Abstract
Background
Cholecystocentesis can be part of the diagnostic workup of hepatobiliary disease in small animals, but literature on cytological evaluation of bile is scant.
Objectives
To determine the diagnostic utility of cytological assessment of bile aspirates.
Animals
Fifty‐six and 78 client‐owned dogs and cats, respectively, with bile collected by cholecystocentesis and submitted to our diagnostic laboratory between 1999 and 2014.
Methods
Retrospective study describing cytological findings of bile, concurrent bacterial culture results, hematological and serum biochemical data, gallbladder biopsy results, as well as final diagnosis and complications after cholecystocentesis.
Results
Infectious agents were found in 30% of canine and 22% of feline bile aspirates, and inflammation in 5% and 19% respectively. Presence of microorganisms was more often detected on cytological examination (24%) than by culture (21%). The most common bacterial isolates were Escherichia coli and Enterococcus spp., isolated from 14.8% and 6.7% of cultured samples respectively. Only increased canine pancreatic lipase immunoreactivity concentration (cPLI) was significantly associated with the presence of microorganisms, inflammatory cells, or both in bile. Clinically relevant complications of cholecystocentesis occurred in 2 dogs. The majority of the animals undergoing cholecystocentesis suffered from hepatic, pancreatic, gastrointestinal disease, or a combination thereof.
Conclusions and Clinical Importance
Cytological examination of bile is inexpensive and straightforward, and yields diagnostically relevant information that precedes and complements bacterial culture.
Keywords: Bactibilia, Cholecystitis, Cholecystocentesis, Hepatobiliary disease
Abbreviations
- ALP
alkaline phosphatase
- ALT
alanine transaminase
- cPLI
canine pancreatic lipase immunoreactivity
- fPLI
feline pancreatic lipase immunoreactivity
- GGT
γ‐Glutamyl transferase
- QMHA
Queen Mother Hospital for Animals
- RVC
Royal Veterinary College
Cholecystocentesis is performed regularly in our institution as part of the complete diagnostic work up of hepatobiliary diseases in small animals. Positive bacterial culture results occur in up to 30%1, 2 of bile samples from dogs and cats with hepatobiliary disorders. Commonly cultured bacteria include normal inhabitants of the gastrointestinal tract, such as Escherichia coli and Enterococcus spp. Ascending migration from the gut has thus been accepted as the most plausible pathomechanism for bactibilia; however, experimental studies have also documented that hematogenous spread of bacteria from the portal vein is possible.3, 4
While the majority of published studies focus on bacterial culture, only scant information on cytological findings of bile aspiration are found in the veterinary literature, and include mainly case reports and small case series; larger scale studies on cytological analysis of bile in animals are lacking. Findings include presence of single and mixed bacteria in both canine and feline bile samples, with or without cytological evidence of inflammation.5, 6, 7, 8 Fungal organisms9 have rarely been documented in the bile of a dog, parasite eggs have been found in bile of a cat and of cows,10, 11 and a single case report documents the presence of atypical lymphocytes in gallbladder aspirates from a cat with lymphoma.12
We hypothesize that cytological evaluation of bile yields clinically relevant information that complements bacterial culture, and that cholecystocentesis has a low complication rate. The objectives of this study were to describe cytological findings of bile aspirates in a large number of dogs and cats, and to compare findings with concurrent bacterial culture results and additional clinicopathological data. Furthermore, we intended to describe working diagnoses and concurrent diseases of the sampled population, and assess for potential complications, in order to determine the usefulness of cytological evaluation.
Materials and Methods
In this retrospective study, a computer‐based search through the laboratory information system of the Diagnostic Laboratory of the Royal Veterinary College (RVC) was performed to identify submissions of canine and feline bile samples for cytological examination between March 1999 and January 2014. Main indications for cholecystocentesis included clinical findings or serum biochemistry results suggestive of hepatic or biliary disease, abnormal appearance of the liver or biliary tree on imaging or during exploratory laparotomy, and follow‐up after previous diagnosis of biliary disease. Gallbladder aspiration was performed either percutaneously under ultrasound guidance, predominantly by right‐sided transhepatic approach, or intraoperatively during laparoscopy or laparotomy. Direct smears and cytocentrifuge preparations of the fluids were usually prepared within 24 h of sampling, and occasionally within 2–3 days if bile was sampled on weekends or holidays, and stained using modified Wright's stain (Hematek, Siemens). Initial cytology reports were reviewed. Archived slides reported to contain cells or microorganisms were re‐assessed when available by a final year clinical pathology resident, and additionally by a board‐certified clinical pathologist if discrepancies from the original report were noted.
Results of concurrent bacterial cultures were recorded. A negative culture was defined by the absence of bacterial growth after 48 h of both aerobic and anaerobic incubation.
