Abstract
Background
Pheochromocytoma is the most common adrenal medullary neoplasm of domestic animals, but it is rare in horses. Antemortem diagnosis in horses is difficult, with clinical signs often being vague or non‐specific.
Objective
The objective of this study was to describe the clinical, laboratory, and pathologic findings of pheochromocytoma in horses.
Animals
Thirty‐seven horses diagnosed with pheochromocytoma based on postmortem examination from 2007 to 2014.
Methods
Retrospective case series.
Results
Pheochromocytoma was identified in 37/4094 horses during postmortem examination. Clinical signs consistent with pheochromocytoma had been observed antemortem in only 7 cases, with the remainder being incidental findings. Colic was the most common presenting complaint (13 of 37 cases) and tachycardia was noted in 95% of cases (median heart rate of 86 bpm in clinical cases). Hyperlactatemia (median, 4.9 mmol/L) and hyperglycemia (median, 184 mg/dL) were the most common clinicopathologic abnormalities. Hemoperitoneum caused by rupture of pheochromocytoma was noted in 4/7 clinical cases. Concurrent endocrine abnormalities (eg, thyroid adenoma, adrenal hyperplasia, pituitary pars intermedia hyperplasia or adenoma, parathyroid C‐cell carcinoma) were found in 27/37 horses, with 8/37 horses having lesions consistent with multiple endocrine neoplasia syndrome as described in humans.
Conclusions
Pheochromocytoma was diagnosed in 0.95% of horses presented for necropsy. The majority of these were incidental findings, but pheochromocytoma was thought to contribute to clinical findings in 19% of cases, and multiple endocrine neoplasms were commonly seen. Usually an incidental finding at necropsy, pheochromocytoma may cause acute death from intraperitoneal exsanguination and should be considered in horses presenting with colic, tachycardia, and hemoperitoneum.
Keywords: Adrenal, Hemoperitoneum, Multiple endocrine neoplasia, Pheochromocytoma
Abbreviations
- MEN
multiple endocrine neoplasia syndrome
- PCV
packed cell volume
- SDHAF2
succinate dehydrogenase complex assembly factor 2
- SDHA
succinate dehydrogenase subunit A
- SDHB
succinate dehydrogenase subunit B
- TMEM127
transmembrane protein 127
- TS
total solids
- WBC
white blood cell
Pheochromocytoma is the most common adrenal medullary neoplasm of domestic animals,1 but it is a relatively rare finding in horses. Only a few cases of pheochromocytoma have been reported in horses,2, 3, 4, 5, 6, 7 but it has been well described in dogs,8 cattle,9 and humans.10
Pheochromocytomas arise from the chromaffin cells of the adrenal medulla, which are postganglionic neurons of the autonomic nervous system.1 Although frequently seen as solitary neoplasms, pheochromocytomas may develop concurrently with parafollicular cell tumors of the thyroid gland11, 12 in association with a multiple endocrine neoplasia (MEN) syndrome. Multiple endocrine neoplasia is defined as simultaneous neoplasia and hyperplasia of multiple endocrine tissues, and has been found to have a genetic basis in humans.13 Such a syndrome previously has been described in horses,14, 15 cattle,12 dogs,16, 17, 18, 19 cats,20, 21 and humans.13
Although bilateral disease has been described, as in humans, reports in horses most commonly describe unilateral, benign pheochromocytomas.6, 22 Functional pheochromocytomas are those that result in clinical signs arising from production and secretion of catecholamines, and are infrequently reported in animals.1 In humans, pheochromocytomas often are functional, and human patients often present with a history of headaches, palpitations, diaphoresis, and severe episodic hypertension.10 In case reports of horses with functional pheochromocytomas, clinical findings include tachycardia, profuse sweating, excitation, and muscle fasciculations.2, 7 Colic and intraperitoneal hemorrhage were reported in 2 affected horses.5
A number of diagnostic tests are available for antemortem diagnosis in humans, including hormone assays (urinary and plasma free metanephrine), computed tomography, magnetic resonance imaging, positron emission tomography, and scintigraphy, as well as testing for associated germline mutations.10, 23 Abdominal ultrasonography and surgical biopsy have been used for antemortem diagnosis in dogs.8 By contrast, antemortem diagnosis of pheochromocytomas in horses is rare, with the majority of diagnoses occurring postmortem. The purpose of this study was to characterize the clinical, laboratory, and pathologic findings in horses with pheochromocytoma diagnosed at necropsy.
