Abstract
Background
Naturally occurring adrenocortical insufficiency (NOAI) in dogs is considered an uncommon disease with good prognosis with hormonal replacement treatment. However, there are no epidemiological studies with estimates for the general dog population.
Objectives
To investigate the epidemiological characteristics of NOAI in a large population of insured dogs.
Animals
Data were derived from 525,028 client‐owned dogs insured by a Swedish insurance company representing 2,364,652 dog‐years at risk (DYAR) during the period between 1995–2006.
Methods
Retrospective cohort study. Incidence rates, prevalences, and relative risks for dogs with NOAI (AI with no previous claim for hypercortisolism), were calculated for the whole dog population, and for subgroups divided by breed and sex. Mortality rates were calculated and compared in dogs with NOAI and the remaining dogs overall.
Results
In total 534 dogs were identified with NOAI. The overall incidence was 2.3 cases per 10,000 DYAR. The relative risk of disease was significantly higher in the Portuguese Water Dog, Standard Poodle, Bearded Collie, Cairn Terrier, and Cocker Spaniel compared with other breeds combined. Female dogs overall were at higher risk of developing AI than male dogs (RR 1.85; 95% CI, 1.55–2.22; P < .001). The relative risk of death was 1.9 times higher in dogs with NOAI than in dogs overall.
Conclusion and Clinical Importance
The data supports the existence of breed‐specific differences in incidence rates of NOAI in dogs.
Keywords: Addison's disease, Hypoadrenocorticism, Incidence
Abbreviations
- AI
adrenocortical insufficiency
- CI
confidence interval
- CKCS
Cavalier King Charles Spaniel
- DYAR
dog‐years at risk
- FCI
Fédération Cynologique Internationale
- IQR
interquartile range
- IR
incidence rate
- MHC
major histocompatibility complex
- NOAI
naturally occurring adrenocortical insufficiency
- NSDTR
Nova Scotia Duck Tolling Retriever
- RR
relative risk
- WHWT
West Highland White Terrier
Naturally occurring adrenocortical insufficiency (NOAI) is generally considered an uncommon disease in dogs. However, there is a paucity of epidemiological studies. One difficulty is to get good access to clinical data on the general population of companion dogs. Previous studies have often been limited to case‐series of dogs presented to veterinary practices or referral clinics,1, 2, 3, 4 breed‐specific studies5, 6, 7 and estimations by breed‐clubs,8, 9 highly dependent on owners' and breeders' voluntarily participation and reporting the disease of their dogs.
The majority of dogs with NOAI have primary AI (ie, Addison′s disease),3 which is most commonly considered to be associated with adrenalitis and adrenocortical atrophy, supported by evidence from pathology‐based reports of dogs with AI.10, 11, 12, 13 Other causes of primary AI include endogenous destruction because of granulomatous processes,14 or infiltration of a primary or metastatic neoplasm.15 Adrenocortical insufficiency can also be caused iatrogenically during treatment of hyperadrenocorticism,16, 17, 18, 19 or by sudden withdrawal of glucocorticoid treatment.
Previous estimates of incidence rates for AI in dogs range from 0.13 to 0.6 per 1,000 dogs per year, depending on the population studied.1 The prevalence of NOAI in a population of dogs presented to veterinary clinics was 0.6 per 1,000 dogs per year.1 The occurrence of NOAI in dogs is reported to be higher in certain breeds including the Standard Poodle, Bearded Collie, and Portuguese Water Dog, with a reported occurrence up to 10% within the study populations.3, 5, 6, 7, 20 Reported sex distributions of NOAI vary by study. Initially, a significant overall female predominance was reported.1, 2, 3, 4 However, subsequent breed‐specific studies on Standard Poodles, Portuguese Water Dogs, and Bearded Collies failed to identify any significant difference by sex.5, 6, 7 The prognosis for dogs with NOAI on hormone replacement treatment is generally believed to be excellent, although there are few studies to support this.21, 22
In Sweden, there is a long tradition for dog owners to insure their animals for veterinary care, loss of function and death. The national dog population has an estimated size of 729,000 (±68,000) dogs. It is estimated that about 30–40% of the entire population is insured by 1 company – Agria Animal Insurance, Sweden.1, 23, 24, 25 Most dogs are first insured as puppies and stay insured for veterinary care during life. Therefore, there are three major advantages to performing epidemiological studies on the insured dog population: large and known population at risk, inclusion of dogs from early in life, and long follow‐up times.
Much remains unclear regarding AI in dogs. For example, it is unknown whether the reported variations in sex distribution represent true differences among breeds or are merely related to how the study‐populations were selected.
In order to characterize the epidemiology of NOAI in dogs in a large, defined population of dogs at risk, data derived from Agria‐insured dogs during an 11‐year period (1995–2006), were analyzed. Incidence rates, relative risks, prevalence, and mortality rates were calculated for dogs with AI, without a previous claim for hypercortisolism.
