Abstract
Background
Acute pancreatitis (AP) occurs frequently in dogs, but most previous studies examining the diagnosis of AP have used data from secondary care hospitals.
Hypothesis/Objectives
The aim of this study was to investigate the clinical utility of diagnostic laboratory tests in dogs with AP in a primary care hospital.
Animals
Sixty‐four dogs with clinical signs suggestive of AP diagnosed with nonpancreatic disease (NP) or AP.
Methods
Medical records were retrospectively reviewed, including diagnostic laboratory tests considered potentially useful in the diagnosis of AP. The diagnostic accuracy of amylase and FUJI DRI‐CHEM lipase (FDC lip) were investigated using receiver operating characteristics (ROC). In addition, we verified whether diagnostic laboratory tests were useful for evaluating duration of hospitalization and as biomarkers for monitoring recovery.
Results
Activities of amylase and FDC lip were significantly higher in the AP group than in the NP group (P = .001, P < .001, respectively). The sensitivity of FDP lip activity for diagnosing AP was 100% (95% confidence interval [CI], 87.7–100%); the specificity was 89.5% (95% CI, 66.9–98.7%). Area under the ROC curve for FDC lip activity was 0.98 (95% CI, 0.93–1). High alanine aminotransferase (ALT) activity was associated with extended duration of hospitalization (P = .04). A significant difference in C‐reactive protein (CRP) concentration before and 5 days after treatment was found (P = .001).
Conclusions and clinical importance
Measurement of FDC lip activity appears useful for diagnosing AP. High ALT activity might be associated with prolonged duration of hospitalization, and CRP might be useful as a biomarker for monitoring recovery from AP.
Keywords: Canine, inflammation, pancreas
Abbreviations
- ALT
alanine aminotransferase
- AP
acute pancreatitis
- CI
confidence interval
- CRP
C‐reactive protein
- FDC lip
FUJI DRI‐CHEM lipase
- IQR
interquartile range
- NP
nonpancreatic disease
- ROC
receiver operating characteristic
- Spec‐cPL
canine pancreas‐specific lipase
Acute pancreatitis (AP) frequently is diagnosed in dogs presenting with acute nonspecific clinical signs such as anorexia, vomiting, weakness, diarrhea, and abdominal pain.1, 2, 3 Observed systemic clinical signs such as fever, respiratory distress, and cardiovascular shock also can be attributed to AP. These clinical signs in patients with pancreatitis are caused by local and systemic effects of pancreatic inflammation.4
In general, diagnosis is made using laboratory testing and diagnostic imaging procedures such as radiography and abdominal ultrasonography, in addition to clinical signs.5 Biomarkers for AP include amylase and lipase activities, serum trypsin‐like immunoreactivity, and canine pancreatic lipase immunoreactivity, which is considered the most reliable.6, 7, 8, 9 Pancreatic biopsy is useful for diagnosing AP and has been performed to evaluate biomarker concentrations.5, 10, 11, 12
Canine pancreas‐specific lipase (Spec‐cPL) is a recently established laboratory test that has been well validated and is now in wide use.5, 13, 14, 15, 16 However, results cannot be obtained immediately, and testing must be performed by a commercial veterinary medical laboratory. Recently, an immunochromatographic test for Spec‐cPL1 was developed to address this problem, but the method is semiquantitative and does not measure concentrations.15, 17 At the same time, a new lipase test slide for dry chemistry analyzers2 has been developed and made commercially available, allowing immediate results to be obtained in hospitals.18 FUJI DRI‐CHEM lipase (FDC lip) activity and Spec‐cPL concentrations have been verified to show good correlation with the diagnosis of AP.18 Diagnostic performance for AP using diagnostic laboratory tests thus has improved in recent years.
Many dogs with AP and nonspecific clinical signs are diagnosed in primary care hospitals. Nevertheless, most previous studies examining the diagnostic accuracy of laboratory tests for AP have used data from secondary care hospitals.1, 2, 17, 19, 20 This study therefore investigated the diagnostic accuracy of laboratory tests for AP used in a primary care hospital. In addition, we verified whether diagnostic tests performed in the hospital are useful for predicting the likely duration of hospitalization and as biomarkers for monitoring recovery from AP.
Materials and methods
Retrospective Study Criteria for Case Selection
Medical records at Yuki Animal Hospital were searched to identify all dogs with clinical signs suggestive of AP diagnosed with nonpancreatic disease (NP) or AP between June 2012 and December 2014. Each dog had undergone evaluation that included physical examination, CBC, serum biochemistry profile, radiography, and ultrasonography. The FDC lip activity was measured using a dry chemistry analyzer2 with dedicated FUJI DRI‐CHEM lipase slides.3 Serum Spec‐cPL concentration was measured using the Spec‐cPL assay in a commercial laboratory4 that reports Spec‐cPL <200 μg/L as normal and >400 μg/L as abnormal. In this study, we divided dogs into 2 groups, with reference to Chartier et al.3 and Steiner et al.13
Selection of dogs with NP
Dogs were included in the NP group if they met all of the following 3 criteria: (1) at least 2 of the following clinical signs consistent with suspected AP: lethargy, inappetence, weakness, vomiting, abdominal pain, diarrhea; (2) serum Spec‐cPL concentration <200 μg/L, not consistent with a diagnosis of pancreatitis; and, (3) results of abdominal ultrasonography that were not consistent with AP.
