Abstract
Background
Canine babesiosis, caused by Babesia canis, is a prevalent and clinically relevant disease in Europe. Severe acute babesiosis is characterized by a high mortality but prognosis is not always correlated with clinical signs nor with the level of parasitemia.
Objective
This study evaluated prognostic markers associated with poor outcomes in acute Babesia canis infections.
Animals and Methods
We compared the results of routine laboratory profiles, hand‐held lactate and glucose analyzer, and the acute phase response in 2 groups of naturally infected dogs (7 survivors and 8 nonsurvivors). Samples were collected at the time of first admission and before any treatment. Subsequently, the course of prognostic markers was followed in 3 dogs experimentally inoculated with B. canis.
Results
Nonsurvivors showed significantly higher concentrations of lactate, triglycerides and phosphate and lower hematocrit, leukocyte counts, total serum protein concentrations, and thrombocyte counts when compared to survivors. All nonsurvivors (8/8) had hyperlactatemia, whereas most survivors (6/7) had values within the reference range. All survivors had leucocyte counts within the reference range, unlike the nonsurvivors, which showed leukopenia. During the course of acute babesiosis, the variables serum lactate, triglyceride, and phosphate concentrations, and thrombocyte count only exceeded a prognostic threshold during acute crisis.
Conclusions and clinical importance
Poor outcome in acute B. canis infection is indicated by changes in the laboratory profile. Intensive care should be considered for dogs presenting with moderate anemia, severe thrombocytopenia, mild to moderate leukopenia, hyperlactatemia, moderately increased serum phosphate, and triglyceride concentrations, and moderately decreased total serum protein concentrations.
Keywords: Biomarker, Canine babesiosis, Dog, Outcome
Abbreviations
- ALT
alanine aminotransferase
- AP
alkaline phosphatase
- APP
acute phase protein
- AST
aspartate aminotransferase
- AUC
area under the curve
- BW
body weight
- CRP
C‐reactive protein
- DIC
disseminated intravascular coagulopathy
- EDTA
ethylenediaminetetraacetic acid
- hpi
hours postinfection
- IFAT
immune fluorescence antibody test
- IQR
interquartile range
- MCH
mean corpuscular hemoglobin
- MCHC
mean corpuscular hemoglobin concentration
- MCV
mean corpuscular volume
- PCR
polymerase chain reaction
- RBC
red blood cell
- ROC
receiver operation characteristic
- SAA
serum amyloid A
- WBC
white blood cell
Canine babesiosis is a tick‐borne disease caused by apicomplexan hemoprotozoan parasites. The 3 distinct large Babesia species, B. canis, B. vogeli, and B. rossi, and the small B. gibsoni, B. conradae, and B. annae have been characterized in dogs.1, 2, 3 Babesia canis is the predominant and clinically relevant canine Babesia species in Europe4 and infection typically is characterized by lethargy, apathy, and pale mucous membranes. The disorder can manifest as a mild or severe form. The clinical signs of the severe form are variable and often related to an excessive inflammatory response syndrome associated with multiple organ dysfunction, shock, and high mortality.3, 5
Hematologic abnormalities in natural and experimental B. canis infections include anemia, thrombocytopenia, and inconsistent leukocyte abnormalities such as leukocytosis, leukopenia, neutrophilia, neutropenia, and eosinophilia. The most common abnormalities in the serum biochemical profile are increases in the activity of aspartate aminotransferase (AST) and alanine aminotransferase (ALT), hyperbilirubinemia, hypoalbuminemia, and electrolyte and acid‐base abnormalities.6, 7, 8, 9, 10 Babesiosis in dogs affects primary and secondary blood coagulation and can induce disseminated intravascular coagulopathy (DIC).11, 12 Furthermore, a systemic inflammatory response syndrome (SIRS) has been described in acute B. canis infection characterized by an acute phase response.12, 13, 14
The clinical manifestations of acute babesiosis are not always proportional to the degree of anemia, and are not correlated with the level of parasitemia, which often remains below 1%.1, 6, 15 Hence, besides mechanical erythrocyte damage, other pathophysiologic mechanisms have been proposed to contribute to hemolysis, such as toxic hemolytic factors and immune‐mediated destruction of erythrocytes.16 Furthermore, disease severity cannot be readily explained as a consequence of hemolysis alone, which often is mild to moderate in acute infections.6 Severe complications of acute Babesia infections have been described such as hemolytic and septic shock, acute renal failure, multiple organ dysfunction syndrome, and other complications.17, 18
The clinical outcome of a B. canis infection is influenced by many factors and the primary pathophysiologic mechanisms of babesiosis in dogs remain unclear.19, 20 In canine B. rossi infections, poor prognosis and mortality are associated with hyperlactatemia, hypoglycemia, clinically compromised circulation, high parasite load, increased serum cortisol concentrations, and signs of consumptive coagulopathy.21, 22, 23, 24, 25 Accordingly in B. canis infections, an excessive inflammatory response with increased concentrations of fibrinogen, C‐reactive protein (CRP) and secreted intracellular adhesion molecule‐1 (sICAM‐1) from erythrocytes, and thrombocytopenia have been associated with poor outcome.19 Furthermore, an increase in lipid mediators has been shown to be associated with severe complications such as development of SIRS and multiple organ dysfunction.5, 26
We aimed to evaluate routine laboratory and rapid in‐clinic laboratory tests for their applicability as prognostic markers associated with poor outcome in acute B. canis infections in dogs.