Material and Methods
Animals
The study population consisted of dogs insured by Agria Animal Insurance, Sweden.1 The insurance process has previously been described in detail.24 Briefly, the insurance offers 2 main options; veterinary health care and life. The insurance for veterinary health care covers the costs exceeding a deductible chosen by the owner. This insurance may be continued throughout most of the life of the dog. When the dog is insured for life, the owner is reimbursed the monetary value of the dog in the case of death caused by disease or accident. Dogs in these data were not eligible for life insurance after 10 years of age. Most insured dogs were covered by both the veterinary care and life insurance. Dogs could enter the insurance program from 6 weeks of age until 6 years of age. Veterinary care events are recorded in the claims database when a preset deductible has been exceeded or in case of reported death with or without accompanying claim for life reimbursement. In cases where more than 1 diagnosis is reported on the same claim, only 1 diagnostic code is recorded in the database. Neuter status was not available. Breeds are classified based on the classification system by Fédération Cynologique Internationale (FCI) and Swedish Kennel Club breed classification system.
The insurance claim records for the period between January 1, 1995, and December 31, 2006 were accessed. Only dogs that were covered for both veterinary care and life were included in the study. Data collected from the records were breed, sex, date of birth, date of life insurance claim, date, and type of insurance claim (eg, veterinary care or life), and diagnostic code. Diagnostic codes were assigned by the attending veterinarian based on a standardized diagnostic register.26 There was only 1 code, addressed as “Addison′s disease” available for all conditions associated with AI. Further discrimination (eg, into primary and secondary AI or spontaneous and iatrogenic) was not possible. To decrease the potential for inclusion of dogs with iatrogenically caused AI, dogs were excluded if there was a history of claims for hypercortisolism before or within 20 days after the first claim for AI. For the remaining dogs (ie, dogs with a claim for AI excluding cases with a previous claim for hypercortisolism) the term NOAI is used in the present study.
For the cohort and breed‐risk analysis, dogs were included, regardless of age at the time of insurance or during the study period (1995–2006). An individual was considered to be an incident case of AI at the time of the first recorded claim.
Analysis
Data analysis was performed with the R version 3.0.1 (www.r-project.org). Incidence rates (IRs) were calculated based on number of new cases and the exact times at risk. Incidence rates were expressed as number of cases per 10,000 dog‐years at risk (DYAR). Relative risks (RRs) were calculated by dividing the IR for the population of interest with the IR of the residual population (excluding the population of interest). Prevalence was calculated at the end of the study period in dogs that were still insured at that time point. Mortality rate was calculated by expressing the number of dogs that were registered as dead within the study per 10,000 DYAR. The 95% confidence interval (CI) for prevalence was calculated with exact tests based on binominal distribution.27 Confidence intervals for IRs, mortality rates, and RRs were calculated with exact methods based on the Poisson distribution using the “epiR” package (version 0.9–58),2 and “exactci” package (version 1.2–1).28 For determination of level of significance, Bonferroni correction for multiple comparisons was applied, based on the number of breeds included in the comparison (n = 110 for RRs for breeds, n = 28 for sex distribution, n = 27 for mortality rates). P‐values <.05 after Bonferroni correction were regarded as significant.
Results
The database included information on 525,028 dogs accounting for 2,364,652 DYAR. The dogs were divided into 354 breed categories. There were 260,552 (49.6%) female dogs, and 264,488 (50.4%) male dogs. Median age at enrollment in insurance was 77 days [interquartile range (IQR), 59–183 days]. Dogs contributed a median of 3.8 DYAR (IQR, 1.7–6.8 DYAR).
Incidence Rate: Overall and by Breed
Of the dogs with at least 1 claim for AI during the study period, 65 dogs had a previous claim for hypercortisolism and were excluded. The remaining dogs (n = 534) represented 110 different breed categories, including each of the 30 largest (based on DYAR) breed categories in the database. There were 74 Standard Poodles, 35 Bearded Collies, 26 mixed breed dogs, 25 Cairn Terriers, and 21 Golden Retrievers. The overall IR for NOAI was 2.26 cases per 10,000 DYAR (95%CI, 2.07–2.46 cases per 10,000 DYAR). A significantly increased RR of NOAI compared with the residual population was seen in the Portuguese Water Dog, Standard Poodle, Bearded Collie, Cairn Terrier, and Cocker Spaniel (Fig 1). A significantly decreased RR for NOAI compared with the residual population was seen in the German Shepherd Dog, and Dachshund.
Figure 1.
