Abstract
Background
Sucralfate impairs absorption of ciprofloxacin and other fluoroquinolones in humans, but no sucralfate–fluoroquinolone interaction has been reported in dogs. Veterinary formularies recommend avoiding concurrent administration of these medications, which might impact compliance, therapeutic success, and resistance selection from fluoroquinolones.
Objectives
To determine whether a drug interaction exists when sucralfate is administered to fed dogs concurrently with ciprofloxacin or enrofloxacin, and whether a 2 hour delay between fluoroquinolone and sucralfate affects fluoroquinolone absorption.
Animals
Five healthy Greyhounds housed in a research colony.
Methods
This was a randomized crossover study. Treatments included oral ciprofloxacin (C) or oral enrofloxacin (E) alone, each fluoroquinolone concurrently with an oral suspension of sucralfate (CS, ES), and sucralfate suspension 2 hours after each fluoroquinolone (C2S, E2S). Fluoroquinolone concentrations were evaluated using liquid chromatography with mass spectrometry.
Results
Drug exposure of ciprofloxacin was highly variable (AUC 5.52–22.47 h μg/mL) compared to enrofloxacin (AUC 3.86–7.50 h μg/mL). The mean relative bioavailability for ciprofloxacin and concurrent sucralfate was 48% (range 8–143%) compared to ciprofloxacin alone. Relative bioavailability of ciprofloxacin improved to 87% (range 37–333%) when sucralfate was delayed by 2 hours. By contrast, relative bioavailability for enrofloxacin and concurrent sucralfate was 104% (94–115%).
Conclusions and Clinical Importance
A possible clinically relevant drug interaction for the relative bioavailability of ciprofloxacin with sucralfate was found. No significant difference in bioavailability was documented for enrofloxacin with sucralfate. Further research is warranted in fasted dogs and clinical cases requiring enrofloxacin or other approved fluoroquinolones in combination with sucralfate.
Keywords: Canine, Drug interaction, Fluoroquinolone, Pharmacokinetics
Abbreviations
- AUC
area under the curve extrapolated to infinity
- AUC extrap
% AUC extrapolated to infinity
- C
treatment group receiving ciprofloxacin alone
- Cl/F
clearance per fraction of the dose absorbed
- CS
treatment group receiving concurrent ciprofloxacin and sucralfate
- C2S
treatment group receiving ciprofloxacin followed 2 hours later by sucralfate
- CMAX
maximum plasma concentration
- CV
coefficient of variation
- E
treatment group receiving enrofloxacin alone
- ES
treatment group receiving concurrent enrofloxacin and sucralfate
- E2S
treatment group receiving enrofloxacin followed 2 hours later by sucralfate
- MRT
mean residence time extrapolated to infinity
- TMAX
time to maximum plasma concentration
- T1/2
terminal half‐life
- μg
microgram
- μL
microliter
Enrofloxacin is a fluoroquinolone with a good spectrum for gram‐negative bacteria, some gram‐positive, and intracellular bacteria.1 Enrofloxacin1 was the first fluoroquinolone developed for and labeled for use in dogs. Recently, an approved bioequivalent generic2 formulation of enrofloxacin has become available. Enrofloxacin is partially metabolized to ciprofloxacin in dogs, which is an active metabolite and contributes to the antimicrobial activity of enrofloxacin.2, 3 Generic formulations of human labeled ciprofloxacin have been investigated in dogs; however, oral absorption of ciprofloxacin tablets by dogs is variable.4
Some dogs require gastroprotection and fluoroquinolones concurrently. Sucralfate is a frequently prescribed gastroprotectant that dissociates into sucrose octasulfate and aluminum hydroxide, which has antacid effects. Dosing recommendations of sucralfate for dogs are derived from human medicine and based on empirical practice in veterinary medicine. Sucralfate is often administered to dogs as an oral suspension, supported by a study finding fragments of sucralfate tablets in canine feces after oral administration of sucralfate tablets.5
A drug interaction is an altered pharmacologic response to a drug caused by concurrent administration of other drugs, and can be pharmacokinetic (absorption, distribution, metabolism, elimination), pharmacodynamic (antagonistic, synergistic), or pharmaceutic (incompatible drugs) in nature.6 Current veterinary formularies cite a potential interaction between sucralfate and both fluoroquinolone and tetracycline antimicrobials, caused by binding of the aluminum component of sucralfate with the antimicrobial, resulting in non‐absorbable chelate complexes and decreased bioavailability of the antimicrobial.7, 8 Interactions between sucralfate and both doxycycline and minocycline have been verified in dogs5, 9; however, research to validate an interaction of sucralfate with fluoroquinolones in dogs has not been reported.
The interaction between fluoroquinolones and sucralfate is well documented in humans. The relative bioavailability of ciprofloxacin is 4% compared to ciprofloxacin alone in human volunteers administered ciprofloxacin with sucralfate concurrently.10 Relative bioavailability improves to 83% when a 2‐hour delay is implemented between oral ciprofloxacin and sucralfate, and to 96% with a 6‐hour delay, leading to the recommendation of at least a 2‐hour delay.10 Similarly, when sucralfate and ciprofloxacin are administered concurrently to healthy human subjects, serum ciprofloxacin concentrations are reduced, with AUC0–12 decreasing from 8.8 to 1.1 μg h/mL (P < .005).11 This interaction in humans occurs with other fluoroquinolones, including norfloxacin, ofloxacin, moxifloxacin, sparfloxacin, and fleroxacin.12, 13, 14, 15, 16 When norfloxacin or ofloxacin is administered 2 hours before sucralfate, no effect on bioavailability is seen.12 Based on extrapolation from these human studies, it has been recommended that sucralfate be delayed up to 2 hours after fluoroquinolone administration to dogs.7, 8
The purpose of this study was to determine whether concurrent administration of sucralfate and fluoroquinolone (ciprofloxacin or enrofloxacin) affects the extent of fluoroquinolone absorption. Ciprofloxacin is not approved for dogs, but was included because of its known drug interaction with sucralfate in humans. Enrofloxacin was chosen because it is approved and commonly used in dogs. A second objective was to determine if administration of the fluoroquinolone 2 hours before sucralfate administration would result in a difference in extent of fluoroquinolone absorption.