Selection of dogs with AP
Dogs were included in the AP group if they met all of the following 3 criteria: (1) at least 2 of the following clinical signs consistent with AP: lethargy, inappetence, weakness, vomiting, abdominal pain, diarrhea; (2) serum Spec‐cPL concentration >400 μg/L, consistent with a diagnosis of pancreatitis; and, (3) results of abdominal ultrasonography supportive of AP, such as hyperechoic peripancreatic fat, hypoechoic pancreatic parenchyma, hypoechoic pancreatic nodules within parenchyma, or unclear pancreatic lesions.
Diagnostic Accuracy of Laboratory Tests in the Diagnosis of AP
White blood cell count; hematocrit; platelet count; concentrations of urea nitrogen, total calcium, albumin, and C‐reactive protein (CRP); and, activities of alanine aminotransferase (ALT), amylase, and FDC lipase were compared between NP and AP groups. Receiver operating characteristic (ROC) curve analysis was used to assess the accuracy of the tests as potentially useful in the diagnosis of AP.
Relationship between Laboratory Tests and Duration of Hospitalization
In AP dogs, we investigated the relationship between laboratory tests measured on the first day and duration of hospitalization. Duration of hospitalization was classified as follows: Grade I, 0–3 days; Grade II, 4–7 days; Grade III, 8–11 days; and Grade IV, death during hospitalization. Treatment during hospitalization5 consisted of IV fluid therapy, antiemetics, antibiotics, gastric acid suppression, and nutritional management.
Usefulness of Laboratory Tests as Biomarkers for Monitoring Recovery
C‐reactive protein concentration and FDC lip activity were measured periodically during hospitalization in dogs diagnosed with AP. Results obtained at the time of diagnosis were compared with those obtained 5 days after treatment.
Data Analysis and Statistics
Each laboratory test was compared between NP and AP groups using the Mann–Whitney U‐test. For comparing sex and breeds between NP and AP groups, Fisher's exact test was used. For the relationships between duration of hospitalization, age, and diagnostic test results, the Kruskal–Wallis one‐way analysis of variance was used. Comparisons between groups for each grade of duration of hospitalization were made using the Steel–Dwass test. Pre‐ and post‐treatment findings were compared between groups using the Wilcoxon signed‐rank test. Values of P < .05 were considered significant. Receiver operating characteristic analysis was used to assess the accuracy of the test. Area under the ROC curve and sensitivity and specificity with 95% confidence intervals (CI) were calculated. Statistical analyses were performed using Easy R software.21
Results
Sixty‐four dogs were enrolled in the study and assigned to either the NP group (n = 20) or the AP group (n = 44). The NP group consisted of 10 females (2 sexually intact, 8 spayed) and 10 males (6 sexually intact, 4 neutered). The median age of NP dogs was 9.5 years (interquartile range [IQR], 5.8–11.3 years) and median body weight was 5.2 kg (IQR, 4.2–7.3 kg; Table 1). Breeds in the NP group were Miniature Dachshund (n = 10), Pomeranian (n = 2), Yorkshire Terrier (n = 1), Shih Tzu (n = 1), Toy Poodle (n = 1), Maltese (n = 1), Jack Russell Terrier (n = 1), Shiba Inu (n = 1), Miniature Schnauzer (n = 1), and mixed breed (n = 1).
Table 1.
Comparison of age, sex, body weight, and laboratory tests in nonpancreatitis and acute pancreatitis groups
| Variable | RI | NP Group (no., IQR) | AP Group (no., IQR) | P value |
|---|---|---|---|---|
| Age, median (years) | 9.5 (n = 20, 5.8–11.3) | 12 (n = 44, 7.8–14.2) | .04 | |
| Sex | ||||
| Male | 10 | 27 | .85 | |
| Female | 10 | 17 | ||
| B.W. (kg) | 5.2 (n = 20, 4.2–7.3) | 5.3 (n = 44, 4.2–6.9) | .87 | |
| WBC (/μL) | 6,000–17,000 | 12,000 (n = 19, 9,750–24,750) | 13,700 (n = 43, 9,500–17,850) | .71 |
| HCT (%) | 37–55 | 51 (n = 19, 46.8–57.0) | 48.6 (n = 43, 43.4–55.9) | .33 |
| Plate (/μL) | 200,000–500,000 | 277,000 (n = 19, 228,500–397,000) | 338,000 (n = 43, 223,500–442,500) | .41 |
| BUN (mg/dL) | 9.2–29.2 | 15 (n = 14, 11.5–36.2) | 21.3 (n = 39, 13.1–38.4) | .35 |
| ALT (U/L) | 17–78 | 55 (n = 13, 48–148) | 81.5 (n = 38, 46–119) | .85 |
| Ca (mg/dL) | 9.3–12.1 | 10.9 (n = 10, 9.9–13.0) | 10.7 (n = 22, 9.7–11.5) | .52 |
| Alb (mg/dL) | 2.6–4.0 | 3.2 (n = 11, 3.0–3.5) | 3.2 (n = 21, 2.8–3.5) | .97 |
| CRP (mg/dL) | 0–1.0 | 8.6 (n = 20, 4.8–15.0) | 9.6 (n = 41, 3.5–19.5) | .53 |
| Amy (U/L) | 200–1,400 | 870 (n = 11, 445–1,190) | 1,674 (n = 32, 1,235–2,500) | .001 |
| FDC lip (U/L) | 10–160 | 60 (n = 17, 37–101) | 904 (n = 42, 360–1,000) | <.001 |
| Spec–cPL (μg/L) | <200 | 88 (n = 20, 36–134) | 809 (n = 44, 812–1,000) | <.001 |
RI, reference intervals; IQR, interquartile range; B.W., body weight; WBC, white blood cell count; HCT, hematocrit; Plate, platelet count; BUN, blood urea nitrogen; ALT, alanine aminotransferase; Ca, calcium; Alb, albumin; CRP, C reactive protein; Amy, amylase; FDC lip, FUJI DRI‐CHEM lipase.