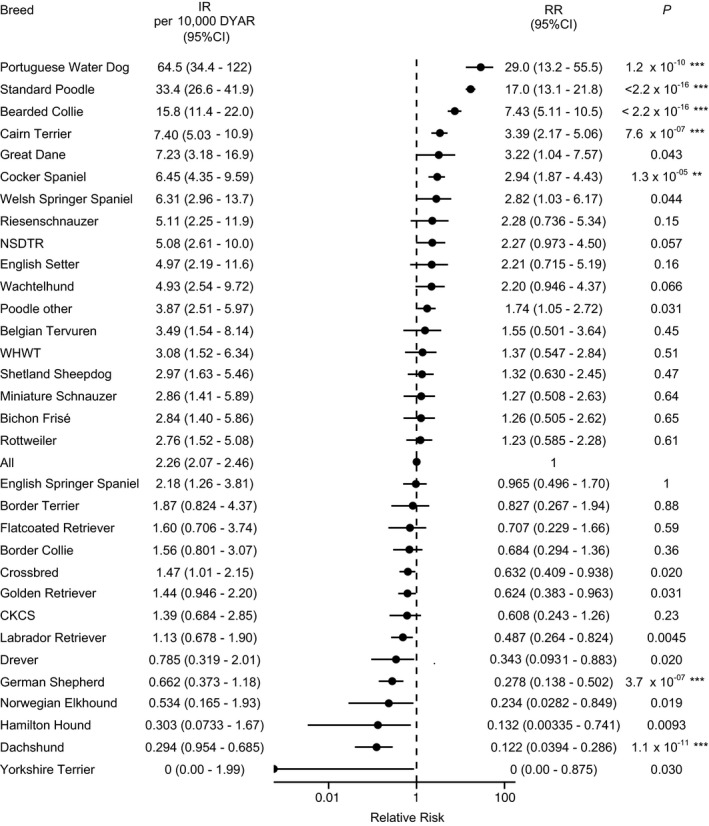
Incidence rate of adrenocortical insufficiency (AI), excluding cases with a previous claim for hypercortisolism, in a population of 525,028 insured dogs. Data from breeds with ≥5 cases of AI, and from breeds that had a reduced relative risk (RR) with P < .05 before Bonferroni correction. NSDTR, Nova Scotia Duck Tolling Retriever; CKCS, Cavalier King Charles Spaniel; WHWT, West Highland White Terrier; DYAR, dog years at risk; IR, incidence rate, cases per 10,000 DYAR; CI, confidence interval; RR, relative risk of AI within the breed in comparison with the other breeds combined. Asterisks represent level of significance after conservative correction (Bonferroni, n = 115) for multiple testing: *P < .05; **P < .01; ***P < .001.
Prevalence
At the end of the study period 191,434 dogs were alive. Of these, 166 dogs had at least 1 claim of NOAI, resulting in an overall prevalence of 0.0867% (95% CI, 0.0740% to 0.101%). The prevalence was 1.17% (95% CI, 1.21% to 2.35%) in Standard Poodles, 1.16% (95% CI, 0.240% to 3.35%) in Portuguese Water Dogs, 0.417% (95% CI, 0.200% to 0.766%) in Cairn Terriers and 0.322% (95% CI, 0.0880% to 0.825%) in Bearded Collies.
Sex
Of 534 dogs with NOAI, 64% (95% CI, 60% to 68%) were females, and 36% (95% CI, 32% to 40%) were males. The overall IR in female dogs was 3.45 cases per 10,000 DYAR (95% CI, 3.10–3.84 cases per 10,000 DYAR). The overall IR in male dogs was 1.86 cases per 10,000 DYAR (95% CI, 1.61–2.15 cases per 10,000 DYAR). The RR in female dogs compared with male dogs overall was 1.85 (95% CI, 1.55–2.22; P < .001) (Fig 2). Sex proportion varied by breed. After Bonferroni correction, female predisposition within the breed was statistically significant in Golden Retrievers. In Standard Poodles and mixed breed dogs among others, incidence rates were not significantly different between sexes (Fig 2).
Figure 2.
