Abstract
Background
Chronic inflammatory diseases are common in cats and mesenchymal stem cells (MSC) are a promising therapeutic approach for management of these disorders. The purpose of this study was to evaluate the safety of intraperitoneal injection of MSC in cats.
Hypothesis
Intrapertioneal injection of autologous MSC in cats is safe.
Animals
Ten healthy adult purpose‐bred cats.
Methods
Mesenchymal stem cells were isolated from subcutaneous adipose tissue collected during ovariohysterectomy and characterized for expression of CD90, CD105 and CD44 and trilineage differentiation. Three weeks postoperatively a complete blood count, serum chemistry profile, urinalysis, and abdominal ultrasound were performed. Five cats then received 1 × 106 of autologous MSC/kg of body weight intraperitoneally with ultrasound guidance; 5 additional cats were sham injected. Cats were monitored for 6 weeks with daily physical examinations and weekly clinicopathological evaluations. Abdominal ultrasonography was repeated at weeks 1 and 5 after injection.
Results
Serious adverse effects were not observed in any MSC‐injected cat. Two animals developed transient lethargy and decreased activity. Jejunal lymph node size was increased in MSC‐injected cats compared to controls at weeks 1 (1.38 ± 0.25 versus 0.88 ± 0.25 cm2; P = .036) and 5 (1.75 ± 0.82 versus 0.79 ± 0.12 cm2; P = .047). A hyperechoic renal segmental cortical lesion was observed in 1 MSC‐injected cat.
Conclusions and Clinical Relevance
Intraperitoneal MSC injection was well tolerated with only mild, self‐limiting adverse effects being observed in 2 cats. This route provides a safe means of administration for cell‐based treatment in cats.
Keywords: Feline, Inflammatory disease, Regenerative medicine, Route of injection
Abbreviations
- MSC
mesenchymal stem cells
- IP
intraperitoneal
- IDO
indoleamine 2,3‐dioxygenase
- IL10
interleukin‐10
- HGF
hepatocyte growth factor
- PGE2
prostaglandin E2
- TGF‐beta
transforming growth factor beta
- IL6
interleukin‐6
- PD‐L1
programmed death ligand 1
- HMOX1
heme‐oxygenase 1
- IBD
inflammatory bowel disease
- CK
creatine kinase
- IBMIR
instant blood mediated inflammatory reaction
- CBC
complete blood count
- APC
allophycocyanin
- PE
phycoerythrin
- FITC
fluorescein isothiocyanate
- FLR
fluorescence intensity
Administration of mesenchymal stem cells (MSC) has been recently proposed as an alternative therapeutic modality for management of chronic inflammatory conditions in cats and other species.1, 2, 3 MSC express a myriad of factors such as IDO, IL10, HGF, PGE2, TGF‐beta, IL6, PD‐L1, FASL or HMOX14, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 and affect the immunomodulating properties of many types of immune cells.12, 16, 17, 18, 19, 20 Interestingly, MSC are capable of inducing T‐regulatory cells, which in turn can provide a long‐lasting immunomodulation.21 In vitro experiments suggest that MSC need to be in close proximity to target cells and in sufficient quantity to induce a therapeutic effect.11, 13, 14, 18, 22 In addition, in vivo experiments also suggest paracrine effects of MSCs on other cells are also important.15, 23, 24
Multiple routes of injection of MSC are described and include intravenous (IV), intraperitoneal (IP) as well as intra‐articular, cardiac, hepatic or nasal.1, 24, 25, 26, 27 IV injection is a frequently reported route of MSC administration for immunomodulation.1, 2, 15, 22, 24, 25, 26, 27, 28, 29 However, IV administration of MSC has potential limitations for treatment of inflammatory conditions localized to the peritoneal cavity. Exposure of IV administered MSC to plasma complement reduces cell survival.30 Retention of MSC in pulmonary vasculature reduces the number of cells reaching the target and increases the risk of pulmonary microthrombus formation.31, 32 Pulmonary thrombosis is a potential serious complication and might increase morbidity and mortality.2 Furthermore, instant blood mediated inflammatory reaction (IBMIR) occurs after injection of MSC and can be cause of decreased cell survival and increased risk of thromboembolism.33, 34, 35, 36 Thus, IV injection can potentially limit the availability of MSC and their migration capabilities, decrease the overall cell survival, and increase risk of adverse effects.30
Intraperitoneal injection is potentially a safer and more effective route of MSC administration for disorders within the abdominal cavity. IP‐injected MSC are placed in close proximity to the target organ and could reach the target in greater numbers and subsequently effect a better outcome. IP injection is simple to perform and has the promise of widespread adoption in clinical settings and high impact on patient management. IP injection of MSC was significantly more effective than IV administration for ameliorating clinical signs in a rodent model of inflammatory bowel disease.37 MSC, administered IP have been studied also for their regenerative potential38 and ability to deliver gene treatment.39, 40
The safety of IP injection of MSC has not been studied in the cats. This study was undertaken to test the hypothesis that IP injection is a safe route of autologous MSC administration in cats.
