Abstract
Background
Hospital‐acquired anemia is commonly described in people but limited information currently is available regarding its prevalence in animals.
Hypothesis/objectives
Assess the prevalence of hospital‐acquired anemia in hospitalized critically ill dogs and cats, and examine its relationship with phlebotomy practices, transfusion administration, and survival to discharge.
Animals
Eight hundred and fifty‐one client‐owned animals (688 dogs and 163 cats).
Methods
A multicenter, observational study was conducted in which packed cell volume (PCV) was recorded at the time of admission and on subsequent hospitalization days. Signalment, number of blood samples obtained, underlying disease, whether or not blood products were administered, duration of hospitalization, and survival to discharge were recorded.
Results
Admission anemia prevalence was 32%, with overall prevalence during the hospitalization period of 56%. The last recorded PCV was significantly lower than the admission PCV for both dogs (admission PCV, 42% [range, 6–67%]; last recorded PCV, 34% [range, 4–64%], P < .0001) and cats (admission PCV, 31% [range, 6–55%]; last recorded PCV, 26% [range, 10–46%], P < .0001). Patients that developed anemia had significantly more blood samples obtained (nonanemic, 5 blood samples [range, 2–54]; anemic, 7 blood samples [range, 2–49], P < .0001). Hospitalized cats were significantly more likely to develop anemia compared to dogs (P < .0001), but anemic dogs were significantly less likely to survive to discharge (P = .0001). Surgical patients were at higher risk of developing hospital‐acquired anemia compared to medical patients (OR, 0.63; 95% CI, 0.4–0.9; P = .01).
Conclusions and Clinical Relevance
Hospital‐acquired anemia occurred frequently, especially in surgical patients. Additional studies focused on the direct effect of phlebotomy practices on the likelihood of anemia development in hospitalized animals are warranted.
Keywords: Canine, Clinical pathology, Feline, Hematology, Intensive care, Transfusion medicine
Abbreviations
- PCV
packed cell volume
- ICU
intensive care unit
- IL‐1
interleukin‐1
- IL‐6
interleukin‐6
- TNF‐α
tumor necrosis factor α
Anemia is a commonly encountered laboratory finding in critically ill dogs and cats. The etiology, magnitude, chronicity, and physiological consequences of anemia influence the therapeutic plan recommended for the patient, which may involve administration of blood products.1, 2, 3, 4 Transfusion of red blood cells is an integral aspect of veterinary critical care as a mean of improving oxygen delivery in variety of disorders associated with hemorrhage, hemolysis, and dyserythropoiesis.5 In people, however, there is increasing awareness of the potential detrimental effects of blood product administration, including but not limited to transfusion reactions and transmission of infectious diseases.6 Concerns also exist about potential adverse effects associated with administration of aged blood products in both people and animals.7, 8, 9 The optimal timing of blood product administration, frequently referred to as the “transfusion trigger” in human medicine, is debatable and will vary with individual cases.10 A better appreciation of the mechanisms underlying the development of anemia in critically ill animals may allow clinicians to implement strategies to avoid its occurrence, and consequently limit transfusion in these patients.
Hospital‐acquired anemia development frequently is encountered in intensive care units (ICU) in human medicine.11, 12, 13, 14 According to a study, up to 95% of people hospitalized in an ICU became anemic by the third day of hospitalization.12 Phlebotomy practices, blunted erythropoietic response to anemia, and a hypoferric state have been suggested as contributing factors to the high prevalence of anemia in critically ill patients.15, 16 Critically, ill people therefore may be more likely to receive transfusions, which may further increase their risk of poor outcome.11 Limited information is available hospital‐acquired anemia development in critically ill animals. One small retrospective study documented anemia in 74% of cats that were not anemic at the time of admission to an ICU.17 These cats tended to be hospitalized longer, and required more blood products.
The purpose of our study was to identify the prevalence of hospital‐acquired anemia in critically ill dogs and cats managed in ICUs of 3 veterinary teaching hospitals. A secondary aim was to investigate the association between development of anemia, and phlebotomy practices, transfusion practices, duration of hospitalization, and outcome.