Materials and Methods
Electronic and hard copy medical records from the New Bolton Center at the University of Pennsylvania and the William R. Pritchard Veterinary Medical Teaching Hospital at the University of California – Davis were searched for horses diagnosed with pheochromocytoma from January 1, 2007 to December 31, 2014. Information was recorded for signalment, history, presenting complaint, physical examination findings, clinicopathologic data, and postmortem examination findings for each horse.
Medical records were reviewed to determine whether the tumor was clinical or an incidental finding. Criteria for including cases in the clinical group were based on previous studies in dogs,8 and included the presence of at least 1 of the following: weakness, collapse, dyspnea or tachypnea, seizures, tachyarrhythmias, colic, hemoperitoneum, or cardiac arrest, with no other obvious cause of death determined on postmortem examination. Definitive diagnosis of pheochromocytoma was based on histopathologic examination of samples collected on postmortem examination.
Statistical Analysis
Descriptive statistics were used to report clinical and clinicopathologic findings. Numerical values are reported as medians and ranges unless otherwise specified.
Results
A search of the medical records identified 37 horses with a confirmed diagnosis of pheochromocytoma on postmortem examination: 13 from the University of Pennsylvania and 24 from the University of California – Davis. A total of 2,262 necropsies were performed on horses at the University of Pennsylvania and 1,832 necropsies were performed at the University of California – Davis during the study period. Lesion prevalence at the University of Pennsylvania and University of California – Davis was 0.6 and 1.3%, respectively, for a combined prevalence of 0.95%.
Of the affected animals, 15 were mares and 22 were geldings. Median age was 28 years (range, 13–38 years). The median age of the horses judged to have clinical pheochromocytomas was 17 years (range, 13–38 years) and the median age of those judged to have incidental pheochromocytomas was 27 years (range, 16–37 years). Breeds represented included Quarter Horse/Quarter Horse cross (8), pony breed (7), Arabian (6), Thoroughbred (4), Warmblood (3), Standardbred (3), Morgan (2), Appaloosa (1), Mustang (1), Paso Fino (1), and 1 of unknown breed. The most common presenting complaint was colic (13/37). Less common presenting complaints included recumbency or inability to stand (n = 6), weight loss (n = 3), chronic laminitis (n = 3), respiratory disease (n = 2), trembling (n = 1), epistaxis (n = 1), and ascites (n = 1). Seven of the horses were presented for euthanasia because of chronic conditions.
Pheochromocytoma was considered to have contributed to clinical signs and death in 7 of 37 cases (19%). Four of these horses had hemoperitoneum associated with a ruptured pheochromocytoma on postmortem examination, and all 4 of them were presented with signs of colic. In no instance was a definitive diagnosis of endocrinologically active pheochromocytoma made based on serum or urinary assays of catecholamines or their metabolites. The remaining 30 animals were determined to have incidental pheochromocytomas (81%).
Tachycardia (heart rate >40 bpm) was identified in 20/21 cases (95%) in which heart rate was recorded, with a median heart rate of 68 bpm (range, 44–136 bpm) in tachycardic horses. Heart rate was not recorded in 16 horses, either as a result of incomplete medical records or because horses were presented solely for necropsy after euthanasia. Median heart rate in the 7 horses judged to have clinical pheochromocytomas was 86 bpm (range, 44–136 bpm). Blood pressure was not measured in any horse, and no horses had cardiac arrhythmias on auscultation, but 8/21 (38%) were noted to have a cardiac murmur. Severe sweating was noted in 2/21 horses, and muscle fasciculations were noted in 6/21.
Antemortem packed cell volume (PCV) and total solids (TS) were available in 12 horses, with a median PCV of 43% (range, 32–65%) and median TS of 6.95 g/dL (range, 5.7–8.2 g/dL). Hyperlactatemia (peripheral lactate concentration >2.0 mmol/L) was noted in 9/10 cases where available, with a median value of 4.9 mmol/L (range, 2.6–16.4 mmol/L). Hyperglycemia (blood glucose concentration >114 mg/dL) was noted in 10/14 cases, with a median glucose concentration of 183.5 mg/dL (range, 96–825 mg/dL). Although 7/16 horses had leukocytosis (WBC >10,500/μL), none had a lymphocytosis as may be expected in an epinephrine‐stimulated leukogram.