P values <.05 were considered statistically significant.
The AP group consisted of 17 females (2 sexually intact, 15 spayed) and 27 males (15 sexually intact, 12 neutered). The median age of AP dogs was 12 years (IQR, 7.8–14.2 years) and median body weight was 5.3 kg (IQR, 4.2–6.9 kg; Table 1). Breeds in the AP group were Miniature Dachshund (n = 17), Yorkshire Terrier (n = 4), Toy Poodle (n = 4), Pomeranian (n = 3), Shih Tzu (n = 2), Maltese (n = 2), mixed breed (n = 2), Papillon (n = 2), Shiba Inu (n = 1), Chihuahua (n = 1), West Highland White Terrier (n = 1), Pekinese (n = 1), Miniature Pinscher (n = 1), Pembroke Welsh Corgi (n = 1), Cavalier King Charles Spaniel (n = 1), and Cairn Terrier (n = 1). No significant differences in sex (P = .85), body weight (P = .87), or breed (P = .98) were identified between the AP and NP groups.
The most common clinical signs in dogs with NP were inappetence (18/20, 90%), vomiting (16/20, 80%), weakness (14/20, 70%), abdominal pain, diarrhea or both (5/20, 25%), and lethargy (1/20, 0.05%). The most common clinical signs in dogs with AP were inappetence (37/44, 84%), vomiting (36/44, 82%), weakness (33/44, 75%), abdominal pain, diarrhea or both (30/44, 68%), and lethargy (3/44, 0.07%).
Diagnoses in NP dogs included gastroenteritis (n = 12), prostatic abscess (n = 2), bacterial pyelonephritis (n = 2), hepatitis (n = 2), hypoadrenocorticism (n = 1), and lymphoma (n = 1). Underlying or concurrent diseases were mitral regurgitation (n = 1) and hyperadrenocorticism (n = 1). In the AP group, underlying or concurrent diseases and complications included mitral regurgitation (n = 5), diabetes mellitus (n = 3), hepatitis (n = 3), cholangiohepatitis (n = 2), immune‐mediated polyarthritis (n = 2), idiopathic epilepsy (n = 1), chronic kidney disease (n = 1), hyperadrenocorticism (n = 1), nonregenerative immune‐mediated anemia (n = 1), pneumonia (n = 1), and pyometra (n = 1).
Diagnostic Accuracy of Laboratory Tests in Diagnosis of AP
Median white blood cell count; hematocrit; blood platelet count; concentrations of blood urea nitrogen, calcium, albumin, CRP, and Spec‐cPL; and, activities ALT, amylase and FDC lip in the NP and AP groups are shown in Table 1. Activities of amylase (P = .001) and FDC lip (P < .001) were significantly higher in the AP group than in the NP group (Table 1; Figs 1 and 2).
Figure 1.
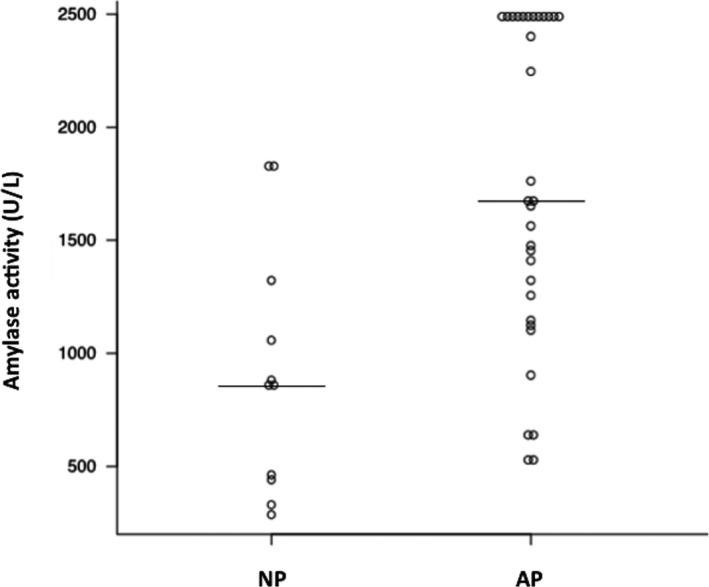
Comparison of amylase activities between nonpancreatitis (NP) and acute pancreatitis (AP) groups. Horizontal bars indicate medians within groups. A significant difference is apparent between groups (P < .05).