Material & Methods
Animals
Naturally infected animals
The prognostic potential of different laboratory tests was evaluated in 15 naturally infected animals, of which 12 dogs were presented to the Clinic for Small Animal Internal Medicine at the Vetsuisse Faculty, University of Zurich, and 3 dogs to private veterinary practices in Switzerland in the years 2011 to 2013. Inclusion criteria were the presence of acute clinical signs consistent with canine babesiosis at admission and the identification of large Babesia species by microscopic evaluation of Giemsa‐stained blood smears. In each dog, B. canis diagnosis was confirmed by PCR27 and direct sequencing of the amplicons.1 At time of admission, blood samples were collected, and all animals were treated with antibabesial therapy (a single dose of 3–6 mg/kg body weight [BW] imidocarb diproprionate IM or combined with 10 mg/kg BW doxycycline PO q12h for at least 10 days, and a second dose of imidocarb diproprionate after 14 days). Dogs were enrolled whenever inclusion criteria were met and adequate samples were available. The animals were categorized into 2 groups according to clinical outcome, which was defined as survival (survivor, n = 7 dogs) or death (nonsurvivor, n = 8 dogs). Six of the nonsurvivors died spontaneously within 24 hours of admission and 2 dogs had to be euthanized within 48 hours because of clinical deterioration within 48 hours. Survivors were considered to be cured based on the absence of parasites 14 days after first admission on evaluation of Giemsa‐stained blood smears and PCR.
Experimentally‐infected animals
The course of laboratory test results was evaluated in experimentally‐infected animals. Three facility‐housed adult beagles (of which 1 was 4 years and 2 were 6 years old) were inoculated IV with approximately 1 × 106 parasitized erythrocytes from an isolate stored in liquid nitrogen. The parasite isolate originated from a naturally infected Bernese mountain dog from Switzerland that had travelled to Hungary. The experiments were terminated at the very first signs of acute crisis (which was defined as weak pulse, shallow breathing, somnolence, and any clinical signs of acute shock or central nervous depression). Experiments with dogs were conducted according to Swiss animal rights and regulations standards and approved by the Cantonal Veterinary Office of Zurich (permission number 122/2012) before the study.
Samples
Venous blood samples from the naturally infected dogs were collected into tubes with and without ethylenediaminetetraacetic acid (EDTA) at the time of first admission and before any treatment. Serum and EDTA‐preserved blood samples were collected through an indwelling catheter from the experimentally inoculated dogs at different times. In addition, citrated plasma samples were collected from these dogs at the end of the experiments.
Analysis of blood samples
Parasitemia was expressed as the percentage of infected erythrocytes in Giemsa‐stained blood smears by manually scanning at least 5000 erythrocytes. Exposure to Ehrlichia canis and Anaplasma phagocytophilum was tested by an immuno‐fluorescence antibody test (IFAT).2 , 3 Complete blood cell counts were performed using EDTA‐anticoagulated blood in an automated analyzer.4 Hematologic analysis included total white blood cell (WBC), thrombocyte and red blood cell (RBC) counts and RBC indices. Serum biochemical profiles were performed using an automated analyzer.5 Laboratory reference intervals are stated as 5% and 95% quantiles. Portable hand‐held devices for rapid in‐clinic testing were used to measure concentrations of lactate6 and glucose7 immediately in freshly collected EDTA samples.28, 29 Serum CRP concentration was determined using a canine‐specific immunoturbidimetric assay8 and serum amyloid A (SAA) concentration was measured using a latex agglutination turbimetric immunoassay on an automated analyzer.5 , 9 In the citrated samples from the experimentally infected animals, fibrinogen concentrations were measured using the Clauss method and a semi‐automated coagulometer.10 D‐dimer concentrations were measured on an automated analyzer.5 , 11
Statistical analysis
Results of the 2 groups (survivor and nonsurvivor) of naturally infected dogs were compared by the Mann–Whitney U test. The initially significant variables then were analyzed with receiver operator characteristic (ROC) curves for which the area under the curve (AUC) was calculated. The ROC analysis was used for determining a prognostic cut‐off value for best differentiating between survivors and nonsurvivors with a maximal Youden's index.30, 31 If the cut‐off value fell within the normal reference range, it was set at the corresponding border of the reference. Statistical analyses were performed using a statistical software package.12 A P‐value < .05 was considered statistically significant. The hematologic and serum biochemical profiles from the samples collected at private practices were excluded from the analysis because these variables were measured with other analytical instruments. Hence, for these variables 6 survivors and 6 nonsurvivors were included. For parasitemia, variables from hand‐held devices, and the acute phase response, all of the naturally infected dogs were included in the analysis (7 survivors and 8 nonsurvivors). Graphs were generated using Graph Pad.13
Results
At admission, all of the naturally infected dogs had diverse clinical signs consistent with canine babesiosis, including lethargy (all 15 dogs), pale mucous membranes (all 15 dogs), pigmenturia (10 of 15), icterus (6 of 15), pyrexia (5 of 15), anorexia (4 of 15), vomiting (4 of 15), “water hammer” pulse (4 of 15), and epistaxis (3 of 15). Although Babesia infection was assumed and antibabesial treatment initiated shortly after admission, 8 of the 15 dogs died or had to be euthanized within 2 days of admission. All of the dogs were positive for B. canis in Giemsa‐stained blood smears and by PCR, and none of these dogs reacted serologically to E. canis or A. phagocytophilum on IFAT. Data on characteristics of the individual dogs (animal description, travel history, and clinical signs) are summarized in supplemental file 1. No statistical difference in age, sex, and clinical signs was identified between survivors and nonsurvivors.