On postmortem examination, pheochromocytoma was determined to be unilateral in 33 (89%) cases (18 right, 12 left, 3 unspecified) and bilateral in 4 (11%) cases. Pheochromocytomas were defined as benign when well encapsulated and confined to the affected adrenal gland (Fig. 1) and as malignant when they effaced normal adrenal architecture and invaded the ipsilateral vasculature. Eight of 37 were classified as malignant (22%) and 29 of 37 were classified as benign (78%). One of the 8 malignant cases was associated with metastasis to the brain and pulmonary parenchyma. Histologically, the majority of tumors were highly vascular, with cuboidal to polygonal neuroendocrine cells arranged in nests and packets surrounded by a fine fibrovascular stroma interspersed with coalescing foci of hemorrhage (Fig. 2).
Figure 1.
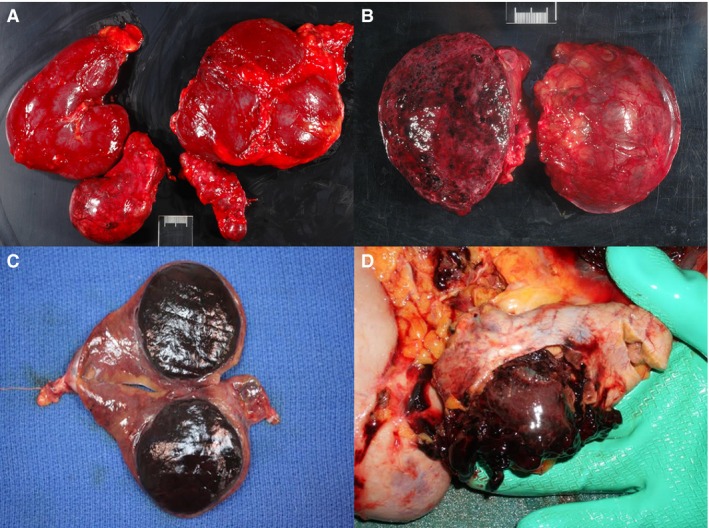
(A) Left and right kidney and adrenal gland of a horse showing pheochromocytoma of the right adrenal gland. (B) Mottled, cut section of the pheochromocytoma seen in image A. (C) Depicted is a well‐demarcated pheochromocytoma with associated compression and atrophy of the adjacent adrenocortical tissue on cut section of an adrenal gland from an adult horse (photo credit Dr. Suzanne Stewart). (D) Ruptured pheochromocytoma causing hemoperitoneum in adult horse (photo credit Dr. Suzanne Stewart).
Figure 2.
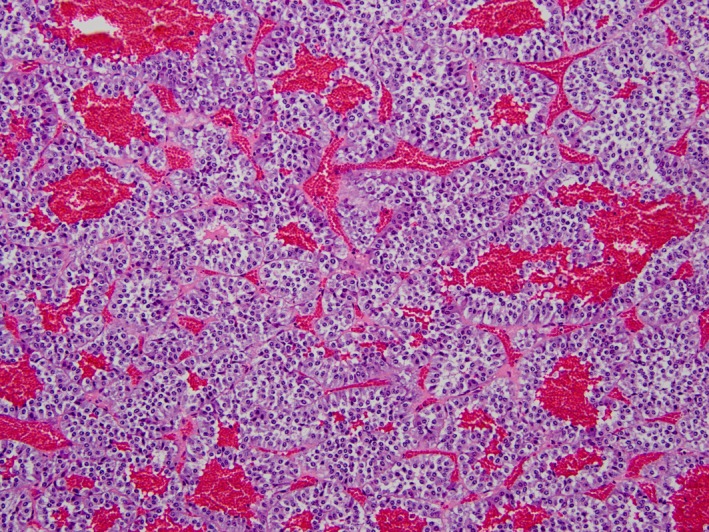
Nests and cords of neoplastic neuroendocrine cells are homogeneously ovoid to polygonal and are intimately associated with blood‐filled vascular spaces. Pheochromocytoma, equine adrenal gland, hematoxylin and eosin‐stained section, 200× magnification.