Hematological and biochemical test results from the same admission to the Queen Mother Hospital for Animals (QMHA) at the RVC were retrieved from the laboratory information system and were included in the pre‐aspiration data if they were submitted before or on the day of bile sampling. Post‐aspiration CBC and biochemistry data were included if submitted within a week after cholecystocentesis. Serum pancreatic lipase immunoreactivity concentration (PLI), clotting times, bile acids, and plasma ammonium concentrations measured at any time as part of the same admission as the respective cholecystocentesis were also included.
Gallbladder tissue was obtained by incisional biopsy technique during exploratory laparotomy or at postmortem examination and routinely processed and stained with hematoxylin and eosin. Histopathology reports were compared to bile cytological findings.
Medical records of the sampled animals admitted to the QMHA were evaluated for information regarding signalment, working or final diagnosis, and complications. The latter were defined as leakage of bile after cholecystocentesis, either documented by direct intraoperative or postmortem visualization, ultrasonographic assessment, or development of peritoneal effusion with bilirubin concentrations higher than serum levels.
Statistical analysis was performed using IBM SPSS Statistics 22. Dogs and cats were assessed separately, and were allocated to Group 1 if no inflammation or microorganisms were detected, or Group 2 if inflammation or microorganisms were detected by microscopic examination, by culture, or by both modalities.
CBC and serum biochemistry results were assessed for normality by visual inspection of histograms. Results of subgroups were compared using the Mann‐Whitney U test for continuous variables and Chi‐square test for categorical variables. In animals with repeated gallbladder aspirates, only blood results taken at time of first cholecystocentesis were included. Variables identified as significant (p‐value ≤.05) were further assessed for correlation using multivariate logistic regression analysis with backwards elimination. Comparison of paired CBC and biochemistry data before and after cholecystocentesis was performed using Wilcoxon Signed Rank Test.
Results
Sample Population
A total of 140 bile samples were submitted to the RVC Diagnostic Laboratory for cytological examination between March 1999 and January 2014, including 59 and 81 samples from 56 dogs and 78 cats respectively. Over 80% of the samples were procured within the last 5 years.
The dog population comprised 26 female spayed, 6 female entire, 19 male neutered, and 5 male entire dogs. Age at sampling ranged from 10 months to 15 years 5 months (median age 8 years 8 months). The cat population included 31 female spayed, one female entire, 44 male neutered, one male entire cat, and one cat of unknown sex. Age at sampling ranged from 6 months to 19 years 4 months (median age 9 years 3 months). Labrador Retriever and cross‐bred dogs were the most prevalent breeds within the dog population (8 each), and domestic short‐hair was the most common cat breed (n = 45).
Cytological Findings
Dogs
All canine bile aspirates had a green to blue to gray, granular background (Fig 1A), with amorphous aggregates of pale blue to purple material additionally found in 12 samples, and occasional eosinophilic globular material in 3 cases. Golden to brown pigment was present in 29 cases, often appearing needle‐shaped or as clumped granules, and was interpreted as bilirubin (Fig 1B). Erythrocytes were present in low numbers in 6 cases, and in high numbers in 5. Collection artifact was suspected for all but one case, where the presence of high numbers of ghost erythrocytes raised the possibility of prior hemorrhage; however, erythrolysis in vitro could not be ruled out.
Figure 1.
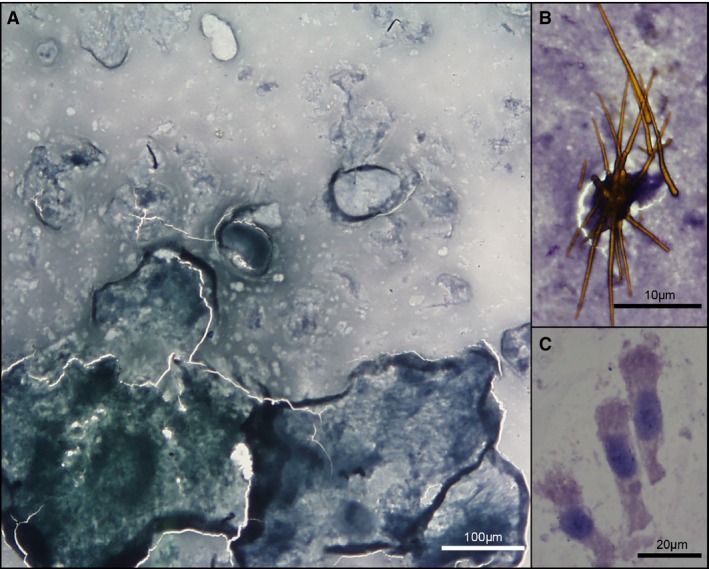
Normal bile. (A) Acellular bile with green‐gray, granular background; dog, 10×. (B) Bilirubin crystals on purple granular background; dog, 100× oil. (C) Columnar biliary epithelial cells; cat, 40× oil. Modified Wright's stain.