The parasitemia ranged between 0.5 and 3.1% (median, 1.2%; interquartile range [IQR], 0.83–1.63), but no statistical difference was identified in the level of parasitemia between the survivors and nonsurvivors. Results of laboratory findings as well as comparison between outcome groups are summarized in Table 1. In both groups of dogs, mild to moderate normochromic normocytic nonregenerative anemia, mild to severe hyperbilirubinemia, mild to moderate azotemia, mild to moderate hypoalbuminemia, mildly increased alkaline phosphatase (AP) activity, moderate to severe hyponatremia, moderate hypocalcaemia, and a mild to moderate increase in CRP concentration were observed commonly. Nonsurvivors had significantly higher concentrations of lactate (P < .001), triglycerides (P < .01), and phosphate (P < .05), and significantly lower hematocrit (P < .05), WBC counts (P < .01), total serum protein concentrations (P < .05), and thrombocyte counts (P < .05) than survivors.
Table 1.
Median values of various variables (minimum–maximum value) in dogs with naturally acute Babesia canis infections: a comparison between survivors and nonsurvivors
| Variable (unit) | Reference range | Survivors | Nonsurvivors | P‐value |
|---|---|---|---|---|
| Parasitemia (%) | 1.2 (0.5–3.1) | 1.25 (0.5–1.9) | 1 | |
| Fast in‐clinic variables | ||||
| Lactate (mmol/L) | <2.5 | 1.6 (0.5–3.6) | 8.35 (4.3–11.8) | <0.001 |
| Glucose (mmol/L) | 3.9–6.7 | 4.8 (4.3–7.6) | 5.2 (3–11.9) | 1 |
| Hematologic variables | ||||
| Hematocrit (%) | 42–55 | 30 (25–40) | 25.5 (17–28) | 0.041 |
| Hemoglobin (g/dL) | 14.4–19.1 | 11.45 (9.2–14.2) | 9.05 (7–18.2) | 0.240 |
| RBC (×106/μL) | 6.1–8.1 | 4.52 (3.95–6.2) | 3.95 (3.05–5.39) | 0.240 |
| MCH (pg) | 23–26 | 24.5 (22–29) | 23 (22–24) | 0.180 |
| MCHC (g/dL) | 34–36 | 35.5 (34–36) | 35 (35–39) | 0.937 |
| MCV (fL) | 64–73 | 67.5 (64–74) | 65 (58–67) | 0.093 |
| WBC (×103/μL) | 4.7–11.3 | 6.85 (5.1–9.2) | 2.65 (1.59–4.5) | 0.002 |
| Thrombocytes (×103/μL) | 130–394 | 45 (17–190) | 14.5 (6–41) | 0.026 |
| Reticulocytes (%) | 0.31 (0–0.81) | 0.53 (0.29–1.17) | 0.132 | |
| Biochemical variables | ||||
| Total bilirubin (μmol/L) | <3.5 | 21.25 (4.7–85.3) | 54.7 (19.7–221.8) | 0.240 |
| Urea (mmol/L) | 3.8–9.4 | 12.65 (4.5–25.7) | 30.45 (6.2–79.4) | 0.065 |
| Creatinine (μmol/L) | 50–119 | 88 (75–133) | 84 (54–651) | 1 |
| Total protein (g/L) | 56–71 | 57 (40–64) | 44 (34–50) | 0.026 |
| Albumin (g/L) | 29–37 | 26.5 (19–38) | 24 (13–26) | 0.240 |
| Cholesterol (mmol/L) | 3.5–8.6 | 6.25 (4.9–7.7) | 6.05 (2.7–9.3) | 0.589 |
| Triglycerides (mmol/L) | 0.4–1.5 | 0.85 (0.8–1.1) | 1.95 (0.9–3.5) | 0.009 |
| Alkaline phosphatase (U/L) | 20–98 | 113.5 (74–184) | 253.5 (61–358) | 0.132 |
| ALT (U/L) | 20–93 | 47.5 (26–72) | 72.5 (30–96) | 0.132 |
| Sodium (mmol/L) | 152–159 | 144.5 (132–154) | 142.5 (140–151) | 0.485 |
| Potassium (mmol/L) | 4.3–5.3 | 4.2 (3.6–4.5) | 4.4 (3.6–5) | 0.394 |
| Chloride (mmol/L) | 113–124 | 113.5 (97–115) | 109 (94–121) | 0.485 |
| Calcium (mmol/L) | 2.4–2.8 | 2.43 (2.11–2.52) | 2.225 (2.1–2.55) | 0.180 |
| Phosphate (mmol/L) | 1.0–1.6 | 1.36 (1.05–1.68) | 2.54 (1.39–3.08) | 0.015 |
| Acute phase response | ||||
| Canine CRP (mg/L) | <5 | 84.7 (3.3–169.8) | 155.55 (22.5–232.8) | 0.189 |
| SAA (mg/L) | <2.19 | 0 (0–2.5) | 0 (0–1.1) | 0.536 |
These 7 initially identified prognostic factors were further analyzed by ROC analysis (Table 2). Of all variables studied, lactate concentrations and WBC counts showed the best prognostic sensitivity and specificity (both 100%) to differentiate between survivors and nonsurvivors. Fig 1 illustrates the significant prognostic variables for dogs in the 2 outcome groups. All nonsurvivors (8 of 8) had moderate to severe hyperlactatemia (median, 8.35 mmol/L; IQR, 7.18–9.13), whereas most survivors (6 of 7) had concentrations within the reference range (median, 1.6 mmol/L; IQR, 1.05–2.3). The WBC counts for all of the survivors (6 of 6) were within the reference range (median, 6.85 × 103/μL; IQR, 6.03–8.2) unlike the group of nonsurvivors, which had mild to moderate leukopenia (6 of 6; median, 2.65 × 103/μL; IQR, 1.7–3.53).