Materials and Methods
Animals
Five healthy Greyhounds were included in the study. Three were neutered males and two were spayed females. Their ages ranged from 4 to 5 years old, and body weights ranged from 30.4 to 42.0 kg. The Institutional Care and Use Committee at Kansas State University approved the study.
Drug Administration and Sample Collection
This study used a randomized crossover design, with three treatment groups for each fluoroquinolone. All 5 dogs received all six treatment groups. Ciprofloxacin crossovers were performed first, and when completed were followed by enrofloxacin crossovers. A random numbers table was used to determine the order of treatments for each dog within each fluoroquinolone crossover. A washout period of at least 2 weeks was included between groups.11 Ciprofloxacin3 treatments included: Group C: ciprofloxacin (25 mg/kg PO) alone; Group CS: sucralfate 1 g suspension administered PO q8h starting 24 hours before ciprofloxacin, administered concurrently with ciprofloxacin (25 mg/kg PO), and sucralfate suspension continued q8h for two additional doses; and Group C2S: sucralfate one 1 g suspension administered PO q8h starting 24 hours before ciprofloxacin, administered 2 hours after ciprofloxacin (25 mg/kg PO), and sucralfate suspension continued q8h for two additional doses.
Enrofloxacin2 treatments included: Group E: enrofloxacin (5 mg/kg PO) alone; Group ES: sucralfate one 1 g suspension administered PO q8h starting 24 hours before enrofloxacin, administered concurrently with enrofloxacin (5 mg/kg PO), and sucralfate suspension continued q8h for two additional doses; and Group E2S: sucralfate one 1 g suspension administered PO q8h starting 24 hours before enrofloxacin, administered 2 hours after enrofloxacin (5 mg/kg PO), and sucralfate suspension continued q8h for two additional doses.
A target dose of 25 mg/kg ciprofloxacin3 was obtained using 250 and 500 mg tablets rounding to the nearest whole tablet/s size. A target dose of 5 mg/kg enrofloxacin2 was obtained using 22.7 and 135 mg flavored tablets, rounding to the nearest whole tablet/s size. All dogs were offered enrofloxacin tablets for consumption; and if they did not ingest them on their own, hand‐administration of pills was performed (pilled) by the researchers as suggested on the drug's package insert. Sucralfate suspension (200 mg/ml) was made by suspending one 1 g tablet of sucralfate4 in 5 mL water and shaking until fully dissolved.7 Each dog received water (10 mL) PO by syringe after administration of all medications to ensure complete swallowing. The dosing protocol for sucralfate was designed to ensure that all potential for interaction would be captured during initial oral absorption of fluoroquinolones as well as absorption that might occur because of enterohepatic circulation. Dogs were not fasted before drug administration; they received a standard maintenance canine diet calculated for their daily requirements; water was provided ad libitum. Throughout the study, all dogs had consistent food consumption patterns daily, and no changes were made in type or amount of diet offered or timing of meals.
Blood samples, 3 mL per time point, were collected by jugular venipuncture before fluoroquinolone administration and at 0.33, 0.67, 1, 2, 3, 4, 8, 12, and 24 hours after fluoroquinolone administration. Whole blood was placed into tubes containing lithium heparin and stored on ice until centrifugation at 3,000 × g for 15 min; plasma was then stored at −70°C before drug analysis.
Liquid Chromatography/Mass Spectrometry Method of Analysis for Ciprofloxacin and Enrofloxacin
Plasma concentrations of enrofloxacin and ciprofloxacin were determined using liquid chromatography5 with mass spectrometry6, according to previously published methods.17 Briefly, 0.1 mL of plasma was added to 0.4 mL methanol containing 0.1% formic acid and 500 ng/mL of the internal standard norfloxacin. The samples were vortexed for 5 seconds, centrifuged for 5 minutes at 15 000 × g, and the supernatant transferred to an injection vial. The mobile phase consisted of 0.1% formic acid in deionized water and acetonitrile. A C18 column7 achieved separation. The mass to charge ratio (m/z) of the qualifying and quantifying ions for enrofloxacin were 360 and 245.2, respectively; for ciprofloxacin were 332.2 and 245.2, respectively; and for norfloxacin were 320.0 and 276.3, respectively. The lower limit of quantification was 0.01 μg/mL and the standard curves were linear from 0.01 to 5 μg/mL. The interday accuracies of the assay for ciprofloxacin were 97, 91 and 95% and the interday coefficients of variation were 9.5, 4.2 and 8.0% determined on replicates of 5 each at 0.01, 0.5 and 5 μg/mL. The interday accuracies of the assay for enrofloxacin were 98, 99 and 94% and the interday coefficients of variation were 6.5, 4.0 and 10.4% determined on replicates of 5 each at 0.01, 0.5 and 5 μg/mL.
Pharmacokinetic Analysis
Pharmacokinetic analyses were performed using computer software.8 The area under the curve extrapolated to infinity (AUC) was determined with the linear trapezoidal method, and this was considered the pivotal parameter for comparison of treatments in this study. The maximum plasma concentration (C MAX) and time to maximum plasma concentration (T MAX) were determined directly from the data. The terminal half‐life (T 1/2), clearance per fraction of the dose absorbed (Cl/F), and mean residence time extrapolated to infinity (MRT) were determined. Relative bioavailability was calculated by comparing the dose‐normalized AUC between the two treatments (fluoroquinolone with and without sucralfate) using a standard equation18:
Statistical Analysis
Statistical analysis was performed with computer software9 using a Friedman repeated measures analysis of variance on ranks to compare pharmacokinetics parameters of the fluoroquinolone when administered with sucralfate (either concurrently or delayed) to when administered alone. A P‐value of <0.05 was considered statistically significant.
