Abstract
An update on the 2005 American College of Veterinary Internal Medicine (ACVIM) Consensus Statement on blood donor infectious disease screening was presented at the 2015 ACVIM Forum in Indianapolis, Indiana, followed by panel and audience discussion. The updated consensus statement is presented below. The consensus statement aims to provide guidance on appropriate blood‐borne pathogen testing for canine and feline blood donors in North America.
Keywords: Blood donor testing, Transfusions
Abbreviations
- BAPGM
Bartonella alpha proteobacteria growth medium
- FeLV
feline leukemia virus
- FIV
feline immunodeficiency virus
- IFA
immunofluorescent antibody
- PCR
polymerase chain reaction
- RSAT
rapid slide agglutination test
- WNV
West Nile virus
A blood or blood component transfusion generally is a life‐saving measure, but absolute safety can never be guaranteed. In addition to immune‐mediated reactions caused by infusion of allogeneic cells or proteins, blood‐borne pathogens can be transmitted by transfusion, potentially causing disease in the transfused recipient. In an effort to minimize pathogen transmission, all blood donors should be appropriately screened for infectious agents.
The following recommendations are based on the information available at the time of this writing. For clarity, the consensus panel subdivided pathogens into the following categories for the dog and cat:
Vector‐borne pathogens—testing recommended
Non vector‐borne pathogens—testing recommended
Other pathogens—testing not recommended
Pathogens for which testing is recommended met at least three of the following criteria: (1) the pathogen has been documented to cause clinical infection in recipients after blood transmission, (2) the pathogen is capable of causing subclinical infection such that carriers might inadvertently be identified as healthy blood donors, (3) the pathogen can be detected using culture or molecular methods from the blood of an infected animal, and (4) the resultant infection in the recipient has the potential to cause life‐threatening illness and be difficult to eliminate with antimicrobial drugs. Using optimal standards (Tables 1 and 2, see below), testing also is recommended for those pathogens that can be experimentally transmitted by blood transfusion, even though clinical illness after transfusion has not been described.
Table 1.
Recommendations for screening of canine blood donors for blood‐borne pathogens
| Agenta | Optimal Standardsb | Minimal Standards | Comments |
|---|---|---|---|
| Vector‐borne pathogens—testing recommended | |||
| Anaplasma phagocytophilum | Seronegative and PCR negative dogs | PCR negative dogs. Seronegative dogs are an acceptable alternative if serologic testing is more economical or yields more rapid turnaround time than PCR. | In areas endemic for Ixodes spp., identification of seronegative donors may be difficult. Therefore, use of seropositive but PCR negative dogs as donors is considered acceptable in this situation. Seronegative dogs are rarely PCR positive and so serological testing alone could be considered if serologic testing is more economical or yields more rapid turnaround time than PCR. |
| Anaplasma platys | Seronegative and PCR negative dogs | PCR negative dogs. Seronegative dogs are an acceptable alternative if serologic testing is more economical or yields more rapid turnaround time than PCR. | In areas endemic for Rhipicephalus tick spp., identification of seronegative donors may be difficult. Therefore, use of seropositive but PCR negative dogs as donors is considered acceptable in this situation. Seronegative dogs are rarely PCR positive and so serological testing alone could be considered if serologic testing is more economical or yields more rapid turnaround time than PCR. Not all serological assays are known to detect A. platys antibodies and so the minimal standard is the PCR. |
| Babesia canis vogeli | Seronegative and PCR negative, especially in high risk dogs | PCR negative | High risk dogs include greyhounds and those with a history of exposure to Rhipicephalus ticks. |
| Babesia gibsoni | Seronegative and PCR negative, especially in high risk dogs | PCR negative | High risk dogs include pitbull terriers and donors that have had a history of aggressive interactions with pitbull terriers. |
| Other Babesia spp. | PCR negative dogs | PCR negative dogs or no screening | Serology is not available; distribution is limited and so screening could be considered optional. |
| Bartonella henselae | Seronegative and BAPGM culture‐PCR negative dogs | PCR negative dogs | Serology is negative in over 50% of clinical cases and should not be used alone for screening. PCR without BAPGM culture enrichment is insensitive for detection of Bartonella bacteremia in dogs, but the overall prevalence of infection in dogs is low. When testing with BAPGM culture‐PCR is not practical because of expense and/or turnaround time, either serology combined with PCR or PCR alone could be considered. |
| Bartonella vinsonii var. berkhoffi | Seronegative and BAPGM culture‐PCR negative dogs | PCR negative dogs | See Bartonella henselae |
| Other Bartonella spp. | BAPGM culture‐PCR negative dogs | No screening | Serologic assays are species‐specific and are not available for many species; most are not as prevalent as B. henselae or B. vinsonii and their pathogenicity is less well established. |
| Ehrlichia canis | Seronegative and PCR negative dogs | Seronegative dogs or PCR negative dogs | All donors should be screened. Seronegative dogs are rarely PCR positive and so serological testing alone could be considered if serologic testing is more economical or yields more rapid turnaround time than PCR. In contrast to A. phagocytophilum, seropositive dogs should not be used as donors, as E. canis is a significant pathogen and PCR assays are insensitive for ruling out the presence of infection in chronically infected dogs. |
| Ehrlichia chaffeensis | Seronegative and PCR negative dogs | PCR negative in dogs from high risk areas; no screening in low risk areas | High risk areas are the southeastern United States and the mid‐Atlantic states. Not all serological assays are known to detect antibodies to E. chaffeensis. |
| Ehrlichia ewingii | Seronegative and PCR negative dogs | Seronegative dogs or PCR negative dogs in high risk areas; no screening in low risk areas | High risk areas are those endemic for Amblyomma americanum ticks. Not all serological assays are known to detect antibodies to E. ewingii. |
| Hepatozoon canis/americanum | PCR negative dogs | No screening | Serologic assays are not available for routine diagnosis in the United States. Testing using PCR is strongly recommended in endemic regions (south‐eastern and south‐central United States). Natural transmission requires ingestion of an infected tick; transmission by blood transfusion has not been documented. |
| Leishmania donovani | Seronegative and PCR negative | Seronegative and PCR negative in high risk dogs; no screening in low risk dogs | High‐risk dogs include foxhounds, foxhound/crosses, or dogs living in or traveling to endemic areas. |
| Mycoplasma haemocanis | PCR negative dogs | PCR negative dogs | Serologic assays are not available. Cytologic examination of blood smears is not accurate. The organism can be a primary pathogen and so PCR screening is recommended. |
| “Candidatus Mycoplasma haematoparvum” | PCR negative dogs | No screening | Serologic assays are not available. Cytologic examination of blood smears is not accurate. The organism is not considered a primary pathogen and so screening could be considered optional. |
| Neorickettsia risticii | PCR negative dogs | No screening | Serologic assays are not available. The organism has only rarely been detected in dogs. |
| Rickettsia felis | PCR negative dogs | No screening | Serologic assays are not available. While R. felis has been detected in the blood of dogs with heavy flea infestations, it has not been associated with disease in dogs and so screening could be considered optional. |
| Trypanosoma cruzi | Seronegative dogs | No screening | Transfusion‐related infections have not been reported in dogs and so screening could be considered optional. Screening is primarily recommended in endemic areas (southern United States, primarily southeastern Texas) |
| Non vector‐borne pathogens—testing recommended | |||
| Brucella canis | Seronegative dogs | No screening | A single negative serology result is considered sufficient in neutered donors, but screening should be repeated in sexually active dogs. Healthy neutered dogs that are not from a kennel and without a breeding history are unlikely to be exposed. |
| Other pathogens—testing not recommended | |||
| Borrelia burgdorferi | No screening | No screening | Transfusion‐related infections not reported |
| Neorickettsia helminthoeca | No screening | No screening | Neorickettsia helminthoeca has not been documented to cause persistent subclinical infections and so is not likely to be transfused from a healthy dog. |
| Rickettsia rickettsii | No screening | No screening | Rickettsia rickettsii has not been documented to cause persistent subclinical infections and so is not likely to be transfused from a healthy dog. |
| West Nile virus | No screening | No screening | No persistent infections; no transfusion‐related infections described. |
See the text for further discussion of geographic distribution and risk factors.
See the text for further discussion of specific tests.
Table 2.
Recommendations for screening of feline blood donors for blood‐borne pathogens
| Agenta | Optimal Standards | Minimal Standardsb | Comments |
|---|---|---|---|
| Vector‐borne pathogens—testing recommended | |||
| Anaplasma phagocytophilum | Seronegative and PCR negative cats | PCR negative cats. Seronegative cats are an acceptable alternative if serologic testing is more economical or yields more rapid turnaround time than PCR. | Seropositive, PCR‐negative cats may be used in endemic regions if no other suitable donor can be identified. |
| A. platys | PCR negative cats | No screening | There is no valid serological assay for cats. Infection of cats has only been occasionally documented. |
| Bartonella henselae | Seronegative and PCR or culture negative cats | PCR negative cats | Around 70% of seropositive cats are PCR negative. In endemic areas, finding seronegative cats can be difficult and so use of seropositive, PCR negative cats may be needed. |
| Other Bartonella spp. | PCR negative cats | No screening | Serologic assays are species‐specific, and assays are not readily available for many species. B. henselae appears to be the most pathogenic species. |
| Cytauxzoon felis | PCR negative cats | No screening | Serology is not available. Testing using PCR is strongly recommended for cats with access to the outdoors that reside in endemic regions; cytologic examination of blood smears is not accurate. |
| Ehrlichia canis and E. canis‐like | PCR negative cats | No screening | Infection of cats is extremely rare |
| Mycoplasma haemofelis | PCR negative cats | PCR negative cats | Serologic assays are not available. Cytologic examination of blood smears is not accurate. The organism is a major primary pathogen and so PCR screening is always optimal. |
| “Candidatus Mycoplasma haemominutum” | PCR negative cats | No screening | Serologic assays are not available. Cytologic examination of blood smears is not accurate. The organism is not considered a primary pathogen and is highly prevalent in the cat population, so screening could be considered optional. |
| “Candidatus Mycoplasma turicensis” | PCR negative cats | No screening | Serologic assays are not available. “Candidatus M. turicensis” has never been detected using cytologic examination of blood smears, and cytology is not accurate for identification of hemoplasmas. The organism is not considered a primary pathogen and so screening could be considered optional. |
| Neorickettsia risticii | PCR negative cats | No screening | Serology is not available. The organism has only rarely been associated with infection in cats |
| Non vector‐borne pathogens—testing recommended | |||
| Feline leukemia virus | Antigen negative and proviral DNA PCR negative cats | Antigen negative cats | Clinically validated proviral DNA assays are not routinely available in the United States. |
| Feline immunodeficiency virus | Antibody negative cats | Antibody negative cats | It is currently not possible to accurately differentiate between an infected cat and an FIV‐vaccinated cat and so all positive cats should be excluded as donors. |
| Other pathogens—testing not recommended | |||
| Feline coronavirus | No screening | No screening | No documentation of virus transmission by blood transfusion. |
| Rickettsia felis | No screening | No screening | While seropositive cats have been detected, the organism has not been found in the blood of cats in the United States. |
| Toxoplasmosis | No screening | No screening | No documentation of virus transmission by blood transfusion. |
See the text for further discussion of geographic distribution and risk factors.
See the text for further discussion of specific tests.