Materials and Methods
Animals
Ten purpose‐bred mixed breed, intact female cats were obtained from the MSU Comparative Ophthalmology Cat Colony and from a licensed commercial vendor,1 5 from each source. The mean age (±SD) at the time of injection of the cats was 20.7 (±5.7) months and the mean (±SD) weight was 3.79 (±0.5) kg. Animals were housed under standard conditions at the Michigan State University College of Veterinary Medicine Vivarium, fed a commercial diet2 and received environmental enrichment during the study period. Cats were determined to be healthy on the basis of physical examination. The study was approved by the Michigan State University Institutional Care and Animal Use Committee.
Isolation and Characterization of MSC
Subcutaneous adipose tissue (2–4 g) from the ventral midline abdomen was collected during ovariohysterectomy was placed in KNAC medium (Keratinocyte SFM medium3 supplemented with 2 mM of N‐acetyl‐L‐cysteine4 and 0.2 mM of l‐ascorbic acid 2‐phosphate4) with 5% of MSC‐grade FBS5 in 50 mL tube for transfer to the laboratory for processing. Each tissue sample was finely minced with sterile scalpel blade and incubated in 1 mg/mL of Collagenase I4 in HBSS4 for 2 hours at 37°C with 5% CO2. After incubation, the cell suspension was repeatedly aspirated with sterile serological pipette to facilitate dissociation and passed through a 70 μm cell strainer6 to remove excess tissue stroma. The resulting cell suspension was washed twice in sterile PBS by centrifugation at 467 × g for 5 minutes at room temperature. Cells were then resuspended in 5 mL of KNAC medium with 5% of MSC‐grade FBS5 and plated in T‐25 plastic tissue culture flask.7 After reaching 80% confluency, cells were trypsinized with 0.05% trypsin,8 resuspended in 20 mL of medium and divided between 2 T‐75 plastic tissue culture flasks.7 All cell cultures were subsequently passaged in a similar fashion until sufficient quantities of cells were available for injection and characterization, which took approximately 3 weeks.
Adherent cells were characterized for cell surface expression of CD90,9 CD105,10 CD44,11 MHCII12 and for differentiation into osteocytes, adipocytes, and chondrocytes.1, 41, 42 Cell membrane marker expression was analyzed by flow cytometry13 and analyzed using commercial software.14 Differentiation of cells was achieved through use of commercial osteogenesis, adipogenesis, and chondrogenesis kits,15 , 16 according to manufacturer's recommendations. For osteogenesis and adipogenesis experiments, 1 × 104 and 1 × 105 cells per well were plated in 24‐well tissue culture plates containing KNAC medium with 5% FBS and incubated at 37°C for 24 hours to allow cells to attach. After incubation, the medium was changed to the specific differentiation medium and cultured for 3 weeks in 37°C with 5% CO2 with medium being changed twice a week. Chondrogenic differentiation was accomplished through culture of at least 1 × 106 cells in micromass. Cells were re‐suspended in 1 mL of cell culture medium and spun down at 325 × g for 5 minutes. Subsequently, the medium was carefully exchanged with chondrogenesis medium, in order not to disrupt the pellet. Cells were then cultured for 3 weeks at 37°C with 5% CO2, with semiweekly medium changes. After 3 weeks of differentiation, the cells were stained with Alizarin Red4 to assess osteogenic differentiation, Alcian Blue4 to assess chondrogenesis and Oil‐o‐Red4 stain to assess adipogenesis.
Procedures
All cats underwent a routine ovariohysterectomy, at which time a 2–4 g sample of ventral midline subcutaneous fat was obtained and processed for MSC isolation and propagation. For ovariohysterectomy, animals were sedated with acepromazine17 (0.05 mg/kg) and buprenorphnine18 (10 mcg/kg), anesthetized with isoflurane19 (0.25–3% isoflurane in 100% oxygen in an induction chamber). For subsequent examinations (ultrasound, blood draw, cystocentesis), all cats were sedated or anesthetized for all procedures with either acepromazine17 (0.05 mg/kg) and buprenorphnine18 (10 mcg/kg) or isoflurane19 (0.25–3% isoflurane in 100% oxygen in an induction chamber). Five cats (3 MSC‐treated and 2 sham‐injected) were sedated using acepromazine and buprenorphine and 5 cats (2 MSC‐treated and 3 sham‐injected) were anesthetized with isofluorane.