Table 2.
Results of the ROC analysis with prognostic cut‐off values of significantly altered variables and respective sensitivity, specificity, area under the curve (AUC), and standard error (SE) associated with the outcome in Babesia canis infected dogs
| Parameter (unit) | Prognostic cut‐off value | Sensitivity (%) | Specificity (%) | AUC | SE |
|---|---|---|---|---|---|
| Lactate (mmol/L) | 3.95 | 100 | 100 | 1.00 | 0.00 |
| Hematocrit (%) | 28.5 | 66.7 | 100 | 0.86 | 0.11 |
| WBC (×103/μL) | 4.7a | 100 | 100 | 1.00 | 0.00 |
| Thrombocytes (×103/μL) | 27.5 | 83.3 | 83.3 | 0.89 | 0.10 |
| Total protein (g/L) | 50.5 | 83.3 | 100 | 0.88 | 0.12 |
| Triglycerides (mmol/L) | 1.5 | 83.3 | 100 | 0.94 | 0.07 |
| Phosphate (mmol/L) | 1.72 | 83.3 | 100 | 0.92 | 0.09 |
Set at the border of the reference range (calculated cut‐off at 4.8 × 103/μL).
Figure 1.
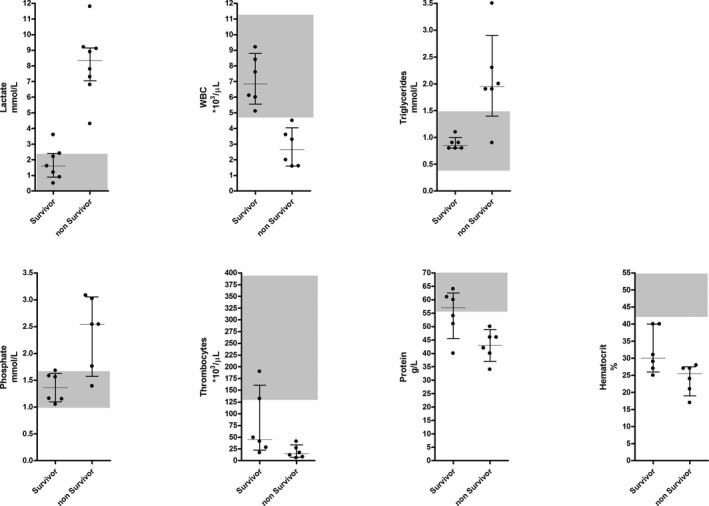
Prognostic markers in acute Babesia canis infected dogs. Median and interquartile range for significant prognostic markers (P < .05) recorded at admission in naturally infected dogs that did or did not survive an acute B. canis infection. Dots correspond to the data from individual dogs; the shaded grey areas represent the reference intervals. WBC: white blood cells.
The course of the prognostic variable, parasitemia, and the acute phase response was followed in the 3 dogs experimentally inoculated with B. canis. The 3 infected dogs became lethargic and showed signs of hemolysis (pale mucous membranes and pigmenturia) 105, 120, and 119 hours postinoculation (on days 4–5), respectively. They had a low grade parasitemia with a maximum of 1.75% of the erythrocytes infected at the end of the experiment, and during the course 2 of the 3 dogs had episodes of pyrexia (Fig 2A). An acute phase response could be observed with a moderate increase in CRP concentration and a moderate decrease in serum albumin concentration (Fig 2B), whereas SAA concentrations remained below the diagnostic limit (data not shown). Follow‐up of prognostic markers is shown in Fig 3. A decrease in the hematologic variables leukocytes, thrombocytes, and hematocrit was found before the identification of parasites in stained blood smears, and resulted in moderate leukopenia, severe thrombocytopenia, and decreased hematocrit. In general, mild to moderate anemia was observed. Changes in lactate, triglyceride, and phosphate concentrations corresponded to the first appearance of parasites, and they only exceeded the prognostic threshold at the first observation of acute crisis. In addition, thrombocytopenia was a common finding and platelet counts exceeded the prognostic threshold toward the end of the experiment. Total serum protein concentrations also decreased over time but passed the threshold only in 2 of the 3 dogs before first signs of an acute breakdown. At the end of the experiment, the 3 dogs showed mildly increased levels of fibrinogen of 2.6 g/L, 3.8 g/L, and 3.2 g/L (reference range, 1.0–2.5 g/L), and D‐dimer concentrations of 0.26 mg/L, 0.44 mg/L, and 0.92 mg/L (reference range, <0.4 mg/L), respectively.
Figure 2.