Results
Actual washout periods between treatment groups in this study ranged from 4 to 9 weeks, meeting the 2‐week minimum time frame. No dog had measurable enrofloxacin or ciprofloxacin in their plasma at time 0 during any treatment group.
The pharmacokinetic parameters for ciprofloxacin administered alone and with sucralfate are presented in Table 1 and Fig 1. No statistically significant (P < 0.05) differences in pharmacokinetic parameters were noted between groups. The AUC of ciprofloxacin had a 4‐fold range (5.52–22.47 h μg/mL) when it was administered alone. This variability continued when ciprofloxacin was administered with concurrent sucralfate (1.57–17.97 h μg/mL) with a mean relative bioavailability of 48% and three dogs having relative bioavailability <55%. The AUC of ciprofloxacin with sucralfate delayed 2 hours was 7.60–17.65 h μg/mL with a mean relative bioavailability of 87%. The dog with the lowest relative bioavailability in the CS group (8%) also had the lowest relative bioavailability in the C2S group (37%); similarly, the dog with the highest relative bioavailability in the CS group (143%) also had the highest relative bioavailability in the C2S group (333%). A post hoc sample size calculation found that 23 dogs would be required to achieve 0.8 power to detect a 50% change in AUC/Dose (mean = 0.631 h μg/mL) of ciprofloxacin with an alpha of 0.05 and standard deviation of 0.350 h μg/mL, which is based on the pharmacokinetics in this study for group C.
Table 1.
Ciprofloxacin pharmacokinetics in dogs (n = 5) administered with ciprofloxacin alone (C), with concurrent sucralfate (CS), and with sucralfate delayed 2 hours (C2S), reported as geometric mean and range
| Variable | Units | Ciprofloxacin (C) | Ciprofloxacin Concurrent Sucralfate (CS) | Ciprofloxacin Sucralfate Delayed 2 hours (C2S) |
|---|---|---|---|---|
| AUC extrap | % | 7.5 (3.0–29.6) | 5.3 (3.3–16.0) | 7.6 (2.9–16.0) |
| AUC | h μg/mL | 13.59 (5.52–22.47) | 6.52 (1.57–17.97) | 11.75 (7.60–17.65) |
| C MAX | μg/mL | 1.68 (1.13–2.90) | 0.86 (0.21–1.72) | 1.58 (1.10–1.61) |
| T MAX | hour | 0.92 (0.67–1.00) | 0.78 (0.67–1.00) | 1.40 (0.67–2.00) |
| T 1/2 | hour | 6.74 (4.66–12.15) | 5.8 (4.9–9.2) | 6.8 (4.5–10.4) |
| MRT | hour | 9.47 (6.05–18.44) | 7.87 (6.44–12.76) | 9.11 (6.69–11.95) |
| Cl/F | mL/min/kg | 31.20 (16.31–81.59) | 64.66 (22.07–269.01) | 35.83 (24.53–55.50) |
| Dose | mg/kg | 25.4 (22.0–27.9) | 25.3 (23.8–27.6) | 25.3 (22.9–28.2) |
| Relative bioavailability | % | N/A | 48 (8–143) | 87 (37–333) |
AUC extrap, % AUC extrapolated to infinity; AUC, area under the curve; C MAX, maximum plasma concentration; T MAX, time to C MAX; T 1/2, terminal half‐life; MRT, mean residence time extrapolated to infinity; Cl/F, clearance per fraction of the dose absorbed; relative bioavailability, fraction of the ciprofloxacin dose absorbed relative to when ciprofloxacin was administered alone.
Figure 1.
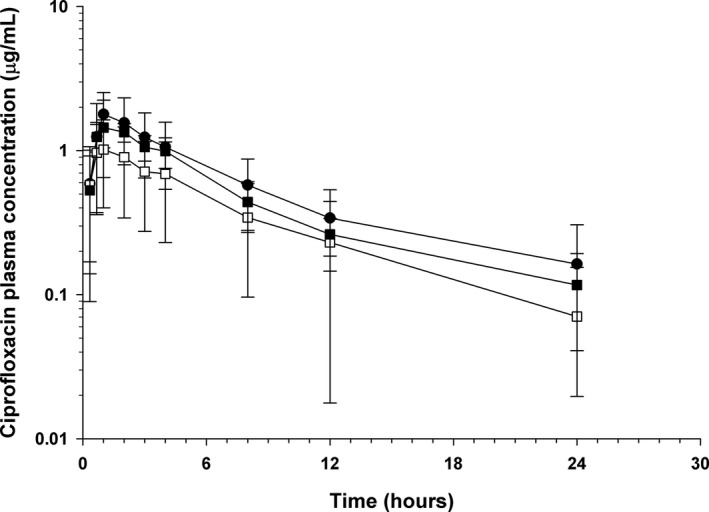
Mean ± SD plasma concentrations of ciprofloxacin in dogs (n = 5) administered ciprofloxacin alone (●), with concurrent sucralfate (□), and with sucralfate delayed by 2 hours (■).
Pharmacokinetic parameters for enrofloxacin administered alone and with sucralfate are presented in Table 2 and Fig 2. Much less variability in exposure to enrofloxacin was present in this population of dogs as compared with ciprofloxacin, with the AUC of enrofloxacin alone having a range of 3.86–7.50 h μg/mL, and enrofloxacin with concurrent sucralfate having a range of 4.15–7.58 h μg/mL. No significant differences were documented in the pharmacokinetics of enrofloxacin when administered with concurrent sucralfate, as compared with enrofloxacin alone. The mean relative bioavailability of 104% confirmed that no drug interaction for relative bioavailability was found when enrofloxacin was administered with concurrent sucralfate. The mean relative bioavailability of enrofloxacin administered with sucralfate delayed by 2 hours was 128%. A post hoc sample size analysis indicated 4 dogs would be sufficient to detect a 50% change in the AUC/Dose of enrofloxacin with a power of 0.8 and alpha = 0.05, which is based on the pharmacokinetics in this study for group E (mean = 1.15 h μg/mL, standard deviation = 0.23 h μg/mL).