After a 3‐week recovery period from ovariohysterectomy, all cats were evaluated at baseline (pretreatment) with a complete physical examination, a CBC, serum chemistry profile, urinalysis, and abdominal ultrasonography. Cats were then randomly divided into 2 groups of 5 animals each. Cats from both sources were represented in each of the groups. Group 1 cats received an ultrasound‐guided injection of 1 × 106 autologous MSC/kg of body weight; Group 2 control cats received an equivalent IP sham injection of sterile phosphate buffered saline (PBS).20 MSCs were suspended in 4 mL of saline for IP injections. Isolated MSCs in passage 4 were grown to about 80–90% confluence, detached using 0.05% trypsin for 10 minutes, washed twice using PBS20 and quantified using an electronic cell counter21 and resuspended in 4 mL of PBS for injection. The suspension was then transferred into a 20 cc syringe14 with a 22F needle14 for injection. After injection of MSC, cats were monitored for 6 weeks with daily physical examinations and weekly clinicopathological evaluations (CBC, serum chemistry profiles, and urinalyses).22 Abdominal ultrasonography was repeated at 1 and 5 weeks after injection23 with the ultrasound examiner being blinded to each cat's treatment status.
For ultrasonographic examinations, cats were placed in dorsal recumbency and the abdomen was imaged using a linear array multifrequency transducer.24 The frequency used varied between 10 and 13 MHz, with the highest frequency chosen, which allowed complete organ evaluation. During each ultrasound evaluation, the entire abdominal contents were surveyed, including the liver, spleen, kidneys, bladder, adrenal glands, pancreas, stomach and small intestines. The medial iliac and jejunal lymph nodes were also imaged at these time periods, and their long axis (cranial to caudal length) and short axis (dorsal to ventral height) were measured. For statistical comparisons, lymph node size was calculated as the multiplication of length times width. At 5 weeks after injection, fine needle aspirate biopsies of selected abdominal lymph nodes were obtained from 3 MSC‐treated cats under ultrasound guidance using a 22 g 1.5 inch needle.
Statistical Analyses
Mean values with standard deviation were calculated for MSC‐treated and control cats at each sampling time for data from physical examination, clinicopathological evaluations, and ultrasound measurements. Comparison between the before and after treatment results within each group were performed using paired t‐test. Continuous quantitative variables that failed normality testing were evaluated with nonparametric analyses. Nonparametric Wilcoxon signed‐rank tests were used for analyses of outcome variables for significant differences between treatment groups. In addition, multivariable ANOVA (MANOVA) was used to evaluate the linear changes over time. All data were analyzed with a commercially accessible statistical software package.25 Results with P < .05 were considered statistically significant.
Results
Each cell preparation displayed the expected MSC‐phenotype. Cells underwent differentiation into adipocytes, chondrocytes, and osteocytes (Fig 1) and strongly expressed the surface markers CD44, CD90, CD105, while lacking expression of MHCII (Fig 2).
Figure 1.
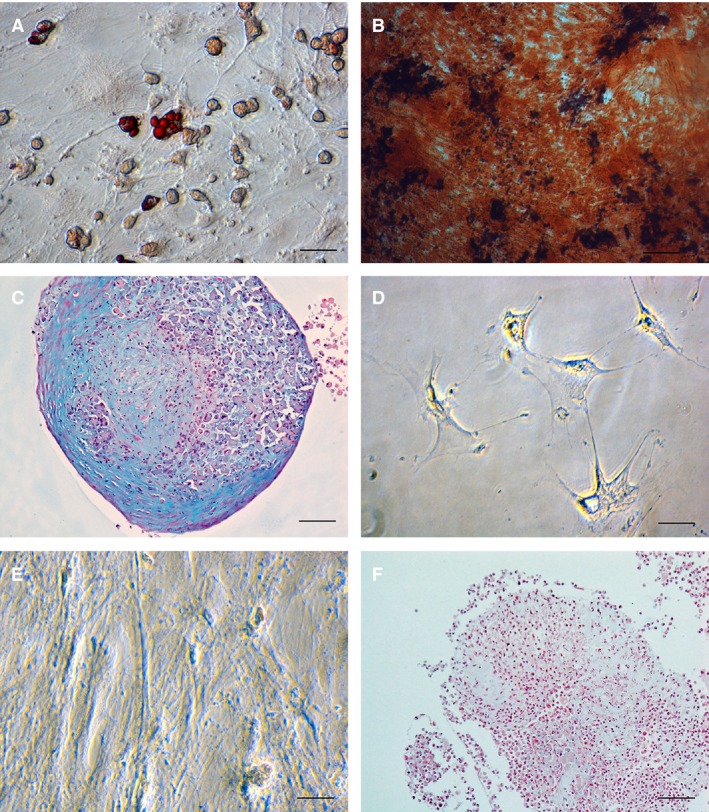
Representative photomicrographs illustrating differentiation characterization of a mesenchymal stem cells cell line isolated from adipose tissue obtained from a 2 year old, female mixed breed cat (magnification 40×). (A) Lipid droplets in differentiated cells are stained red with Oil‐o‐red demonstrating adipogenesis, (B) Red‐colored calcium deposits inside cells stained with Alizarin red stain demonstrating osteogenesis, (C) Blue‐colored in glycosaminoglycan deposits in cells stained with Alcian Blue stain after chondrogenic differentiation. (D–F) Control photomicrographs after incubation of the same cell line in KNAC medium and stained with Oil‐o‐red (D), Alizarin red (E) and Alcian Blue (F). Note lack of uptake of the stains in both micrographs (D,E) and no blue staining extracellular matrix in the chondrogenic control stain (F).