Numbers of leukocytes exceeding that expected from blood content alone were identified in 3/59 cases (5%). Poorly preserved or degenerate neutrophils predominated in all, with lower numbers of macrophages and lymphocytes also observed in one sample. Only one of the 3 cases had cytological evidence of bacteria. Deterioration of cellularity and cell preservation was observed in smears made in our laboratory, compared to fresh smears, when the sample was over 12 h old.
Cells other than leukocytes, including hepatocytes, mesothelial cells, or columnar biliary epithelial cells were found in 3 cases, and were considered an incidental finding.
Microorganisms were visualized in 15/59 (25%) canine bile aspirates, 14 of which were consistent with bacteria (including 3 repeated samples from the same dog). Bacterial populations appeared as mixed rods and cocci in 9 samples (Fig 2A), whereas 5 were monomorphic. Filamentous and spiral shaped bacteria were identified in 2 cases each. One sample contained banana‐shaped protozoal organisms, identified as Isospora spp. zoites (Fig 2B) by polymerase chain reaction. A total of 42/59 (71%) canine bile samples revealed neither microorganisms nor inflammation on cytological examination.
Figure 2.
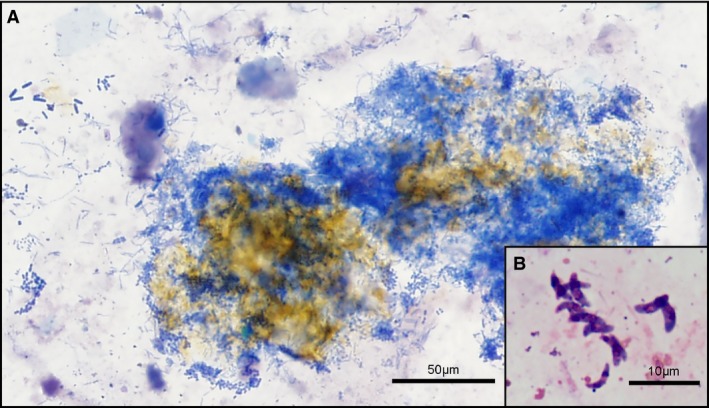
(A) Mats of variable bacteria admixed with golden bile pigment; dog, 20×. (B) Banana‐shaped zoites identified as Isospora spp.; dog, 100× oil. Modified Wright's stain.
Cats
The background of feline bile samples was similar to canine specimens. Aggregates and lakes of pale basophilic material were found in 16 cases, and golden to brown pigment was seen in 23 cases, predominantly found as fine needles or granular clumps. Rhomboid golden crystals, consistent with hematoidin, were rarely also observed. Variable numbers of erythrocytes were found in 19 cases, including ghost cells in 3, potentially suggesting in vivo hemorrhage into the gallbladder lumen. One sample consisted of clotted blood only, consistent with sampling artifact, and was thus considered non‐diagnostic.
Leukocytes were observed in excess of that expected from blood content in 15/81 feline samples (19%). All had neutrophilic inflammation with cells often being degenerate or lysed, resulting in streaming of purple nuclear material (Fig 3A). Lymphocytes were additionally observed in lower numbers in 8 samples, and macrophages in 7, the latter occasionally displaying phagocytic activity. Eosinophils were identified in 5 preparations, comprising up to 10% of nucleated cells. Other cells, interpreted as incidental findings, included variably preserved cuboidal to columnar biliary epithelial cells in 6 cases (Fig 1C), occasional spindle cells in 2 cases, and low numbers of hepatocytes in one.
Figure 3.
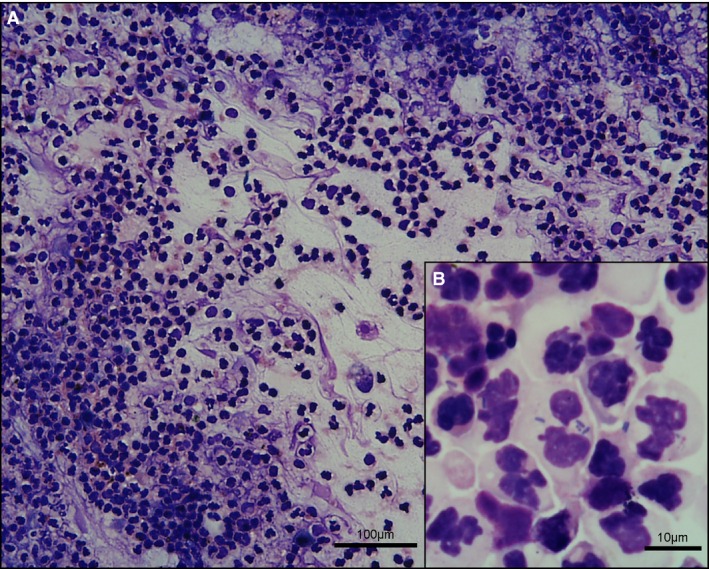
(A) Marked neutrophilic inflammation with nuclear streaming; bile, cat, 10×. (B) Degenerate neutrophils with intracellular bacteria; cat, 100× oil. Modified Wright's stain.