Twenty‐seven horses also had other endocrine tumors identified on postmortem examination, including thyroid adenoma (n = 8), parathyroid C‐cell carcinoma (n = 1), adrenal hyperplasia (n = 9), and pituitary pars intermedia hyperplasia or adenoma (n = 18). Of these, 8 had at least 3 disorders of growth within the endocrine system.
Discussion
Our study indicates that pheochromocytomas are rare in horses, with a postmortem examination incidence of 0.95%, and that generally they are clinically silent. An incidence of approximately 3% has been reported in humans in a study evaluating patients presented for adrenal incidentalomas,24 with pheochromocytoma occurring in 0.2–0.6% of human patients with hypertension.10 Pheochromocytomas appeared to contribute to clinical disease in 20% of horses examined, with the remainder being incidental. This observation is in contrast to dogs, in which 34% were reported to be clinical.8 However, the distinction between clinical and incidental pheochromocytomas is indistinct because many of the signs that could be attributed to excess catecholamine secretion from an active pheochromocytoma (eg, increased heart rate, hyperlactatemia, pain) also could be caused by hemorrhagic shock, other forms of colic, or systemic disease. The study population of horses was geriatric, with a median age of 28 years. A study in dogs found that pheochromocytomas developed in middle‐aged to older dogs and found no sex or breed predisposition.8 Similarly, no breed predilection was noted in our study.
The most common presenting complaint was colic, a vague clinical sign that may be associated with a number of diseases. Although nonspecific, tachycardia was seen in 95% of horses, with the median heart rate in horses with putatively clinical pheochromocytoma being markedly increased at 86 bpm. Tachycardia may be associated with the adrenergic effects of catecholamines produced by neoplastic cells of the adrenal medulla, but alternatively could be caused by pain or hemorrhagic shock. Sweating and muscle tremors also were seen in our study and may be caused by β‐adrenergic stimulation. Catecholamine release by pheochromocytomas may result in systemic hypertension, and such findings have been reported in affected dogs and humans.6, 8, 10 Unfortunately, no blood pressure measurements were available for the horses in our study, and none of them had any diagnostic testing performed to confirm the presence or endocrine activity of the tumor. Other signs of pheochromocytoma in humans include weakness, nausea, abdominal pain, tremors, and anxiety or a “sensation of doom.” Anxiety is, however, difficult to characterize in horses. The intraperitoneal hemorrhage seen in 4 horses is of particular note. Previous case reports in horses have described intraperitoneal hemorrhage in association with pheochromocytomas, suggesting that vascular integrity within these tumors is poor, which may result in bleeding.5 Other causes of intraperitoneal hemorrhage in horses include splenic rupture, rupture of the uterine artery in periparturient mares, coagulopathy, or other neoplasia.25
Although most pheochromocytomas are associated with the adrenal glands, in humans, approximately 10% are found in extra‐adrenal sites. In humans, pheochromocytomas are sometimes referred to as the “10% tumor,” because “approximately 10% occur above the diaphragm, 10% of intra‐abdominal pheochromocytomas are extra‐adrenal, 10% are bilateral, 10% are multiple, 10% are familial, 10% are malignant, and 10% recur post‐operatively”.26 The most common extra‐adrenal site of pheochromocytoma in humans is the para‐aortic region between the diaphragm and caudal pole of the kidney.22 Other extra‐adrenal sites include the uterus, ovary, retroperitoneum, lumbar spine, bladder, ocular orbit, heart, mediastinum,27 and also in the Organ of Zuckerkandl, a chromaffin body at the aortic bifurcation that acts to regulate blood pressure by catecholamine release during fetal development.28 In our study, no extra‐adrenal pheochromocytomas were identified in affected horses.