Figure 2.
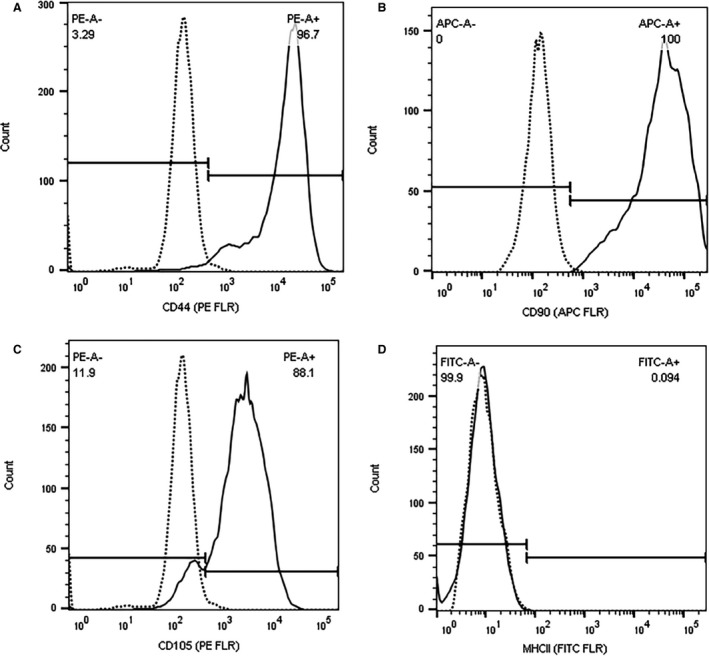
Flow cytometric analysis of surface markers expressed by a representative cell line from the same Animal 1 as in Fig 1. Gray lines represent the negative controls, while in black is the population of cells stained with antibody specific for each epitope. The X‐axis represents the fluorescence intensity of the fluorophore (APC, PE or FITC) while the Y‐axis represents the cell counts. Note strong expression of all markers (A) CD90, (B) CD105, (C) CD44 in each cell line (continuous line), compared to negative control (dashed line) and lack of expression of MHCII (D).
Severe adverse effects were not observed in any cat after injection of MSC. There were no significant differences in mean temperature, respiratory rate, heart rate, and body weight between groups over the course of the study. Two MSC‐treated cats were lethargic and were less interactive with their caretakers immediately after injection. No pain was elicited by abdominal palpation in either cat. Both cats spontaneously recovered within 1 to 3 days after injection of cells.
Results of clinicopathologic evaluations revealed significant differences between study group for creatine kinase (CK) enzyme activity. CK activity was significantly higher in MSC‐injected cats on weeks 1 (271.4 ± 154.36 IU/L versus 150.6 ± 54.79; P = .047, Wilcoxon) and 3 (196 ± 49.75 versus 125.8 ± 34.72; normal reference range = 59–354 IU/L; P = .047, Wilcoxon). Individual cat CK values were above the reference range at various times in 3 MSC‐injected cats and 2 sham‐injected cats. Abdominal ultrasonography revealed a significant increase in jejunal lymph node size in MSC‐injected cats compared to controls at weeks 1 (1.38 ± 0.25 versus 0.88 ± 0.25 cm2; P = .036) P = .036, Wilcoxon) and 5 (1.75 ± 0.82 versus 0.79 ± 0.12 cm2; P = .047, Wilcoxon) after cell injection (Fig 3). A significant increase in lymph node size over time was also identified in MSC‐injected cats when comparing the before injection lymph node size with size at week 5 (1.1 ± 0.52 cm2 before injection versus 1.75 ± 0.82 cm2 after injection; P = .033). A hyperechoic renal segmental cortical lesion consistent with a renal infarct was identified sonographically in 1 MSC‐injected cat 1 week after injection. Cytologic examination of lymph node aspirates obtained from 3 MSC‐injected animals revealed normal cell populations consisting primarily of small lymphocytes with low numbers of medium to large lymphocytes and occasional plasma cells and macrophages; there was no cytologic evidence of disease.
Figure 3.