Figure 2.
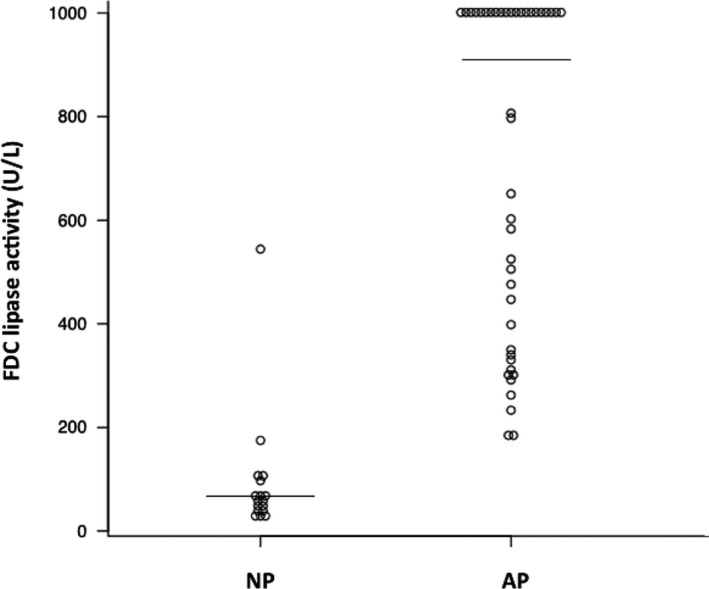
Comparison of FUJI DRI‐CHEM lipase activities between nonpancreatitis (NP) and acute pancreatitis (AP) groups. Horizontal bars indicate medians within groups. A significant difference is apparent between groups (P < .05).
The sensitivity and specificity of amylase activity (cut‐off, 1400 U/L) for a diagnosis of AP were 68.9% (95% CI, 50.0–83.9%) and 81.8% (95% CI, 48.2–97.7%), respectively (Fig 3). The sensitivity and specificity of FDC lip activity (cut‐off, 160 U/L) for a diagnosis of AP were 100% (95% CI, 87.7–100%) and 89.5% (95% CI, 66.9–98.7%), respectively (Fig 4). Area under the ROC curve for amylase activity was 0.82 (95% CI, 0.68–0.97; Fig 3). Area under the ROC curve for FDC lip activity was 0.98 (95% CI, 0.93–1; Fig 4).
Figure 3.
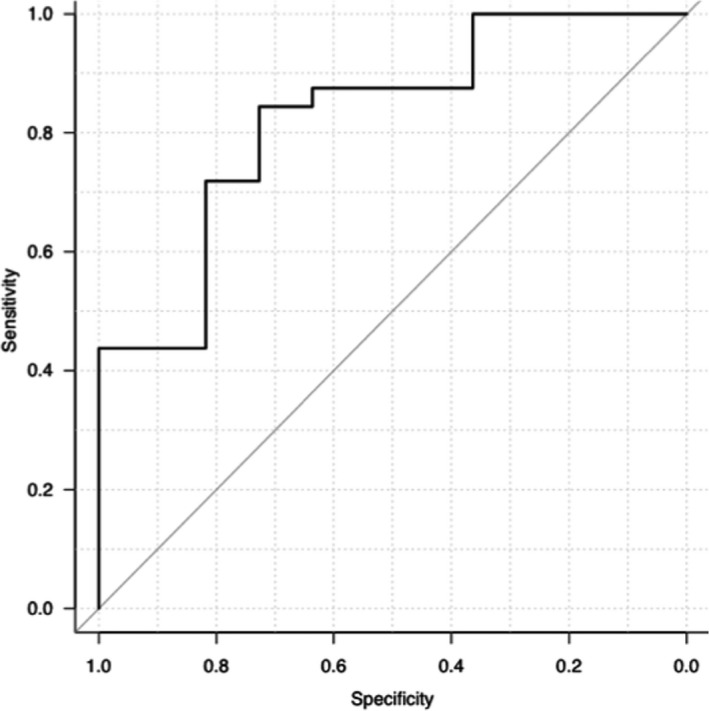
Receiver operator characteristic (ROC) curve for amylase activity in acute pancreatitis. The sensitivity and specificity of amylase activity (cut‐off, 1400 U/L) for a diagnosis of AP were 68.9% (95% CI, 50.0–83.9%) and 81.8% (95% CI, 48.2–97.7%), respectively. Area under the ROC curve for amylase activity was 0.82 (95% CI, 0.68–0.97).
Figure 4.