Materials and Methods
A multicenter, observational, cohort study was conducted between May 2012 and May 2013. Dogs and cats >6 months of age admitted to the ICU of the teaching hospitals of the Cummings School of Veterinary Medicine at Tufts University, Cornell University, and the University of Illinois were eligible for inclusion, provided a packed cell volume (PCV) was recorded at time of admission to the ICU and ≥1 result was recorded in the patient record during hospitalization. A PCV was obtained from the medical record daily when available and in event of >1 PCV being performed that day, the first value was recorded. Blood was obtained from either direct venipuncture, or by use of blood obtained from preplaced IV catheters in awake animals housed in these ICUs. If blood was obtained from an IV catheter, a 3‐mL presample was obtained by use of a syringe containing 0.3‐mL heparinized saline, to insure no contamination of the blood product with IV fluids or medications. This presample was returned to the patient after collection of the blood sample. Blood was collected either into a plastic tube containing 17 IU/mL of lithium heparin or 7.5% potassium EDTA. Anticoagulated blood was transferred to microhematocrit tubes for centrifugation and PCV was estimated by use of a percentage scale rather than laboratory‐derived hematocrit. Dogs were considered anemic if their PCV was <35% and cats were considered anemic if their PCV was <30%.18 Data included in the study were collected prospectively at 2 centers (Tufts and Illinois) using a standardized data collection sheet. Data were retrospectively collected at the final center (Cornell). Data were recorded each day from the patient medical record at Tufts and Illinois. For the retrospective center, patients were identified by reviewing the census of patients hospitalized in the ICU during the study period, with data being collected retrospectively from the electronic medical record. All collected data were subsequently collated and reviewed by 2 authors (A.L. and M.R.).
Patient data including signalment, body weight, presence of medical or surgical disease, number of blood samples obtained during hospitalization, duration of hospitalization, survival to discharge, and cause of death were recorded. Information regarding blood product administration, as well as any transfusion reactions that occurred, was recorded when applicable.
Statistical Analysis
Normality was assessed by the Kolomogorov–Smirnov test. Normally distributed data are presented as mean ± SD, whereas non‐normally distributed data are presented as median (range). The Kruskal–Wallis nonparametric test of central tendency was used to compare first and last recorded PCV, number of blood samples obtained in patients with hospital‐acquired anemia compared to those that did not develop anemia, number of blood samples indexed to the duration of hospitalization with respect to anemia development, number of blood samples obtained with respect to transfusion administration, as well as the difference between duration of hospitalization with respect to transfusion administration. The Chi‐square test for independence was used to assess the relationship between survival to discharge with respect to anemia development, survival to discharge with respect to transfusion administration, and type of disease (medical or surgical) with respect to anemia development. Spearman's correlation coefficients were used to measure the correlation between duration of hospitalization and number of blood samples obtained. Logistic regression was used to model the risk of anemia development with respect to disease category (ie, medical or surgical). Statistical analyses were performed using a commercially available statistical program.1 A 2‐sided P‐value (P < .05) was considered statistically significant.
Results
Data from 851 client‐owned animals, consisting of 688 dogs and 163 cats, were collected during the study period. The median age of the dogs was 8 years (range, 0.5–18 years), and median age of cats was 9 years (range, 0.5–20 years). There were 342 female dogs (295 spayed), and 346 male dogs (279 neutered), and 68 spayed female cats and 95 male cats (92 neutered male). In total, 45 different dog breeds were included, with the most common breeds being mixed breeds (n = 132), Labrador retrievers (n = 74), Golden retrievers (n = 39), and German shepherd dogs (n = 29). The majority of cats were domestic short hairs (n = 132), with the remainder being domestic long hairs (n = 16), and pure‐bred cats (n = 15). The median weight of dogs was 22.3 kg (range, 0.3–94 kg), and the median weight of cats was 4.7 kg (range, 0.5–6.8 kg). The majority of patients had medical diseases (n = 567) and 284 patients had surgical diseases. The most common medical conditions in dogs were neoplastic disease, respiratory disease, gastrointestinal disease, and cardiovascular disease. In cats, renal disease, cardiovascular disease, neoplastic disease, and hepatobiliary disease were most commonly identified. Abdominal surgery, thoracic surgery, and repair of traumatic wounds were the most common surgical procedures performed.