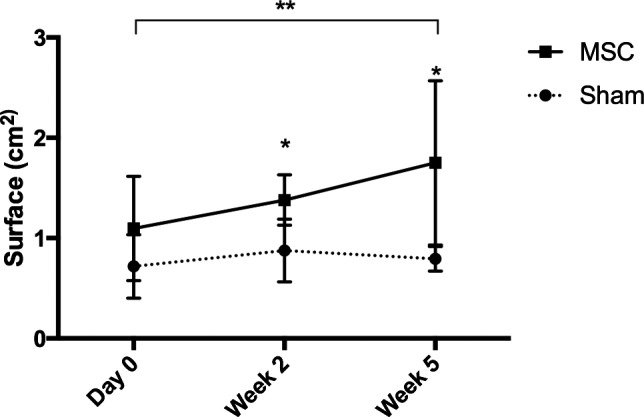
Changes in mean (±SD) jejunal lymph node size in cats injected intraperitoneally with either 1 × 106 mesenchymal stem cells (MSC) (n = 5) or a sham preparation (n = 5) over the course of study. *Statistically significant difference between treatment groups (MSC treated versus sham treated animals) at the weeks indicated (P < .5). **Statistically significant difference between pre‐injection and 5 week after inject lymph node size (as presented by the black line over the graph) within the group treated with MSCs (P = .033).
Discussion
This study evaluated the safety of IP injection of autologous MSC in cats. Our findings show that IP administration of autologous MSCs appears to be a safe and technically feasible approach to cell‐based treatment in cats. Limited adverse effects were observed, manifesting as transient lethargy and decreased activity in 2 cats. The cause of these behaviors in uncertain, but might be related to discomfort associated with a host response to MSC or other components of the cell preparation. Although abdominal pain was not elicited during palpation, increased abdominal lymph node size in MSC‐injected cats is consistent with a heightened host response to MSC injection. Alternatively, this reaction might have been caused by the sedative or anesthesia used. However, the fact that this behavior was observed only after cell injection and not at other times the cats underwent anesthesia, suggests that an adverse anesthetic reaction is less likely the cause of lethargy.
All clinicopathological results were similar between MSC‐injected and sham‐injected animals with the exemption of CK. Importantly, CK values were only mildly outside of the reference ranges. Activity of this activity can be induced by multiple factors. Cats in this study received intramuscular injections of sedatives, which can increase the CK values.43 Some animals required additional physical restraint, which could result in increase in CK as well.43
A hyperechoic renal segmental cortical lesion was observed in 1 MSC‐injected cat 1 week after injection, which was assumed to be a renal infarct. These changes are incidentally encountered in cats in the clinical practice setting and have been observed more frequently in Ragdoll cats and cats with cardiomyopathy.44, 45 In the MSC‐injected cat with the renal segmental cortical lesion in this study, no heart murmur was identified on cardiac auscultation. An echocardiogram was performed to investigate for cardiomyopathy at a recheck appointment 1 year after completion of this study and no abnormalities were noted within the heart by a board certified cardiologist. Interestingly, the animal developed other similar infarcts in the other kidney at the 1 year follow‐up, which suggests that this animal is prone to this kind of changes and suggest that the infarcts were not related to the injected MSCs.
We also observed a significant increase in the size of jejunal lymph nodes in MSC‐injected cats over the course of the study. We speculate that secretion of IL6 by MSC,14 which is known to cause T‐cell expansion, may be responsible for the differences in lymph node size observed between the 2 groups. Cytological examination of lymph nodes of a limited number of MSC‐injected animals did not reveal any pathological process. However, proof of that hypothesis would require histopathological evaluation of the jejunal lymph nodes. Necropsy examinations were not part of the experimental design, so it could not be assessed. Another explanation might be a complement‐mediated reaction to injected MSCs, similar to IBMIR reaction to injected cells and subsequent activation of innate immune system. Elements of complement are constitutively expressed by mesothelial cells.46 C3 complement component expressed by peritoneal mesothelial cells has been previously implicated to be responsible for IBMIR reaction after MSC IV injection in vivo and subsequent activation of innate immune system.47 Interestingly, the complement binding also enhances the immunomodulatory properties of MSCs.47 Regardless, follow‐up ultrasonograms performed on 2 MSC‐injected cats approximately 12 months after injection, revealed that all abdominal lymph nodes had returned to pre‐injection size (data not shown). These observations suggest that abdominal lymphadenopathy associated with MSC injection is be transient in nature. Further studies are needed to determine the precise cause of the increased lymph node size observed in MSC‐injected cats.
A limitation of this study is that only a single dose of MSC was evaluated. Responses to IP MSC injection may vary by dose and frequency of administration, as suggested by in vitro studies in other species.12, 14, 18, 48, 49, 50 Future studies evaluating a higher dose of MSC than the one evaluated in this study may be necessary to fully evaluate the safety profile of the IP injection in cats.
Although the efficacy of IP MSC injection has been previously studied in a variety of species and disease models,37, 38, 39, 40, 51 there have been few studies reported that comprehensively investigate the safety of IP administration of MSC in healthy control animals. In one study evaluating the safety and distribution of amniotic fluid stem cells injected IP in neonatal rats,52 no adverse effects were noted in MSC‐injected rats during the 21‐day after injection observation period. However, neither lymph node size nor behavioral changes were investigated in the rat study.