Diagnosis of pheochromocytoma in human patients is based on characteristic history and clinical signs, plasma metanephrine testing, urinary catecholamine measurement, and advanced imaging.10 Plasma metanephrine testing has not been well described in the horse and is limited by assay availability and likely a lack of clinical suspicion prompted by the impression that pheochromocytomas are a rare or implausible differential diagnosis. Venous norepinephrine concentrations have been proposed for the diagnosis of pheochromocytoma in horses, but use of this assay has not been reported in horses with pheochromocytoma.6 Hyperglycemia is a common finding in human patients with pheochromocytoma,23 and was a notable feature in our study, with marked hyperglycemia of 825 mg/dL being observed in 1 horse with ruptured pheochromocytoma, and a median blood glucose concentration in clinical cases of 422 mg/dL. In humans, marked hyperglycemia is attributed to catecholamine stimulation of lipolysis, leading to subsequent inhibition of glucose uptake by muscle cells.23 By contrast, in a study of affected dogs, only 7 of 45 had documented hyperglycemia.8 Hyperlactatemia also was a notable clinicopathologic finding in our study and may have been associated with catecholamine‐mediated vasoconstrictive ischemia, the metabolic effects of epinephrine on gluconeogenesis, or hemorrhagic shock in horses with intraperitoneal hemorrhage. In humans, hyperlactatemia is a rare but recognized effect of pheochromocytoma,29, 30 especially in association with tumor manipulation during adrenalectomy.31
Abdominal ultrasonography is used in dogs to visualize the adrenal glands, but a positive diagnosis of pheochromocytoma cannot be made exclusively by use of ultrasonography.8 Transcutaneous and transrectal abdominal ultrasonography has the potential to identify adrenal tumors in horses if the tumors are large enough and in a location that is sonographically accessible. Gene sequencing may have potential application in the future for the diagnosis, treatment choices and prognosis of pheochromocytomas in humans and animals. Mutations in the succinate dehydrogenase complex assembly factor 2 (SDHAF2), succinate dehydrogenase subunit A (SDHA), and transmembrane protein 127 (TMEM127) genes have been associated with pheochromocytoma in humans.32 Germline mutations of the succinate dehydrogenase subunit B (SDHB) gene are most frequently associated with aggressive pheochromocytomas that result in poor survival, and 50% of patients with metastatic pheochromocytoma carry SDHB mutations.33
Eight horses in our study had pathologic findings consistent with MEN. This syndrome has been well described in humans34 and has been reported in horses.14, 15 Multiple endocrine neoplasia is defined as simultaneous neoplasia and hyperplasia of multiple endocrine tissues. These neoplasms arise from cells that share the ability for amine precursor uptake and decarboxylation.14 Although isolated case reports of MEN have been described in dogs, the clinical relevance of genetic mutations associated with this finding have not been determined.16, 17, 18, 19 Our findings seem most similar to Type 2 MEN described in humans, an autosomal dominant inherited syndrome that may be subdivided into 2 forms.34 Type 2a MEN (Sipple's syndrome) is characterized by the concurrent appearance of medullary thyroid carcinoma, hyperparathyroidism, and pheochromocytoma. In Type 2b MEN, hyperparathyroidism is absent.34 Multiple endocrine neoplasia may be associated with C‐cell carcinomas and parathyroid gland hyperplasia. Unfortunately, the parathyroid gland rarely is identified during postmortem examinations in horses. The occurrence of thyroid adenomas, pheochromocytoma, and C‐cell carcinomas has been described in horses with suspected MEN.14 In bulls, adrenal medullary hyperplasia was noted to precede the development of pheochromocytoma.11, 12 In our study, 49% of horses with pheochromocytomas also had pituitary pars intermedia hyperplasia or adenomas on postmortem examination. This frequency is considerably higher than described in a recent study, which reported a prevalence of 21.2% in horses >15 years,35 a discrepancy possibly due to the fact that the median age of horses in our study was 28 years. Nonetheless, it is possible that these pituitary adenomas are a form of type 1 MEN, which in humans includes the presence of pituitary adenomas.36
Additional research is required to evaluate the diagnosis of metabolically active (catecholamine‐secreting) pheochromocytomas in horses using serum and urine assays or imaging modalities. Doing so requires increased awareness of the expected clinical presentation of pheochromocytoma so that it is included in the differential diagnosis by practitioners evaluating horses with compatible clinical signs. In conclusion, although a fairly rare finding in horses, pheochromocytoma may contribute to clinical disease and should be considered in horses presented for colic or intraperitoneal hemorrhage.
Acknowledgments
Grant support: The study was not supported by a grant.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
All work was done at the University of Pennsylvania New Bolton Center, using records from New Bolton Center and UC Davis.
This study has been submitted as a research abstract for presentation at the AAEP Annual Convention 2015.
