12 of 14 TLI values increased, including at baseline. This dog had no clinical signs of pancreatitis at the beginning or end of the study. These maximal biomarker increases did not correlate with the highest creatinine concentration. When analyzing the data as a group, there was moderate and nonsignificant correlation between serum creatinine and TLI in the 5 dogs (r = 0.458, P = .056).
Figure 4.
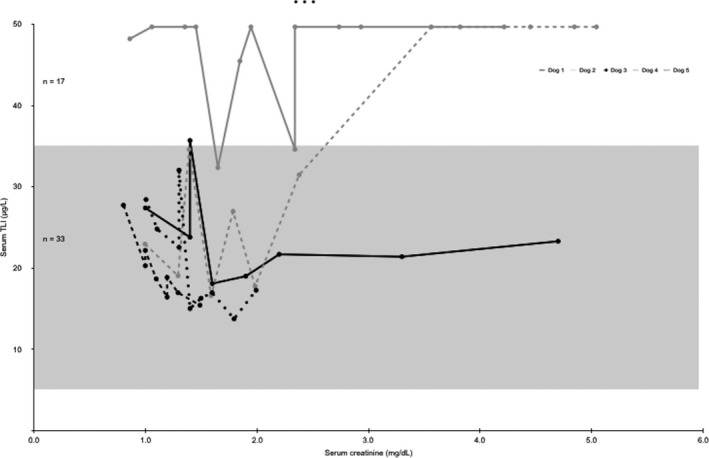
Serum creatinine and TLI concentrations for each dog (N = 5). Creatinine reference range (0.8–1.5 mg/dL). TLI reference range (5–35 μg/L) denoted by the shaded box.
Recovery from AKI
All 5 dogs recovered from AKI, with final creatinine concentrations at day 60 ranging from 0.9 to 1.4 mg/dL (reference range 0.8–1.5 mg/dL), and final USG measurements ranging from 1.022 to 1.039. Spec cPL and lipase activities were within the normal reference range in all dogs at the final time point evaluated. One dog had an increased TLI at the final time point (day 60) and represented the same dog with an increased TLI at baseline.
Discussion
This study showed that experimentally induced AKI did not cause consistent and correlated increases in Spec cPL, lipase activity, or TLI in dogs. Results also showed that decreased renal excretion during AKI, as documented by increases in serum creatinine, did not affect measurement of these biomarkers in this cohort of dogs. This finding is similar to previous studies in dogs with CKD.14, 21 Because of the complex metabolism of these pancreatic biomarkers and the possible effects of extrapancreatic factors on their measurement, it is important to evaluate them under various disease conditions, including AKI, so that their use in clinical practice can be optimized.
A previous study evaluated serum amylase and lipase activities in dogs with either naturally acquired or surgically induced CKD.21 Results of that study demonstrated a 3‐fold increase in mean serum lipase activity in 18 dogs with surgically induced CKD and a 4‐fold increase in mean serum lipase activity in 41 dogs with naturally occurring CKD; however, these increases were not correlated with serum creatinine. Another study evaluated serum lipase activity and serum cPLI in 17 dogs with experimentally induced CKD.14 In that study, serum lipase activity and cPLI concentrations were measured at a single time point for each dog and tested serum samples were stored frozen for approximately 25 years before analysis. No significant increases in serum lipase activity were found; however, statistically significant, but not clinically relevant (ie, not outside the reference range) increases in serum cPLI concentrations were observed.