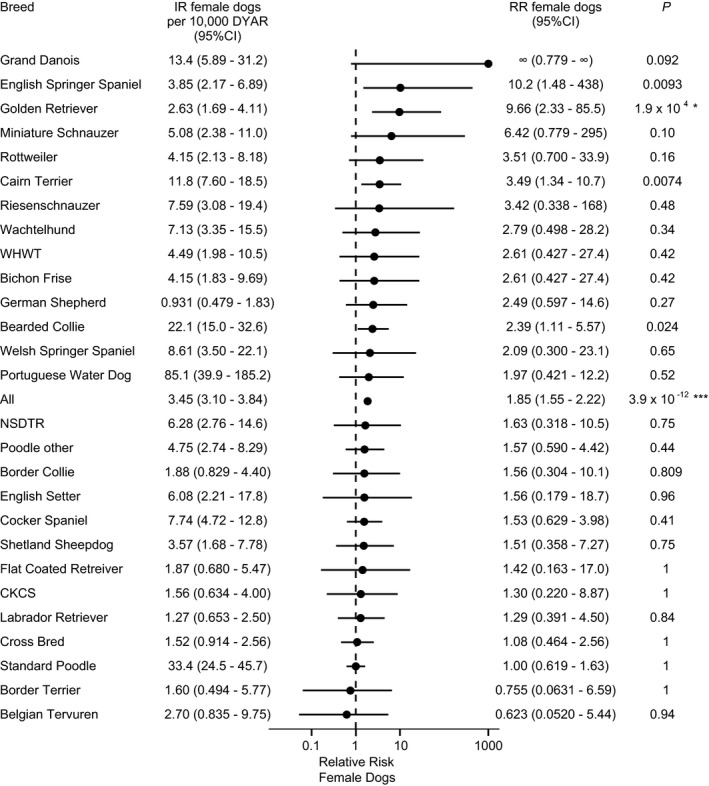
Incidence rate of adrenocortical insufficiency (AI), excluding cases with a previous claim for hypercortisolism, in female dogs by breed. Relative risk (RR) represents the RR of AI in female dogs compared to male dogs within the same breed. Data is shown for breeds with ≥5 cases of AI. NSDTR, Nova Scotia Duck Tolling Retriever; CKCS, Cavalier King Charles Spaniel; WHWT, West Highland White Terrier; DYAR, dog years at risk; IR, incidence rate; CI, confidence interval; RR, relative risk. Asterisks represent level of significance after conservative correction (Bonferroni, n = 28) for multiple testing: *P < .05; **P < .01; ***P < .001.
Mortality Rates
Of the dogs that were registered as dead within the study period, median age at death for dogs with NOAI was 6.90 years (IQR, 4.33–8.46 years) and median age at death for the other dogs overall in the same period was 6.53 years (IQR, 3.56–8.60 years). Of the 534 dogs with NOAI, 212 dogs were reported to die within the study period. The mortality rate because of all causes in dogs with NOAI was 664 (95% CI, 578–760) dogs per 10,000 DYAR. The mortality rate in the residual dogs was 346 (95% CI, 344–348) dogs per 10,000 DYAR. The relative risk of death was significantly higher in dogs with NOAI compared with dogs without AI overall (RR, 1.92; 95% CI, 1.67–2.20; P < 2.2 × 10−16). Cairn Terriers and Bearded Collies with NOAI had a significantly increased relative risk of death compared to other dogs without NOAI within the breed (Fig 3). The RR of death for male dogs with NOAI was 1.78 (95% CI, 1.41–2.22) times that of male dogs without NOAI overall. The RR of death for female dogs with NOAI was 2.11 (95% CI, 1.77–2.50) compared with female dogs without NOAI overall.
Figure 3.
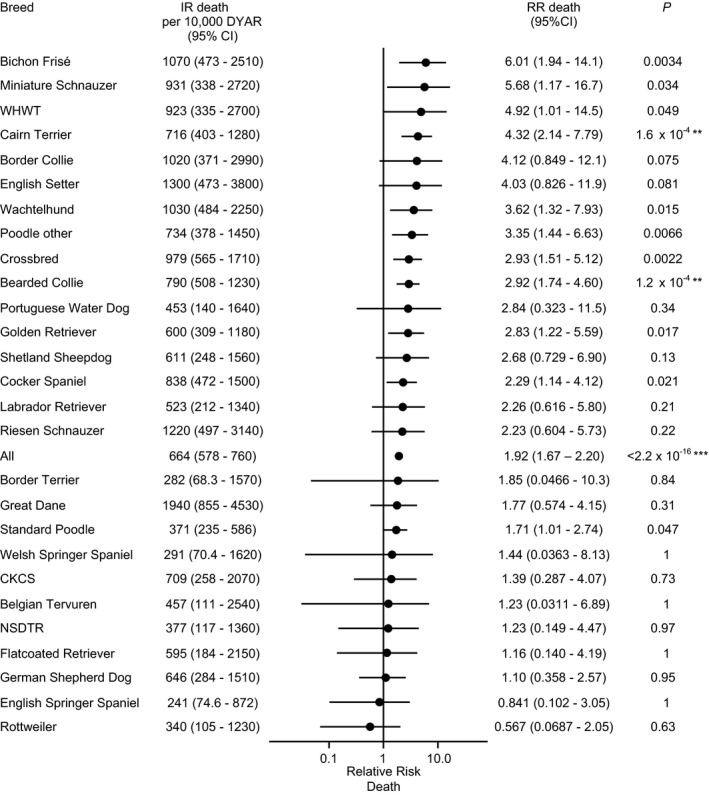
Mortality rate of adrenocortical insufficiency (AI) by breed in a population of 528,028 insured dogs. Dogs with a previous claim for hypercortisolism were excluded. Data are shown for breeds with ≥5 cases of AI. NSDTR, Nova Scotia Duck Tolling Retriever; CKCS, Cavalier King Charles Spaniel; WHWT, West Highland White Terrier; IR, incidence rate; CI, confidence interval; RR, relative risk of death of AI dogs in comparison with overall within the breed. Asterisks represent level of significance after conservative correction (Bonferroni, n = 28) for multiple testing: *P < .05; **P < .01; ***P < .001.