At the time of admission, the median PCV for all patients was 44% (range, 9–67%), with the median PCV for dogs being 43% (range, 6–67%), and the median PCV for cats being 31% (range, 6–55%). At admission, the prevalence of anemia was 32% (272/851), with 30.5% (210/688) of dogs and 49.7% (81/163) of cats being anemic. The last recorded PCV was significantly lower for both dogs (median, 34%; range, 4–64%; P < .0001), and cats (median, 26%; range, 10–46%; P < .0001). Figures 1 and 2 illustrate the trend in PCV over the duration of hospitalization for dogs and cats with medical and surgical diseases, respectively. The overall prevalence of anemia during hospitalization, based on last recorded PCV, was 56.3% (479/851), with 57.1% (393/688) of dogs and 52.7% (86/163) of cats being anemic by the end of their hospitalization. Cats were significantly more likely to become anemic during hospitalization compared to dogs (P < .0001). The risk of developing anemia compared to admission PCV was significantly higher in patients with surgical disease compared to those with medical disorders (OR, 0.63; 95% CI, 0.4–0.9; P = .01).
Figure 1.
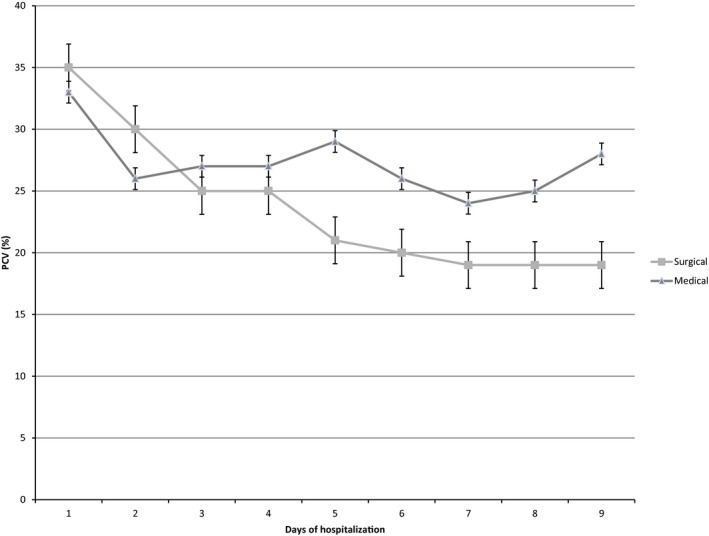
Median and interquartile range for packed cell volume versus time for dogs with medical and surgical disease.
Figure 2.
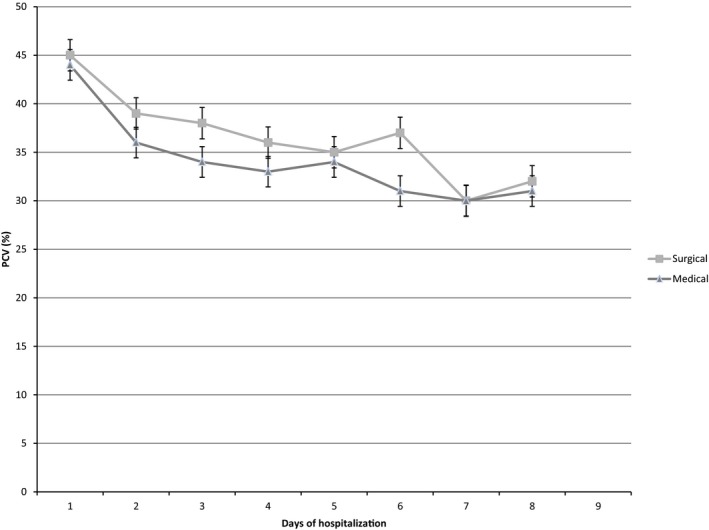
Median and interquartile range for packed cell volume for cats with medical and surgical disease.