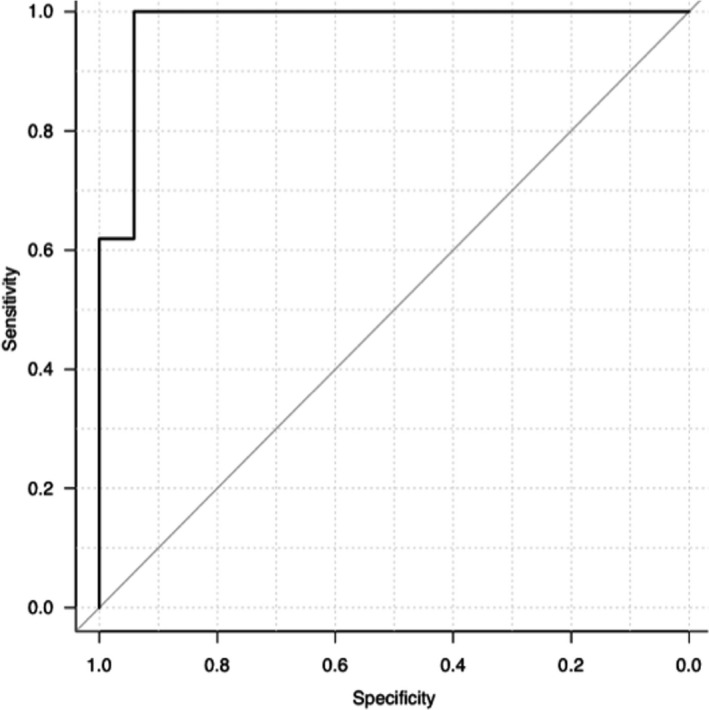
Receiver operator characteristic (ROC) curve for FUJI DRI‐CHEM lipase activity in acute pancreatitis. The sensitivity and specificity of FDC lip activity (cut‐off, 160 U/L) for a diagnosis of AP were 100% (95% CI, 87.7–100%) and 89.5% (95% CI, 66.9–98.7%), respectively. Area under the ROC curve for FDC lip activity was 0.98 (95% CI, 0.93–1).
Relationship between Laboratory Tests and Duration of Hospitalization
Overall duration of hospitalization in the AP group ranged from 0 to 10 days (median, 4 days). Six dogs (14%) died during hospitalization. Eighteen dogs were classified as having Grade I disease; their median age was 8.5 years (IQR, 5.5–12) and median time in the hospital was 2 days (IQR, 1.3–2.8). Fifteen dogs were classified as having Grade II disease; their median age was 14 years (IQR, 8–16) and median time in the hospital was 4 days (IQR, 4–6). Five dogs were classified as having Grade III disease; their median age was 14 years (IQR, 12–15) and median time in the hospital was 9 days (IQR, 9–9). Six dogs were classified as having Grade IV disease; their median age was 12 years (IQR, 11.3–12.8) and median time in the hospital was 4.5 days (IQR, 3.3–5.8). Laboratory tests at the time of AP diagnosis were compared using Kruskal–Wallis one‐way analysis, and identified a significant difference in ALT activity (P = .01). Multiple comparison analysis identified a significant difference between Grade I and Grade III cases (P = .04; Fig 5). Kruskal–Wallis one‐way analysis showed no significant differences in age (P = .17); white blood cell count (P = .58); hematocrit (P = .25); platelet count (P = .38); concentrations of blood urea nitrogen (P = .23), total calcium (P = .40), albumin (P = .98), CRP (P = .14), or Spec‐cPL (P = .84); or activities of amylase (P = .56) or FDC lipase (P = .87) among different grades of hospitalization duration.
Figure 5.
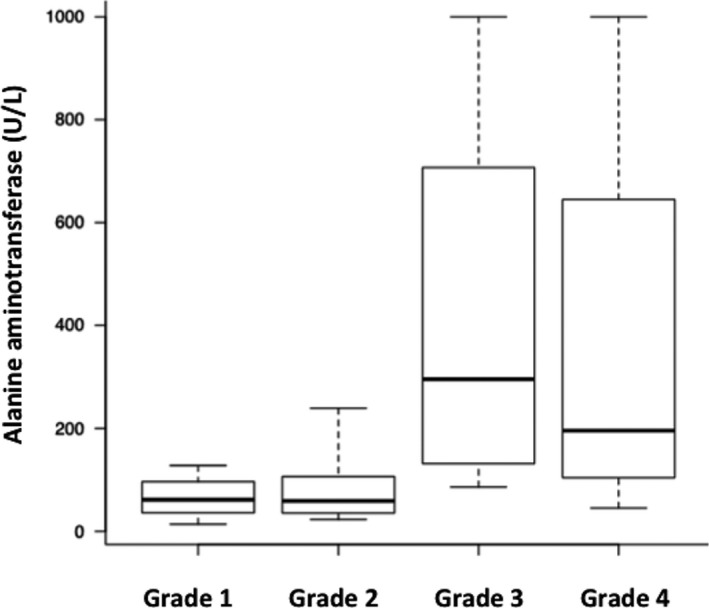
Comparison of alanine aminotransferase activities between groups for each grade of duration of hospitalization in dogs with acute pancreatitis. Grade I (IQR, 1.3–2.8 days, n = 18), Grade II (IQR, 4–6 days, n = 15), Grade III (IQR, 9–9 days, n = 5), Grade IV (death during hospitalization, n = 6). A significant difference is apparent between Grade I and Grade III (P < .05). Box plots show the median, range, and 25th and 75th quartiles for alanine aminotransferase activity.