Discussion
This study reports epidemiological data for NOAI during an 11‐year period in a population of more than 500,000 insured dogs. The uniqueness of the study lies in it being based on a large population of insured dogs. Overall and breed‐specific incidence rates are presented. Sex variation was only shown in some breeds, and a higher mortality rate was identified in dogs with AI compared with other dogs. The results from this study support the existence of breed‐specific differences in the behavior of AI in dogs.
The overall incidence rate of AI in this study was 2.3 cases per 10,000 DYAR. The overall prevalence was almost 0.09%, which is in the magnitude of previous prevalence estimates of 0.09–0.32% for both naturally occurring and iatrogenic AI, and of 0.28% for NOAI.1, 2 This also matches a previous statement of a 100 times higher prevalence in the dog than in humans.6, 29 The highest IRs and RRs were, as anticipated, found in the Portuguese Water Dog, Standard Poodle, and the Bearded Collie.5, 6, 7 However, the figures for estimated prevalence by breed in the present report are lower than previously reported occurrences; 1.2% versus 8.6% for the Standard Poodle,5 0.32% versus 4.5% to 9.4% in the Bearded Collie,6, 8 and 1.2% versus 1.7% to 7.0% in the Portuguese Water Dog.9, 20 The most probable explanation for this difference is that the different studies look at different subsets of the overall AI picture. The present study is based on a large cohort of dogs, which represent an insured dog population comprising approximately 30–40% of the national population during the time of the study. Dogs were included at an early age (median less than 3 months of age, upper interquartile range about 6 months of age), which is reflected by the median contribution of 3.8 DYAR per dog. Previous calculations were positively biased with the respect to proportion of dogs with NOAI, although other factors may contribute such as differences in family/bloodlines within a breed between countries. The present results affirm the previously reported increased risk of developing NOAI in the Cairn Terrier.3 In contrast to previous reports, the Cocker Spaniel breed was associated in the present study with a significantly increased risk of developing NOAI.1, 3 The Cocker Spaniel is known to be predisposed for other immune‐mediated diseases such as immune‐mediated hemolytic anemia, immune‐mediated thrombocytopenia, and reportedly hypothyroidism.30, 31, 32, 33, 34 Therefore, it is reasonable to assume that the adrenal gland would be another target of the increased tendency for autoimmune reactions seen in the Cocker Spaniel breed. The German Shepherd Dog is another breed known to be predisposed to immune‐related disorders, for example, atopic dermatitis, unspecific dermatitis, enteritis, and exocrine pancreatic atrophy.35 In contrast to Cocker Spaniels, however, the German Shepherd dogs had a low risk of NOAI. A possible explanation for the difference in the risk of AI between the Cocker Spaniel and German Shepherd Dog may be explained by dissimilarities in the underlying pathophysiological mechanisms for the immunological reactions. For example, it was recently shown that atopic dermatitis may be related to a defect in skin integrity because of an altered expression of the desmosome protein Plakophilin 2.36 In Cocker Spaniels, however, NOAI may be related to the class of major histocompatibility complex (MHC) which in humans is shown to increase the likelihood of the development of autoimmune AI29, 37, 38, 39 and autoimmune diseases in general.40
In this study, NOAI was generally more frequently diagnosed in female dogs. However, incidence rates between sexes varied by breed ranging from an almost 10 times higher risk of developing NOAI in female Golden Retrievers than in male Golden Retriever dogs to an equal risk of developing NOAI between sexes in the Standard Poodle. As the calculations in this study are based on dogs in the same population, the evidence is strong that there is a true difference in sex predisposition between some breeds. Furthermore, for the first time we show that in the Golden Retriever more females than male dogs get NOAI although the breed was not at increased risk of developing NOAI compared with all breeds, combined. This may have important future implications. In people, it is estimated that approximately 40% of human patients with the autoimmune adrenalitis have sporadically occurring, so‐called “isolated” form of primary AI.29 Adrenocortical insufficiency in the Golden Retriever thereby may prove to be a model for isolated AI in women. Likewise the Standard Poodle and Bearded Collie may prove to be models for other forms of inherited AI in man.41 Information about castration status of the dogs in the present study is unknown. However, most Swedish dogs are intact (78%).42 It can therefore be assumed that most of the dogs in the insurance database are intact, which may have influenced the risks of developing disease positively or negatively.1, 3
The mortality rate was significantly higher in dogs with NOAI compared with dogs overall, which may indicate that there may be problems encountered during the management of the disease that may lead to the early death of the dog or may make the owner inclined to opt for euthanasia. After Bonferroni correction, Cairn Terriers, and Bearded Collies with NOAI had a significantly higher mortality rate than dogs without NOAI of the same breed. For breeds with small number of NOAI cases, lack of power can be a reason for not being able to detect a difference. However, in the breed with most registered cases with NOAI, the Standard Poodle, mortality rate in NOAI dogs was not significantly higher than for other dogs of that breed after Bonferroni correction. Breed‐dependent differences may exist. Our findings in the dog reflect what has earlier been reported in humans. After an initial report on excellent survival on glucocorticoid and mineralocorticoid hormone replacement treatment,43 there was paucity of prognostic studies for AI in people. Recently, 2 Scandinavian studies on long‐term prognosis were published, reporting a higher risk of mortality during the early time‐period after detection of Addison′s disease,44 and an increased risk of mortality was identified in patients younger than 40 years.45 Additionally, a reduction in life expectancy was reported in both women (reduction by 3.2 years) and in men (reduction by 11.2 years).45 Further studies are needed to investigate the underlying characteristics, and breed differences in the increased mortality rate in dogs with AI.