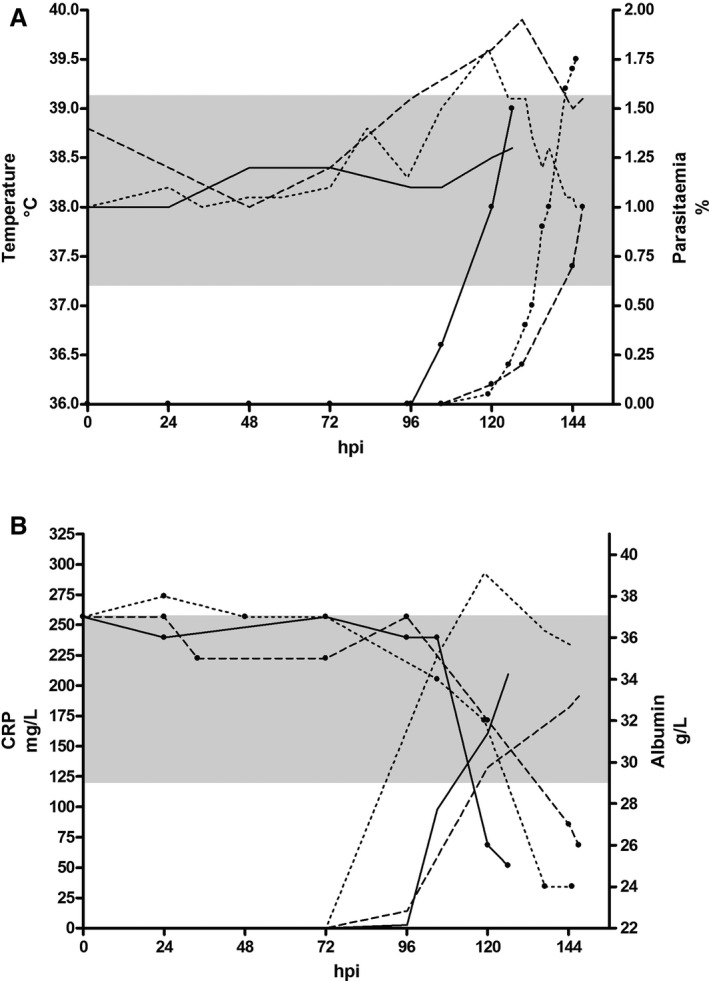
Course of selected variables in 3 experimentally infected dogs. Dog 1: solid line; dog 2: broken line; dog 3: dotted line. (A) Body temperature (left y‐axis) and parasitemia (right y‐axis; lines with dots). The shaded grey area represents the reference interval for the body temperature. (B) CRP (left y‐axis) as a marker for positive acute phase response and albumin (right y‐axis; lines with dots) as a marker for negative acute phase response. The shaded grey area represents the reference interval for albumin. hpi: hours postinfection, CRP: C‐reactive protein.
Figure 3.
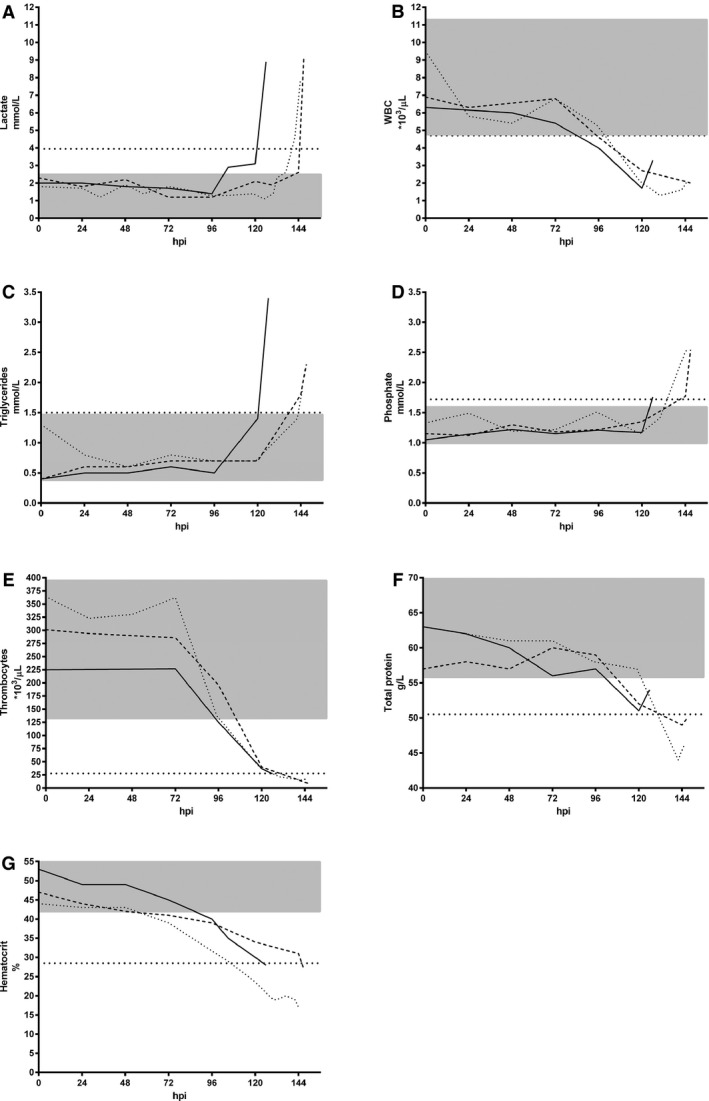
Course of significant prognostic markers in 3 experimentally‐infected dogs. Dog 1: solid line; dog 2: broken line; dog 3: dotted line. The shaded grey areas represent the reference intervals. The horizontal broken lines represent the corresponding prognostic cut‐off. (A) blood lactate, (B) WBC, (C) triglycerides, (D) phosphate, (E) thrombocyte count, (F) total protein, (G) hematocrit. hpi: hours postinfection, WBC: white blood cells.