We conclude that IP injection of autologous MSC in cats is overall safe and is associated only with mild, self‐limiting, and short‐lasting adverse effects and provides a safe alternative route of administration for cell‐based treatment in cats. Given the apparent safety of IP administration of MSC and the potential of IP administered MSC to reach intra‐abdominal target sites, the effectiveness of the IP route for MSC‐based treatment of chronic inflammatory disorders of the peritoneal cavity such as chronic idiopathic cystitis, hepatitis, pancreatitis, or inflammatory bowel disease warrant further investigations.
Acknowledgments
Authors acknowledge Marlee Richter, LVT, Janice Querubin, LVT, Ramona Stambaugh and Kyle Anderson, BSc, for their technical assistance during the study and Drs. Simon Petersen‐Jones and Laurence Occelli for their assistance.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐Label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This study was supported by Morris Animal Foundation Fellowship Award (D13FE‐405), and grants from the Michigan State University Center for Feline Health and Well‐Being and the Michigan State University, College of Veterinary Medicine Endowed Research Funds.
Footnotes
Liberty Research, Inc., Waverly, NY
Adult Optimal Care, Hill's Pet Nutrition, Inc., Topeka, KS
Life Technologies, Thermo Fisher Scientific Inc, Grand Island, NY
Sigma‐Aldrich, St. Louis, MO
Fetal Bovine Serum‐mesenchymal stem cell‐qualified, Gibco, Grand Island, NY
Falcon™ Cell Strainers, Thermo Fisher Scientific Inc., Waltham, MA
CytoOne, USA Scientific, Ocala, FL
Gibco, Life Technologies, Thermo Fisher Scientific Inc
Anti‐human CD90, clone 5E10, BD Biosciences, San Jose, CA
Anti‐human CD105, clone SN6, Invitrogen, Grand Island, NY
Anti‐mouse CD44, clone IM7.8.1, Invitrogen
Anti‐human HLA‐DR, clone Tu39, BD Biosciences
BD Biosciences
BD Medical, Franklin Lakes, NJ
FlowJo, LLC, Ashland, OR
StemPro Chondrogenesis, Adipogenesis, Osteogenesis Differentiation Kit, Gibco
Boehringer Ingelheim, Ridgefield, CT
Reckitt Benckiser, Hull, England
IsoFlo, Abbott Laboratories, Abbott Park, IL
Dulbecco Phosphate Buffered Saline, Sigma‐Aldrich
Countess, Life Technologies, Thermo Fisher Scientific Inc
DCPAH, Michigan State University, East Lansing, MI
Veterinary Medical Center, Michigan State University, East Lansing, MI
GE Logiq 9, General Electric, Princeton, NJ
SAS 9.3, SAS Institute Inc., Cary, NC
References
- 1. Quimby JM, Webb TL, Gibbons DS, et al. Evaluation of intrarenal mesenchymal stem cell injection for treatment of chronic kidney disease in cats: A pilot study. J Feline Med Surg 2011;13:418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Quimby JM, Webb TL, Habenicht LM, et al. Safety and efficacy of intravenous infusion of allogeneic cryopreserved mesenchymal stem cells for treatment of chronic kidney disease in cats: Results of three sequential pilot studies. Stem Cell Res Ther 2013;4:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Trzil JE, Masseau I, Webb TL, et al. Long‐term evaluation of mesenchymal stem cell therapy in a feline model of chronic allergic asthma. Clin Exp Allergy 2014;44:1546–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Meisel R, Zibert A, Laryea M, et al. Human bone marrow stromal cells inhibit allogeneic T‐cell responses by indoleamine 2,3‐dioxygenase‐mediated tryptophan degradation. Blood 2004;103:4619–4621. [DOI] [PubMed] [Google Scholar]
- 5. Piantadosi CA, Withers CM, Bartz RR, et al. Heme oxygenase‐1 couples activation of mitochondrial biogenesis to anti‐inflammatory cytokine expression. J Biol Chem 2011;286:16374–16385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. English K, Barry FP, Field‐Corbett CP, et al. IFN‐gamma and TNF‐alpha differentially regulate immunomodulation by murine mesenchymal stem cells. Immunol Lett 2007;110:91–100. [DOI] [PubMed] [Google Scholar]
- 7. Chang CP, Chio CC, Cheong CU, et al. Hypoxic preconditioning enhances the therapeutic potential of the secretome from cultured human mesenchymal stem cells in experimental traumatic brain injury. Clin Sci 2013;124:165–176. [DOI] [PubMed] [Google Scholar]
- 8. Wang PP, Xie DY, Liang XJ, et al. HGF and direct mesenchymal stem cells contact synergize to inhibit hepatic stellate cells activation through TLR4/NF‐kB pathway. PLoS One 2012;7:e43408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Prasanna SJ, Gopalakrishnan D, Shankar SR, et al. Pro‐inflammatory cytokines, IFNgamma and TNFalpha, influence immune properties of human bone marrow and Wharton jelly mesenchymal stem cells differentially. PLoS One 2010;5:e9016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang A, Wang Y, Ye Z, et al. Mechanism of TNF‐alpha‐induced migration and hepatocyte growth factor production in human mesenchymal stem cells. J Cell Biochem 2010;111:469–475. [DOI] [PubMed] [Google Scholar]