There are a number of limitations to this study some that may, depending on cause, have contributed to either an increased or decreased incidence rate. In order to include dogs with a lifetime longer than the total duration of the study period (ie, 10 years), the study also includes dogs that were born before the study period. Therefore, for some dogs, the first claim of diagnosis within the study may not be truly the first claim of that dog, which may contribute to a slight overestimation of the incidence rates. The main aim of the study was to investigate the incidence rate of NOAI, therefore dogs with a previous history of claims for hypercortisolism were excluded. Still there may be dogs included that may have been iatrogenically caused by sudden withdrawal of glucocorticoid treatment. There are no pathognomonic signs for AI. Therefore, the diagnosis of AI is dependent upon a test measuring the adrenocortical hormone‐producing capacity, most commonly the ACTH‐stimulation test.4 Because the present study is based on data from many different veterinarians, irrespective of level of specialization, there is a risk that dogs with AI have been misdiagnosed as other more common disorders such as renal failure or gastrointestinal disease, alternatively AI could have been erroneously diagnosed. Although the study period starts at a time when important and extensive articles on AI were published,1, 3, 21 which most probably contributed to an increased awareness of the existence of the disorder among veterinarians world‐wide, the study period extended over a decade, during which there may have been variation in the degree of awareness of and capability to diagnose the disease. Thus, the true incidence in the general dog population may be higher or lower than presented here. There may also be a negative bias for the diagnosis in breeds that is not usually associated with NOAI, and young dogs that died before 6 weeks of age, which is the age limit for individual dog insurance. Other potential risks for underestimation of disease incidence are that dogs were diagnosed with the disease without exceeding the set deductible, or that AI was not registered as first diagnosis, and therefore not included in the database. However, the expected costs for initial work up including veterinary consult, blood work, ACTH‐stimulation test, and control visits will likely exceed the standard deductible. Likewise, due to its severity, AI is likely to be chosen as first diagnosis at time of diagnosis or at control visits. With increased awareness of the disease and recognition of its many faces of clinical presentation, it can be assumed that increased testing for the disease will result in increased incidence rates. Finally, even though the interquartile ranges are largely overlapping, the slightly higher median age at death of the NOAI dogs may have contributed to some degree to the overall increased mortality rate found. However, at the end of the study, the median age of breeds contributing with NOAI cases was higher than the median age of all dogs (data not shown). Thus, we do not expect differences in age distribution to be a major determining factor for the increased mortality rate observed in NOAI dogs in this study.
In summary, in addition to being the largest epidemiological study of NOAI in dogs reported in the literature, the present study is based on a large and well‐defined population of dogs that are reasonably representative of the general dog population. Importantly, we present data supporting the presence of breed‐specific differences in NOAI regarding incidence rates, sex distribution and mortality rates, suggesting the existence of different subtypes of NOAI in the dog, analogous to what is known in people. More studies are needed to further characterize AI in dogs with respect to possible breed and sex differences, and long‐term survival during treatment.
Acknowledgments
Financial Support: This study was supported by grants from the Swedish Research Council and the Foundation for Research, Agria Insurance Company. The contribution of the Agria Insurance Company by making part of their database available for the research is highly appreciated. The authors thank Prof. Agneta Egenvall, Mikael Andersson Franko, Marcin Kierczak for statistical advice, and Hanna Bremer for input and discussions.