Discussion
In this study, several variables were shown to be associated with poor outcome in acute Babesia canis infections. By including 2 rapid in‐clinic tests, standard hematologi and biochemical variables, and acute phase proteins, we found the variables lactate, WBC, triglycerides, phosphate, thrombocytes, total serum protein, and hematocrit to be significant prognostic markers. Thus, nonsurvivors at admission had more severe anemia, leukopenia, and thrombocytopenia in addition to alterations in their serum biochemical profile results.
Lactate concentrations were significantly lower in survivors and showed a clear difference from the nonsurvivors. This finding is similar to what is observed in dogs infected with Babesia rossi, the agent of severe canine babesiosis in South Africa, where serum lactate concentration is used for post‐treatment monitoring,21 and high blood lactate concentrations correlate with poor outcome.32, 33, 34 Nevertheless, the pathogenesis of hyperlactatemia in dogs with acute babesiosis is not well established, and it might not be caused by hypoxia as a consequence of anemia, which remains mild to moderate in most B. canis infected animals.34 Hence, hypoxia in canine babesiosis may be the consequence of alterations in the macro‐ and micro‐cirulation triggered by protozoal sepsis, hypotension, DIC, and SIRS, all of which are well known in B. canis infections18, 35. Indeed, increased lactate concentrations have prognostic value in SIRS caused by various conditions.36, 37
The second variable that clearly differentiates between the 2 studied groups was WBC count. Nonsurvivors had mild to moderate leukopenia in contrast to the survivors with WBC counts in the reference range. Although the WBC count was a significant marker for outcome in our study, leukopenia was reported in 60% of mild cases of acute canine babesiosis.17 Indeed, the WBC count fell below the prognostic cut‐off before any clinical signs were observed in the experimentally inoculated dogs. Severely affected dogs had mild to moderate neutropenia, with an overall degenerative tendency and lacking a left shift (see supplemental file 2). Furthermore, lymphopenia seems to be a hallmark of acute canine babesiosis.17, 19 A markedly increased serum cortisol concentration was found in dogs with lethal B. rossi infections, indicating a potential immunosuppressed state in these animals, which also is indicated by an unexpected mild to moderate regenerative response of lymphocytes in dogs that survived.24, 38 Furthermore, studies in humans with acute malaria infections with Plasmodium falciparum and P. vivax, which are related to Babesia spp., identified mechanisms that could explain a depletion of lymphocytes from the peripheral blood by acute sequestration of the cells in the lymph nodes or other parts of the body or by immune cell exhaustion and abnormal cell death through parasite‐induced apoptosis.39, 40 Similarly, toxic parasitic factors have been shown to be involved in canine B. gibsoni infection.41
Hemolytic anemia and thrombocytopenia are the most frequent abnormalities associated with a diagnosis of B. canis in naturally infected dogs and thrombocytopenia usually is the most dramatic hematologic abnormality in the course of babesiosis.12, 42, 43, 44 Our data indicate that severe thrombocytopenia is associated with poor outcome by a prognostic cut‐off of 27,500 thrombocytes per μL, although a sensitivity and specificity of 83.3% for each indicates limited prognostic value. Presumably, several factors are involved in the origin of thrombocytopenia in canine babesiosis including increased platelet activation and consumption by a SIRS (hypercoagulable state), increased platelet sequestration and aggregation, and a decreased platelet production.19, 45, 46 Comparable in B. rossi infections, poor outcome was associated with a consumptive coagulopathy, although even severe thrombocytopenia was not accompanied by apparent bleeding diathesis and hemorrhage.25, 47, 48
Increased phosphate concentrations often are associated with metabolic acidosis characterized by tissue hypoxia and high blood lactate concentrations, although the underlying mechanisms have not been completely explained.49 Hemorrhage, hypovolemia, and shock as cause or consequence of tissue hypoperfusion could further explain changes in altered variables, also including azotemia and potential protein‐losing nephropathy caused by hypoxic renal damage.35 Complications related to hemolytic anemia, coagulation disorders and hypotension, SIRS, and secondary impaired renal function likely account for the severe outcome of the infection.6, 9, 10, 12 Furthermore, in other studies, acute respiratory distress syndrome, renal failure, immune‐mediated hemolytic anemia, cerebral syndrome, and DIC were associated with increased mortality in acute B. canis infections.17, 50
Acute phase proteins were used as prognostic factors for different inflammatory processes,51 and an acute phase response also was observed in acute B. canis infections.12, 13, 14, 20 We measured the acute phase proteins CRP and SAA, because they are considered major APP in dogs52 and are not significantly affected by hyperbilirubinemia, which is commonly present in acute babesiosis.51 We found an increase in CRP before parasite detection as previously observed,12 without any significant difference between the outcome groups. This finding is in accordance with findings in B. rossi infections in which no prognostic value for CRP concentrations was observed.53 Furthermore, the SAA concentrations did not increase significantly in naturally and experimentally infected animals. This finding is in contrast to other observations of increased SAA concentrations in dogs with babesiosis on the day of admission.14 As another indicator, serum albumin concentration could serve as a negative APP.51 With the onset of acute infection, we observed a moderate decrease in serum albumin concentration and it had no prognostic relevance. Although differences between survivors and nonsurvivors were absent for an acute phase response, APP (among other variables) could serve as important variables for monitoring response to therapy.14, 54
In the course of validating prognostic markers in 3 experimentally inoculated dogs, we observed low grade parasitemia with a maximum of 1.75% of infected erythrocytes, which was comparable to the group of naturally infected animals. Even in infections with serious clinical signs, low parasitemia is a common finding in B. canis infections.1, 6, 12 The course in the infected dogs highlights the prognostic value of lactate, triglycerides, and phosphate concentrations, and thrombocyte counts, because these factors only crossed the prognostic threshold in an acute crisis. Referring to the ROC analysis of these variables, only lactate showed optimal characteristics. Therefore, any prognosis based on individual variables should be interpreted with caution.