References
- 1. Capen CC. Tumors of the endocrine glands In: Meuten DJ, ed. Tumors in Domestic Animals, 4th ed Iowa State Press, Ames, Iowa, USA; 2002:632–636. [Google Scholar]
- 2. Buckingham JD. Case report. Pheochromocytoma in a mare. Can Vet J 1970;11:205–208. [PMC free article] [PubMed] [Google Scholar]
- 3. Gelberg H, Cockerell GL, Minor RR. A light and electron microscopic study of a normal adrenal medulla and a pheochromocytoma from a horse. Vet Pathol 1979;16:395–404. [DOI] [PubMed] [Google Scholar]
- 4. Froscher BG, Power HT. Malignant pheochromocytoma in a foal. J Am Vet Med Assoc 1982;181:494–496. [PubMed] [Google Scholar]
- 5. Johnson PJ, Goetz TE, Foreman JH, Zachary JF. Pheochromocytoma in two horses. J Am Vet Med Assoc 1995;206:837–841. [PubMed] [Google Scholar]
- 6. Yovich JV, Horney FD, Hardee GE. Pheochromocytoma in the horse and measurement of norepinephrine levels in horses. Can Vet J 1984;25:21–25. [PMC free article] [PubMed] [Google Scholar]
- 7. Yovich JV, Ducharme NG. Ruptured pheochromocytoma in a mare with colic. J Am Vet Med Assoc 1983;183:462–464. [PubMed] [Google Scholar]
- 8. Barthez PY, Marks SL, Woo J, et al. Pheochromocytoma in dogs: 61 cases (1984–1995). J Vet Intern Med 1997;11:272–278. [DOI] [PubMed] [Google Scholar]
- 9. West JL. Bovine pheochromocytoma: Case report and review of literature. Am J Vet Res 1975;36:1371–1373. [PubMed] [Google Scholar]
- 10. Pappachan JM, Raskauskiene D, Sriraman R, et al. Diagnosis and management of pheochromocytoma: A practical guide to clinicians. Curr Hypertens Rep 2014;16:442. [DOI] [PubMed] [Google Scholar]
- 11. Black HE, Capen CC, Young DM. Ultimobranchial thyroid neoplasms in bulls. A syndrome resembling medullary thyroid carcinoma in man. Cancer 1973;32:865–878. [DOI] [PubMed] [Google Scholar]
- 12. Sponenberg DP, McEntee K. Pheochromocytomas and ultimobranchial (C‐cell) neoplasms in the bull: Evidence of autosomal dominant inheritance in the guernsey breed. Vet Pathol 1983;20:396–400. [DOI] [PubMed] [Google Scholar]
- 13. Hoff AO, Cote GJ, Gagel RF. Multiple endocrine neoplasias. Annu Rev Physiol 2000;62:377–411. [DOI] [PubMed] [Google Scholar]
- 14. De Cock HE, MacLachlan NJ. Simultaneous occurrence of multiple neoplasms and hyperplasias in the adrenal and thyroid gland of the horse resembling multiple endocrine neoplasia syndrome: Case report and retrospective identification of additional cases. Vet Pathol 1999;36:633–636. [DOI] [PubMed] [Google Scholar]
- 15. Germann SE, Rutten M, Derungs SB, Feige K. Multiple endocrine neoplasia‐like syndrome in a horse. Vet Rec 2006;159:530–532. [DOI] [PubMed] [Google Scholar]
- 16. Kiupel M, Mueller PB, Ramos Vara J, et al. Multiple endocrine neoplasia in a dog. J Comp Pathol 2000;123:210–217. [DOI] [PubMed] [Google Scholar]
- 17. Peterson ME, Randolph JF, Zaki FA, Heath H 3rd. Multiple endocrine neoplasia in a dog. J Am Vet Med Assoc 1982;180:1476–1478. [PubMed] [Google Scholar]
- 18. Proverbio D, Spada E, Perego R, et al. Potential variant of multiple endocrine neoplasia in a dog. J Am Anim Hosp Assoc 2012;48:132–138. [DOI] [PubMed] [Google Scholar]
- 19. Thuroczy J, van Sluijs FJ, Kooistra HS, et al. Multiple endocrine neoplasias in a dog: Corticotrophic tumour, bilateral adrenocortical tumours, and pheochromocytoma. Vet Q 1998;20:56–61. [DOI] [PubMed] [Google Scholar]
- 20. Reimer SB, Pelosi A, Frank JD, et al. Multiple endocrine neoplasia type I in a cat. J Am Vet Med Assoc 2005;227:101–104, 86. [DOI] [PubMed] [Google Scholar]