The panel separated screening recommendations into optimal and minimal standards, which are included in the text and in Tables 1 and 2. These recommendations were made using available evidence from human and veterinary medical literature, and, where evidence was lacking, the combined opinions and clinical experiences of the panel members were used to develop recommendations. The goal of the optimal standards is to minimize risk to the best of our ability by application of currently available diagnostic tests. However, the panel acknowledged that application of all diagnostic tests might not be relevant for all geographic locations and donor backgrounds (eg, breed, environment) and some diagnostic tests have limited availability or could be cost prohibitive for some programs. Therefore, the minimal standards were developed taking into account these factors. In some cases, this approach unfortunately could result in movement of infected animals into the donor pool. The panel also discussed alternative acceptable strategies for geographic regions where the prevalence of infection may be high and identification of suitable donors is difficult as well as screening of potential donors when blood is required in an emergency situation and time does not permit thorough screening before donation. An apparently healthy donor may be acceptable in that situation given the low risk of transmission of infection when weighed alongside a high risk of death of the recipient in the absence of blood product transfusion. However, pre‐emptive identification and screening of healthy blood donors remains an important strategy of safe blood banking.
In human blood collections, individual units of whole blood collected for transfusion purposes typically are screened for infectious agents. By contrast, economic factors in veterinary medicine often limit testing to the blood donor animals themselves. The consensus panel recommends a minimum of yearly testing of blood donors, with consideration of more frequent retesting for some pathogens in endemic areas and in donors with repeated exposure to risk factors (eg, tick exposure). The consensus panel agreed that prevention of infections by proper handling and storage of blood products techniques also should be considered, recommendations for which are included in the consensus statement.
General Comments on Infectious Agent Screening
Screening of blood donors should always follow a thorough history and physical examination to evaluate for factors that may make the animal a poor blood donor choice (see donor selection and care section). No tests for infectious agents have 100% analytical or clinical sensitivity and specificity. When screening for blood‐borne pathogens in a potential blood donor, the test or tests with the greatest analytical sensitivity should be used. However, in situations where the prevalence of infection is low (as is the case for some pathogens in healthy animals compared with sick animals), positive results are more likely to represent false positives than in regions of high prevalence (low positive predictive value), and consideration should be given to verifying such positive results with a second test, preferably using a reference laboratory. Also, testing decisions should be based on the value of individual tests for a given pathogen, rather than choosing tests simply because they are present in blood donor panels offered by commercial laboratories.
Following is a brief discussion of the basic utility and limitations of these tests:
Organism or Antigen Tests
Light Microscopy
Documentation of an infectious agent in blood smears by cytologic examination requires skilled personnel, is time consuming (an adequate blood film examination can take 20–30 minutes), and lacks sensitivity for most pathogens. False positives can also occur, such as when staining artifacts are confused with micro‐organisms. Therefore, cytologic examination of blood smears is not recommended as the sole means of screening blood donors for infection.
Culture
Positive blood culture results indicate the presence of cultivable bacteria in the blood. Although transient bacteremia can occur in healthy animals after disruption of mucosal barriers, transfusion of blood from animals with transient bacteremia has not been documented to cause disease in a recipient. Therefore, routine blood culture generally is not indicated for screening potential blood donors, with rare exceptions (see Bartonella section). In transfusion medicine, routine blood culture is more appropriate for screening individual units of blood for bacteria if contamination is suspected.
Serum Antigen Tests
Assays that detect antigens of several blood‐borne pathogens in whole blood or serum are commercially available. Dirofilaria immitis (dogs and cats) and feline leukemia virus (FeLV) antigen tests are used most frequently for donor screening and health assessment. Point‐of‐care tests are available for both organisms, and the potential for inaccurate results from operator error is small.
Molecular Assays
Because the immune system generally clears nonviable microbes quickly, amplification of specific microbial nucleic acids using assays like polymerase chain reaction (PCR) generally indicates the presence of viable microbes, provided laboratory quality assurance is high. These techniques can provide high analytic sensitivity and specificity and the potential to rapidly test for more than 1 pathogen. Disadvantages include the current lack of point‐of‐care nucleic acid‐based assays in veterinary medicine; lack of standardization of assays among laboratories, which results in variable sensitivities and specificities; lack of assay availability for some infectious agents; and expense. Also, high analytical sensitivity of a PCR assay does not necessarily imply high clinical sensitivity. In other words, an assay may detect minute quantities of DNA in the laboratory but have poor sensitivity for detection of a pathogen in a blood specimen. Most PCR assays utilize 10–200 μL of blood, and animals can receive over 10,000 times that volume during a transfusion. Although most molecular assays have high analytical sensitivity, these assays cannot amplify microbes that are not in the specimen collected; thus, false negative results can occur with some agents found in very low quantities in the bloodstream, such as Ehrlichia canis and feline immunodeficiency virus (FIV).
Animals that have overt microscopic evidence of infection, are culture‐positive, are antigen‐positive, or are positive by PCR assay for clinically relevant blood‐borne pathogens should be excluded from the donor pool. Whether some pathogens that cause chronic, persistent infections can be eliminated by antimicrobial therapy is not certain, and animals with a history of previous positive test results for these pathogens should not be used as blood donors.
Serum Antibody Tests
Positive serum antibody assay results suggest previous infection with the pathogen in question but do not prove current infection. Negative results of antibody testing generally suggest lack of infection, but serum antibodies can be undetectable even in the presence of an active infection. This situation is likely most common when the antibody test is performed during the acute stage of infection, which is well documented with several vector‐borne pathogens (see Ehrlichia and Anaplasma sections). Other agents, such as Bartonella, are stealth organisms that evade the immune system and may not induce detectable serum antibodies. Severely immunocompromised animals also may fail to mount a specific antibody response (eg, cats with advanced FIV infection). These dogs and cats generally are ill, and hopefully would be excluded from the donor pool based on other findings.
Point‐of‐care assays available for detection of the antibody response to some pathogens have the advantages of being rapid and inexpensive, and the potential for operator error with most assays is small. No standardization of serological tests offered by commercial laboratories for infectious agents is available. Differences in antigen, antigen preparation, reagents, and protocols can influence the results of serological assays among laboratories and point‐of‐care assays. In addition, inherent subjectivity in interpretation of immunofluorescent antibody (IFA) assays can be problematic.
For some pathogens, a combination of both serological and organism demonstration techniques (cytology, culture, PCR) may be required to maximize diagnosis of infection.1
Screening of Blood Donors for Blood‐borne Pathogens
Canine Blood‐borne Pathogens
Vector‐borne Pathogens—Testing Recommended
Dirofilaria immitis does not meet the criteria used to categorize other vector‐borne pathogens because transfusion of microfilaria from an infected donor cannot lead to heartworm disease in the recipient. However, filaremic blood transfused to a recipient has the potential to interfere with diagnostic testing, can be infectious to mosquito vectors, and can carry Wolbachia spp.2 In addition, a donor infected with D. immitis would not be considered a healthy donor, and collection of large amounts of blood from such a donor could be unsafe. Therefore, it is recommended that dogs and cats to be used as blood donors in heartworm endemic areas be screened for D. immitis infection and placed on heartworm prophylaxis.
Anaplasma spp. A. phagocytophilum and A. platys are the causative agents of canine granulocytic anaplasmosis and infectious canine cyclic thrombocytopenia, respectively. Transmission of A. phagocytophilum occurs via Ixodes scapularis and Ixodes pacificus ticks in the United States. Widespread subclinical infections followed by pathogen clearance appear common in both humans and dogs. A further pathway for transmission is via infected blood, either experimentally or by blood transfusion.3 In human medicine, several reports of transfusion‐transmission of A. phagocytophilum via different blood products (non‐leukoreduced/leukoreduced RBCs, leukoreduced platelets) and also transfusion‐transmitted granulocytic anaplasmosis have been documented.4, 5 Widespread subclinical infections followed by pathogen clearance appear common in both humans and dogs, and in immunocompromised or elderly people such infections can cause severe disease. Donation screening or inactivation by pathogen reduction technologies is considered in human medicine.5 Anaplasmosis occurred in a splenectomized dog on chemotherapy after a packed RBC transfusion; both the donor and recipient tested positive by PCR (Kohn unpublished data). PCR positive dogs can be seronegative and can have clinical and hematologic variables within reference intervals.6, 7 Antibodies to Anaplasma species can be detected using IFA assays, automated fluorescence‐based systems, a point‐of‐care lateral flow ELISA assay,1 or laboratory‐based ELISA assays.2,7, 8 Serologic cross‐reactivity among Anaplasma species occurs in some assays, but not all (Table 3). The seroprevalence (IFA or ELISA) is high in endemic areas (up to 50%) and antibody titers may persist for several months or even years.6, 9
Table 3.
Laboratoriesa offering point of care or laboratory‐based assays for potential use in screening blood donors
| Laboratory | Services |
|---|---|
|
Abaxis 510‐675‐6500 www.abaxis.com/veterinary/products/rapid-tests.html |
Several serological assays, including some for point‐of‐care |
|
Animal Blood Resources International (ABRI) 1‐800‐243‐5759 www.abrint.net/ |
Blood donor typing PCR assays for some blood‐borne agents Laboratory‐based serological assays for some blood‐borne agents |
|
ANTECH Diagnostics 1‐800‐745‐4725 (West) 1‐800‐872‐1001 (East) www.antechdiagnostics.com/main/TestingServices.aspx |
PCR assays for some blood‐borne agents Laboratory‐based serological assays for some blood‐borne agents |
|
Biogal http://www.biogal.co.il/products/immunocomb/pet-technical-information |
Ehrlichia canis point‐of‐care antibody assay |
|
Specialized Infectious Diseases Laboratory Colorado State University www.dlab.colostate.edu |
PCR panel and individual assays for some blood‐borne agents Combination feline Bartonella spp. PCR and serology Laboratory‐based serological assays for some blood‐borne agents |
|
Galaxy Diagnostics, Inc. 919‐313‐9672 http://www.galaxydx.com/web/animal-health/ |
Laboratory‐based Bartonella spp. serology for dogs and cats. PCR assay for Bartonella spp. BAPGM culture for Bartonella spp. Combination of serology, PCR, and culture for dogs or cats |
|
IDEXX Laboratories 1‐800‐548‐6733 https://www.idexx.com/smallanimal/reference-laboratories/directory-tests-services.html |
PCR assays for some blood‐borne agents Laboratory‐based serological assays for some blood‐borne agents Point‐of‐care serological assays for some blood‐borne agents |
|
Michigan State University Diagnostic Center for Population and Animal Health 517‐353‐1683 http://www.animalhealth.msu.edu |
Blood typing PCR assays for some blood‐borne agents Laboratory‐based serological assays for some blood‐borne agents |
|
National Veterinary Laboratories 201‐891‐2992 http://www.natvetlab.com/ |
Laboratory‐based serological assays for some blood‐borne agents |
| New York State Veterinary Diagnostic Laboratory/Animal Health Diagnostic Center 607‐253‐3900 https://ahdc.vet.cornell.edu/ |
PCR assays for some blood‐borne agents Laboratory‐based serological assays for some blood‐borne agents |
|
North Carolina State University Vector Borne Disease Laboratory 919‐513‐8279 http://www.cvm.ncsu.edu/vhc/csds/ticklab.html |
PCR panel and individual assays for some blood‐borne agents Combination Bartonella spp. serology and PCR/culture for dogs and cats Laboratory‐based serological assays for some blood‐borne agents |
|
Protatek Reference Laboratory 480‐545‐8499 http://www.protatek.com/ref_services.html |
PCR assays for some blood‐borne agents Laboratory‐based serological assays for some blood‐borne agents |
|
Real‐time PCR Research and Core Diagnostics Facility, University of California, Davis, CA 530‐752‐7991 http://www.vetmed.ucdavis.edu/vme/taqmanservice/ |
PCR assays for some blood‐borne agents Laboratory‐based serological assays for some blood‐borne agents |
|
Zoetis 888‐963‐8471 https://www.zoetisus.com/products/index.aspx |
Point‐of‐care serological assays for some blood‐borne agents |
|
Zoologix 818‐717‐8880 http://www.zoologix.com/dogcat/index.htm |
PCR panel and individual assays for some blood‐borne agents |
The laboratories selected for inclusion either provide standard operating procedures for critical review, have published peer reviewed articles documenting quality control and assay specifics, are standard operating procedures monitored state‐accredited laboratories, are laboratories producing kits licensed by the USDA, or are laboratories that panel members have worked with directly providing evidence of quality assurance.