The median number of blood samples obtained during hospitalization, which included blood samples for PCV estimation and other routine laboratory tests, was 5 (range, 2–54) for dogs and cats. Patients that became anemic had significantly more blood samples obtained (median, 7 samples; range, 2–49 samples) compared to patients that did not develop anemia (median, 5 samples; range, 2–54 samples; P < .0001). Likewise, patients that received blood products had significantly more blood samples obtained (8 samples; range, 2–54 samples) compared to those that did not become anemic (5 samples; range 2–48 samples (P < .0001). When the number of blood samples obtained was indexed to the duration of hospitalization, the median number of blood samples obtained per day was significantly higher for patients that developed anemia (anemia: 1.67 (range, 1–8.8), no anemia: 1.25 (range, 1–9.5), P < .0001) and those that received blood products (blood products: 2 (range, 1–9), no blood products: 1.4 (range, 1–9.5), P < .0001). Blood products were administered to 198 patients (158 dogs and 40 cats), with packed red blood cells being the most commonly administered product in 129 cases (100 dogs and 29 cats).
The mean duration of hospitalization was 3.9 ± 2 days. There was a moderate correlation between the total number of blood draws (r = 0.62, P < .0001) and anemia development in both dogs and cats. Hospitalization duration was not significantly different between dogs and cats with respect to transfusion administration (P > .05). Survival to discharge was 85.7% (729/851). The majority of nonsurvivors (102 of 144) were euthanized, with only 20 animals dying of natural causes. Dogs that developed anemia were less likely to survive to discharge compared to those that did not develop hospital‐acquired anemia (P = .001), but survival in cats was not significantly different between cats with respect to anemia development (P = .3). Dogs that did not receive blood products were significantly more likely to survive to discharge compared to those that did (P < .0001), but survival was not significantly different for cats that received blood products compared to those that did not (P = .23).
Discussion
This study describes the development of hospital‐acquired anemia in critically ill dogs and cats managed in the ICUs of 3 veterinary teaching hospitals. Anemia was relatively common in this patient population at the time of admission to the ICU with a prevalence of 32%. By the end of hospitalization, anemia was more common however with a prevalence of 56.3%. The extent of hospital‐acquired anemia described in this study is lower than previously reported in critically ill people (70% within 48 hours of admission to an ICU)12 and in a retrospective study of cats (84.4%).17 This apparent lack of agreement may be because of disparity in the severity of patient illness, with the previous studies in people and cats potentially describing a cohort of more severely affected individuals. Regrettably, an illness severity score was not determined for the animals enrolled in our study because of inconsistent availability of data required to formulate such a score. People with higher illness severity scores tend to have overall lower baseline hemoglobin concentrations and higher transfusion requirements during hospitalization.12 It is a limitation of this study that illness severity was not stratified with respect to PCV and transfusion administration. Interestingly, blood product administration has been considered an independent predictor of worse outcome in people, regardless of baseline anemia, implying that transfusion in itself carries certain degree of risk.11, 12 Emergency treatment provided for people before admission to the ICU (eg, fluid resuscitation) have been of a greater magnitude than the treatment provided to the patients included in our study.
Our study identified that hospitalized cats became anemic more commonly than dogs, whereas patients undergoing a surgical procedure also were more at risk of developing anemia in hospital. No relationship was apparent however between the development of anemia and overall outcome in cats. In contrast, dogs less commonly developed hospital‐acquired anemia, but dogs that developed anemia were significantly less likely to survive to discharge. The outcome of a hospitalized critically ill patient depends on many factors, with hospital‐acquired anemia being unlikely to be the sole cause of poor outcome in these patients with complex medical disorders influenced by multiple confounding factors.