Missing data about the course of disease before admission and the time point of infection in the naturally infected dogs is a limitation of this study. Generally, practitioners inquire about the duration of illness and the appearance of the first clinical signs, and they can estimate the time of the infection in affected dogs. In this respect, the prognostic markers are helpful for guiding clinical decision making. To get an overall picture of individual cases, a systematic collection of clinical, laboratorial, and other individual factors must be emphasized. For example, in our cohort of infected dogs, circulatory disturbances were detected in 4 relatively young dogs (7 month to approximately 3 years), of which 3 dogs died (see supplemental file 1). Such clinical variables could affect outcome in the laboratory test results and the likely progression of a patient's infection.55 In any case, outcome depends on a rapid diagnosis and early treatment.
Mortality in the investigated group of dogs was higher as compared to an endemic area.5 This finding reflects a typical situation for nonendemic areas such as Switzerland, where dogs became infected from local Babesia outbreaks or have traveled to an endemic area. These dogs likely never have had contact with the parasite and therefore did not develop partial immunity.46, 56 Nonetheless, findings on mortality rates should not be over interpreted because of the small sample size. In our cohort, we included every possible case for which we could obtain comparable clinicopathologic data.
Unfortunately, we did not have precise data about infection rates in dogs in Switzerland. However, during the sampling period, 2 indigenous outbreaks were reported in 44 dogs, of which 10 died.57, 58 Most indigenous cases in our cohort originated from these areas (4 survivors and 1 nonsurvivor), whereas 1 dog originated from Geneva, a known endemic region in Switzerland.59 The remaining 9 infected dogs had a positive travel history. Information about infection rate in dogs in Switzerland that have travelled is rare. For example, from 2011 to 2013, the diagnostic unit of the Institute of Parasitology in Zurich (which offers a travel screening panel) identified 2.1% of 804 samples as positive on blood smears for large Babesia species (F. Grimm, personal communication). This observation is in agreement with observed cases in dogs in Germany that have travelled, with 3.7% (19/508) of animals positive for large Babesia spp. in Giemsa‐stained blood or buffy coat smears.60 Hence, to compensate for the small sample size, prognostic markers were cross‐validated in the course of experimental babesiosis.
Although a significant prognostic marker is not necessarily clinically relevant, the pathophysiologic reason for death would be of interest. With this in mind, additional studies should include postmortem examination, and more prognostic factor studies should be conducted including other nonroutine variables. This study focused on rapid in‐practice tests (e.g. lactate and glucose determined by hand‐held analyzers) and routine laboratory variables, and the associated findings summarize the prognostic value of these variables. Nevertheless, additional research is needed to evaluate what additional evaluation and intensive care is needed for dogs with a poor prognosis. In this context, several markers have been demonstrated as good variables for follow‐up and post‐treatment monitoring after antibabesial therapy, such as APP, lactate, thrombocytes, and leukocytes.13, 14, 21, 43, 54
Supporting information
Supplemental File 1. Characteristics of the individual dogs (animal description, travel history, and clinical signs).
Supplemental File 2. Data on differential WBC count. Differential WBC count in the course of 3 experimentally inoculated dogs, and in dogs that did or did not survive a naturally acquired acute Babesia canis infection.
Acknowledgments
We thank the animal keepers, A. Rüdemann and B. Brändle, for their support throughout the study. We thank veterinary practitioners Drs. C. Boller, and B. and R. Pool for providing samples and data on Babesia cases. We are indebted to Robert A. Walker (James Cook University, Cairns) for linguistic revision. RME was a recipient of grants from the “Forschungskredit” of the University of Zurich (grant nos. 55080506 and FK‐13‐053), and this study is part of his PhD thesis.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Ethical Standards Statement: Animal experiments were carried out at the experimental units of the Vetsuisse Faculty at the University of Zurich after approval by the Cantonal Veterinary Office of Zurich (permission number 122/2012) according to Swiss animal rights and regulation standards.