Table 2.
Enrofloxacin pharmacokinetics in dogs (n = 5) administered with enrofloxacin alone (E), with concurrent sucralfate (ES), and with sucralfate delayed 2 hours (E2S), reported as geometric mean and range
| Variable | Units | Enrofloxacin (E) | Enrofloxacin Concurrent Sucralfate (ES) | Enrofloxacin Sucralfate Delayed 2 hours (E2S) |
|---|---|---|---|---|
| AUC extrap | % | 1.4 (0.8–2.5) | 1.7 (0.9–2.5) | 1.9 (1.0–5.1) |
| AUC | h μg/mL | 5.58 (3.86–7.50) | 5.78 (4.15–7.58) | 7.27 (4.09–10.32) |
| C MAX | μg/mL | 0.77 (0.48–1.24) | 1.07 (0.86–1.23) | 1.14 (0.85–1.83) |
| T MAX | hour | 2.50 (1–8) | 0.98 (0.67–2) | 1.52 (0.67–4) |
| T 1/2 | hour | 3.71 (3.46–4.04) | 4.07 (3.39–4.61) | 3.99 (3.40–5.16) |
| MRT | hour | 6.47 (4.49–9.12) | 5.46 (4.39–6.10) | 6.07 (4.11–8.50) |
| Cl/F | mL/min/kg | 14.77 (11.57–20.31) | 14.18 (10.54–19.45) | 11.57 (8.22–21.25) |
| Dose | mg/kg | 4.94 (4.71–5.21) | 4.92 (4.79–5.10) | 5.04 (4.93–5.22) |
| Relative bioavailability | % | N/A | 104 (94–115) | 128 (96–186) |
AUC extrap, % AUC extrapolated to infinity; AUC, area under the curve; C MAX, maximum plasma concentration; T MAX, time to C MAX; T 1/2, terminal half‐life; MRT, mean residence time extrapolated to infinity; Cl/F, clearance per fraction of the dose absorbed; relative bioavailability, fraction of the dose absorbed relative to when enrofloxacin was administered alone.
Figure 2.
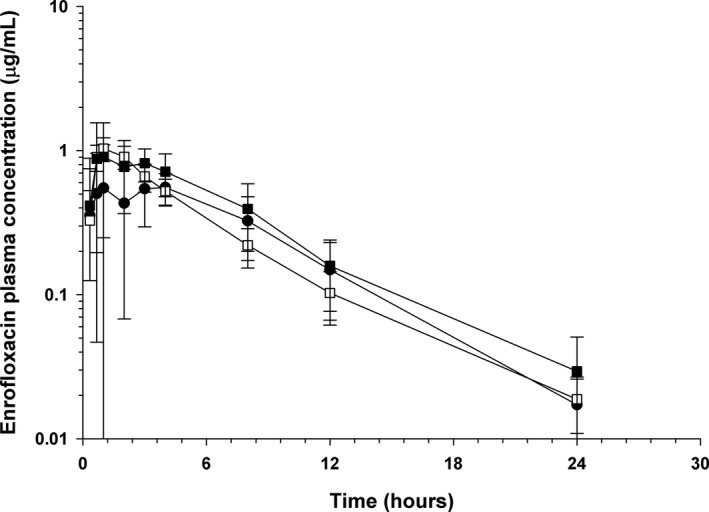
Mean ± SD plasma concentrations of enrofloxacin in dogs (n = 5) administered enrofloxacin alone (●), with concurrent sucralfate (□), and with sucralfate delayed by 2 hours (■).
Ciprofloxacin was identified in all dogs after enrofloxacin administration (Table 3, Fig 3). The relative bioavailability of ciprofloxacin after enrofloxacin administration was similar when enrofloxacin was administered with sucralfate (86%) and when enrofloxacin was delayed 2 hours (101%). Likewise, the total exposure (AUC) of fluoroquinolones (enrofloxacin + ciprofloxacin) and C MAX of total fluoroquinolones after enrofloxacin was not significantly different with regard to timing of sucralfate administration (Table 4).
Table 3.
Ciprofloxacin pharmacokinetics in dogs (n = 5) administered with enrofloxacin alone (E), with concurrent sucralfate (ES), and with sucralfate delayed 2 hours (E2S), reported as geometric mean and range
| Variable | Units | Enrofloxacin (E) | Enrofloxacin Concurrent Sucralfate (ES) | Enrofloxacin Sucralfate Delayed 2 hours (E2S) |
|---|---|---|---|---|
| AUC extrap | % | 12.1 (7.0–23.3) | 14.1 (9.6–18.3) | 15.3 (9.0–27.8) |
| AUC | h μg/mL | 3.55 (2.97–4.49) | 3.07 (2.35–4.35) | 3.57 (2.30–4.79) |
| C MAX | μg/mL | 0.23 (0.17–0.29) | 0.22 (0.17–0.28) | 0.24 (0.23–0.25) |
| T MAX | hour | 4.59 (4.00–8.00) | 1.84 (0.67–4.00) | 3.48 (1.00–8.00) |
| T 1/2 | hour | 7.31 (5.76–9.84) | 8.13 (6.79–9.22) | 8.27 (6.71–11.2) |
| MRT | hour | 12.34 (9.31–17.36) | 12.17 (9.77–14.07) | 12.99 (9.47–18.59) |
| Relative bioavailability | % | N/A | 86 (74–123) | 101 (74–141) |
AUC extrap, % AUC extrapolated to infinity; AUC, area under the curve; C MAX, maximum plasma concentration; T MAX, time to C MAX; T 1/2, terminal half‐life; MRT, mean residence time extrapolated to infinity; Relative bioavailability, fraction of the dose absorbed relative to when enrofloxacin was administered alone.