Usefulness of Laboratory Tests as Biomarkers for Monitoring Recovery
A significant difference in CRP concentration at the time of diagnosis and 5 days after treatment (n = 17) was identified (P = .001; Fig 6). No significant difference in FDC lip activity at the time of diagnosis and after treatment (n = 10) was identified (P = .08).
Figure 6.
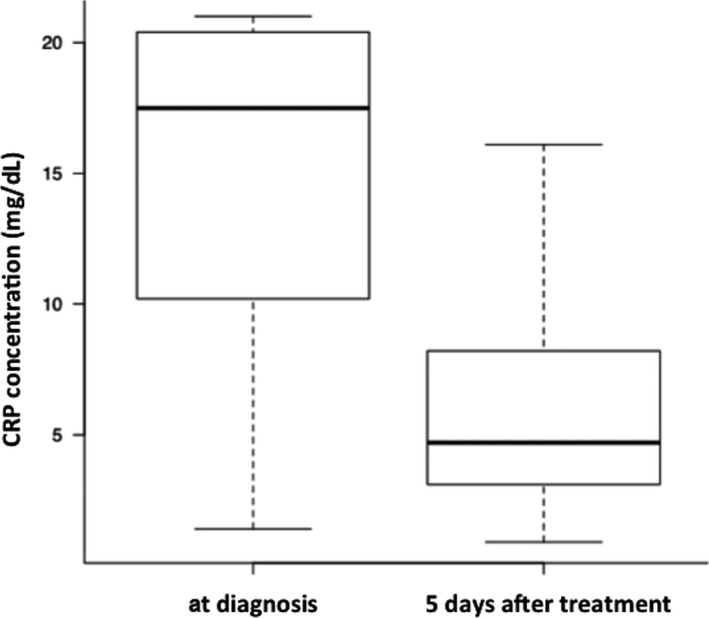
Comparison of CRP concentrations at the time of diagnosis and after treatment in dogs with acute pancreatitis. CRP concentration differs significantly at the time of diagnosis and 5 days after treatment (P < .05). Box plots show the median, range, and 25th and 75th quartiles for CRP concentration.
Discussion
Severe pancreatitis in dogs has been reported to be associated with thrombocytopenia, hypovolemia due to dehydration, anemia, hypoalbuminemia, hypocalcemia, azotemia, and increased activities of hepatic enzymes and concentrations of CRP.1, 2, 19 However, these variables were not significantly different between the NP and AP groups in our study, indicating that these variables were not useful in the diagnosis of AP. It is also possible that none or only a few of our AP dogs had severe pancreatitis. On the other hand, activities of amylase and FDC lip were significantly different between the NP and AP groups, with higher sensitivities and specificities than previously reported.4 Area under the ROC curve also was sufficient, potentially indicating that these laboratory tests are useful for immediate measurement in the hospital.
Activities of amylase and lipase are influenced by azotemia and glucocorticoid administration. These factors may complicate the diagnosis of AP,22, 23 and activities of amylase and lipase have been found to be within the reference ranges in many dogs with severe or fatal pancreatitis.1, 24 In addition, multiple isozymes such as gastric lipase, hepatic lipase, and lipoprotein lipase are produced outside of the pancreas and are present in the circulating blood; lipase activity represents a sum of this activity. FUJIFILM (Tokyo, Japan) developed FDC lip, offering high specificity to measure pancreatic lipase. A good correlation between FDC lip activity and Spec‐cPL concentration already has been identified in dogs diagnosed with AP.18 Therefore, unlike previous measures of lipase activity, FDC lip activity should be very useful as an immediately obtainable diagnostic test for AP in the hospital. However, in our study, amylase activity provided higher sensitivity and specificity than previously reported.4 These results may be attributed to relatively small number of the cases with underlying factors affecting amylase activity, such as chronic kidney disease, hyperadrenocorticism, and glucocorticoid administration. The SNAP cPL™ test was not evaluated in our study. This omission is a limitation of our study, because SNAP cPL™ test currently is the best in‐house test to diagnose AP in dogs. The SNAP cPL™ test can provide immediate results in the hospital and does not require specific chemistry analyzers.15, 17 It should be included in future comparisons of the accuracy of tests for amylase or FDC lip activities or both.