- 21. Roccabianca P, Rondena M, Paltrinieri S, et al. Multiple endocrine neoplasia type‐I‐like syndrome in two cats. Vet Pathol 2006;43:345–352. [DOI] [PubMed] [Google Scholar]
- 22. Appleby EC, Sohrabi I. Pathology of the adrenal glands and paraganglia. Vet Rec 1978;102:76–78. [DOI] [PubMed] [Google Scholar]
- 23. Ayala‐Ramirez M, Feng L, Johnson MM, et al. Clinical risk factors for malignancy and overall survival in patients with pheochromocytomas and sympathetic paragangliomas: Primary tumor size and primary tumor location as prognostic indicators. J Clin Endocrinol Metab 2011;96:717–725. [DOI] [PubMed] [Google Scholar]
- 24. Kasperlik‐Zaluska AA, Roslonowska E, Slowinska‐Srzednicka J, et al. 1,111 patients with adrenal incidentalomas observed at a single endocrinological center: Incidence of chromaffin tumors. Ann N Y Acad Sci 2006;1073:38–46. [DOI] [PubMed] [Google Scholar]
- 25. Pusterla N, Fecteau ME, Madigan JE, et al. Acute hemoperitoneum in horses: A review of 19 cases (1992–2003). J Vet Intern Med 2005;19:344–347. [DOI] [PubMed] [Google Scholar]
- 26. Montemurro S, Ruggieri E, Maselli E, et al. A rare case of extra‐adrenal pheochromocytoma masquerading as an ovarian mass treated by laparoscopic surgery. Eur J Gynaecol Oncol 2007;28:491–496. [PubMed] [Google Scholar]
- 27. Liu H, Li WZ, Wang XY, et al. A rare case of extra‐adrenal pheochromocytoma localized to the ovary and detected via abdominal computed tomography angiography. Oncol Lett 2015;9:774–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dossett LA, Rudzinski ER, Blevins LS, Chambers EP Jr. Malignant pheochromocytoma of the organ of zuckerkandl requiring aortic and vena caval reconstruction. Endocr Pract 2007;13:493–497. [DOI] [PubMed] [Google Scholar]
- 29. Zaludik J, Schuitemaker F, DeWaal R, et al. Severe lactate acidosis and cardiogenic shock: A rare manifestation of a phaeochromocytoma. Anaesth Intensive Care 2010;38:593–594. [PubMed] [Google Scholar]
- 30. Madias NE, Goorno WE, Herson S. Severe lactic acidosis as a presenting feature of pheochromocytoma. Am J Kidney Dis 1987;10:250–253. [DOI] [PubMed] [Google Scholar]
- 31. Suzuki K, Tanaka S, Uchida T, et al. Catecholamine release induces elevation in plasma lactate levels in patients undergoing adrenalectomy for pheochromocytoma. J Clin Anesth 2014;26:616–622. [DOI] [PubMed] [Google Scholar]
- 32. Gimenez‐Roqueplo AP. New advances in the genetics of pheochromocytoma and paraganglioma syndromes. Ann N Y Acad Sci 2006;1073:112–121. [DOI] [PubMed] [Google Scholar]
- 33. Brouwers FM, Eisenhofer G, Tao JJ, et al. High frequency of SDHB germline mutations in patients with malignant catecholamine‐producing paragangliomas: Implications for genetic testing. J Clin Endocrinol Metab 2006;91:4505–4509. [DOI] [PubMed] [Google Scholar]
- 34. Gagel RF. Multiple endocrine neoplasia type II and familial medullary thyroid carcinoma. impact of genetic screening on management. Cancer Treat Res 1997;89:421–441. [DOI] [PubMed] [Google Scholar]
- 35. McGowan TW, Pinchbeck GP, McGowan CM. Prevalence, risk factors and clinical signs predictive for equine pituitary pars intermedia dysfunction in aged horses. Equine Vet J 2013;45:74–79. [DOI] [PubMed] [Google Scholar]
- 36. Alband N, Korbonits M. Familial pituitary tumors. Handb Clin Neurol 2014;124:339–360. [DOI] [PubMed] [Google Scholar]