This is not meant as an exhaustive list and many other laboratories, especially state accredited laboratories, also provide quality laboratory services.
Direct comparisons among different tests for infectious disease agents are generally not available unless published. See the reference list of the main document.
The extent to which A. phagocytophilum can persist in tissues and contribute to chronic disease manifestations in humans and dogs currently is unknown. In 1 study, treatment of experimentally infected dogs with prednisolone up to 6 months after infection was followed by development of positive PCR results for the organism, and in some dogs, thrombocytopenia and reappearance of morulae on blood smears.10 In another study, dogs infected with A. phagocytophilum by exposure to wild‐caught Ixodes scapularis ticks were PCR positive for at least 12 weeks.7 In light of the above information, the panel recommends that optimal standards are to screen donors using both serology and PCR, and dogs that test positive with 1 or both assays should be excluded. Exclusion of all seropositive dogs might limit the donor pool in endemic areas (see comments in Table 1).
Anaplasma platys is thought to be transmitted by Rhipicephalus sanguineus, and infections are common in regions endemic for this tick. Anaplasma platys can establish a chronic, persistent subclinical infection, sometimes accompanied by mild thrombocytopenia.11 Dogs infected experimentally with blood developed severe thrombocytopenia within 7 days after inoculation.12 Transfusion‐transmitted infection or disease has been reported neither in humans nor in dogs. An IFA assay for detection of serum antibodies is commercially available, but cross‐reactions occur with A. phagocytophilum.13 Species‐specific PCR testing of blood samples is the diagnostic method of choice. Assays for A. phagocytophilum antibodies may or may not detect A. platys antibodies (Table 3).7 Donor dogs that are negative for antibodies and negative using species‐specific PCR are optimal (Table 1).
Babesia spp
Babesiosis is caused by organisms of the genus Babesia. Babesia (canis) vogeli and Babesia gibsoni are the most common species diagnosed in North American dogs. Several other Babesia spp. have been identified in dogs in North America (Babesia sp. Coco and Babesia conradae) and other parts of the world (B. canis, B. rossi, B. microti‐like and un‐named Babesia sp.). Transmission of Babesia spp. by transfusion is well documented in both humans14, 15 and dogs.16, 17 The resulting disease in dogs can be peracute, acute, chronic, or subclinical. A high seroprevalence of B. canis occurs in greyhounds,18 and an increased prevalence of B. gibsoni occurs in American pit bull terriers and American Staffordshire terriers, as detected by PCR.19, 20 Optimal screening should include serology against B. vogeli and B. gibsoni and broad range PCR screening (ie, that which detects all known Babesia spp. that infect dogs). Minimal screening may include broad‐range PCR screening only. For all “pit bull” type dogs, Greyhounds, dogs with known tick exposure or dogs with bite exposure from a “pit bull” type dog, 1 additional PCR screening should be considered beyond the optimal recommendations to enhance sensitivity.
Bartonella spp
Dogs can be infected by several Bartonella spp., most of which are proven or suspected to be vectored by fleas or, potentially, ticks.21 Bartonella vinsonii subspecies berkhoffi and B. rochalimae were the most common species amplified from dogs and their fleas in a shelter in Florida.22 Bartonella henselae, which is most common in cat blood and Ctenocephalides felis collected from cats, also has been grown or amplified from the blood of dogs.23 Although Bartonella spp. transmission has not been documented by blood transfusion in a client‐owned dog, clinically ill dogs that are positive for Bartonella spp. have been detected, healthy dogs can harbor Bartonella spp., and the organisms can be transmitted by inoculation.24 Thus, it is plausible that Bartonella spp. could be transmitted by blood transfusion and result in clinical illness, and the panel therefore believes testing is indicated for this genus.
Validated serological assays, PCR assays, and culture are available for both B. henselae and B. vinsonii subspecies berkhoffi, and these are the most likely pathogens in dogs (Table 3). The most sensitive way to document Bartonella spp. in the blood of dogs is by the concurrent use of specialized culture media (Bartonella alpha Proteobacteria Growth Medium [BAPGM]) and PCR assay in multiple blood samples, and this combination of tests will detect all known Bartonella spp. of dogs.25 However, BAPGM‐PCR can be negative even in infected dogs if only 1 sample is tested. The panel believes the optimal standard is to use B. henselae and B. vinsonii subspecies berkhoffi seronegative and BAPGM‐PCR negative dogs as donors (Table 1).
In 1 canine blood donor candidate study, although 18% of screened dogs were positive for a Bartonella spp., only 11% were seropositive.26 In addition, serological cross‐reactivity is variable among Bartonella spp. and assays are not routinely available for all species. Thus, the panel believes that Bartonella spp. serum antibody tests alone should not be used exclusively for assessment of dogs to be used as blood donors.
Several laboratories offer broad range PCR assays to amplify DNA of multiple Bartonella spp. or offer specific primers for B. henselae and B. vinsonii subspecies berkhoffi (Table 3). When testing with serology and BAPGM culture‐PCR is not practical because of expense, turnaround time, or both, the panel believes the minimal standard is to use dogs that are PCR negative for DNA of B. henselae and B. vinsonii subspecies berkhoffi.
Ehrlichia spp
Ehrlichia canis, E. ewingii, and E. chaffeensis are vector‐borne agents belonging to the family Rickettsiaceae. All are capable of causing disease in dogs.
Of these pathogens, E. canis is of greatest importance for blood donor screening because of its high prevalence worldwide and its propensity to cause chronic, persistent infections. Recent studies suggest that E. ewingii also is capable of causing persistent infection in dogs,27 but it has a more restricted geographic distribution. Experimentally, SC inoculation of E. canis results in dose‐dependent infection and positive blood cultures.28 Screening of potential donor dogs for antibodies to some Ehrlichia antigens can be performed by IFA assay, 1 of several commercial point‐of‐care assays,1,3,4 or other laboratory‐based assays.b Variable serologic cross‐reactivity occurs among some Ehrlichia species. In 1 of the point‐of‐care assays,a recombinant peptide antigens of E. canis and E. ewingii are combined together in a single spot, so a positive result reflects either seroreactivity to E. canis, E. ewingii, or both pathogens. The sensitivity and specificity of this assay compared to IFA have been reported.8 The clinical sensitivity and specificity of other available assays require further study. Because serological reactivity against E. canis, E. ewingii, and E. chaffeensis is variable and not known for all assays, and because infection can be documented by broad‐range PCR assay before seroconversion, the panel believes the optimal standard is to use donors that are seronegative and PCR negative (Table 1). This is most important for E. canis because it is the most important primary pathogen.
Hemoplasmas
Dogs can be infected with several hemoplasma species, including Mycoplasma haemocanis, “Candidatus M. haematoparvum,” and possibly also “Candidatus M. haemominutum” or a related organism.29, 30, 31 Although ticks have been implicated in transmission of M. haemocanis, the mechanism of transmission has not been proven. Kenneled dogs and research animals appear to be at higher risk for infection by M. haemocanis. Diagnosis of infection is based on PCR assay of whole blood.32 No serologic assay for hemoplasma infection is commercially available. In general, dogs are subclinically infected with these organisms, but M. haemocanis can cause anemia in splenectomized dogs, with a few case reports of infected dogs with other immunocompromising comorbidities. Only a single clinical infection with “Candidatus M. haematoparvum” has been reported in a splenectomized dog with hemic neoplasia being treated with chemotherapy, and it was unclear to what extent the hemoplasma played a role in development of anemia.33 This dog received several units of blood products, some of which tested positive using PCR for “Candidatus M. haematoparvum,” and tested negative before transfusion (Sykes et al, unpublished data). Therefore, optimally, donor dogs should be screened for all hemoplasma by PCR assay and excluded if positive (Table 1). However, until more is learned about the risk of transfusing blood testing positive for “Candidatus M. haemominutum” and “Candidatus M. haematoparvum”, testing for these pathogens could be considered optional. It also should be kept in mind that the prevalence of hemoplasma infection in the general client‐owned pet dog population in North America appears to be low (<5%).34 Whether the viability of canine hemoplasma species is lost during storage of blood products (see feline hemoplasmas) requires further study. Because antimicrobial therapy does not reliably eliminate hemoplasmas, the panel does not recommend treating potential donors with antimicrobials in an attempt to eliminate infection.
Hepatozoon canis and Hepatozoon americanum
Hepatozoon canis and Hepatozoon americanum are tickborne protozoal pathogens that are transmitted primarily by ingestion of Rhipicephalus sanguineus or Amblyomma maculatum ticks, respectively.35 In North America, the distribution of these infections primarily is limited to the south‐central and south‐eastern United States, with occasional H. americanum infections identified in other states, including Washington, Vermont, California, and Nebraska.36 Most H. canis infections are subclinical. H. americanum can cause lethargy, fever, locomotory abnormalities, hyperesthesia, and protein‐losing nephropathy. The organisms circulate in the peripheral blood as gamonts in leukocytes. There are no reports of transmission by blood transfusion. Screening could be considered in endemic areas using specific PCR assays for H. canis or H. americanum. Serologic assays for routine diagnostic purposes are not available in North America.
Leishmania spp
Leishmaniosis is caused by protozoal organisms of the genus Leishmania and is transmitted in Mediterranean regions by the bite of an infected female sandfly. The vector in North America is not known; Lutzomyia shannoni is the most highly suspected vector in the United States, but dog‐to‐dog transmission also has been hypothesized.37 Visceral leishmaniosis, caused by Leishmania donovani, is considered an exotic disease in dogs in North America, with the exception of the foxhound population in which it is endemic.38 Dogs in North America also have acquired the infection during travel to foreign countries months to years before diagnosis. Visceral leishmaniosis has been transmitted by blood transfusion to dogs, with clinically healthy foxhounds as blood donors.39 A retrospective study (2000–2003) performed on 12,000 serum samples from foxhounds and other canids in the United States reported an 8.9% seroprevalence in foxhounds, but no other randomly selected domestic dogs or wild canids were seropositive.40 Because the infection appears to be only endemic within foxhounds in North America, screening of every potential blood donor is not necessary. However, all foxhounds and dogs with travel history to or from endemic countries should be screened for Leishmania spp infection using IFA serology performed by a reputable laboratory. Because IFA serology may lack sensitivity in subclinically infected dogs,41 foxhounds or dogs living in or traveling to endemic areas, and found to be seronegative should then be screened additionally by Leishmania PCR.38, 42, 43 The IFA assay for Leishmania spp. can cross‐react with Trypanosoma cruzi.44 Although dogs with either infection should be excluded as blood donors, Leishmania spp. seropositive dogs can be evaluated for the presence of specific antibodies to T. cruzi if further clinical information is desired.44
Neorickettsia risticii
Dogs can be experimentally infected with N. risticii, and antibodies to N. risticii have been detected in pet dogs.45, 46, 47 Polymerase chain reaction was used in 1 study to identify N. risticii obtained from blood cultures of 2 clinically ill dogs.48 There is no documentation of transmission of N. risticii to dogs by blood transfusion.45 Although the committee believes that optimally dogs should test PCR‐negative for Neorickettsia risticii infections, no screening is also acceptable.