When evaluating the effects of renal dysfunction on these biomarkers, it is useful to understand their renal handling under normal physiologic conditions. Serum concentrations of these biomarkers are affected by their generation (ie, prerenal factors, such as pancreatic production and enzyme activation) and by both renal and nonrenal metabolism. Renal handling is affected by biomarker size and charge (ie, factors affecting permselectivity and glomerular ultrafiltration), and by renal tubular handling, (ie, reabsorption). Canine pancreas‐specific lipase (~50,700 MW), trypsinogen (~25,000 MW), and lipase (~50,000 MW) have a molecular size smaller than serum albumin, and with regard to glomerular permselectivity are therefore considered to be low molecular weight molecules. Consequently, the filtration rate and sieving coefficients of these biomarkers is relatively high.22 A reduction of glomerular filtration in association with renal dysfunction would therefore be expected to decrease their relative filtration and increase their serum concentration. Nevertheless, several studies in dogs with CKD have not supported this prediction.14, 21 In addition to alterations in filtration, renal tubular damage can lead to a decrease in tubular uptake of these biomarkers, with a subsequent increase in their concentration in the urine.23
In this study, serum biomarkers used currently (Spec cPL) or historically (lipase and TLI) for the diagnosis of pancreatitis were evaluated. Although lipase activity and TLI have poor performance for diagnosis of pancreatitis, they are still included on common biochemical and gastrointestinal panels and their historical use as biomarkers of pancreatitis prompted their inclusion in this study. In addition, TLI is used to diagnosis EPI, and the effects of AKI on this biomarker have not been evaluated. These 3 biomarkers were evaluated at multiple time points and severities of AKI, during both AKI induction, and recovery from AKI. The spectrum of renal injury achieved in these dogs spanned International Renal Interest Society (IRIS) Grades I–IV of AKI, representing a broad range of disease severity.17 It was possible to correlate changes in creatinine, as an estimation of GFR, with assessment of these biomarkers.20
Spec cPL concentrations were increased >400 μg/L in only 4 of 52 samples (8%), derived from 2 dogs. In contrast, AKI was documented in 35 of 52 (67%) samples. Group analysis revealed a minimal and nonsignificant correlation between serum creatinine concentration and Spec cPL. Three of 5 dogs demonstrated no increases in Spec cPL concentration above the reference range despite development of AKI, evidenced by maximum serum creatinine concentrations that were at least double the baseline value. The increased serum creatinine concentrations represent significant kidney dysfunction and are predictive of significant decreases in GFR.20 The absence of Spec cPL increases in these 3 dogs suggests that acute decreases in renal excretion did not affect the measurement of serum Spec cPL in dogs with the grades of AKI achieved in this study. Additionally, in the 2 dogs with a total of only 4 increased Spec cPL concentrations, maximum Spec cPL concentrations did not correlate with maximum creatinine concentrations, as would be expected if Spec cPL increases were caused by decreased renal excretion. This finding, combined with the observation that 3 dogs maintained normal Spec cPL concentrations throughout the study and the absence of significant statistical correlation between creatinine and Spec cPL, suggests nonrenal mechanisms (including the possibility of active pancreatitis) for the Spec cPL increases in these 2 dogs. In these same 2 dogs, the Spec cPL increases occurred sequentially, further supporting that the increases may have been caused by the transient development of pancreatitis. If measurement of Spec cPL were significantly affected by decreased renal excretion, all dogs would have been expected to demonstrate consistent increases in Spec cPL correlated with the increase in serum creatinine concentrations. The results of this study demonstrate that the measurement of Spec cPL is not mechanistically influenced by acute decreases in kidney function. This suggests that increases of Spec cPL in dogs with the degree of AKI achieved in this study could be specific for pancreatitis, provided that false positive results are excluded, including other comorbidities that might affect Spec cPL.
Likewise, the measurement of serum lipase activity and TLI was not consistently affected by decreased renal excretion in this cohort of dogs, similar to previous experimental studies in dogs with CKD.24, 25 It important to note, however, that the correlation between serum creatinine and TLI in the 5 dogs as a group, nearly achieved statistical significance at P = .056, and it is possible that a larger number of dogs would have led to significant positive correlation between these variables. Nonetheless, results of this study demonstrate that the grades of AKI achieved, did not consistently influence the circulating concentrations of TLI. It is well documented that trypsin (the active form of trypsinogen) can bind to inhibitory proteins, and the large molecular weight of these circulating complexes prevents ultrafiltration by an intact glomerular membrane.26 In disease states such as renal failure, it is likely that there are complex physiologic alterations, including increased reticulendothelial clearance, alterations in glomerular permselectivity and the presence of trypsin‐protease complexes that influence the measured serum concentration of this biomarker. Studies in human patients with chronic renal disease have documented a marked increase in serum trypsinogen concentration relative to healthy controls.27 In addition, the mean circulating trypsinogen concentration is increased more frequently and to a higher level than amylase or lipase in these human patients and this is thought to be secondary to increased pancreatic release, decreased renal clearance, or a combination of both; despite this speculation, a definitive causal relationship between increased serum trypsinogen and decreased renal clearance has not been made. Differences in size and charge among amylase, lipase, and trypsinogen did not fully explain the differences observed in the magnitude of the increase of these enzymes in humans, and it is highly plausible that extrarenal clearance mechanisms and net rates of marker production may be different for each enzyme.28, 29
There were several limitations to this study including the enrollment of only 5 dogs. Second, pancreatic ultrasonography was not performed to evaluate for the presence of pancreatitis, making it difficult to definitively explain the causes for the increases in Spec cPL that occurred in 4 of the 52 serum samples. It is reasonable to speculate that these observations in 2 dogs represented either false positive results or the development of mild pancreatitis after the experimental procedure and development of AKI, or the development of AKI. In addition, it would have been optimal to quantify water intake in all dogs to more accurately assess for the presence of hypodipsia.