Our study investigated the relationship between diagnostic tests including Spec‐cPL and duration of hospitalization. Only high ALT activity was associated with prolonged hospitalization, but these results showed no relationship with mortality outcomes during hospitalization. Activity of ALT does not represent a negative prognostic factor for AP.2 Increased ALT activity thus might be a factor associated simply with extended duration of hospitalization and not specifically with disease severity. However, the relevance of underlying or concurrent diseases and high ALT activity cannot be excluded. No associations were observed between duration of hospitalization and the variables most likely to be involved, such as concentrations of CRP and Spec‐cPL and activities of amylase and FDC lip. Previous reports have suggested that assessing the severity of AP using pancreatic enzyme activity potentially is inaccurate. In 1 report, the use of a severity score based upon organ system compromise was more accurate in determining the likelihood of mortality in AP.25 The CRP concentration appeared to be associated with disease severity. In another study, no relationship was observed between CRP concentration and outcome.20 However, within 2 days after the onset of clinical signs, serum CRP concentrations differed significantly between survivors and nonsurvivors.20 In our study, CRP concentrations were not reassessed within 2 days after the onset of clinical signs. C‐reactive protein concentration at the time of diagnosis of AP seems to be unrelated to duration of hospitalization and mortality outcomes during hospitalization in the primary care hospital.
A previous study documented that CRP concentrations were significantly lower on day 5 than on day 1 in dogs with AP, suggesting that CRP offers a useful laboratory measure for monitoring clinical progression and response to treatment.19 Similar results were obtained in our study. Because optimal methods for treating dogs with AP have yet to be established, monitoring CRP cannot be said to have useful therapeutic applications, but this biomarker may be useful for monitoring recovery. On the other hand, FDC lip was not useful as a biomarker for monitoring recovery. This observation may be attributed to the fact that the half‐life of lipase in dogs is 1–3 hours,26 and much shorter than the CRP half‐life of 162 hours.27 C‐reactive protein is an inflammatory marker, and lipase is released into the circulation because of damage and necrosis of the pancreas, thus, the pathophysiology of these 2 markers differs.
In previous studies, Miniature Schnauzers, Toy Poodles, Yorkshire Terriers, Cocker Spaniels, and Dachshunds have been reported to be at increased risk of pancreatitis.2, 22, 23 In our study, Miniature Dachshunds, Toy Poodles, and Yorkshire Terriers were relatively common in the AP group, but no significant breed differences were found with the NP group. Although the sample size was small, these breeds might be more common among dogs with clinical signs suggestive of AP rather than with actual AP.
Several limitations of the present investigation should be considered. This study classified the NP and AP groups in accordance with previous reports.3, 13 Although tissue biopsy is more useful than any other examination for diagnosing pancreatitis, pancreatic pathology can be regional, and the diagnosis could be missed on biopsy.11 Obtaining tissue biopsy in every case also could be difficult in primary care hospitals. Another limitation is that some dogs could have been incorrectly classified as having AP because ultrasound reports or images were not available for review in all dogs. In addition, our study was retrospective in design and the control group was not matched for age, treatment, or duration of hospitalization. The results for sensitivity and specificity might therefore be limited. Finally, not all laboratory tests were assessed at all time points, which could have affected the statistical power of this investigation.
In conclusion, FDC lip activity, when combined with clinical signs and clinical laboratory findings, seems to be a useful laboratory test for AP screening. This test also offers the advantage that results can be obtained immediately in the hospital. However, future studies must investigate whether the accuracy of FDP lip activity is equal to or superior to that of SNAP cPL™ test. Increased ALT activity may be linked to extended duration of hospitalization. C‐reactive protein might be a useful biomarker for monitoring recovery from AP 5 days after treatment. To the best of our knowledge, ours is the first study to analyze data from a primary care hospital with a focus on diagnostic accuracy for dogs with AP.
Acknowledgments
We acknowledge the assistance of Dr K. Kojima with the statistical analysis.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Footnotes
SNAP cPL™; IDEXX Laboratories, North Grafton, MA
FUJI DRI‐CHEM 7000V; FUJIFILM Corporation, Tokyo, Japan
FUJI DRI‐CHEM lipase slides; FUJIFILM Corporation, Tokyo, Japan
Spec‐cPL assay; IDEXX Laboratories, Tokyo, Japan
References
- 1. Hess RS, Saunders HM, Van Winkle TJ, et al. Clinical, clinicopathologic, radiographic, and ultrasonographic abnormalities in dogs with fatal acute pancreatitis: 70 cases (1986‐1995). J Am Vet Med Assoc 1998;213:665–670. [PubMed] [Google Scholar]