Rickettsia felis
Rickettsia felis is a member of the spotted fever group rickettsiae in dogs in the United States. These agents are likely to induce serological cross reactivity in the R. rickettsii assay. It is currently assumed that these agents are not associated with illness in dogs but further study is needed. There is evidence that dogs are the reservoir for R. felis 49 and Ctenocephalides felis collected from cats in the United States are commonly positive for R. felis DNA.50 It is currently unknown whether transfusion of a large volume of blood from a R. felis carrier dog to an ill dog in need of a transfusion would have clinical sequelae. A PCR assay is the only way currently to prove R. felis infection in dogs. Although the panel believes that optimally dogs should test PCR‐negative for R. felis infections, no screening is also acceptable (Table 1).
Trypanosoma cruzi
American trypanosomiasis (Chagas disease) is caused by Trypanosoma cruzi, a hemoflagellate protozoan. Transmission most commonly occurs through a feces‐contaminated bite from, or ingestion of, triatomine bug vectors.51 A small number of transfusion‐acquired T. cruzi infections have been reported in people in North America. All patients were immunocompromised at the time of infection, and 6 of the donors were from countries where T. cruzi is endemic (South and Central America).52 In 2010, the Food and Drug Administration recommended that all presenting human blood donors be asked about a history of Chagas disease in addition to being tested at least once using a licensed screening test.53, 54 Infection in dogs can result in acute or chronic myocarditis, but in 1 study,55 dogs that were experimentally inoculated with T. cruzi were parasitemic but only developed transient lymphadenopathy. Survivors of acute disease can remain subclinically infected for several months until chronic myocarditis develops. Infection is characterized by detectable concentrations of specific antibodies and low concentrations of circulating parasites.56
Most dogs that develop trypanosomiasis in the United States reside in Texas or in the southwestern states. The seroprevalence in a 2014 study of 205 dogs from 7 shelters in diverse ecoregions in Texas was 8.8%.57 Transmission to dogs by blood transfusion has not been reported. Dogs with a history of travel to and from endemic areas (Texas, New Mexico, Arizona, southern California, Mexico, Central America and South America) should be considered for serological screening by IFA, indirect hemagglutination assays (IHA), ELISA, or immunochromatographic dipstick tests, and seropositive donors should be excluded from the donor pool. Serologic cross‐reactions between T. cruzi and Leishmania spp. have been documented.58 A PCR assay to detect T. cruzi in whole blood also could be considered.59
Non Vector‐borne Pathogens—Testing Recommended
Brucella canis
Brucella canis, a zoonotic pathogen, is a gram‐negative bacterium that causes brucellosis in dogs. Venereal transmission can occur during breeding, or transmission can follow oronasal contact with vaginal discharges, aborted material, and urine from infected dogs. In humans, only a few transfusion‐transmitted Brucella infections have been documented worldwide.60, 61, 62 Transmission of B. canis by blood transfusion has not been documented in dogs. However, infection is associated with prolonged bacteremia that may be subclinical, and thus the potential for transmission by transfusion exists.63
Because it is commercially available and results can be obtained in minutes, serological screening of potential donors for antibodies using the rapid slide agglutination test (RSAT) initially is recommended.5 Positive dogs should be excluded as blood donors and additional confirmatory diagnostic assays performed using blood culture, PCR on whole blood, tube agglutination tests, agarose gel immunodiffusion tests, or ELISA tests. A single negative RSAT is sufficient for neutered donors to meet optimal standards, but RSAT screening should be repeated in sexually active intact dogs. This approach is supported by numerous studies that have concluded that RSAT is highly sensitive, but lacks specificity.64 In recent studies, PCR assays performed on whole blood or genitourinary secretions were found to be more sensitive than serologic tests, notably in the early phase of infection.65, 66, 67, 68, 69
Other Pathogens—Testing Not Recommended
Borrelia burgdorferi
Lyme borreliosis is caused by the spirochete Borrelia burgdorferi, which is vectored by Ixodes spp.70 Many dogs in the northeastern and upper Midwest regions of the United States are seropositive, and a small percentage of dogs develop polyarthritis or nephritis.71 Transfusion‐related infections have not been reported. Despite the ability to culture B. burgdorferi from human blood,72 studies in humans have demonstrated that the risk of acquiring Lyme disease from a transfused unit of packed red blood cells or platelets is negligible.73, 74 In a study in dogs, only 1.6% of 576 blood samples from experimentally infected dogs tested positive for B. burgdorferi by PCR.75 The consensus of the panel is that healthy canine blood donors should not be screened for B. burgdorferi. If a screening testa,b that detects seroreactivity to other pathogens is used and the donor is seropositive to B. burgdorferi, that animal need not be excluded from the donor pool.
Neorickettsia helminthoeca
Testing of blood donor dogs for N. helminthoeca is not recommended because the pathogen produces acute disease, without evidence of a carrier state in healthy dogs and without evidence of blood‐borne transmission.76
Rickettsia rickettsii
Rocky mountain spotted fever (RMSF), caused by Rickettsia rickettsii, is an acute systemic infection of vascular endothelial cells. The organism is rapidly eliminated from dogs that survive clinical illness, and chronic carrier states have not been reported. The consensus of the panel is that healthy blood donors do not need to be screened for antibodies to R. rickettsii, because infected dogs are acutely ill and no subclinical carrier state is known to exist. Dogs that are seropositive for Rickettsia spp. need not be excluded as blood donors.
West Nile virus
West Nile virus (WNV) is a mosquito‐borne zoonotic arbovirus (genus Flavivirus). Most infected humans are asymptomatic or have mild disease characterized by fever, headache, muscle ache, and skin rash; meningoencephalitis develops in <1% of cases. Although dogs can become viremic after infection with WNV, they typically develop a subclinical viremia of low magnitude followed by clearance of the virus. Only rare reports of clinical disease exist.77 Therefore, canine blood donors do not need to be tested for WNV.78
Feline Pathogens
Vector‐borne Pathogens—Testing Recommended
Anaplasma spp
Cats with A. phagocytophilum infections can develop mild clinical illness that resolves quickly with administration of doxycycline.79, 80 Research cats infested with field‐caught I. scapularis from endemic areas become PCR positive before seroconversion, develop antibodies that can be detected by a commercially available assay used with dog sera,a and maintain rickettsemia for weeks before administration of doxycycline.81 In addition, some cats with A. phagocytophilum infections will have morulae visualized cytologically in the cytoplasm of neutrophils.79 Because this organism causes illness in cats, can be transmitted experimentally by blood inoculation, results in persistent infection, and is associated with illness, the panel recommends that optimally healthy cats from endemic areas be screened for A. phagocytophilum infection by serology and PCR and be negative in both tests (Table 2). However, in endemic areas, seropositive cats may be common, limiting the blood donor pool. Thus, the committee believes that if blood donor cats that are A. phagocytophilum seronegative and PCR negative are not available, cats that are A. phagocytophilum seropositive but PCR (or culture) negative could be used (Table 2). Because infection of cats with A. platys occasionally has been documented, cats living in areas endemic to Rhipicephalus spp. ticks should be screened with PCR (Table 2).
Bartonella spp
A number of Bartonella spp. have been grown or amplified from the blood of cats, most commonly B. henselae, B. clarridgeiae, B. koehlerae, and B. quintana.21 Cats are the reservoirs and Ctenocephalides felis is the vector for B. henselae, B. clarridgeiae, and B. koehlerae; these agents are extremely common in the blood of cats and their fleas.82 Bartonella henselae appears to be the most likely to be pathogenic, but more studies are needed to determine disease associations with other species. Bartonella henselae infection was only documented by PCR assay in 2 of 117 (1.7%) community source cats used as blood donors in the United States, which likely reflects the use of flea control products.83 Infected cats typically have a prolonged, subclinical bacteremia, but a number of clinical sequelae also occur.21, 84 Bartonella spp. can be transmitted by blood transfusion and storing blood does not inactivate the organism.6
The most sensitive way to document Bartonella spp. in the blood of cats is by the concurrent use of specialized culture media and PCR assay using several blood samples.25 Although this approach frequently is needed to prove the presence of Bartonella spp. in the blood of clinically ill dogs, whether the increased sensitivity is needed for screening cats to be used as blood donors remains to be proven. Because cats are the definitive host for B. henselae, high levels of bacteremia often are detected even when the cats are healthy. Thus, broad range PCR assays or PCR assays using specific primers that are used widely in commercial laboratories in the United States are likely to detect most infected cats (Table 3). Bacteremia in cats infected with B. henselae by exposure to infected C. felis precedes seroconversion by 7–42 days and thus serology alone is inadequate as a screening test.84 However, after immune responses develop, bacteremia can be intermittent in cats and a false negative result could occur only if a single sample is assayed.85 Thus, the panel recommends as the optimal standard to use cats that are seronegative and PCR assay or culture negative (Table 2).
Because the majority of states in the United States are endemic for C. felis, Bartonella spp. seroprevalence rates can be as high as 93%.86 Thus, for many states, requiring that community‐based blood donor cats be Bartonella spp. seronegative and PCR or culture negative could make it very difficult to find adequate numbers of donors. Although seropositive, many healthy cats infected by B. henselae‐infected C. felis will limit bacteremia over time. Thus, the panel believes the minimal standard is to use a Bartonella spp. PCR negative cat (Table 2). Use of antibiotics does not consistently eliminate Bartonella spp. infections in cats, and thus PCR or culture positive cats should be excluded from the blood donor program.87
Cytauxzoon felis
Cytauxzoon felis is a tickborne protozoal pathogen in the order Piroplasmida and family Theileriidae. In this section, cytauxzoonosis is used to denote the acute illness (ie, systemic inflammatory response syndrome, cytopenias, multi‐organ failure) associated with C. felis infection. After tick transmission, the organism undergoes schizogony in myeloid cells (specific lineage is unknown) followed by merogony in erythrocytes. The schizogenous stage is associated with illness and disease can be transmitted experimentally by transmission of blood from a cat with cytauxzoonosis to a naive cat.88 The majority of cats with cytauxzoonosis that are presented to veterinary hospitals develop severe febrile illness, cytopenias, and often die within 5 days of presentation if appropriate treatments are not given. A carrier state (erythrocyte infection only) has been identified that is not associated with clinical disease.89 In fact, transfusion of blood from a chronically infected cat into a naive cat does not result in illness. A theoretical risk for transmission of cytauxzoonosis exists because parasitemia can precede illness. In experimental infection, parasite DNA can be detected as early as 7 days post‐infection but clinical signs do not begin until 10–21 days post‐infection. Serological assays are not available. Use of indoor only cats receiving appropriate ectoparasite prophylaxis is advised to avoid this disease in donor animals. Appropriate donor selection and pre‐donation physical examinations should minimize transmission risk. The panel recommends as the optimal standard to use PCR negative cats in endemic areas (Table 2).