Bacteria were identified cytologically in 18/81 cases (22%), with 9 preparations suggesting a mixed bacterial population. Bacteria were accompanied by inflammation in 10 aspirates, and were found intracellularly within neutrophils in 5 (Fig 3B). Neither microorganisms nor inflammatory cells were found in 57/81 cases (70%).
The measured total nucleated cell count failed to correlate with cytological findings in both canine and feline bile samples (data not shown).
Culture Results
Dogs
Bacterial culture was performed in 58/59 samples, and bacterial growth was documented in 13 cultured bile fluids (22%). Isolates included Escherichia coli in 6 cases, Enterococcus spp. (including E. faecalis) in 5, and one isolate each of coliform bacteria, Campylobacter jejuni, Bacillus licheniformis, Clostridium perfringens, Proteus spp., and coagulase‐negative Staphylococcus spp. Mixed populations were identified in 4 cases, and often contained Enterococci and E. coli.
Cats
Bacterial culture was performed on 77/81 bile fluids, and yielded positive results in 16 aspirates (21%). Escherichia coli was the most commonly cultured organism (14 cases), and was predominantly found as a pure growth (9 cases).Other cultured strains included Enterococcus spp. (including E. faecalis; 4 cases), Clostridium spp. (2), and one each of Proteus spp., Peptostreptococcus spp., and α‐hemolytic Streptococcus.
Comparison of Cytology and Culture
Dogs
In 11 cases, the presence of bacteria was detected both cytologically and by means of bacterial culture. Mixed populations were identified by both methods in 5 cases, whereas in the remaining 6 samples single bacterial populations were cultured while cytological examination revealed a mixed bacterial picture. Three samples suggested presence of bacteria cytologically, but no bacterial growth was observed. These included 2 cases with long chains of cocci, and one with spiral shaped bacteria. Medical history was lacking in all three cases, thus prior antimicrobial treatment could not be ruled out. Conversely, two cultures yielded positive results when no microorganisms were detected cytologically. Cultured organisms in these cases were Bacillus licheniformis in one and coagulase‐negative Staphylococcus spp. in the other; the latter was considered likely to be a contaminant. No bacteria were isolated from the sample containing protozoal zoites.
Cats
Bacteria were found on both cytological evaluation and bacterial culture in 16 bile samples. Only in one sample were bacteria observed cytologically but could not be cultured, although no antimicrobial treatment had been administered before cholecystocentesis. Cytological and culture results concordantly identified single versus mixed bacterial populations in 9 cases. Six samples with cytological suspicion of mixed bacterial population yielded a pure growth (E. coli in all cases), whereas one sample suggesting uniform bacterial morphology grew a mixed population.
Gallbladder Histopathology and Comparison to Cytology
Histopathological sections of the gallbladder wall were examined in 6 dogs and 6 cats, with evidence of inflammation found in 4 and 6 sections, respectively. In dogs, inflammation was predominantly composed of lymphocytes and plasma cells, occasionally forming lymphoid follicles, with lower numbers of neutrophils additionally found in 2 cases and macrophages in one. Inflammation was not evident on concurrent cytological examination of bile in any of these cases, but bacteria were observed cytologically and confirmed by bile culture in 2 cases with histopathological evidence of inflammation; bacteria were detected histologically in one of these cases. Other findings included calcification of arteriolar walls, mucosal necrosis and mucocele in individual cases.
All 6 gallbladder sections from cats were diagnosed with cholecystitis, including 3 cases with mainly suppurative, 2 cases with mixed, and one case displaying lymphoplasmacytic inflammation. All 3 suppurative specimens had cytological evidence of neutrophilic inflammation and bacteria, and growth of E. coli from both gallbladder tissue and bile. One case with mixed inflammation on histopathology had evidence of mainly neutrophilic inflammation on cytological evaluation of bile, but no microorganisms were seen or cultured from either bile or gallbladder wall. The remaining 2 cases, one with lymphoplasmacytic and one with mixed inflammation on histopathological review, were allocated to Group 1 based on bile findings. Other findings included gallbladder wall edema in 3 cases, mucosal hyperplasia in 2, and necrosis, mucosal ulceration and granulation tissue in one section.
Comparing CBC and Biochemistry Results Between Group 1 and Group 2
Group 2 included 19/59 canine (32%) and 23/81 feline (28%) samples from 17 and 20 animals, respectively. CBC results before cholecystocentesis were available in 50/56 dogs and 67/78 cats, and serum biochemistry was performed in 51/56 and 65/78 respectively. Data with medians outside our reference intervals or with statistically significant differences between groups are listed in Table 1. For complete CBC and serum biochemical profiles, see supportive Table 1.
Table 1.