Figure 3.
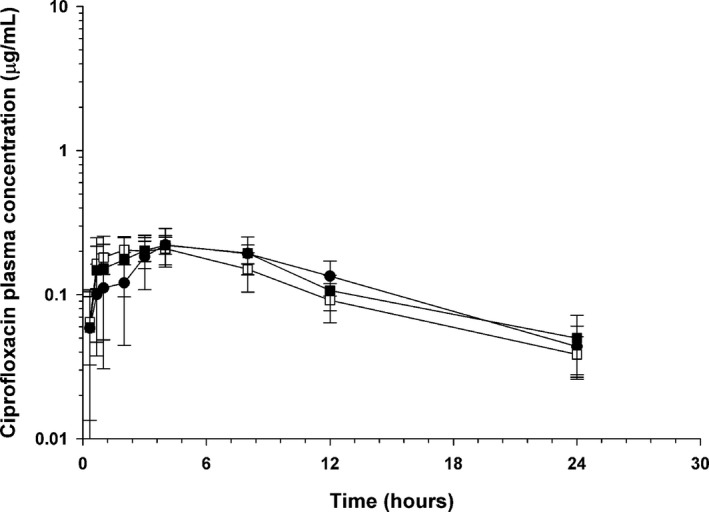
Mean ± SD plasma concentrations of ciprofloxacin in dogs (n = 5) administered enrofloxacin alone (●), with concurrent sucralfate (□), and with sucralfate delayed by 2 hours (■).
Table 4.
Total fluoroquinolone (enrofloxacin + ciprofloxacin) pharmacokinetics in dogs (n = 5) administered with enrofloxacin alone (E), with concurrent sucralfate (ES), and with sucralfate delayed 2 hours (E2S), reported as geometric mean and range
| Variable | Units | Enrofloxacin (E) | Enrofloxacin Concurrent Sucralfate (ES) | Enrofloxacin Sucralfate Delayed 2 hours (E2S) |
|---|---|---|---|---|
| AUC | h μg/mL | 9.15 (6.99–12.00) | 8.88 (6.74–11.19) | 10.85 (6.39–14.50) |
| C MAX | μg/mL | 0.99 (0.65–1.46) | 1.97 (1.09–2.85) | 2.36 (1.88–2.71) |
| T MAX | hour | 2.49 (1.00–8.00) | 0.78 (0.67–1.00) | 1.06 (0.67–2.00) |
| Relative bioavailability | % | N/A | 97 (87–120) | 119 (91–171) |
AUC, area under the curve; C MAX, maximum plasma concentration; T MAX, time to C MAX; relative bioavailability, fraction of the dose absorbed relative to when enrofloxacin was administered alone.
Discussion
In this study, the AUCs and relative bioavailability of ciprofloxacin were quite variable. This variability could have been influenced in part by factors related to food consumption and digestion since dogs were not fasted. However, similar variability in ciprofloxacin pharmacokinetics occurs in dogs fasted for 18 hours, and thus it is not believed to be related to an interaction from food and might instead be dependent on formulation, drug solubility, and tablet disintegration in the small intestine.4 Similar to this study using Greyhounds in a research facility, it is known that Beagles with relatively uniform weight and identical housing and feeding conditions have wide ranges in absorption of ciprofloxacin (32–80%), and thus it is difficult to predict if an individual dog would be able to absorb ciprofloxacin adequately and consistently to achieve clinical success with this medication. Variability in AUC and bioavailability means that with the same dosing protocol to target bacteria with a particular MIC, some dogs might be underdosed and others overdosed, leading to potential therapeutic failure or increased adverse effects, respectively. Furthermore, achieving subtherapeutic fluoroquinolone concentrations and drug exposure in some dogs might contribute to selection or promotion of antimicrobial resistant organisms within these canine hosts. Ciprofloxacin might be a greater risk for therapeutic failure and selection of resistant bacteria than fluoroquinolones that are consistently absorbed.4 Owing to the documented variability in absorption, ciprofloxacin is not a fluoroquinolone of choice for dogs; fluoroquinolones labeled for use in dogs should be used clinically.
This study documented a decrease in mean AUC from 13.59 to 6.52 h μg/mL and mean C MAX from 1.68 to 0.86 μg/mL when comparing administration of ciprofloxacin alone to administration of ciprofloxacin with concurrent sucralfate. Ranges for these values were wide, as would be expected based on variability in ciprofloxacin alone, and this likely contributed to lack of significant differences between these groups. When relative bioavailability was calculated, the ranges were also wide as expected, with mean 48% (8–143%) for concurrent sucralfate and 87% (37–333%) for C2S. Although outliers influence the mean with a small sample size, 3/5 dogs had relative bioavailabilities low enough to suggest that they could have had an interaction with sucralfate, which would be important clinically; further studies with increased sample size are needed to determine if this interaction would be statistically significant. Bioavailability >100% can occur because this is a calculated value based on dose‐normalized AUC from two treatments (with and without sucralfate) owing to intra‐individual daily variability in drug absorption. The maximal value of 333% for C2S is notably high; however, maximal AUC and C MAX for C2S group were within range of CS values, suggesting delayed sucralfate administration does not increase total drug exposure compared to the ciprofloxacin group but was likely individual variability in ciprofloxacin absorption. On the contrary, one dog had only 8% relative bioavailability when ciprofloxacin was administered concurrently with sucralfate and his C MAX was decreased to 10% compared to when he received ciprofloxacin alone. Therefore, despite lack of a statistically significant interaction, these data support a clinically relevant interaction for the relative bioavailability and verify that ciprofloxacin has a potential drug interaction with sucralfate in dogs.