Ehrlichia canis
Client‐owned cats with clinical signs of disease have been documented with E. canis,90, 91 and E. canis‐like organism DNA in their blood.90 Studies evaluating for E. canis or E. canis‐like organisms in healthy cats in the United States have yielded negative results.82, 83, 92 Thus, although the committee believes that optimally cats should test PCR‐negative for Ehrlichia spp. infections, no screening also is acceptable (Table 2).
Hemoplasmas
Hemoplasmosis or “feline infectious anemia” is caused by Mycoplasma haemofelis. Other species of hemoplasmas that infect cats in North America are “Candidatus M. haemominutum” (Mhm) and “Candidatus M. turicensis” (Mtc), but these organisms are substantially less pathogenic and frequently detected (10–25% prevalence) using PCR in the blood of non‐anemic client‐owned pet cats that are either apparently healthy or brought to veterinary clinics for reasons other than anemia.83, 93, 94, 95 By contrast, in North America, M. haemofelis is rarely (<1%) detected in non‐anemic cats using PCR.94, 95 When cats that were chronically infected with “Candidatus M. haemominutum” were splenectomized and concurrently treated with high doses of glucocorticoids, persistent subclinical infection occurred in the absence of anemia.96 Although fleas have been suggested to be involved in transmission, the evidence for flea‐borne transmission is weak. Epidemiologic evidence suggests that aggressive interactions between cats may lead to transmission.95 Intravenous inoculation of infected blood has been used to produce experimental infections.97 No serologic assay for infection is commercially available. Blood smear evaluation is insensitive (especially for chronic carrier cats) and also lacks specificity (organisms can be easily confused with stain precipitate or drying artifacts), and thus the diagnostic test of choice for screening blood donors is PCR. Because of the pathogenicity of M. haemofelis, the panel agreed that all cats should be tested for M. haemofelis and those that test positive should be excluded from the blood donor pool. Optimally, cats should be tested for “Candidatus M. haemominutum” and “Candidatus M. turicensis” and excluded from the donor pool, but given the high prevalence of these organisms in the cat population and the lack of strong evidence that they are associated with disease even in immunosuppressed cats, the panel agreed that screening for these pathogens could be considered optional and positive cats could be used as donors in the absence of a source of negative blood (Table 2). Because antimicrobial therapy does not reliably eliminate hemoplasmas (and appears particularly ineffective for treatment of “Candidatus M. haemominutum” infections), the panel does not recommend treating potential donors with antimicrobials in an attempt to eliminate infection.
Importantly, M. haemofelis appears to be inactivated during storage of whole blood for 1 week.98 “Candidatus M. haemominutum” may remain viable in blood products for >1 week, although inactivation appears to occur after 1 month of storage.
Neorickettsia risticii can infect cats after experimental inoculation but has not been detected in naturally exposed cats.99 Although the committee believes that optimally cats should test PCR‐negative for Neorickettsia risticii infections, no screening also is acceptable (Table 2).
Non Vector‐borne Pathogens—Testing Recommended
Feline Leukemia Virus
Transmission of Feline Leukemia Virus (FeLV) occurs primarily through saliva, but the virus is present in the blood and can be transmitted by blood transfusion.100 Screening of donor cats for FeLV using an ELISA that detects soluble circulating core viral antigen p27 in the peripheral blood is recommended, and all cats testing antigen‐positive should be excluded as blood donors. With the recognition that cats exposed to FeLV can develop regressive infection (defined as transient or undetectable antigenemia with proviral DNA in the blood), optimally real‐time PCR testing for proviral DNA should be performed, because FeLV provirus is infectious.101, 102 However, PCR assays for FeLV proviral DNA currently available in the United States have not been evaluated for clinical sensitivity and specificity. Regressor cats have detectable proviral DNA and viral RNA in many tissues, including the bone marrow, many years after FeLV exposure, indicating that these cats do not completely clear the virus.103 Regressor cats can transmit FeLV infection to recipient cats through blood transfusions, and the recipients can then go on to develop progressive infection.7 Furthermore, reactivation of FeLV infection in regressor cats with and without immune suppression has been reported.103 Reactivation of a regressive FeLV infection has the potential to place FeLV‐negative donor cats at risk if housed together in a colony situation if reactivation occurs in the interval between routine screening tests (eg, annual screening) and is not detected. Free‐roaming cats have constant potential exposure and should be excluded from blood donor programs.
Feline Immunodeficiency Virus
Feline Immunodeficiency Virus (FIV) is transmitted primarily through bite wounds, but it can be readily transmitted via inoculation of infected blood.104, 105 Testing of donor cats for FIV‐specific antibodies by ELISA (which uses immobilized FIV core proteins p24gag and p15 to capture antibodies in blood) is recommended, and all seropositive cats should be excluded from the donor pool. Cats vaccinated against FIV also will be seropositive. Definitive differentiation of infected from vaccinated cats is challenging, because virus isolation, the gold standard for diagnosis of FIV infection, is time‐consuming and expensive. Real‐time quantitative PCR assays for FIV are moderately sensitive and highly specific for FIV infection, but positive results have occurred in uninfected, vaccinated cats.106 Therefore, until reliable approaches are available that conclusively discriminate naturally infected cats from vaccinated cats, the panel recommends that all seropositive cats, including vaccinated cats, be excluded from the donor pool. Free‐roaming cats also should be excluded from donor programs.
Other Pathogens—Testing Not Recommended
Feline Coronavirus
Although cats can be subclinically infected with feline coronavirus, documentation of transmission of the virus by blood transfusion does not exist at this time. Screening of blood donor cats with serology or reverse transcriptase (RT)‐PCR is not recommended, because healthy cats can have antibody titers against feline coronavirus and also can be RT‐PCR positive. The consensus of the panel was not to screen for coronavirus antibodies or RNA in clinically healthy cats being considered as blood donors.
Rickettsia felis
The dog has recently been documented to be the reservoir for this agent, and studies have failed to amplify R. felis DNA from the blood of cats. Thus, the panel does not recommend screening for this organism in cats.
Toxoplasmosis
Cats are the definitive host for Toxoplasma gondii, an intracellular coccidian parasite. Although Toxoplasma gondii antigens and DNA have been detected in healthy cats by PCR assay, transmission by blood transfusion has not been documented.107 For purposes of blood safety, the consensus of the panel is that there is no indication for screening healthy potential donor cats for T gondii antigens, antibodies, or DNA.
General Recommendations
Tables 1 and 2 present current recommendations for blood donor screening. The consensus panel hopes that its findings will lead to reevaluation of the current infectious disease screening process for potential canine and feline donors. Diagnostic laboratories should reexamine their donor screening panels, offering assays for those pathogens that are of most concern in blood‐borne disease transmission.
Management Techniques
The panel agreed on several management techniques designed to decrease the risk of disease transmission by blood transfusion.
Donor Selection and Care
Standardized forms (Tables 4, 5, 6, 7) should be completed at enrollment of potential blood donors and before each donation.
A complete history and thorough physical examination, including determination of rectal temperature of the donor animal, should be completed before each blood collection.
Use of antimicrobial drugs to prevent or treat possible infection is not acceptable as a substitute for laboratory testing of potential blood donors.
Initial clinicopathologic screening of potential donors is advised (eg, CBC, serum biochemistry, urinalysis, fecal examination). Determination of the PCV is advised before each collection.
Table 4.
Potential canine blood donor evaluation form

Table 5.
Potential feline blood donor evaluation form

Table 6.
Pre‐donation history questionnaire for canine blood donors
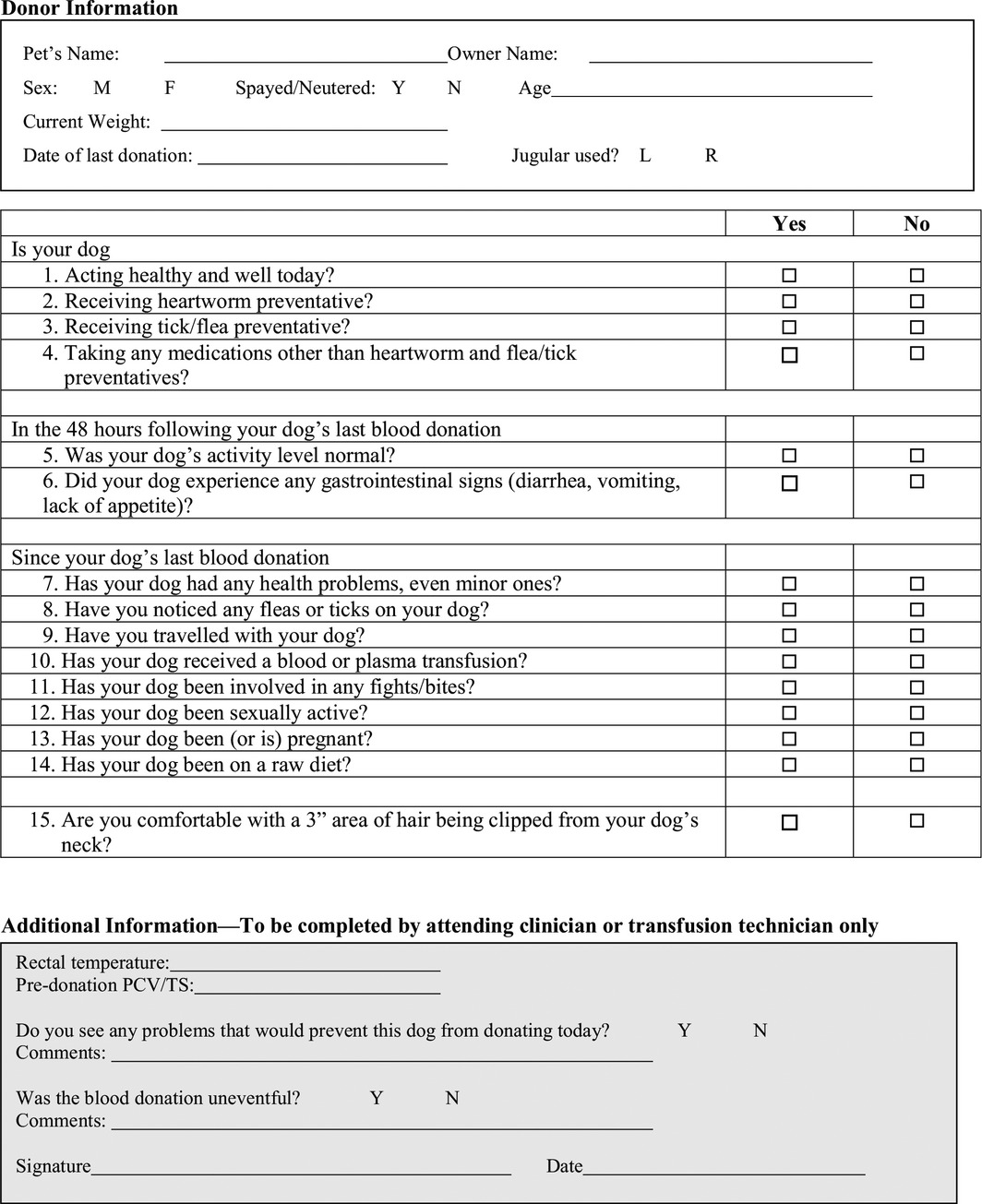
Table 7.