- 11. Ren G, Zhang L, Zhao X, et al. Mesenchymal stem cell‐mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2008;2:141–150. [DOI] [PubMed] [Google Scholar]
- 12. Spaggiari GM, Capobianco A, Abdelrazik H, et al. Mesenchymal stem cells inhibit natural killer‐cell proliferation, cytotoxicity, and cytokine production: Role of indoleamine 2,3‐dioxygenase and prostaglandin E2. Blood 2008;111:1327–1333. [DOI] [PubMed] [Google Scholar]
- 13. Ryan JM, Barry F, Murphy JM, et al. Interferon‐γ does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin Exp Immunol 2007;149:353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Najar M, Rouas R, Raicevic G, et al. Mesenchymal stromal cells promote or suppress the proliferation of T lymphocytes from cord blood and peripheral blood: The importance of low cell ratio and role of interleukin‐6. Cytotherapy 2009;11:570–583. [DOI] [PubMed] [Google Scholar]
- 15. Akiyama K, Chen C, Wang D, et al. Mesenchymal‐stem‐cell‐induced immunoregulation involves FAS‐ligand‐/FAS‐mediated T cell apoptosis. Cell Stem Cell 2012;10:544–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beyth S, Borovsky Z, Mevorach D, et al. Human mesenchymal stem cells alter antigen‐presenting cell maturation and induce T‐cell unresponsiveness. Blood 2005;105:2214–2219. [DOI] [PubMed] [Google Scholar]
- 17. Liu WH, Liu JJ, Wu J, et al. Novel mechanism of inhibition of dendritic cells maturation by mesenchymal stem cells via interleukin‐10 and the JAK1/STAT3 signaling pathway. PLoS One 2013;8:e55487. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18. Luz‐Crawford P, Noel D, Fernandez X, et al. Mesenchymal stem cells repress Th17 molecular program through the PD‐1 pathway. PLoS One 2012;7:e45272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brown JM, Nemeth K, Kushnir‐Sukhov NM, et al. Bone marrow stromal cells inhibit mast cell function via a COX2‐dependent mechanism. Clin Exp Allergy 2011;41:526–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Najar M, Raicevic G, Fayyad‐Kazan H, et al. Impact of different mesenchymal stromal cell types on T‐cell activation, proliferation and migration. Int Immunopharmacol 2013;15:693–702. [DOI] [PubMed] [Google Scholar]
- 21. Ge W, Jiang J, Arp J, et al. Regulatory T‐cell generation and kidney allograft tolerance induced by mesenchymal stem cells associated with indoleamine 2,3‐dioxygenase expression. Transplantation 2010;90:1312–1320. [DOI] [PubMed] [Google Scholar]
- 22. Duijvestein M, Vos AC, Roelofs H, et al. Autologous bone marrow‐derived mesenchymal stromal cell treatment for refractory luminal Crohn's disease: Results of a phase I study. Gut 2010;59:1662–1669. [DOI] [PubMed] [Google Scholar]
- 23. Nemeth K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)‐dependent reprogramming of host macrophages to increase their interleukin‐10 production. Nat Med 2009;15:42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu L, Mao Q, Chu S, et al. Intranasal versus intraperitoneal delivery of human umbilical cord tissue‐derived cultured mesenchymal stromal cells in a murine model of neonatal lung injury. Am J Pathol 2014;184:3344–3358. [DOI] [PubMed] [Google Scholar]
- 25. Carrade DD, Owens SD, Galuppo LD, et al. Clinicopathologic findings following intra‐articular injection of autologous and allogeneic placentally derived equine mesenchymal stem cells in horses. Cytotherapy 2011;13:419–430. [DOI] [PubMed] [Google Scholar]
- 26. Spriet M, Hunt GB, Walker NJ, et al. Scintigraphic tracking of mesenchymal stem cells after portal, systemic intravenous and splenic administration in healthy beagle dogs. Vet Radiol Ultrasound 2015;56:327–334. [DOI] [PubMed] [Google Scholar]
- 27. Zhao Y, Li T, Wei X, et al. Mesenchymal stem cell transplantation improves regional cardiac remodeling following ovine infarction. Stem Cells Transl Med 2012;1:685–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Constantin G, Marconi S, Rossi B, et al. Adipose‐derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis. Stem Cells 2009;27:2624–2635. [DOI] [PubMed] [Google Scholar]
- 29. Morigi M, Rota C, Montemurro T, et al. Life‐sparing effect of human cord blood‐mesenchymal stem cells in experimental acute kidney injury. Stem Cells 2010;28:513–522. [DOI] [PubMed] [Google Scholar]