In conclusion, serum Spec cPL, lipase activity, and TLI appear to be unaffected by decreased renal excretion in experimental AKI. Additional studies are warranted in a larger cohort of dogs with naturally occurring AKI.
Acknowledgments
The study was supported in part by a grant from the Center for Companion Animal Health (CCAH), School of Veterinary Medicine, University of California‐Davis. The authors acknowledge the support of Dr. Jane Robertson and Michelle Clemens of IDEXX Laboratories Inc., Sacramento, CA.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Gentamicin was used for induction of acute kidney injury and not for treatment of a susceptible infection.
Work completed at the University of California‐Davis, School of Veterinary Medicine, One Shields Avenue, Davis, CA 95616.
This study was presented as an oral abstract at the 2014 ACVIM Forum, Nashville, TN.
Footnotes
University of California‐Davis, Veterinary Medical Teaching Hospital Laboratory, Davis, CA
IDEXX Laboratories Inc., Sacramento, CA
Beckman Coulter, Inc., Brea, CA
Siemens Medical Solutions USA, Inc., Malvern, PA
Stata/IC 13.1 software, StataCorp LP, College Station, TX
References
- 1. Hess RS, Saunders HM, Van Winkle TJ, et al. Clinical, clinicopathologic, radiographic, and ultrasonographic abnormalities in dogs with fatal acute pancreatitis: 70 cases (1986–1995). J Am Vet Med Assoc 1998;213:665–670. [PubMed] [Google Scholar]
- 2. Steiner JM, Williams DA. Development and validation of a radioimmunoassay for the measurement of canine pancreatic lipase immunoreactivity in serum of dogs. Am J Vet Res 2003;64:1237–1241. [DOI] [PubMed] [Google Scholar]
- 3. Steiner JM, Teague SR, Williams DA. Development and analytic validation of an enzyme‐linked immunosorbent assay for the measurement of canine pancreatic lipase immunoreactivity in serum. Can J Vet Res 2003;67:175–182. [PMC free article] [PubMed] [Google Scholar]
- 4. Kook PH, Kohler N, Hartnack S, et al. Agreement of serum Spec cPL with the 1,2‐o‐dilauryl‐rac‐glycero glutaric acid‐(6′‐methylresorufin) ester (DGGR) lipase assay and with pancreatic ultrasonography in dogs with suspected pancreatitis. J Vet Intern Med 2014;28:863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huth SP, Relford R, Steiner JM, et al. Analytical validation of an ELISA for measurement of canine pancreas‐specific lipase. Vet Clin Pathol 2010;39:346–353. [DOI] [PubMed] [Google Scholar]
- 6. Newman S, Steiner J, Woosley K, et al. Localization of pancreatic inflammation and necrosis in dogs. J Vet Intern Med 2004;18:488–493. [DOI] [PubMed] [Google Scholar]
- 7. Junge W, Mályusz M, Ehrens HJ. The role of the kidney in the elimination of pancreatic lipase and amylase from blood. J Clin Chem Clin Biochem 1985;23:387–392. [DOI] [PubMed] [Google Scholar]
- 8. Xenoulis PG, Steiner JM. Canine and feline pancreatic lipase immunoreactivity. Vet Clin Pathol 2012;41:312–324. [DOI] [PubMed] [Google Scholar]
- 9. Trivedi S, Marks SL, Kass PH, et al. Sensitivity and specificity of canine pancreas‐specific lipase (cPL) and other markers for pancreatitis in 70 dogs with and without histopathologic evidence of pancreatitis. J Vet Intern Med 2011;25:1241–1247. [DOI] [PubMed] [Google Scholar]
- 10. McCord K, Morley PS, Armstrong J, et al. A multi‐institutional study evaluating the diagnostic utility of the spec cPL™ and SNAP® cPL™ in clinical acute pancreatitis in 84 dogs. J Vet Intern Med 2012;26:888–896. [DOI] [PubMed] [Google Scholar]