When sucralfate administration was delayed 2 hours after ciprofloxacin, the AUC, C MAX and relative bioavailability were improved but not equivalent to those from ciprofloxacin alone. It is possible that a longer delay, such as 3 or 4 hours, might be more ideal than the tested 2 hours to minimize this drug interaction and allow for maximal relative bioavailability of ciprofloxacin, if both drugs were clinically indicated. However, it is also possible that the difference in magnitude between ciprofloxacin alone compared to ciprofloxacin with sucralfate delayed 2 hours is just because of the large variability in ciprofloxacin absorption and pharmacokinetics in dogs. Repeating this study with an enhanced sample size (23 dogs) to account for the variability in bioavailability could be performed to determine if this interaction also reaches statistical significance; however, obtaining funding for such a large study would be challenging with a drug that is not approved for use in dogs.
It is an interesting observation that the dog who had the lowest relative bioavailability in the CS treatment (8%) also had the lowest relative bioavailability (37%) in the C2S treatment. In addition, the dog who had the highest relative bioavailability in the CS treatment (143%) also had the highest in the C2S treatment (333%). These observations might indicate that individual animals will have differing degrees of drug interactions between sucralfate and ciprofloxacin, even when the administration is separated by 2 hours, furthering the suggestion that sucralfate and ciprofloxacin should not be administered to the same animal (concurrently or even delayed) as the interaction in an individual dog is difficult to predict.
The findings of this study represent a clinically relevant interaction for the relative bioavailability of ciprofloxacin because drug efficacy for fluoroquinolones is best correlated with AUC (as compared to bacterial MIC or minimum inhibitory concentration); drug exposure (AUC) is correlated with clinical efficacy because ideal therapeutic target is having AUC:MIC exceed 100 to achieve clinical success.4 The lower mean AUC (6.52 h μg/mL) and minimum AUC (1.57 h μg/mL) for the CS group, compared with the C group (mean 13.59 h μg/mL, minimum 5.52 h μg/mL), could directly impact treatment success since the AUC:MIC is correlated with clinical success. Therefore, the dog with the lowest drug exposure for CS (AUC=1.57 h μg/mL) would have a predicted clinical cure for bacterial MICs of 0.016 μg/mL or lower compared to the lowest drug exposure in the C group (AUC = 5.52 h μg/mL) with predicted clinical cures for MICs of 0.06 μg/mL or lower. With ciprofloxacin having a susceptible breakpoint of 1 μg/mL for human infections caused by Enterobacteriaceae, drug exposure in this study was approximately 100‐fold lower than what would be expected to meet targeted plasma concentration. There is not a CLSI breakpoint available for ciprofloxacin for canine infections; thus, the human breakpoint is typically used. In addition, the AUC:MIC has been associated with selection of resistant strains when it is <100.19 Therefore, the dog with the minimum AUC value for CS (1.57 h μg/mL) having a 4‐fold lower AUC than the dog with the minimum AUC for the C group (5.52 h μg/mL) would have an increased potential for treatment failure as well as selection and propagation of resistant bacteria.
Throughout the study, no dog had measurable fluoroquinolone in their plasma at time 0 for any treatment group, verifying that no carryover effect was documented from previous therapy. This was not unexpected, as we had targeted and maintained a minimum 2 week washout time between treatment groups.11
No significant differences were seen when AUC and relative bioavailability were compared between groups receiving enrofloxacin alone and receiving enrofloxacin with concurrent or delayed sucralfate. Although Table 2 shows higher C MAX, AUC, and relative bioavailability means for the concurrent (ES) and delayed (E2S) sucralfate groups than the enrofloxacin alone group (E), these findings were not statistically different and their ranges overlapped. Since post hoc sample size analysis found that only 4 dogs would have been needed to identify a statistical difference if it was present for a 50% change in the AUC, caution should be used not to overinterpret these trends or to conclude that bioavailability is actually improved with sucralfate administration. Lack of difference in AUC or relative bioavailability when sucralfate was administered concurrently with enrofloxacin compared to enrofloxacin alone suggests a potential lack of a clinically relevant drug interaction on the relative bioavailability of enrofloxacin. This was an unexpected finding but has clinical importance, suggesting that canine owners might no longer need to separate administration of enrofloxacin and sucralfate, which could lead to better compliance for both medications.
It is unclear why enrofloxacin absorption was not affected by sucralfate in dogs, even though ciprofloxacin appeared to be affected in dogs, and many fluoroquinolones have been documented to have this interaction in human studies.10, 11, 12, 13, 14, 15, 16 The authors hypothesize this difference could be related to the chemical structure of enrofloxacin, which might make it less vulnerable than other fluoroquinolones to complex formation with the aluminum in sucralfate. Specifically, it is hypothesized that the additional methyl group on enrofloxacin might interfere with the chelation site of aluminum. A similar explanation, related to the additional fluorine atoms at positions 8 and 1 reducing potential for complex formation, has been proposed for why fleroxacin was found to have only a modest interaction with sucralfate when administered to human volunteers.16, 20 A sample size analysis indicated only 4 dogs would be needed to achieve a power of 0.8 to document a 50% change in AUC (relative bioavailability) with an alpha of 0.05, suggesting this study had enough dogs enrolled to document a clinically relevant interaction if it was truly present. However, further studies with larger numbers of dogs with naturally occurring bacterial infections should be performed to confirm this finding. Further studies with additional fluoroquinolones approved for use in dogs would also be helpful to further evaluate for existence of this interaction and need for separation of these medications in the clinical setting.