CBC and serum biochemistry results comparing Group 1 and Group 2
| Variable (RI) | Group 1 | Group 2 | P value | ||
|---|---|---|---|---|---|
| Sample size | Median (min‐max) | Sample size | Median (min‐max) | ||
| Dogs | |||||
| Total bilirubin (0–0.14 mg/dL) | 38 | 1.96 (0.02–54.34) | 13 | 0.81 (0.02–7.79) | .048a |
| ALT (13–88 U/L) | 38 | 800 (34–3233) | 13 | 582 (95–2065) | .40 |
| ALP (19–285 U/L) | 38 | 808 (39–8838) | 13 | 1515 (209–7899) | .18 |
| cPLI (<200 μg/L) | 16 | 130 (30–620) | 9 | 388 (125–650) | .013a |
| Cats | |||||
| Total bilirubin (0–0.18 mg/dL) | 49 | 1.15 (0–20) | 16 | 2.88 (0.07–15.1) | .39 |
| ALT (25–130 U/L) | 49 | 157 (21–2287) | 16 | 259 (34–1642) | .41 |
| GGT (0–2 U/L) | 48 | 2 (0–28) | 16 | 4 (1–13) | .061 |
ALT, Alanine transaminase; ALP, alkaline phosphatase; cPLI, canine pancreatic lipase immunoreactivity; GGT, γ‐glutamyl transferase.
Mann–Whitney U Test, significant difference between groups (P ≤ .05).
Using logistic regression analysis, only cPLI proved significantly different (P = .031; Fig 4) between groups.
Figure 4.
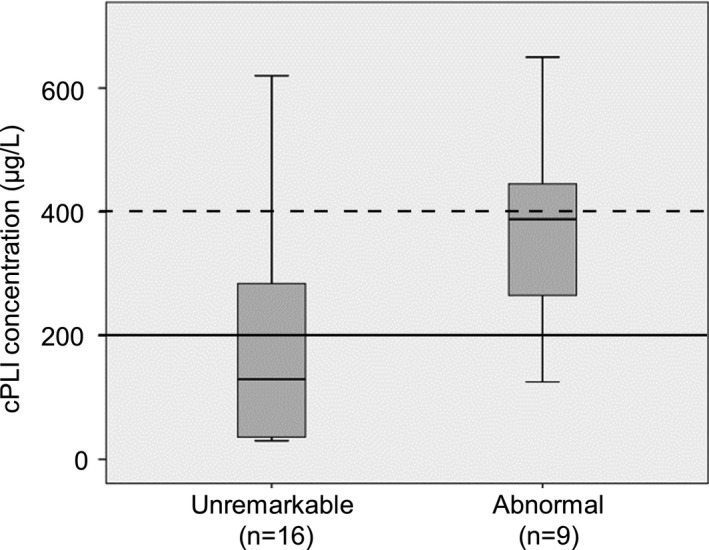
cPLI concentrations in canine Group 1 (median 130 μg/L; 30–620) and Group 2 (median 388 μg/L; 125–650). Logistic regression analysis, P = .031. Reference interval <200 μg/L (solid line), value suggestive of pancreatitis >400 μg/L (dashed line).
None of the additionally recorded measurements, including neutrophil left shift, toxicity, coagulation times, platelet concentrations, plasma ammonium, or bile acid concentrations were significantly associated with cytological bile findings or bile culture (data not shown).
Complications
Clinical follow‐up after cholecystocentesis was available for 53/59 dogs and 70/81 cats. Repeat CBC, serum biochemistry, or both after bile aspiration was available for 18 dogs and 36 cats, but because of concurrent procedures (i.e. laparoscopy, laparotomy, biopsies or placement of feeding tubes), changes in measured variables could not be directly attributed to cholecystocentesis (data not shown). Clinical complications directly associated with cholecystocentesis were identified in 4 dogs (8%) and 1 cat (1%). Two dogs developed bile peritonitis after ultrasound‐guided percutaneous cholecystocentesis, as documented by accumulation of peritoneal fluid with high proportions of neutrophils and bilirubin concentrations higher than serum bilirubin concentrations. Necropsy revealed a perforated gallbladder in the first case, and while histopathological examination of the gallbladder wall yielded no abnormalities, a neuroendocrine carcinoma involving the pancreas, duodenum and liver was detected. The gallbladder was intact in the second case and biopsies revealed lymphoplasmacytic cholecystitis, but concurrent acute pancreatitis with pancreatic and hepatic necrosis were considered the main pathological processes. Bile samples from both dogs were allocated to Group 1 based on cytological review and bacterial culture. One additional dog (with bactibilia) displayed slight accumulation of fluid around the gallbladder on ultrasound examination after percutaneous cholecystocentesis, and mild leakage from the gallbladder was observed in another dog (from Group 1) immediately after laparoscopic cholecystocentesis, but both animals recovered uneventfully from their procedures.
One cat experienced mild leakage from the gallbladder during exploratory laparotomy, but remained clinically stable until discharge with conservative treatment.
Underlying Diagnoses and Concurrent Diseases
Working or final diagnosis was recorded for 85% of animals. Only one dog and one cat had evidence of microorganisms or inflammation in bile as the only documented pathological process, whereas the remainder of the animals suffered from comorbidities. Results are summarized in Table 2.