Conflict of Interest Declaration: Brenda N. Bonnett consults with Agria insurance company on various projects. The Agria insurance company funded work leading to the development of the insurance data base used as data source in the study. This study was supported by grants from the Swedish Research Council and the Foundation for Research, Agria Insurance Company.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Footnotes
Agria Pet Insurance, Stockholm, Sweden
EpiR, An R package for the analysis of epidemiological data. Stevenson M, Sanchez J, Thornton, R. http://epicentre.massey.ac.nz
References
- 1. Kelch WJ. Canine Hypoadrenocorticism (Canine Addison′s disease): History, Contemporary Diagnosis by Practicing Veterinarians, and Epidemiology [dissertation]. Knowville: University of Tennessee; 1996. [Google Scholar]
- 2. Kelch WJ, Lynn RC, Smith CA, New JC. Canine hypoadrenocorticism (Addison′s disease). Comp Small Anim Pract 1998;20:921–934. [Google Scholar]
- 3. Peterson ME, Kintzer PP, Kass PH. Pretreatment clinical and laboratory findings in dogs with hypoadrenocorticism: 225 cases (1979–1993). J Am Vet Med Assoc 1996;208:85–91. [PubMed] [Google Scholar]
- 4. Feldman EC, Nelson RW. Hypoadrenocorticism (Addison's disease) In Canine and Feline Endocrinology and Reproduction, 3rd ed St. Louis: Saunders; 2004:394–439. [Google Scholar]
- 5. Famula TR, Belanger JM, Oberbauer AM. Heritability and complex segregation analysis of hypoadrenocorticism in the standard poodle. J Small Anim Pract 2003;44:8–12. [DOI] [PubMed] [Google Scholar]
- 6. Oberbauer AM, Benemann KS, Belanger JM, et al. Inheritance of hypoadrenocorticism in bearded collies. Am J Vet Res 2002;63:643–647. [DOI] [PubMed] [Google Scholar]
- 7. Oberbauer AM, Bell JS, Belanger JM, Famula TR. Genetic evaluation of Addison's disease in the Portuguese water dog. BMC Vet Res 2006;2:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sell E. Health Committee Report. Bearded Collie Club America National Speciality; 1998. [Google Scholar]
- 9. Slater MR. The 2005 Portuguese Water Dog Health Survey: Final Report. Portuguese Water Dog Club of America; 2006. [Google Scholar]
- 10. Hadlow WJ. Adrenal cortical atrophy in the dog; report of three cases. Am J Pathol 1953;29:353–361. [PMC free article] [PubMed] [Google Scholar]
- 11. Siegel ET, Schryver HF, Fidler I. Clinico‐pathologic conference. J Am Vet Med Assoc 1967;150:423–433. [PubMed] [Google Scholar]
- 12. Frank CB, Valentin SY, Scott‐Moncrieff JCR, Miller MA. Correlation of inflammation with adrenocortical atrophy in canine adrenalitis. J Comp Pathol 2013;149:268–279. [DOI] [PubMed] [Google Scholar]
- 13. Mulnix JA. Hypoadrenocorticism in the dog. J Am Anim Hosp Assoc 1971;7:220–241. [Google Scholar]
- 14. Bistner S, de Lahunta A, Lorenz M. Generalized cryptococcosis in a dog. Cornell Vet 1971;61:440–457. [PubMed] [Google Scholar]
- 15. Kook PH, Grest P, Raute‐Kreinsen U, et al. Addison's disease due to bilateral adrenal malignancy in a dog. J Small Anim Pract 2010;51:333–336. [DOI] [PubMed] [Google Scholar]
- 16. Willard MD, Schall WD, Nachreiner RF, Shelton DG. Hypoadrenocorticism following therapy with o, p‐DDD for hyperadrenocorticism in four dogs. J Am Vet Med Assoc 1982;180:638–641. [PubMed] [Google Scholar]
- 17. Chapman PS, Kelly DF, Archer J, et al. Adrenal necrosis in a dog receiving trilostane for the treatment of hyperadrenocorticism. J Small Anim Pract 2004;45:307–310. [DOI] [PubMed] [Google Scholar]
- 18. Ramsey IK, Richardson J, Lenard Z, et al. Persistent isolated hypocortisolism following brief treatment with trilostane. Aust Vet J 2008;86:491–495. [DOI] [PubMed] [Google Scholar]
- 19. Hanson JM, Teske E, Voorhout G, et al. Prognostic factors for outcome after transsphenoidal hypophysectomy in dogs with pituitary‐dependent hyperadrenocorticism. J Neurosurg 2007;107:830–840. [DOI] [PubMed] [Google Scholar]
- 20. Chase K, Lawler DF, McGill LD, et al. Age relationships of postmortem observations in Portuguese water dogs. Age (Dordr) 2011;33:461–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kintzer PP, Peterson ME. Treatment and long‐term follow‐up of 205 dogs with hypoadrenocorticism. J Vet Intern Med 1997;11:43–49. [DOI] [PubMed] [Google Scholar]
- 22. Bates JA, Shott S, Schall WD. Lower initial dose desoxycorticosterone pivalate for treatment of canine primary hypoadrenocorticism. Aust Vet J 2013;91:77–82. [DOI] [PubMed] [Google Scholar]
- 23. Bonnett BN, Egenvall A. Age patterns of disease and death in insured Swedish dogs, cats and horses. J Comp Pathol 2010;142(Suppl 1):S33–S38. [DOI] [PubMed] [Google Scholar]
- 24. Egenvall A, Bonnett BN, Olson P, Hedhammar Å. Gender, age, breed and distribution of morbidity and mortality in insured dogs in Sweden during 1995 and 1996. Vet Rec 2000;146:519–525. [DOI] [PubMed] [Google Scholar]
- 25. Statistiska Centralbyrån . Förekomst av sällskapsdjur ‐ främst hund och katt ‐ i svenska hushåll. Statistiska Centralbyrån 2006. Swedish.