Our study also identified an apparent association between phlebotomy practices, specifically the number of blood samples obtained from a patient, and the development of hospital‐acquired anemia and administration of blood products. This finding is similar to those of previous studies in people and cats, in which more frequent blood sampling was associated with anemia development.11, 12, 17 Consideration of the number of blood samples obtained in critically ill people is important, because this has been implicated in hospital‐acquired anemia development.15, 16, 19 The absolute number of blood samples obtained may be less crucial than the absolute volume of blood removed. It is therefore a limitation of our study that the volume of blood obtained from patients could not be accurately determined. Consequently, any association between phlebotomy practices in our study with anemia development does not necessarily reflect causation. In critically ill people, collecting smaller volumes of blood however has been associated with decreased transfusion requirements.19 Obtaining smaller volumes of blood in small animals, especially small‐breed dogs and cats would be sensible, and may be associated with less hospital‐acquired anemia. In our study, as has been found in people,12 an association also existed between transfusion requirements and increased frequency of blood sampling. Animals receiving transfusions frequently have blood samples collected both before and after the transfusion, which may in part explain this observation. Additional work focused specifically on phlebotomy practices in animals that are nonanemic at admission and subsequently receive blood products in response to new anemia may improve our understanding of the apparent association between phlebotomy and transfusion administration. A significant relationship also existed between increased frequency of blood draws and longer duration of hospitalization. Underlying disease state and severity likely impact this observation, and more compromised patients likely require closer monitoring, undergo more phlebotomy and may be hospitalized longer compared to less sick individuals.
Other explanations for the high prevalence of hospital‐acquired anemia in critically ill people include a blunted erythropoietic response to anemia and a hypoferric state. The anemia of critical illness in people is characterized by a failure of circulating erythropoietin concentrations to increase appropriately in response to lower hemoglobin concentration.20 In veterinary medicine, the anemia commonly noted in cats and dogs with chronic kidney disease21 and in some endocrinopathies (eg, canine hypothyroidism) is part of the spectrum of the anemia of inflammatory disease.22 In addition to impaired erythropoiesis by the kidney in cases of chronic kidney disease, a wide array of cytokines, including TNF‐α, IL‐1, IL‐6, interferon‐α, interferon‐β, and interferon‐γ, have been implicated in the pathogenesis of the anemia of inflammatory disease.21, 23 Hepcidin is produced as part of the acute phase response, particularly in response to IL‐6, and potentially in response to other molecules such as lipopolysaccharide. Hepcidin overexpression leads to a state of iron retention, and a relative deficiency in circulating iron, which contributes to an overall blunted erythropoietic response.22 Administration of exogenous erythropoiesis stimulating agents (eg, darbepoietin) is common in the management of chronic kidney disease in cats, although this practice is not without controversy.24, 25 This strategy has been documented in people with anemia after trauma, but with inconsistent results.20, 26 Erythropoietin concentrations were not measured in the animals included in our study, but future work investigating the prevalence of erythropoietin deficiency and the role of hepcidin in the pathogenesis of hospital‐acquired anemia in critically illness in dogs and cats may be warranted.
In our study, hospitalized cats were more likely to become anemic than dogs, but this did not appear to be associated with outcome. This finding is similar to that of a retrospective study of 180 anemic cats in which overall survival to discharge was reported to be good (62.2%), and outcome was linked more closely to the underlying disease than anemia itself, given the observation in that study that cats with neoplasia did worse.27 The potential for our study failing to identify an association between hospital‐acquired anemia and outcome in cats should be considered. In critically ill dogs, careful consideration of the cause of an unexpected decrease in PCV in hospitalized patients, including occult gastrointestinal bleeding, coagulopathy, and use of drugs with ulcerogenic potential (eg, nonsteroidal anti‐inflammatory drugs) is important.28
The impact of exogenous factors on the PCV in individual patients should be considered. For example, based on our data, surgical patients were at increased risk of developing hospital‐acquired anemia, which likely is in part associated with perioperative hemorrhage and fluid resuscitation. The impact of dehydration is relevant, because it plausibly could artifactually increase PCV in dehydrated patients, which may have falsely decreased the apparent prevalence of anemia at the time of admission. Institution of fluid therapy also lowers PCV in dehydrated patients, and may effectively unmask anemia. This may partly explain the development of anemia in some animals in our study. The impact of fluid therapy, ongoing fluid losses (eg, vomiting, diarrhea, polyuria) and the use of diuretics on an individual animal's PCV is complex and challenging to accurately assess. General anesthesia and sedation may lower PCV, skewing the apparent prevalence of anemia. In our study, PCV was recorded in awake patients in the ICU and therefore anesthesia is unlikely to contribute to the results reported in our study. The relative contribution of these exogenous factors in individual animals is considered another limitation of this study. The method of obtaining the PCV result was standardized (ie, microcentrifugation) in this study to decrease the impact of laboratory variation with hematology benchtop analyzers among institutions. An additional limitation of this study is that some data were collected prospectively and some retrospectively. Although measures were taken to standardize data collection, by using a single data collection sheet with unambiguous objective data entry points, a universal prospective approach would have allowed greater control of data (eg, insuring highest accuracy of recording phlebotomy practices).