Table 2.
Working diagnoses from dogs and cats with cholecystocentesis
| Dogs Group 1 (n = 38) | Dogs Group 2 (n = 14) |
|---|---|
| Extrahepatic biliary tree (n = 2) | Extrahepatic biliary tree (n = 9) |
| Extrahepatic bile tract obstruction: 1 | Inflammation/infection: 8 |
| Gallbladder mucocele: 1 | w/cholelithiasis: 1 |
| Gallbladder mucocoele & bile peritonitis: 1 | |
| Hepatic (n = 27) | Hepatic (n = 8) |
| Chronic (active) hepatitis: 11 | Cholangiohepatitis: 3 |
| w/accumulation of copper: 7 | w/positive liver culture: 2 |
| Cholangiohepatitis: 5 | Chronic hepatitis: 3 |
| Vacuolar hepatopathy: 6 | Fibrosis: 1 |
| Failure: 3 | Metastatic carcinoma w/insufficiency: 1 |
| Fibrosis: 3 | |
| Necrosis: 2 | |
| Hepatocellular carcinoma: 1 | |
| Metastatic neuroendocrine tumor: 1 | |
| Toxic hepatopathy: 1 | |
| Pancreatic (n = 7) | Pancreatic (n = 4) |
| Pancreatitis: 7 | Pancreatitis: 4 |
| Gastrointestinal (n = 7) | Gastrointestinal (n = 1) |
| Inflammatory bowel disease: 3 | Inflammatory bowel disease: 1 |
| Protein‐losing enteropathy: 3 | |
| w/lymphangiectasia: 1 | |
| Ulcerations: 2 | |
| Colonic plasmacytoma: 1 | |
| Pyloric obstruction: 1 | |
| Others | Others |
| Acute kidney injury: 1 | Hypercholesterolemia: 1 |
| Chronic myeloid leukemia: 1 | Vaginal undifferentiated carcinoma: 1 |
| Granulomatous‐necrotising cerebellitis: 1 | |
| Immune‐mediated thrombocytopenia: 1 | |
| Myxomatous mitral valve disease: 1 |
| Cats Group 1 (n = 51) | Cats Group 2 (n = 16) |
|---|---|
| Extrahepatic biliary tree (n = 3) | Extrahepatic biliary tree (n = 16) |
| Extrahepatic bile tract obstruction: 2 | Inflammation/infection: 16 |
| Cholelithiasis: 1 | w/cholelithiasis: 4 |
| w/extrahepatic bile tract obstruction: 2 | |
| Hepatic (n = 32) | Hepatic (n = 12) |
| Cholangiohepatitis: 26 | Cholangiohepatitis: 10 |
| As part of “triaditis”: 5 | w/positive liver culture: 1 |
| Lipidosis: 7 | As part of “triaditis”: 2 |
| w/hepatic encephalopathy: 1 | Lipidosis: 1 |
| Fibrosis: 3 | Polycystic liver disease: 1 |
| Failure: 3 | |
| Necrosis: 2 | |
| Hepatocellular carcinoma: 1 | |
| Neuroendocrine tumor metastasis: 1 | |
| Toxic hepatopathy: 1 | |
| Pancreatic (n = 17) | Pancreatic (n = 7) |
| Pancreatitis: 15 | Pancreatitis: 7 |
| w/amyloidosis: 1 | |
| Adenocarcinoma: 2 | |
| Gastrointestinal (n = 13) | Gastrointestinal (n = 2) |
| Inflammatory bowel disease: 11 | Inflammatory bowel disease: 2 |
| Foreign body: 1 | |
| Gastric ulceration: 1 | |
| Others | Others |
| Lymphoma: 5 | Chronic kidney disease: 1 |
| Cardiac disease: 2 | Histiocytic sarcoma: 1 |
| Chronic kidney disease: 2 | |
| Immune‐mediate hemolytic anemia: 2 | |
| Acute kidney injury: 1 | |
| Diabetic ketoacidosis: 1 | |
| Epilepsy: 1 | |
| Feline infectious peritonitis: 1 | |
| Feline immunodeficiency virus: 1 | |
| Hyperadrenocorticism: 1 | |
| Hyperthyroidism: 1 | |
| Retroperitoneal abscess: 1 | |
| Septic peritonitis: 1 | |
| Thrombus in vena cava caudalis: 1 |
Discussion
A total of 32% of canine and 28% of feline aspirates were allocated to Group 2, with presence of microorganisms documented in 24% of all cases, as demonstrated by cytological evaluation, bacterial culture, or both modalities. Our results are comparable to previously published prevalence of bactibilia based on bacterial culture.1, 2 The majority of dogs with bactibilia in our study lacked cytological evidence of concurrent inflammation, raising the possibility of transient colonization rather than true infection. Transient, self‐limiting bactibilia can occur in clinically healthy dogs,5 but lack of concurrent inflammation in the bile or gallbladder wall despite bactibilia also occurs in clinically sick dogs.2, 7, 9 Although most of the animals had clinical signs of hepatobiliary disease, these could not be clearly attributed to gallbladder disease in cases from Group 2, as comorbidities were present in most cases. The current literature suggests that bactibilia can be silent until biliary obstruction occurs, hypothesizing that biliary‐venous reflux might lead to systemic sepsis and manifestation of clinical illness.3 Unfortunately, there was insufficient follow‐up information to document resolution of clinical signs or bactibilia after antibiotic treatment, which would have helped to distinguish incidental from clinically important bactibilia.