Pre‐donation history questionnaire for feline blood donors
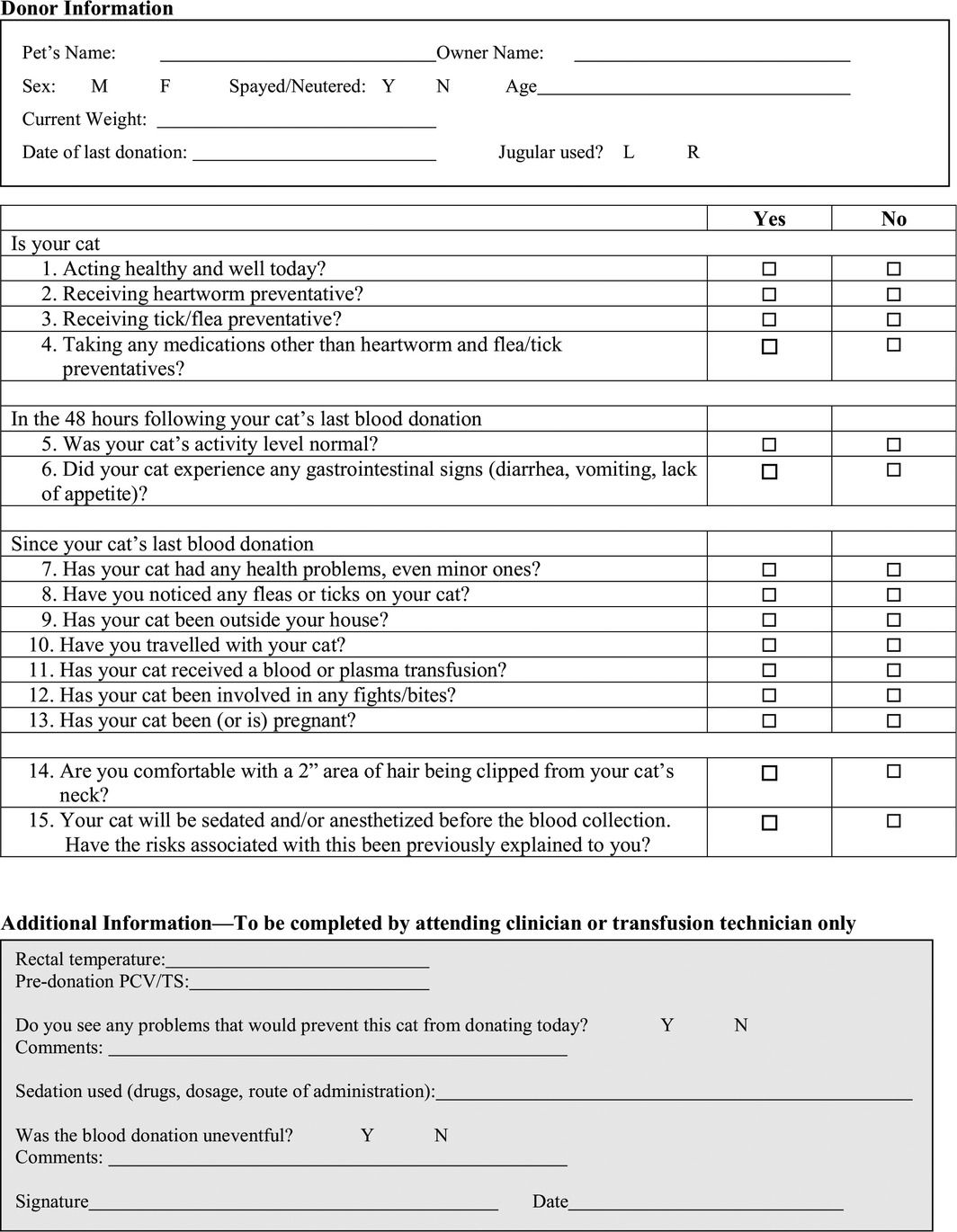
Blood Collection Procedure Recommendations
All blood for transfusion should be collected in an aseptic manner.
Before administration, the label of the blood product should be examined for the following: collection and expiration dates, donor species, product type, and blood type.
Before administration, blood products should be visually inspected. Bacterial contamination should be suspected if bag segments appear much lighter in color than the bag itself, the red blood cell mass appears purple, a zone of hemolysis is observed just above the red cell mass, clots are visible, or the plasma or supernatant fluid is murky, purple, brown, or red. In the presence of any of these findings, culture of the blood should be performed to determine whether contamination has occurred. If the unit appears abnormal, it should not be administered.
Currently, screening of individual units for infectious disease is not practical in veterinary medicine because of the turnaround time and costs of testing. However, this model should be considered the gold standard.
An aliquot of plasma and whole blood tube segments from each donated unit of blood should be stored. This practice allows retrospective testing in cases of suspected transfusion‐associated disease transmission.
Records
Records should be kept on all transfusions, documenting both the donor unit used and the recipient of the transfusion. Appropriate records must be kept so that all recipients receiving blood from a given donor can be easily contacted should that donor be found to carry an infectious disease agent.
Development of consent forms detailing potential disease transmission risks should be considered for owners of patients receiving transfusions.
Summary
Thousands of blood transfusions are performed each year on dogs and cats, and the demand for blood products continues to grow. Risks associated with transfusions include the risk of disease transmission. Appropriate screening of blood donors for blood‐borne infectious disease agents should be performed to decrease this risk. Geographic restrictions of disease, breed predilection, and documentation of actual disease transmission by transfusion all are factors that might need to be considered when making a decision on what screening program to use. In addition, factors involving general health care and management of blood donors should be employed to further ensure blood safety.
Acknowledgments
Conflict of Interest Declaration: Dr. Lappin has been a paid consultant and researcher for IDEXX and Antech Diagnostics. Dr. Sykes has been a paid consultant for UC Davis Tagman Laboratory and IDEXX. Dr. Sykes has received research grants from UC Davis Center for Companion Animal Health.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Consensus Statements of the American College of Veterinary Internal Medicine (ACVIM) provide the veterinary community with up‐to‐date information on the pathophysiology, diagnosis, and treatment of clinically important animal diseases. The ACVIM Board of Regents oversees selection of relevant topics, identification of panel members with the expertise to draft the statements, and other aspects of assuring the integrity of the process. The statements are derived from evidence‐based medicine whenever possible and the panel offers interpretive comments when such evidence is inadequate or contradictory. A draft is prepared by the panel, followed by solicitation of input by the ACVIM membership which may be incorporated into the statement. It is then submitted to the Journal of Veterinary Internal Medicine, where it is edited before publication. The authors are solely responsible for the content of the statements.
Footnotes
Canine SNAP 4DX Plus Test, IDEXX Laboratories Inc, Westbrook, ME
Accuplex4 BioCD system, Antech Diagnostics, Lake Success, NY
WITNESS®Ehrlichia, Zoetis, Florham Park, NJ
Immunocomb Canine Ehrlichia Kit®, BIOGAL, Galed Labs. Acs Ltd, Israel
D‐TEC® CB Canine Brucellosis Antibody Test Kit, Zoetis, Florham Park, NJ
Bradbury CA, Green M, Brewer M, et al Survival of Bartonella henselae in the blood of cats used for transfusion. Proceedings of the American College of Veterinary Internal Medicine Forum (abstract), Anaheim, CA, June 11, 2010 (poster).
Hofmann‐Lehmann R. Feline Leukemia Virus Infection: Where Do We Stand? Proceedings of the Second International Society for Companion Animal Infectious Diseases Symposium, San Francisco, CA, 2012.
References
- 1. Maggi RG, Birkenheuer AJ, Hegarty BC, et al. Comparison of serological and molecular panels for diagnosis of vector‐borne diseases in dogs. Parasit Vectors 2014;7:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dingman P, Levy JK, Kramer LH, et al. Association of Wolbachia with heartworm disease in cats and dogs. Vet Parasitol 2010;170:50–60. [DOI] [PubMed] [Google Scholar]
- 3. Egenvall A, Bjöersdorff A, Lilliehöök I, et al. Early manifestations of granulocytic ehrlichiosis in dogs inoculated experimentally with a Swedish Ehrlichia species isolate. Vet Rec 1998;143:412–417. [DOI] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention . Anaplasma phagocytophilum transmitted through blood transfusion – Minnesota, 2007. MMWR Morb Mortal Wkly Rep 2008;57:1145–1148. [PubMed] [Google Scholar]
- 5. Townsend RL, Moritz ED, Fialkow LB, et al. Probable transfusion‐transmission of Anaplasma phagocytophilum by leukoreduced platelets. Transfusion 2014;54:2828–2832. [DOI] [PubMed] [Google Scholar]
- 6. Kohn B, Silaghi C, Galke D, et al. Infections with Anaplasma phagocytophilum in dogs in Germany. Res Vet Sci 2011;91:71–76. [DOI] [PubMed] [Google Scholar]
- 7. Moroff S, Sokolchik I, Woodring T, et al. Detection of antibodies against Anaplasma phagocytophilum in dogs using an automated fluorescence based system. Vet J 2014;202:348–352. [DOI] [PubMed] [Google Scholar]
- 8. Stillman BA, Monn M, Liu J, et al. Performance of a commercially available in‐clinic ELISA for detection of antibodies against Anaplasma phagocytophilum, Anaplasma platys, Borrelia burgdorferi, Ehrlichia canis, and Ehrlichia ewingii and Dirofilaria immitis antigen in dogs. J Am Vet Med Assoc 2014;245:80–86. [DOI] [PubMed] [Google Scholar]
- 9. Bowman D, Little SE, Lorentzen L, et al. Prevalence and geographic distribution of Dirofilaria immitis, Borrelia burgdorferi, Ehrlichia canis, and Anaplasma phagocytophilum in dogs in the United States: Results of a national clinic‐based serologic survey. Vet Parasitol 2009;160:138–148. [DOI] [PubMed] [Google Scholar]
- 10. Egenvall A, Lilliehook I, Bjoersdorff A, et al. Detection of granulocytic Ehrlichia species DNA by PCR in persistently infected dogs. Vet Rec 2000;146:186–190. [DOI] [PubMed] [Google Scholar]
- 11. Harvey JW. Anaplasma platys infection (thrombocytotropic anaplasmosis) In: Greene CE, ed. Infectious Diseases of the Dog and Cat, 4th ed St. Louis, MO: Elsevier; 2012:256–259. [Google Scholar]
- 12. Gaunt SD, Beall MJ, Stillman BA, et al. Experimental infection and co‐infection of dogs with Anaplasma platys and Ehrlichia canis: Hematologic, serologic and molecular findings. Parasit Vectors 2010;3:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Santos AS, Alexandre N, Sousa R, et al. Serological and molecular survey of Anaplasma species infection in dogs with suspected tickborne disease in Portugal. Vet Rec 2009;164:168–171. [DOI] [PubMed] [Google Scholar]
- 14. Herwaldt BL, Neitzel DF, Gorlin JB. Transmission of Babesia microti in Minnesota through four blood donations from the same donor over a 6 month period. Transfusion 2002;42:1154–1158. [DOI] [PubMed] [Google Scholar]
- 15. Dobroszycki J, Herwaldt BL, Boctor F. A cluster of transfusion‐associated babesiosis cases traced to a single asymptomatic donor. J Am Med Assoc 1999;281:927–930. [DOI] [PubMed] [Google Scholar]
- 16. Freeman MJ, Kirby BM, Panciera DL, et al. Hypotensive shock syndrome associated with acute Babesia canis infection in a dog. J Am Vet Med Assoc 1994;204:94–96. [PubMed] [Google Scholar]