Our study indicates that hospital‐acquired anemia is common in ICUs, despite a relatively high prevalence at the time of admission to the hospital. There was an apparent association of anemia with phlebotomy practices, which warrants further investigation to assess the impact of frequent blood sampling on outcome. The association between hospital‐acquired anemia and survival was species‐specific based on this data, with anemic dogs having an worse outcome as compared to cats, which likely is multifactorial in nature. It seems prudent to institute measures to limit hospital‐acquired anemia in critically ill patients (eg, consideration for small volume blood sampling) pending further data characterizing this association more completely. Additional studies on the pathophysiology of the anemia of critical illness in dogs and cats, including the role of erythropoietin and hepcidin, are warranted.
Acknowledgments
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐Label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
(The work was performed at the teaching hospitals of the Cummings School of Veterinary Medicine at Tufts University, Cornell University College of Veterinary Medicine, and the College of Veterinary Medicine at the University of Illinois. No financial support was provided for this study. This study was presented in part as a poster at the ACVIM Forum 2013, Seattle, WA. The authors acknowledge the statistical support of Shicheng Weng and Bruce Barton of the University of Massachusetts Medical School, Worcester, MA.).
Footnote
SAS 9.3, SAS Institute, Inc., Cary, NC
References
- 1. Kerl ME, Hohenhaus AE. Packed red blood cell transfusions in dogs: 131 cases (1989). J Am Vet Med Assoc 1993;202:1495–1499. [PubMed] [Google Scholar]
- 2. Stone E, Badner D, Cotter SM. Trends in transfusion medicine in dogs at a veterinary school clinic: 315 cases (1986–1989). J Am Vet Med Assoc 1992;200:1000–1004. [PubMed] [Google Scholar]
- 3. Callan MB, Oakley DA, Shofer FS, et al. Canine red blood cell transfusion practice. J Am Anim Hosp Assoc 1996;32:303–311. [DOI] [PubMed] [Google Scholar]
- 4. Barfield D, Adamantos S. Feline blood transfusions: A pinker shade of pale. J Feline Med Surg 2011;13:11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kisielewicz C, Self I. Canine and feline blood transfusions: Controversies and recent advances in administration practices. Vet Anaesth Analg 2014;41:233–242. [DOI] [PubMed] [Google Scholar]
- 6. Vincent JL, Piagnerelli M. Transfusion in the intensive care unit. Crit Care Med 2006;34(5 Suppl):S96–S101. [DOI] [PubMed] [Google Scholar]
- 7. Koch CG, Figueroa PI, Li L, et al. Red blood cell storage: How long is too long? Ann Thorac Surg 2013;96:1894–1899. [DOI] [PubMed] [Google Scholar]
- 8. Soloman SB, Wang D, Sun J, et al. Mortality increases after massive exchange transfusion with older stored blood in canines with experimental pneumonia. Blood 2013;12:1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smith SA, McMichael M, Herring JM, et al. Serum biomarkers indicate hemolysis and inflammatory response to transfusion of autologous stored erythrocyte concentrates. J Vet Int Med 2012;26:778. [Google Scholar]
- 10. Lelubre C, Vincent JL. Red blood cell transfusion in the critically ill patient. Ann Intensive Care 2011;1:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vincent JL, Baron JF, Reinhart K, et al. Anemia and blood transfusion in critically ill patients. JAMA 2002;288:1499–1507. [DOI] [PubMed] [Google Scholar]