- 30. Li Y, Lin F. Mesenchymal stem cells are injured by complement after their contact with serum. Blood 2012;120:3436–3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Eggenhofer E, Benseler V, Kroemer A, et al. Mesenchymal stem cells are short‐lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol 2012;3:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Harting Matthew T, Jimenez Fernando, Xue Hasan, et al. Intravenous mesenchymal stem cell therapy for traumatic brain injury. J Neurosurg 2009;110:1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gustafson EK, Elgue G, Hughes RD, et al. The instant blood‐mediated inflammatory reaction characterized in hepatocyte transplantation. Transplantation 2011;91:632–638. [DOI] [PubMed] [Google Scholar]
- 34. Moll G, Hult A, von Bahr L, et al. Do ABO blood group antigens hamper the therapeutic efficacy of mesenchymal stromal cells? PLoS One 2014;9:e85040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moll G, Rasmusson‐Duprez I, von Bahr L, et al. Are therapeutic human mesenchymal stromal cells compatible with human blood? Stem Cells 2012;30:1565–1574. [DOI] [PubMed] [Google Scholar]
- 36. Bennet W, Sundberg B, Groth CG, et al. Incompatibility between human blood and isolated islets of Langerhans: A finding with implications for clinical intraportal islet transplantation? Diabetes 1999;48:1907–1914. [DOI] [PubMed] [Google Scholar]
- 37. Castelo‐Branco MT, Soares ID, Lopes DV, et al. Intraperitoneal but not intravenous cryopreserved mesenchymal stromal cells home to the inflamed colon and ameliorate experimental colitis. PLoS One 2012;7:e33360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chamberlain J, Yamagami T, Colletti E, et al. Efficient generation of human hepatocytes by the intrahepatic delivery of clonal human mesenchymal stem cells in fetal sheep. Hepatology 2007;46:1935–1945. [DOI] [PubMed] [Google Scholar]
- 39. Porada CD, Sanada C, Kuo CJ, et al. Phenotypic correction of hemophilia A in sheep by postnatal intraperitoneal transplantation of FVIII‐expressing MSC. Exp Hematol 2011;39:1124–1135 , e1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mader EK, Butler G, Dowdy SC, et al. Optimizing patient derived mesenchymal stem cells as virus carriers for a phase I clinical trial in ovarian cancer. J Transl Med 2013;11:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Neupane M, Chang C‐C, Kiupel M, et al. Isolation and characterization of canine adipose‐derived mesenchymal stem cells. Tissue Eng Part A 2008;14:1007–1015. [DOI] [PubMed] [Google Scholar]
- 42. Webb TL, Quimby JM, Dow SW. In vitro comparison of feline bone marrow‐derived and adipose tissue‐derived mesenchymal stem cells. J Feline Med Surg 2012;14:165–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Aroch I, Keidar I, Himelstein A, et al. Diagnostic and prognostic value of serum creatine‐kinase activity in ill cats: A retrospective study of 601 cases. J Feline Med Surg 2010;12:466–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hickey MC, Jandrey K, Farrell KS, et al. Concurrent diseases and conditions in cats with renal infarcts. J Vet Intern Med 2014;28:319–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Paepe D, Bavegems V, Combes A, et al. Prospective evaluation of healthy Ragdoll cats for chronic kidney disease by routine laboratory parameters and ultrasonography. J Feline Med Surg 2013;15:849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tang S, Leung JC, Chan LY, et al. Regulation of complement C3 and C4 synthesis in human peritoneal mesothelial cells by peritoneal dialysis fluid. Clin Exp Immunol 2004;136:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Moll G, Jitschin R, von Bahr L, et al. Mesenchymal stromal cells engage complement and complement receptor bearing innate effector cells to modulate immune responses. PLoS One 2011;6:e21703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Le Blanc K, Tammik L, Sundberg B, et al. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol 2003;57:11–20. [DOI] [PubMed] [Google Scholar]
- 49. Flemming A, Schallmoser K, Strunk D, et al. Immunomodulative efficacy of bone marrow‐derived mesenchymal stem cells cultured in human platelet lysate. J Clin Immunol 2011;31:1143–1156. [DOI] [PubMed] [Google Scholar]
- 50. Spaggiari GM, Capobianco A, Becchetti S, et al. Mesenchymal stem cell‐natural killer cell interactions: Evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL‐2‐induced NK‐cell proliferation. Blood 2006;107:1484–1490. [DOI] [PubMed] [Google Scholar]
- 51. Semon J, Maness C, Zhang X, et al. Comparison of human adult stem cells from adipose tissue and bone marrow in the treatment of experimental autoimmune encephalomyelitis. Stem Cell Res Ther 2014;5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ghionzoli M, Cananzi M, Zani A, et al. Amniotic fluid stem cell migration after intraperitoneal injection in pup rats: Implication for therapy. Pediatr Surg Int 2010;26:79–84. [DOI] [PubMed] [Google Scholar]