- 17. Stegeman JR, Birkenheuer AJ, Kruger JM, Breitschwerdt EB. Transfusion‐associated Babesia gibsoni infection in a dog. J Am Vet Med Assoc 2003;222:959–963. [DOI] [PubMed] [Google Scholar]
- 18. Taboada J, Harvey JW, Levy MG, Breitschwerdt EB. Seroprevalence of babesiosis in Greyhounds in Florida. J Am Vet Med Assoc 1992;200:47–50. [PubMed] [Google Scholar]
- 19. Macintire DK, Boudreaux MK, West GD, et al. Babesia gibsoni infection among dogs in the southeastern United States. J Am Vet Med Assoc 2002;220:325–329. [DOI] [PubMed] [Google Scholar]
- 20. Birkenheuer AJ, Levy MG, Stebbins M, et al. Serosurvey of anti‐Babesia antibodies in stray dogs and American Pit Bull Terriers and American Staffordshire Terriers from North Carolina. J Am Anim Hosp Assoc 2003;39:551–557. [DOI] [PubMed] [Google Scholar]
- 21. Breitschwerdt EB, Maggi RG, Chomel BB, Lappin MR. Bartonellosis: An emerging infectious disease of zoonotic importance to animals and human beings. J Vet Emerg Crit Care (San Antonio) 2010;20:8–30. [DOI] [PubMed] [Google Scholar]
- 22. Yore K, DiGangi B, Brewer M, et al. Flea species infesting dogs in Florida and Bartonella spp. prevalence rates. Vet Parasitol 2014;199:225–229. [DOI] [PubMed] [Google Scholar]
- 23. Cherry NA, Diniz PP, Maggi RG, et al. Isolation or molecular detection of Bartonella henselae and Bartonella vinsonii subsp. berkhoffii from dogs with idiopathic cavitary effusions. J Vet Intern Med 2009;23:186–189. [DOI] [PubMed] [Google Scholar]
- 24. Pappalardo BL, Brown TT, Tompkins M, Breitschwerdt EB. Immunopathology of Bartonella vinsonii (berkhoffii) in experimentally infected dogs. Vet Immunol Immunopathol 2001;83:125–147. [DOI] [PubMed] [Google Scholar]
- 25. Duncan AW, Maggi RG, Breitschwerdt EB. A combined approach for the enhanced detection and isolation of Bartonella species in dog blood samples: Pre‐enrichment liquid culture followed by PCR and subculture onto agar plates. J Microbiol Methods 2007;69:273–281. [DOI] [PubMed] [Google Scholar]
- 26. Balakrishnan N, Musulin S, Varanat M, et al. Serological and molecular prevalence of selected canine vector borne pathogens in blood donor candidates, clinically healthy volunteers, and stray dogs in North Carolina. Parasit Vectors 2014;7:116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Starkey LA, Barrett AW, Beall MJ, et al. Persistent Ehrlichia ewingii infection in dogs after natural tick infestation. J Vet Intern Med 2015;29:552–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gaunt SD, Corstvet RE, Berry CM, Brennan B. Isolation of Ehrlichia canis from dogs following subcutaneous inoculation. J Clin Microbiol 1996;34:1429–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Obara H, Fujihara M, Watanabe Y, et al. A feline hemoplasma, “Candidatus Mycoplasma haemominutum”, detected in dog in Japan. J Vet Med Sci 2011;73:841–843. [DOI] [PubMed] [Google Scholar]
- 30. Valle SF, Messick JB, Dos Santos AP, et al. Identification, occurrence and clinical findings of canine hemoplasmas in southern Brazil. Comp Immunol Microbiol Infect Dis 2014;37:259–265. [DOI] [PubMed] [Google Scholar]
- 31. Willi B, Novacco M, Meli M, et al. Haemotropic mycoplasmas of cats and dogs: Transmission, diagnosis, prevalence and importance in Europe. Schweiz Arch Tierheilkd 2010;152:237–244. [DOI] [PubMed] [Google Scholar]
- 32. Brinson J, Messick J. Use of a polymerase chain reaction assay for detection of Haemobartonella canis in a dog. J Am Vet Med Assoc 2001;218:1943–1945. [DOI] [PubMed] [Google Scholar]
- 33. Sykes JE, Baliff NL, Ball LM, et al. Identification of a novel hemotropic mycoplasma in a splenectomized dog with hemic neoplasia. J Am Vet Med Assoc 2004;224:1946–1951. [DOI] [PubMed] [Google Scholar]
- 34. Compton SM, Maggi RG, Breitschwerdt EB. Candidatus Mycoplasma haematoparvum and Mycoplasma haemocanis infections in dogs from the United States. Comp Immunol Microbiol Infect Dis 2012;35:557–562. [DOI] [PubMed] [Google Scholar]
- 35. Allen KE, Johnson EM, Little SE. Hepatozoon spp infections in the United States. Vet Clin North Am Small Anim Pract 2011;41:1221–1238. [DOI] [PubMed] [Google Scholar]
- 36. Li Y, Wang C, Allen KE, et al. Diagnosis of canine Hepatozoon spp. infection by quantitative PCR. Vet Parasitol 2008;157:50–58. [DOI] [PubMed] [Google Scholar]
- 37. Maroli M, Gradoni L, Oliva G, et al. Guidelines for prevention of leishmaniasis in dogs. J Am Vet Med Assoc 2010;236:1200–1206. [DOI] [PubMed] [Google Scholar]
- 38. Peterson CA. Leishmaniosis, an emerging disease found in companion animals in the United States. Top Companion Anim Med 2009;24:182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Owens SD, Oakley DA, Marryott K, et al. Transmission of visceral leishmaniasis through blood transfusions from infected English Foxhounds to anemic dogs. J Am Vet Med Assoc 2001;219:1081–1088. [DOI] [PubMed] [Google Scholar]
- 40. Duprey ZH, Steurer FJ, Rooney JA, et al. Canine visceral leishmaniasis, United States and Canada, 2000–2003. Emerg Infect Dis 2006;12:440–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mettler M, Grimm F, Capelli G, et al. Evaluation of enzyme‐linked immunosorbent assays, an immunofluorescent‐antibody test, and two rapid tests (immunochromatographic‐dipstick and gel tests) for serological diagnosis of symptomatic and asymptomatic Leishmania infections in dogs. J Clin Microbiol 2005;43:5515–5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Baneth G, Koutinas AF, Solano‐Gallego L, et al. Canine leishmaniosis – New concepts and insights on an expanding zoonosis: Part one. Trends Parasitol 2008;24:324–330. [DOI] [PubMed] [Google Scholar]
- 43. Solano‐Gallego L, Koutinas A, Miro G. Directions for the diagnosis, clinical staging, treatment and prevention of canine leishmaniosis. Vet Parasitol 2009;165:1–18. [DOI] [PubMed] [Google Scholar]
- 44. Grosjean NL, Vrable RA, Murphy AJ, Mansfield LS. Seroprevalence of antibodies against Leishmania spp among dogs in the United States. J Am Vet Med Assoc 2003;222:603–606. [DOI] [PubMed] [Google Scholar]
- 45. Reine NJ. Infection and blood transfusion: A guide to blood donor screening. Clin Tech Small Anim Pract 2004;19:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ristic M, Dawson J, Holland CJ, Jenny A. Susceptibility of dogs to infection with Ehrlichia risticii, causative agent of equine monocytic ehrlichiosis (Potomac horse fever). Am J Vet Res 1988;49:1497–1500. [PubMed] [Google Scholar]
- 47. Amusategui I, Tesouro MA, Kakoma I, Sainz A. Serological reactivity to Ehrlichia canis, Anaplasma phagocytophilum, Neorickettsia risticii, Borrelia burgdorferi and Rickettsia conorii in dogs from northwestern Spain. Vector Borne Zoonotic Dis 2008;8:797–803. [DOI] [PubMed] [Google Scholar]
- 48. Kakoma I, Hansen RD, Anderson BE, et al. Cultural, molecular, and immunological characterization of the etiologic agent for atypical canine ehrlichiosis. J Clin Microbiol 1994;32:170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hii SF, Kopp SR, Abdad MY, et al. Molecular evidence supports the role of dogs as potential reservoirs for Rickettsia felis . Vector Borne Zoonotic Dis 2011;11:1007–1012. [DOI] [PubMed] [Google Scholar]
- 50. Hawley JR, Shaw SE, Lappin MR. Prevalence of Rickettsia felis DNA in the blood of cats and their fleas in the United States. J Feline Med Surg 2007;9:258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bern C, Kjos S, Yabsley MJ, Montgomery SP. Trypanosoma cruzi and Chagas’ disease in the United States. Clin Microbiol Rev 2011;24:655–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Young C, Losikoff P, Chawla A, et al. Transfusion‐acquired Trypanosoma cruzi infection. Transfusion 2007;47:540–544. [DOI] [PubMed] [Google Scholar]
- 53. Food and Drug Administration . Guidance for industry: Use of serological tests to reduce the risk of transmission of Trypanosoma cruzi infection in whole blood and blood components intended for transfusion. 2010. Available from: http://www.fda.gov/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/blood/ucm235855.htm. Accessed June 1, 2015.