In contrast to dogs, cats in our study commonly had a neutrophilic inflammatory response on cytological evaluation, a finding described in a small case series.6 This observation could be associated with the relatively high frequency of cholangitis in cats. Neutrophils predominated in all feline bile fluids with cytological evidence of inflammation, despite only half the available gallbladder biopsies displaying suppurative inflammation. The remaining sections showed lymphocytic or mixed inflammation, and concurrent cytological findings were normal or exhibited neutrophilic inflammation only, suggesting that neutrophils either exfoliate much more readily into the gallbladder lumen than mononuclear cells, or, less likely, that the latter are more susceptible to lysis in bile fluid. These hypotheses could also partially explain the relative lack of inflammation in canine bile, where lymphocytic inflammation predominated on histopathological examination of the gallbladder wall. However, more concurrent gallbladder biopsies need to be examined to allow more definitive conclusions to be made.
Commonly observed discrepancies between culture and cytology included culture of single organisms where microscopic examination suggested a mixed population, which was likely attributable to in vitro overgrowth of a single organism, mainly E. coli in this study. Absence of cytological evidence of bacteria with positive culture was rarely observed, and could be explained by insufficient numbers of microorganisms, or contamination of the sample submitted for culture, as was suspected in one case. Possible causes for negative bacterial culture despite cytological evidence of bactibilia include prior administration of antibiotic treatment, a bacteriostatic effect of bile, or the presence of bacteria that are difficult to culture, such as Helicobacter spp.,13 as was potentially suggested by the presence of spiral shaped bacteria in the bile of one dog in our study. Antibiotic treatment was administered before cholecystocentesis in at least a third of all cases, but did not appear to be associated with the absence of bacteria (data not shown).
Furthermore, although a rare occurrence, cytological evaluation allows for identification of non‐bacterial microorganisms such as protozoal zoites found in one canine bile sample, which would have been missed by submitting bile for bacterial culture alone.
Serum cPLI concentration was significantly higher in Group 2. However, review of medical records revealed that only about half the dogs in Group 2 with serum cPLI concentration over 200 μg/L had a clinical diagnosis of pancreatitis, suggesting that a disease process other than clinically apparent pancreatitis might have led to leakage of pancreatic enzymes in the remaining dogs. Clinical signs are often similar between both conditions: cholangitis could result in peri‐pancreatic inflammation, and pancreatitis can lead to cholestasis, predisposing to ascending bacterial colonization.3 Small group sizes, retrospective nature of the study and lack of a non‐invasive gold standard for diagnosis of pancreatitis hindered a conclusive examination of a possible causative relationship between bactibilia and increased serum cPLI concentration in dogs. Lack of a similar relationship between Group 2 and fPLI concentrations in cats was unexpected, given the high prevalence of concurrent cholangitis and pancreatitis in this species,14, 15 but the possibility of type II error cannot be ruled out given small sample size of subgroups.
Complications directly attributable to cholecystocentesis were low in this study, and comparable to previous reports,5, 16, 17 but nonetheless 2 dogs (<2% of all cases with available follow‐up information) suffered from iatrogenic bile peritonitis. However, necropsy findings suggested that bile peritonitis was not the predominant disease process in either case.
Concurrent diseases identified in sampled dogs and cats did not reveal unexpected results, with hepatopathies being mainly reported, along with lower numbers of animals suffering from pancreatic and gastrointestinal disease. While the anatomical proximity of these organs makes a relationship between bactibilia or inflammatory bile findings and these comorbidities plausible, it is not clear whether pancreatitis, hepatitis and enteritis were the cause, or the consequence of the bile findings in Group 2. The high prevalence of liver disease in the sampled population likely represents a sampling bias, given that the suspicion for hepatobiliary disease was the main indication for cholecystocentesis.
The retrospective nature of our study is a major limitation. This might have led to inconsistent sample acquisition and handling, as well as non‐standardized clinical work up. An additional shortcoming, given that no definition of normal bile exists up to date, is the lack of a healthy control group, without which we could not assess the prevalence of subclinical bactibilia nor establish the clinical importance of bacteria and inflammatory cells in bile.