- 12. Corwin HL, Gettinger A, Pearl RG, et al. The CRIT study: Anemia and blood transfusion in the critically ill—current clinical practice in the United States. Crit Care Med 2004;32:39–52. [DOI] [PubMed] [Google Scholar]
- 13. Thomas J, Jensen L, Nahirniak S, et al. Anemia and blood transfusion practices in the critically ill: A prospective cohort review. Heart Lung 2010;39:217–225. [DOI] [PubMed] [Google Scholar]
- 14. Rodriguez RM, Corwin HL, Gettinger A, et al. Nutritional deficiencies and blunted erythropoietin response as causes of the anemia of critical illness. J Crit Care 2011;16:36–41. [DOI] [PubMed] [Google Scholar]
- 15. Branco BC, Inaba K, Doughty R, et al. The increasing burden of phlebotomy in the development of anaemia and need for blood transfusion amongst trauma patients. Injury 2021;43:78–83. [DOI] [PubMed] [Google Scholar]
- 16. Hobisch‐Hagen P, Wiederman F, Fries Andreas, et al. Blunted erythropoietic response to anemia in multiple traumatized patients. Crit Care Med 2001;29:s157–s161. [DOI] [PubMed] [Google Scholar]
- 17. Balakrishnan A, Drobatz K, Reineke E. Anemia, phlebotomy practices and blood transfusion requirements in 45 critically ill cats: 2009–2011. J Vet Emerg Crit Care (San Antonio) 2012;22(s2):s3. [DOI] [PubMed] [Google Scholar]
- 18. Latimer K.S., Mahaffey E.A., Prasse K.W., Duncan and Prasse's Veterinary Laboratory Medicine: Clinical Pathology, 4th ed Ames, Iowa, USA: Wiley‐Blackwell; 2003. [Google Scholar]
- 19. Chant C, Wilson G, Friedrich JO. Anemia, transfusion, and phlebotomy practices in critically ill patients with prolonged ICU length of stay: A cohort study. Crit Care 2006;10:R140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Napolitano LM, Fabian TC, Kelly KM, et al. Improved survival of critically ill trauma patients treated with recombinant human erythropoietin. J Trauma 2008;65:285–297. [DOI] [PubMed] [Google Scholar]
- 21. Chalhoub S, Langston C, Eatroff A. Anemia of renal disease: What it is, what to do and what's new. J Feline Med Surg 2011;13:629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grimes CN, Giori L, Fry MM. Role of hepcidin in iron metabolism and potential clinical applications. Vet Clin North Am Small Anim Pract 2012;42:85–96. [DOI] [PubMed] [Google Scholar]
- 23. Stenvinkel P. The role of inflammation in the anaemia of end‐stage renal disease. Nephrol Dial Transplant 2001;16(suppl 7):36–40. [DOI] [PubMed] [Google Scholar]
- 24. Langston CE, Reine NJ, Kittrell D. The use of erythropoietin. Vet Clin North Am Small Anim Pract 2003;33:1245–1260. [DOI] [PubMed] [Google Scholar]
- 25. Chalhoub S, Langston SE, Farrelly J. The use of darbepoeitin to stimulate erythropoiesis in anemia of chronic kidney disease in cats: 25 cases. J Vet Int Med 2012;26:363–369. [DOI] [PubMed] [Google Scholar]
- 26. Luchette FA, Pasquale MD, Fabian TC, et al. A randomized, double‐blind, placebo‐controlled study to assess the effect of recombinant human erythropoietin on functional outcomes in anemic, critically ill, trauma subjects: The Long Term Trauma Outcomes Study. Am J Surg 2012;203:508–516. [DOI] [PubMed] [Google Scholar]
- 27. Korman RM, Hetzel N, Knowles TG, et al. A retrospective study of 180 anemic cats: Features, aetiologies and survival data. J Feline Med Surg 2012;15:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Waldrop JE, Rozanski EA, Freeman LM, et al. Packed red blood cell transfusion in dogs with gastrointestinal hemorrhage: 55 cases (1999–2001). J Am Anim Hosp Assoc 2003;39:523–527. [DOI] [PubMed] [Google Scholar]