One dog in the enrofloxacin alone group had a lower than expected C MAX (0.48 μg/mL). The low C MAX appears to have occurred because of slow drug absorption with a T MAX at 8 hours. However, the extent of absorption appeared to be unaffected, as this dog had an AUC of 5.55 h μg/mL for enrofloxacin, and this was the pivotal parameter measured for comparison of bioavailability in this study. All dogs were fed before drug administration; this dog ate his meal quickly and completely, whereas other dogs ate more slowly throughout the morning during sample collection. It is hypothesized that this difference in meal consumption might have resulted in the slower than expected drug absorption in this dog. A veterinary formulary and the website of a manufacturer recommend administration of enrofloxacin on an empty stomach or before feeding.8, 21 However, the approved label does not state to administer enrofloxacin to dogs fasted, and specifically states that the tablets2 used in this study (ANADA 200–551) can be offered with food which is the same recommendation as in the package insert for the pioneer product1 (NADA 140–441).22, 23 For this study, dogs were not fasted in attempt to reflect clinical use and to identify a potential clinical drug interaction. Dogs maintained consistent feeding patterns throughout the study; thus, if a food–drug interaction existed it would be expected to affect each treatment groups' results equally. Lack of a fasted group for each treatment is a limitation of the study and further studies in both fed and fasted dogs are warranted to fully eliminate a food interaction as the cause of decreased AUC and bioavailability of ciprofloxacin in this study.
Ciprofloxacin is an unapproved drug in dogs in the United States. Ciprofloxacin was used in this study because of the well‐documented interaction with sucralfate in humans, and the authors' hypothesis that ciprofloxacin might also interact with sucralfate in dogs.
Ciprofloxacin is an active metabolite of enrofloxacin in dogs and contributes to the antimicrobial efficacy after administration of enrofloxacin. The AUC of ciprofloxacin after enrofloxacin (4.94 mg/kg PO) was similar in this study (mean = 3.55, range 2.97–4.49 h μg/mL) compared to previous studies in dogs (means 2.66 ± 1.03 and 2.27 ± 0.64 h μg/mL)1, 3 administered 5 mg/kg PO. Since effects of sucralfate on enrofloxacin pharmacokinetics were not observed, it was expected that no effects on the formation and pharmacokinetics of ciprofloxacin after enrofloxacin administration would occur. As expected, there were no significant differences in the AUC or C MAX of ciprofloxacin after enrofloxacin when sucralfate was administered concurrently or delayed by 2 hours. Similarly, the total exposure of enrofloxacin and ciprofloxacin was not significantly affected either, again suggesting sucralfate does not produce a clinically relevant drug interaction when administered with enrofloxacin. However, clinical trials in dogs with naturally occurring disease should be performed to confirm these findings.
The stability of enrofloxacin and ciprofloxacin in frozen (−70°C) canine plasma was not assessed, but studies in human plasma demonstrate ciprofloxacin stability for at least 6 months when stored at −20°C.24 Plasma samples were analyzed within 6 months of collection in this study. It is possible that the stability of enrofloxacin and ciprofloxacin in canine plasma frozen at −70°C is less than 6 months. However, as previously stated, the pharmacokinetics of ciprofloxacin and enrofloxacin in this study are similar to previous studies. Further studies should assess the long‐term stability of fluoroquinolones in canine plasma.
In conclusion, the mean relative bioavailability of ciprofloxacin was decreased to 48%, with individual variability, when administered concurrently with suc‐ralfate, but was 87% when sucralfate was delayed by 2 hours after ciprofloxacin suggesting a clinically relevant drug interaction for relative bioavailability. The C MAX and AUC of ciprofloxacin were quite variable in this group of dogs, suggesting variable effects are expected which is consistent with previous studies. By contrast, enrofloxacin was not affected by concurrent sucralfate administration, and enrofloxacin had more consistent C MAX and AUC within this study group. The rate, but not extent of enrofloxacin absorption, might be affected by concurrent food; therefore, administration fasted might produce more consistent rates of absorption, but further studies are needed to confirm the effect of food on rates of absorption for oral enrofloxacin in dogs. Further research is warranted to investigate the presence of an interaction between enrofloxacin or other fluoroquinolones labeled for use in dogs and concurrent sucralfate in a clinical setting.
Acknowledgments
The authors thank Putney, Inc for donation of enrofloxacin for this study. This study was supported in part by Dean's Fund of Kansas State University ‐ Veterinary Research Scholars Program, the Analytical Pharmacology Laboratory, and the Department of Clinical Sciences at Kansas State University. Publication of this article was funded in part by the Kansas State University Open Access Publishing Fund.
Conflict of Interest Declaration: Putney, Inc donated enrofloxacin for this study. Dr Butch KuKanich has provided consulting and continuing education lectures for Putney, Inc, receiving speaking honoraria and reimbursement of travel expenses. He has also provided consulting for Bayer Animal Health, but not in the past 3 years.
Off‐label Antimicrobial Declaration: Ciprofloxacin was chosen in this study because of its documented interaction with sucralfate in human research. Ciprofloxacin is not approved for use in dogs in the United States and the authors do not advocate its use in dogs.
This work was performed at Kansas State University, Manhattan, KS.
This study has been presented at the Merial NIH National Veterinary Scholars Symposium, Ithaca, NY Aug 2014, the Kansas State University Phi Zeta Research Day, Manhattan, KS, Mar 2015, and the ACVIM Forum, Indianapolis, IN, Jun 2015.