In summary, we describe the presence of microorganisms, inflammation, or both in about 30% of bile aspirates from a large group of dogs and cats. We document that cholecystocentesis is associated with relatively little risk, and that cytological analysis of bile yields clinically relevant results that culture alone cannot provide. Because of relevant decline in cellularity and bacterial overgrowth in bile fluids with delayed analysis, we strongly recommend submitting fresh smears along with bile fluid to the laboratory. Given the relatively high prevalence of potentially clinically relevant cytological features observed in bile during our study, we encourage a wider recognition of the diagnostic utility of cholecystocentesis in small animal practice.
Supporting information
Table S1. Complete CBC and serum biochemistry results comparing Group 1 and Group 2.
Acknowledgments
The authors thank Dr. Ruby Chang for her help with statistical analysis, and the anatomic pathologists of the Royal Veterinary College for biopsy and necropsy reporting.
Conflict of Interest Declaration
Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
Funding
No sources of funding were used for this study.
This study was conducted at the Royal Veterinary College, United Kingdom.
A part of this study was presented at the ACVP/ASVCP annual meeting in November 2014 in Atlanta, GA, USA.
References
- 1. Wagner KA, Hartmann FA, Trepanier LA. Bacterial culture results from liver, gallbladder, or bile in 248 dogs and cats evaluated for hepatobiliary disease: 1998‐2003. J Vet Intern Med 2007;21:417–424. [DOI] [PubMed] [Google Scholar]
- 2. Crews LJ, Feeney DA, Jessen CR, et al. Clinical, ultrasonographic, and laboratory findings associated with gallbladder disease and rupture in dogs: 45 cases (1997‐2007). J Am Vet Med Assoc 2009;234:359–366. [DOI] [PubMed] [Google Scholar]
- 3. Center SA. Hepatobiliary infections In: Greene CE, ed. Greene's Infectious Diseases of He Dog and Cat. St. Louis, MO: Elsevier; 2012:981–1012. [Google Scholar]
- 4. Sung JY, Shaffer EA, Olson ME, et al. Bacterial invasion of the biliary system by way of the portal‐venous system. Hepatology 1991;14:313–317. [PubMed] [Google Scholar]
- 5. Kook PH, Schellenberg S, Grest P, et al. Microbiologic evaluation of gallbladder bile of healthy dogs and dogs with iatrogenic hypercortisolism: a pilot study. J Vet Intern Med 2010;24:224–228. [DOI] [PubMed] [Google Scholar]
- 6. Brain PH, Barrs VR, Martin P, et al. Feline cholecystitis and acute neutrophilic cholangitis: clinical findings, bacterial isolates and response to treatment in six cases. J Feline Med Surg 2006;8:91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ramery E, Papakonstantinou S, Pinilla M, et al. Bacterial cholangiohepatitis in a dog. Can Vet J 2012;53:423–425. [PMC free article] [PubMed] [Google Scholar]
- 8. Lawrence YA, Ruaux CG, Nemanic S, et al. Characterization, treatment, and outcome of bacterial cholecystitis and bactibilia in dogs. J Am Vet Med Assoc 2015;246:982–989. [DOI] [PubMed] [Google Scholar]
- 9. Neel JA, Tarigo J, Grindem CB. Gallbladder aspirate from a dog. Vet Clin Pathol 2006;35:467–470. [DOI] [PubMed] [Google Scholar]
- 10. Flatland B. If you have the gall. Vet Clin Pathol 2009;38:280. [DOI] [PubMed] [Google Scholar]
- 11. Braun U, Gerber D. Percutaneous ultrasound‐guided cholecystocentesis in cows. Am J Vet Res 1992;53:1079–1084. [PubMed] [Google Scholar]
- 12. Geigy CA, Dandrieux J, Miclard J, et al. Extranodal B‐cell lymphoma in the urinary bladder with cytological evidence of concurrent involvement of the gall bladder in a cat. J Small Anim Pract 2010;51:280–287. [DOI] [PubMed] [Google Scholar]
- 13. Boomkens SY, Kusters JG, Hoffmann G, et al. Detection of Helicobacter pylori in bile of cats. FEMS Immunol Med Microbiol 2004;42:307–311. [DOI] [PubMed] [Google Scholar]
- 14. Clark JEC, Haddad JL, Brown DC, et al. Feline cholangitis: a necropsy study of 44 cats (1986‐2008). J Feline Med Surg 2011;13:570–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weiss DJ, Gagne JM, Armstrong PJ. Relationship between inflammatory hepatic disease and inflammatory bowel disease, pancreatitis, and nephritis in cats. J Am Vet Med Assoc 1996;209:1114–1116. [PubMed] [Google Scholar]
- 16. Voros K, Sterczer A, Manczur F, et al. Percutaneous ultrasound‐guided cholecystocentesis in dogs. Acta Vet Hung 2002;50:385–393. [DOI] [PubMed] [Google Scholar]
- 17. Savary‐Bataille KC, Bunch SE, Spaulding KA, et al. Percutaneous ultrasound‐guided cholecystocentesis in healthy cats. J Vet Intern Med 2003;17:298–303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Complete CBC and serum biochemistry results comparing Group 1 and Group 2.