- 26. Svenska Djursjukhusföreningen . Diagnosregistret. Olson P, Kängström LE, eds. Taberg: Diagnosregister 1993. Swedish.
- 27. Collett D. Exact methods In Modelling Binary Data, 2nd ed Boca Raton: Chapman & Hall/CRC; 2003:303–324. [Google Scholar]
- 28. Fay MP. Two‐sided exact tests and matching confidence intervals for discrete data. R J 2010;2:53–58. [Google Scholar]
- 29. Charmandari E, Nicolaides NC, Chrousos GP. Adrenal insufficiency. Lancet 2014;383:2152–2167. [DOI] [PubMed] [Google Scholar]
- 30. Reimer ME, Troy GC, Warnick LD. Immune‐mediated hemolytic anemia: 70 cases (1988–1996). J Am Anim Hosp Assoc 1999;35:384–391. [DOI] [PubMed] [Google Scholar]
- 31. Miller SA, Hohenhaus AE, Hale AS. Case‐control study of blood type, breed, sex, and bacteremia in dogs with immune‐mediated hemolytic anemia. J Am Vet Med Assoc 2004;224:232–235. [DOI] [PubMed] [Google Scholar]
- 32. Grindem CB, Breitschwerdt EB, Corbett WT, Jans HE. Epidemiologic survey of thrombocytopenia in dogs: a report on 987 cases. Vet Clin Pathol 1991;20:38–43. [DOI] [PubMed] [Google Scholar]
- 33. Lewis DC, Meyers KM. Canine idiopathic thrombocytopenic purpura. J Vet Intern Med 1996;10:207–218. [DOI] [PubMed] [Google Scholar]
- 34. Nachreiner RF, Refsal KR, Graham PA, Bowman MM. Prevalence of serum thyroid hormone autoantibodies in dogs with clinical signs of hypothyroidism. J Am Vet Med Assoc 2002;220:466–471. [DOI] [PubMed] [Google Scholar]
- 35. Vilson A, Bonnett B, Hansson‐Hamlin H, Hedhammar Å. Disease patterns in 32,486 insured German shepherd dogs in Sweden: 1995–2006. Vet Rec 2013;173:116. [DOI] [PubMed] [Google Scholar]
- 36. Tengvall K, Kierczak M, Bergvall K, et al. Genome‐wide analysis in German shepherd dogs reveals association of a locus on CFA 27 with atopic dermatitis. PLoS Genet 2013;9:e1003475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Massey J, Boag A, Short AD, et al. MHC class II association study in eight breeds of dog with hypoadrenocorticism. Immunogenetics 2013;65:291–297. [DOI] [PubMed] [Google Scholar]
- 38. Short AD, Boag A, Catchpole B, et al. A candidate gene analysis of canine hypoadrenocorticism in 3 dog breeds. J Hered 2013;104:807–820. [DOI] [PubMed] [Google Scholar]
- 39. Mitchell AL, Pearce SHS. Autoimmune Addison disease: pathophysiology and genetic complexity. Nat Rev Endocrinol 2012;8:306–316. [DOI] [PubMed] [Google Scholar]
- 40. Tsai S, Santamaria P. MHC class II polymorphisms, autoreactive T‐cells, and autoimmunity. Front Immunol 2013;4:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Husebye ES, Allolio B, Arlt W, et al. Consensus statement on the diagnosis, treatment and follow‐up of patients with primary adrenal insufficiency. J Intern Med 2014;275:104–115. [DOI] [PubMed] [Google Scholar]
- 42. Statistiska Centralbyrån . Hundar, katter och andra sällskapsdjur 2012. Statistiska Centralbyrån 2012. Swedish.
- 43. Dunlop D. Eighty‐six cases of Addison′s disease. Br Med J 1963;2:887–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bergthorsdottir R, Leonsson‐Zachrisson M, Odén A, Johannsson G. Premature mortality in patients with Addison's disease: a population‐based study. J Clin Endocrinol Metab 2006;91:4849–4853. [DOI] [PubMed] [Google Scholar]
- 45. Erichsen MM, Løvås K, Fougner KJ, et al. Normal overall mortality rate in Addison's disease, but young patients are at risk of premature death. Eur J Endocrinol 2009;160:233–237. [DOI] [PubMed] [Google Scholar]