Footnotes
Baytril, Bayer Animal Health, Shawnee Mission, KS
Enrofloxacin Flavored Tablets, Putney, Inc. Portland, ME
Ciprofloxacin, Pack Pharmaceuticals, Buffalo Grove, IL
Sucralfate, Nostrum Laboratories, Inc., Kansas City, MO
Shimadzu Prominence, Shimadzu Scientific Instruments, Columbia, MD
API 3000, Applied Biosystems, Foster City, CA
Waters XBridge, 50 × 2.1 mm, 5 μM, Waters Corporation, Milford, MA
Phoenix WinNonlin 5.2, Certara, Princeton, NJ
SigmaStat 12.5, Systat Software, Inc., San Jose, CA
References
- 1. Bidgood TL, Papich MG. Plasma and interstitial fluid pharmacokinetics of enrofloxacin, its metabolite ciprofloxacin, and marbofloxacin after oral administration and a constant rate infusion in dogs. J Vet Pharmacol Ther 2005;28:329–341. [DOI] [PubMed] [Google Scholar]
- 2. Cester CC, Toutain PL. A comprehensive model for enrofloxacin to ciprofloxacin transformation and disposition in dog. J Pharm Sci 1997;86:1148–1155. [DOI] [PubMed] [Google Scholar]
- 3. Kung K, Riond JL, Wanner M. Pharmacokinetics of enrofloxacin and its metabolite ciprofloxacin after intravenous and oral administration of enrofloxacin in dogs. J Vet Pharmacol Ther 1993;16:462–468. [DOI] [PubMed] [Google Scholar]
- 4. Papich MG. Ciprofloxacin pharmacokinetics and oral absorption of generic ciprofloxacin tablets in dogs. J Vet Pharmacol Ther 2012;73:1085–1091. [DOI] [PubMed] [Google Scholar]
- 5. KuKanich K, KuKanich B. The effect of sucralfate tablets vs. suspension on oral doxycycline absorption in dogs. J Vet Pharmacol Ther 2015;38:169–173. [DOI] [PubMed] [Google Scholar]
- 6. Hansten PD. Appendix II: Important drug interactions In Katzung BG, ed. Basic & Clinical Pharmacology. 6th ed Norwalk: Appleton & Lange, 1995:986–995. [Google Scholar]
- 7. Papich MG, ed. Saunders Handbook of Veterinary Drugs, 3rd ed St. Louis: Elsevier Saunders; 2011. [Google Scholar]
- 8. Plumb DC, ed. Veterinary Drug Handbook, 7th ed Ames: Blackwell Publishing; 2011. [Google Scholar]
- 9. KuKanich K, KuKanich B, Harris A, Heinrich E. Effect of sucralfate on oral minocycline absorption in healthy dogs. J Vet Pharmacol Ther 2014;37:451–456. [DOI] [PubMed] [Google Scholar]
- 10. Van Slooten AD, Nix DE, Wilton JH, et al. Combined use of ciprofloxacin and sucralfate. DICP 1991;25:578–582. [DOI] [PubMed] [Google Scholar]
- 11. Garrelts JC, Godley PJ, Peterie JD, et al. Sucralfate significantly reduces ciprofloxacin concentrations in serum. Antimicrob Agents Chemother 1990;34:931–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lehto P, Kivisto KT. Effect of sucralfate on absorption of norfloxacin and ofloxacin. Antimicrob Agents Chemother 1994;38:248–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Parpia SH, Nix DE, Hejmanowski LG, et al. Sucralfate reduces gastrointestinal absorption of norfloxacin. Antimicrob Agents Chemother 1989;33:99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stass H, Schuhly U, Moller JG, Delesen H. Effects of sucralfate on the oral bioavailability of moxifloxacin, a novel 8‐methoxyfluoroquinolone, in healthy volunteers. Clin Pharmacokinet 2001;40:49–55. [DOI] [PubMed] [Google Scholar]
- 15. Kamberi M, Nakashima H, Ogawa K, et al. The effect of staggered dosing of sucralfate on oral bioavailability of sparfloxacin. Br J Clin Pharmacol 2000;49:98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lubowski TJ, Nightingale CH, Sweeney K, Quintiliani R. Effect of sucralfate on pharmacokinetics of fleroxacin in healthy volunteers. Antimicrob Agents Chemother 1992;36:2758–2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wack AN, KuKanich B, Bronson E, Denver M. Pharmacokinetics of enrofloxacin after single dose oral and intravenous administration in the African penguin (Spheniscus demersus). J Zoo Wildl Med 2012;43:309–316. [DOI] [PubMed] [Google Scholar]
- 18. Riviere JE. Comparative Pharmacokinetics: Principles, Techniques, and Applications. Ames: Iowa State University Press; 1999. [Google Scholar]
- 19. Thomas JK, Forrest A, Bhavnani SM, et al. Pharmacodynamic evaluation of factors associated with the development of bacterial resistance in acutely ill patients during therapy. Antimicrob Agents Chemother 1998;37:521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vancutsem PM, Babish JG, Schwark WS. The fluoroquinolone antimicrobials: Structure, antimicrobial activity, pharmacokinetics, clinical use in domestic animal and toxicity. Cornell Vet 1990;80:173–186. [PubMed] [Google Scholar]
- 21. Baytril FAQs . What is the best time to applicate Baytril, before feeding or just added to the food? Available at: http://animalhealth.bayer.com/ah/5192.0.html#c14566. Accessed July 4, 2015.
- 22. Enrofloxacin flavored tablets package insert. Putney, Inc. Dosage and administration. Available at: http://putneyvet.com/images/docs/Enrofloxacin_Package_Insert.pdf. Accessed July 4, 2015.
- 23. Baytril® package insert. Bayer Healthcare; Available at: http://bayer.naccvp.com/?e=LSdew7K4HnVeeL2EuzyjnfL5DhrACqQQ&m=product_basic_view&id=1040012. Accessed July 4, 2015. [Google Scholar]
- 24. Grondin C, Zhao W, Fakhoury M, Jacqz‐Aigrain E. Determination of ciprofloxacin in plasma by micro‐liquid chromatography‐mass spectrometry: An adapted method for neonates. Biomed Chromatogr 2011;25:827–832. [DOI] [PubMed] [Google Scholar]