- 54. Steele WR, Hewitt EH, Kaldun AM, et al. Donors deferred for self‐reported Chagas disease history: Does it reduce risk? Transfusion 2014;54:2092–2097. [DOI] [PubMed] [Google Scholar]
- 55. Barr SC, Gossett KA, Klei TR. Clinical, clinicopathologic, and parasitologic observations of trypanosomiasis in dogs infected with North American Trypanosoma cruzi isolates. Am J Vet Res 1991;52:954–960. [PubMed] [Google Scholar]
- 56. Bradley KK, Bergman DK, Woods JP. Prevalence of American trypanosomiasis (Chagas disease) among dogs in Oklahoma. J Am Vet Med Assoc 2000;217:1853–1857. [DOI] [PubMed] [Google Scholar]
- 57. Tenney TD, Curtis‐Robies R, Snowden KF, Hamer SA. Shelter dogs as sentinels for Trypanosoma cruzi transmission across Texas, USA. Emerg Infect Dis 2014;20:1323–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Troncarelli MZ, Camargo JB, Machado JG, et al. Leishmania spp. and/or Trypanosoma cruzi diagnosis in dogs from endemic and nonendemic areas for canine visceral leishmaniasis. Vet Parasitol 2009;164:118–123. [DOI] [PubMed] [Google Scholar]
- 59. Enriquez GF, Cardinal MV, Orozco MM, et al. Detection of Trypanosoma cruzi infection in naturally infected dogs and cats using serological, parasitological and molecular methods. Acta Trop 2013;126:211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. al‐Kharfy TM. Neonatal brucellosis and blood transfusion: Case report and review of the literature. Ann Trop Paediatr 2001;21:349–352. [DOI] [PubMed] [Google Scholar]
- 61. Akcakus M, Esel D, Cetin N, et al. Brucella melitensis in blood cultures of two newborns due to exchange transfusion. Turk J Pediatr 2005;47:272–274. [PubMed] [Google Scholar]
- 62. Ertem M, Kurekci AE, Aysev D, et al. Brucellosis transmitted by bone marrow transplantation. Bone Marrow Transplant 2000;26:225–226. [DOI] [PubMed] [Google Scholar]
- 63. Hollett B. Canine brucellosis: Outbreaks and compliance. Theriogenology 2006;66:575–587. [DOI] [PubMed] [Google Scholar]
- 64. Wanke MM. Canine brucellosis. Anim Reprod Sci 2004;82–83:195–207. [DOI] [PubMed] [Google Scholar]
- 65. Keid LB, Soares RM, Vasconcellos SA, et al. A polymerase chain reaction for the detection of Brucella canis in semen of naturally infected dogs. Theriogenology 2007;67:1203–1210. [DOI] [PubMed] [Google Scholar]
- 66. Keid LB, Soares RM, Vasconcellos SA, et al. A polymerase chain reaction for detection of Brucella canis in vaginal swabs of naturally infected bitches. Theriogenology 2007;68:1260–1270. [DOI] [PubMed] [Google Scholar]
- 67. Keid LB, Soares RM, Vieira NR, et al. Diagnosis of canine brucellosis: Comparison between serological and microbiological tests and a PCR based on primers to 16S‐23S rDNA interspace. Vet Res Commun 2007;31:951–965. [DOI] [PubMed] [Google Scholar]
- 68. Keid LB, Soares RM, Vasconcellos SA, et al. Comparison of agar gel immunodiffusion test, rapid slide agglutination test, microbiological culture and PCR for the diagnosis of canine brucellosis. Res Vet Sci 2009;86:22–26. [DOI] [PubMed] [Google Scholar]
- 69. Kauffman LK, Bjork JK, Gallup JM, et al. Early detection of Brucella canis via quantitative polymerase chain reaction analysis. Zoonoses Public Health 2014;61:48–54. [DOI] [PubMed] [Google Scholar]
- 70. Fritz CL, Kjemtrup AM. Lyme borreliosis. J Am Vet Med Assoc 2003;223:1261–1270. [DOI] [PubMed] [Google Scholar]
- 71. Magnarelli LA, Anderson JF, Schreier AB. Persistence of antibodies to Borrelia burgdorferi in dogs in New York and Connecticut. J Am Vet Med Assoc 1990;196:1064–1068. [PubMed] [Google Scholar]
- 72. Wormser GP, Bittker S, Cooper D, et al. Yield of large‐volume blood cultures in patients with early Lyme disease. J Infect Dis 2001;184:1070–1072. [DOI] [PubMed] [Google Scholar]
- 73. Gerber MA, Shapiro ED, Krause PJ, et al. The risk of acquiring Lyme disease or babesiosis from a blood transfusion. J Infect Dis 1994;170:231–234. [DOI] [PubMed] [Google Scholar]
- 74. Ginzburg Y, Kessler D, Kang S, et al. Why has Borrelia burgdorferi not been transmitted by blood transfusion? Transfusion 2013;53:2822–2826. [DOI] [PubMed] [Google Scholar]
- 75. Straubinger RK. PCR‐based quantification of Borrelia burgdorferi organisms in canine tissues over a 500‐day postinfection period. J Clin Microbiol 2000;38:2191–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sykes JE, Marks SL, Mapes S, et al. Salmon poisoning disease in dogs: 29 cases. J Vet Intern Med 2010;24:504–513. [DOI] [PubMed] [Google Scholar]
- 77. Cannon AB, Luff JA, Brault AC, et al. Acute encephalitis, polyarthritis, and myocarditis associated with West Nile virus infection in a dog. J Vet Intern Med 2006;20:1219–1223. [DOI] [PubMed] [Google Scholar]
- 78. Bowen RA, Rouge MM, Siger L, et al. Pathogenesis of West Nile virus infection in dogs treated with glucocorticoids. Am J Trop Med Hyg 2006;74:670–673. [PubMed] [Google Scholar]
- 79. Savidge C, Ewing P, Andrews J, et al. Anaplasma phagocytophilum infection of domestic cats: 16 cases from the northeastern USA. J Feline Med Surg 2015; doi:10.1177/1098612X15571148 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lappin MR, Breitschwerdt EB, Jensen WA, et al. Molecular and serological evidence of Anaplasma phagocytophilum infection of cats in North America. J Am Vet Med Assoc 2004;225:893–896. [DOI] [PubMed] [Google Scholar]
- 81. Lappin MR, Chandrashekar R, Stillman B, et al. Evidence of Anaplasma phagocytophilum and Borrelia burgdorferi infection in cats after exposure to wild‐caught adult Ixodes scapularis ticks. J Vet Diagn Invest 2015;27:522–525. [DOI] [PubMed] [Google Scholar]
- 82. Lappin MR, Griffin B, Brunt J, et al. Prevalence of Bartonella spp., haemoplasma spp., Ehrlichia spp., Anaplasma phagocytophilum, and Neorickettsia risticii DNA in the blood of cats and their fleas in the United States. J Feline Med Surg 2006;8:85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hackett TB, Jensen WA, Lehman TL, et al. Prevalence of DNA of Mycoplasma haemofelis, “Candidatus Mycoplasma haemominutum”, Anaplasma phagocytophilum, and species of Bartonella, Neorickettsia, and Ehrlichia in cats used as blood donors in the United States. J Am Vet Med Assoc 2006;229:700–705. [DOI] [PubMed] [Google Scholar]
- 84. Bradbury CA, Lappin MR. Evaluation of topical application of 10% imidacloprid‐1% moxidectin to prevent Bartonella henselae transmission from cat fleas. J Am Vet Med Assoc 2010;236:869–873. [DOI] [PubMed] [Google Scholar]
- 85. Kordick DL, Breitschwerdt EB. Relapsing bacteremia after blood transmission of Bartonella henselae to cats. Am J Vet Res 1997;58:492–497. [PubMed] [Google Scholar]
- 86. Nutter FB, Dubey JP, Levine JF, et al. Seroprevalences of antibodies against Bartonella henselae and Toxoplasma gondii and fecal shedding of Cryptosporidium spp, Giardia spp, and Toxocara cati in feral and pet domestic cats. J Am Vet Med Assoc 2004;225:1394–1398. [DOI] [PubMed] [Google Scholar]
- 87. Lappin MR, Miller W, Sellins D. Effect of doxycycline or orbifloxacin administration on Bartonella spp. and Hemoplasma assay results in naturally exposed cats. Intern J Appl Res. Vet Med 2012;10:225–233. [Google Scholar]
- 88. Wagner JE, Ferris DH, Kier AB, et al. Experimentally induced cytauxzoonosis‐like disease in domestic cats. Vet Parasitol 1980;6:305–311. [Google Scholar]
- 89. Meinkoth J, Kocan AA, Whitworth L, et al. Cats surviving natural infection with Cytauxzoon felis: 18 cases (1997–1998). J Vet Intern Med 2000;14:521–525. [DOI] [PubMed] [Google Scholar]
- 90. Breitschwert EB, Abrams‐Ogg AC, Lappin MR, et al. Molecular evidence supporting Ehrlichia canis‐like infection in cats. J Vet Intern Med 2002;16:642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hegarty BC, Qurollo BA, Thomas B, et al. Serological and molecular analysis of feline vector‐borne anaplasmosis and ehrlichiosis using species‐specific peptides and PCR. Parasit Vectors 2015;8:320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Eberhardt JE, Neal K, Shackelford T, Lappin MR. Prevalence of select infectious disease agents in cats from Arizona. J Feline Med Surg 2006;8:164–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Sykes JE, Drazenovich NL, Ball LM, Leutenegger CM. Use of conventional and real‐time polymerase chain reaction to determine the epidemiology of hemoplasma infections in anemic and nonanemic cats. J Vet Intern Med 2007;21:685–693. [DOI] [PubMed] [Google Scholar]
- 94. Jensen WA, Lappin MR, Kamkar S, Reagan WJ. Use of a polymerase chain reaction assay to detect and differentiate two strains of Haemobartonella felis in naturally infected cats. Am J Vet Res 2001;62:604–608. [DOI] [PubMed] [Google Scholar]
- 95. Sykes JE. Feline hemotropic mycoplasmas. J Vet Emerg Crit Care (San Antonio) 2010;20:62–69. [DOI] [PubMed] [Google Scholar]
- 96. Sykes JE, Henn JB, Kasten RW, et al. Bartonella henselae infection in splenectomized domestic cats previously infected with hemotropic Mycoplasma species. Vet Immunol Immunopathol 2007;116:104–108. [DOI] [PubMed] [Google Scholar]
- 97. Berent LM, Messick JB, Cooper SK. Detection of Haemobartonella felis in cats with experimentally induced acute and chronic infections, using a polymerase chain reaction assay. Am J Vet Res 1998;59:1215–1220. [PubMed] [Google Scholar]
- 98. Gary AT, Richmond HL, Tasker S, et al. Survival of Mycoplasma haemofelis and “Candidatus Mycoplasma haemominutum” in blood of cats used for transfusions. J Feline Med Surg 2006;8:321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Dawson JE, Abeygunawardena I, Holland CJ, et al. Susceptibility of cats to infection with Ehrlichia risticii, causative agent of equine monocytic ehrlichiosis. Am J Vet Res 1988;49:2096–2100. [PubMed] [Google Scholar]
- 100. Hardy WD, Old LJ, Hess PW, et al. Horizontal transmission of feline leukaemia virus. Nature 1973;244:266–269. [DOI] [PubMed] [Google Scholar]
- 101. Levy J, Crawford C, Hartmann K, et al. 2008 American Association of Feline Practitioners’ feline retrovirus management guidelines. J Feline Med Surg 2008;10:300–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Chen H, Bechtel MK, Shi Y, et al. Pathogenicity induced by feline leukemia virus, Rickard Strain, subgroup A plasmid DNA (pFRA). J Virol 1998;72:7048–7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Helfer‐Hungerbuehler AK, Widmer S, Kessler Y, et al. Long‐term follow up of feline leukemia virus infection and characterization of viral RNA loads using molecular methods in tissues of cats with different infection outcomes. Virus Res 2015;197:137–150. [DOI] [PubMed] [Google Scholar]
- 104. Goldkamp CE, Levy JK, Edinboro CH, Lachtara JL. Seroprevalences of feline leukemia virus and feline immunodeficiency virus in cats with abscesses or bite wounds and rate of veterinarian compliance with current guidelines for retrovirus testing. J Am Vet Med Assoc 2008;232:1152–1158. [DOI] [PubMed] [Google Scholar]
- 105. Boudreaux CE, Lockett NN, Chemerys DN, et al. Maternal hematological and virological characteristics during early feline immunodeficiency virus (FIV) infection of cats as predictors of fetal infection and reproductive outcome at early gestation. Vet Immunol Immunopathol 2009;131:290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ammersbach M, Little S, Bienzle D. Preliminary evaluation of a quantitative polymerase chain reaction assay for diagnosis of feline immunodeficiency virus infection. J Feline Med Surg 2013;15:725–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Burney DP, Lappin MR, Spilker M, McReynolds L. Detection of Toxoplasma gondii parasitemia in experimentally inoculated cats. J Parasitol 1999;5:947–951. [PubMed] [Google Scholar]