- 11. Steiner JM, Newman S, Xenoulis P, et al. Sensitivity of serum markers for pancreatitis in dogs with macroscopic evidence of pancreatitis. Vet Ther 2008;9:263–273. [PubMed] [Google Scholar]
- 12. Mansfield C. Pathophysiology of acute pancreatitis: potential application from experimental models and human medicine to dogs. J Vet Intern Med 2012;26:875–887. [DOI] [PubMed] [Google Scholar]
- 13. Satake K, Kanazawa G, Hiura A, et al. Renal function in experimentally induced acute pancreatitis in dogs: how it is affected by the nephrotoxic substance in pancreatic exudate from ascitic fluid. Jpn J Surg 1991;21:88–95. [DOI] [PubMed] [Google Scholar]
- 14. Steiner JM, Finco DR, Williams DA. Serum lipase activity and canine pancreatic lipase immunoreactivity (cPLI) concentration in dogs with experimentally induced chronic renal failure. Vet Res 2010;3:58–63. [Google Scholar]
- 15. Grauer GF, Greco DS, Behrend EN, et al. Effects of dietary protein conditioning on gentamicin‐induced nephrotoxicosis in healthy male dogs. Am J Vet Res 1994;55:90–97. [PubMed] [Google Scholar]
- 16. Grauer GF, Greco DS, Behrend EN, et al. Estimation of quantitative enzymuria in dogs with gentamicin‐induced neprhotoxicosis using urine enzyme/creatinine ratios from spot urine samples. J Vet Intern Med 1995;9:324–327. [DOI] [PubMed] [Google Scholar]
- 17. International Renal Interest Society . [Internet]. Grading of acute kidney injury. 2013. Available from: http://www.iris-kidney.com/guidelines/grading.shtml. Accessed January 20, 2015.
- 18. Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004;8:204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Finco DR, Brown SA, Vaden SL, Ferguson DC. Relationship between plasma creatinine concentration and glomerular filtration rate in dogs. J Vet Pharmacol Ther 1995;18:418–421. [DOI] [PubMed] [Google Scholar]
- 21. Polzin DJ, Osborne CA, Stevens JB, Hayden DW. Serum amylase and lipase activities in dogs with chronic primary renal failure. Am J Vet Res 1983;44:404–410. [PubMed] [Google Scholar]
- 22. Fabris C, Basso D, Naccarato R. Urinary enzymes excretion in pancreatic diseases: clinical role and pathophysiological considerations. J Clin Gastroenterol 1992;14:281–284. [PubMed] [Google Scholar]
- 23. Maack T, Johnson V, Kau ST, et al. Renal filtration, transport, and metabolism of low‐molecular‐weight proteins: a review. Kidney Int 1979;16:251–279. [DOI] [PubMed] [Google Scholar]
- 24. Hudson EB, Strombeck DR. Effects of functional nephrectomy on the disappearance rates of canine serum amylase and lipase. Am J Vet Res 1978;39:1316–1321. [PubMed] [Google Scholar]
- 25. Dozzi DL. Origin of blood amylase and blood lipase in the dog. Arch Intern Med 1941;68:232–240. [Google Scholar]
- 26. Fabris C, Piccoli A, Farini R, et al. Trypsin plasma‐urine transfer – a preliminary study. Clin Chim Acta 1981;114:101–105. [DOI] [PubMed] [Google Scholar]
- 27. Fahrenkrug J, Staun‐Olsen P, Magid E. Immunoreactive trypsin and pancreatic isoamylase activity in serum of patients with chronic renal failure or hepatic cirrhosis. Clin Chem 1981;27:1655–1657. [PubMed] [Google Scholar]
- 28. Kimmel PL, Tenner S, Habwe VQ, et al. Trypsinogen and other pancreatic enzymes in patients with renal disease: a comparison of high‐efficiency hemodialysis and continuous ambulatory peritoneal dialysis. Pancreas 1995;10:325–330. [DOI] [PubMed] [Google Scholar]
- 29. Ohlsson K, Tegner H. Anionic and cationic dog trypsin: isolation and partial characterization. Biochim Biophys Acta 1973;317:328–337. [DOI] [PubMed] [Google Scholar]