- 2. Pápa K, Máthé A, Abonyi‐Tóth Z, et al. Occurrence, clinical features and outcome of canine pancreatitis (80 cases). Acta Vet Hung 2011;59:37–52. [DOI] [PubMed] [Google Scholar]
- 3. Chartier MA, Hill SL, Sunico S, et al. Pancreas‐specific lipase concentrations and amylase and lipase activities in the peritoneal fluid of dogs with suspected pancreatitis. Vet J. 2014;201:385–389. [DOI] [PubMed] [Google Scholar]
- 4. Steiner JM. Diagnosis of pancreatitis. Vet Clin North Am Small Anim Pract. 2003;33:1181–1195. [DOI] [PubMed] [Google Scholar]
- 5. Mansfield C. Acute pancreatitis in dogs: advances in understanding, diagnostic, and treatment. Top Companion Anim Med. 2012;27:123–132. [DOI] [PubMed] [Google Scholar]
- 6. Simpson KW, Batt RM, McLean L, et al. Circulating concentrations of trypsin‐like immunoreactivity and activities of lipase and amylase after pancreatic duct ligation in dogs. Am J Vet Res 1989;50:629–632. [PubMed] [Google Scholar]
- 7. Whitney MS. The laboratory assessment of canine and feline pancreatitis. Vet Med 1993;85:1045–1052. [Google Scholar]
- 8. Steiner JM, Williams DA. Development and validation of a radioimmunoassay for the measurement of canine pancreatic lipase immunoreactivity in serum of dogs. Am J Vet Res 2003;64:1237–1241. [DOI] [PubMed] [Google Scholar]
- 9. Steiner JM, Teague SR, Williams DA. Development and analytic validation of an enzyme‐linked immunosorbent assay for the measurement of canine pancreatic lipase immunoreactivity in serum. Can J Vet Res 2003;67:175–182. [PMC free article] [PubMed] [Google Scholar]
- 10. Mansfield CS, Anderson GA, O'Hara AJ. Association between canine pancreatic‐specific lipase and histologic exocrine pancreatic inflammation in dogs: assessing specificity. J Vet Diagn Invest 2012;24:312–318. [DOI] [PubMed] [Google Scholar]
- 11. Newman SJ, Steiner JM, Woosley K, et al. Histologic assessment and grading of the exocrine pancreas in the dog. J Vet Diagn Invest 2006;18:115–118. [DOI] [PubMed] [Google Scholar]
- 12. Watson PJ, Roulois AJ, Scase T, et al. Prevalence and breed distribution of chronic pancreatitis at post‐mortem examination in first‐opinion dogs. J Small Anim Pract 2007;48:609–618. [DOI] [PubMed] [Google Scholar]
- 13. Steiner JM, Newman S, Xenoulis P, et al. Sensitivity of serum markers for pancreatitis in dogs with macroscopic evidence of pancreatitis. Vet Ther. 2008;9:263–273. [PubMed] [Google Scholar]
- 14. Huth SP, Relford R, Steiner JM, et al. Analytical validation of an ELISA for measurement of canine pancreas‐specific lipase. Vet Clin Pathol 2010;39:346–353. [DOI] [PubMed] [Google Scholar]
- 15. Beall MJ, Cahill R, Pigeon K, et al. Performance validation and method comparison of an in‐clinic enzyme‐linked immunosorbent assay for the detection of canine pancreatic lipase. J Vet Diagn Invest 2011;23:115–119. [DOI] [PubMed] [Google Scholar]
- 16. Trivedi S, Marks SL, Kass PH, et al. Sensitivity and specificity of canine pancreas‐specific lipase (cPL) and other markers for pancreatitis in 70 dogs with and without histopathologic evidence of pancreatitis. J Vet Intern Med 2011;25:1241–1247. [DOI] [PubMed] [Google Scholar]
- 17. McCord K, Morley PS, Armstrong J, et al. A multi‐institutional study evaluating the diagnostic utility of the spec cPL™ and SNAP® cPL™ in clinical acute pancreatitis in 84 dogs. J Vet Intern Med 2012;26:888–896. [DOI] [PubMed] [Google Scholar]
- 18. Ishioka K, Hyakawa N, Nakamura K, et al. Patient‐side assay of lipase activity correlating with pancreatic lipase immunoreactivity in the dog. J Vet Med Sci 2011;73:1481–1483. [DOI] [PubMed] [Google Scholar]
- 19. Holm JL, Rozanski EA, Freeman LM, et al. C‐reactive protein concentrations in canine acute pancreatitis. J Vet Emerg Crit Care. 2004;14:183–186. [Google Scholar]
- 20. Mansfield CS, James FE, Robertson ID. Development of a clinical severity index for dogs with acute pancreatitis. J Am Vet Med Assoc 2008;233:936–944. [DOI] [PubMed] [Google Scholar]
- 21. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013;48:452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cook AK, Breitschwerdt EB, Levine JF, et al. Risk factors associated with acute pancreatitis in dogs: 101 cases (1985‐1990). J Am Vet Med Assoc 1993;203:673–679. [PubMed] [Google Scholar]
- 23. Hess RS, Kass PH, Shofer FS, et al. Evaluation of risk factors foratal acute pancreatitis in dogs. J Am Vet Med Assoc 1999;214:46–51. [PubMed] [Google Scholar]
- 24. Strombeck DR, Farver T, Kaneko JJ. Serum amylase and lipase activities in the diagnosis of pancreatitis in dogs. Am J Vet Res 1981;42:1966–1970. [PubMed] [Google Scholar]
- 25. Ruaux CG, Atwell RB. A severity score for spontaneous canine acute pancreatitis. Aust Vet J 1998;76:804–808. [DOI] [PubMed] [Google Scholar]
- 26. Hudson EB, Strombeck DR. Effects of functional nephrectomy on the disappearance rates of canine serum amylase and lipase. Am J Vet Res 1978;39:1316–1321. [PubMed] [Google Scholar]
- 27. Kuribayashi T, Seita T, Momotani E, et al. Elimination Half‐Lives of acute phase proteins in rats and beagle dogs during acute inflammation. Inflammation 2015;38:1401–1405. [DOI] [PubMed] [Google Scholar]