Footnotes
Synergene GmbH, Schlieren, Switzerland
Mega Screen Fluoehrlichia c., MegaCor Diagnostik GmbH, Hörbranz, Austria
E. equi FA substrate slide, VMRD, Inc. Pulma, Washington, USA
Sysmex XT‐2000iV, Sysmex Corporation, Kobe, Japan
Cobas Integra 800, Roche Diagnostics, Rotkreuz, Switzerland
Lactate Pro, Axon Lab AG, Baden, Switzerland
Accu‐Chek, Roche Diagnostics AG, Rotkreuz, Switzerland
Gentian cCRP; Gentian AS, Moss, Norway
LZ Test SAA; Eiken Chemical Co., Ltd., Tokyo, Japan
STart 4, Roche Diagnostics AG, Rotkreuz, Switzerland
Tina‐quant D‐Dimer Gen.2, Roche Diagnostics AG, Rotkreuz, Switzerland
IBM SPSS statistics, 20.0.0, IBM Corp. Armonk, NY, USA
Graph Pad Prism 4, Graph Pad Software, San Diego, USA
References
- 1. Schetters TP, Moubri K, Precigout E, et al. Different Babesia canis isolates, different diseases. Parasitology 1997;115:485–493. [DOI] [PubMed] [Google Scholar]
- 2. Uilenberg G, Franssen FF, Perie NM, et al. Three groups of Babesia canis distinguished and a proposal for nomenclature. Vet Q 1989;11:33–40. [DOI] [PubMed] [Google Scholar]
- 3. Boozer AL, Macintire DK. Canine babesiosis. Vet Clin North Am Small Anim Pract 2003;33:885–904. [DOI] [PubMed] [Google Scholar]
- 4. Halos L, Lebert I, Abrial D, et al. Questionnaire‐based survey on the distribution and incidence of canine babesiosis in countries of Western Europe. Parasite 2014;21:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Matijatko V, Torti M, Schetters TP. Canine babesiosis in Europe: how many diseases? Trends Parasitol 2012;28:99–105. [DOI] [PubMed] [Google Scholar]
- 6. Furlanello T, Fiorio F, Caldin M, et al. Clinicopathological findings in naturally occurring cases of babesiosis caused by large form Babesia from dogs of northeastern Italy. Vet Parasitol 2005;134:77–85. [DOI] [PubMed] [Google Scholar]
- 7. Lobetti R. Changes in the serum urea: creatinine ratio in dogs with babesiosis, haemolytic anaemia, and experimental haemoglobinaemia. Vet J 2011;191:253–256. [DOI] [PubMed] [Google Scholar]
- 8. Zygner W, Gojska‐Zygner O, Norbury LJ, et al. Increased AST/ALT ratio in azotaemic dogs infected with Babesia canis . Pol J Vet Sci 2012;15:483–486. [DOI] [PubMed] [Google Scholar]
- 9. Zygner W, Gojska‐Zygner O, Wedrychowicz H. Strong monovalent electrolyte imbalances in serum of dogs infected with Babesia canis . Ticks Tick Borne Dis 2012;3:107–113. [DOI] [PubMed] [Google Scholar]
- 10. Ruiz de Gopegui R, Penalba B, Goicoa A, et al. Clinico‐pathological findings and coagulation disorders in 45 cases of canine babesiosis in Spain. Vet J 2007;174:129–132. [DOI] [PubMed] [Google Scholar]
- 11. Barić Rafaj R, Matijatko V, Kĭs I, et al. Alterations in some blood coagulation parameters in naturally occurring cases of canine babesiosis. Acta Vet Hung 2009;57:295–304. [DOI] [PubMed] [Google Scholar]
- 12. Schetters TP, Kleuskens JA, Van De Crommert J, et al. Systemic inflammatory responses in dogs experimentally infected with Babesia canis; a haematological study. Vet Parasitol 2009;162:7–15. [DOI] [PubMed] [Google Scholar]
- 13. Ulutas B, Bayramli G, Ulutas PA, et al. Serum concentration of some acute phase proteins in naturally occurring canine babesiosis: a preliminary study. Vet Clin Pathol 2005;34:144–147. [DOI] [PubMed] [Google Scholar]
- 14. Matijatko V, Mrljak V, Kĭs I, et al. Evidence of an acute phase response in dogs naturally infected with Babesia canis . Vet Parasitol 2007;144:242–250. [DOI] [PubMed] [Google Scholar]
- 15. Irwin PJ. Canine babesiosis. Vet Clin North Am Small Anim Pract 2010;40:1141–1156. [DOI] [PubMed] [Google Scholar]
- 16. Carli E, Tasca S, Trotta M, et al. Detection of erythrocyte binding IgM and IgG by flow cytometry in sick dogs with Babesia canis canis or Babesia canis vogeli infection. Vet Parasitol 2009;162:51–57. [DOI] [PubMed] [Google Scholar]
- 17. Máthé A, Vörös K, Nemeth T, et al. Clinicopathological changes and effect of imidocarb therapy in dogs experimentally infected with Babesia canis . Acta Vet Hung 2006;54:19–33. [DOI] [PubMed] [Google Scholar]
- 18. Matijatko V, Kĭs I, Torti M, et al. Septic shock in canine babesiosis. Vet Parasitol 2009;162:263–270. [DOI] [PubMed] [Google Scholar]
- 19. Barić Rafaj R, Kules J, Selanec J, et al. Markers of coagulation activation, endothelial stimulation, and inflammation in dogs with babesiosis. J Vet Intern Med 2013;27:1172–1178. [DOI] [PubMed] [Google Scholar]
- 20. Kuleš J, Mrljak V, Rafaj RB, et al. Identification of serum biomarkers in dogs naturally infected with Babesia canis canis using a proteomic approach. BMC Vet Res 2014;10:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nel M, Lobetti RG, Keller N, et al. Prognostic value of blood lactate, blood glucose, and hematocrit in canine babesiosis. J Vet Intern Med 2004;18:471–476. [DOI] [PubMed] [Google Scholar]
- 22. Keller N, Jacobson LS, Nel M, et al. Prevalence and risk factors of hypoglycemia in virulent canine babesiosis. J Vet Intern Med 2004;18:265–270. [DOI] [PubMed] [Google Scholar]
- 23. Böhm M, Leisewitz AL, Thompson PN, et al. Capillary and venous Babesia canis rossi parasitaemias and their association with outcome of infection and circulatory compromise. Vet Parasitol 2006;141:18–29. [DOI] [PubMed] [Google Scholar]
- 24. Schoeman JP, Herrtage ME. Adrenal response to the low dose ACTH stimulation test and the cortisol‐to‐adrenocorticotrophic hormone ratio in canine babesiosis. Vet Parasitol 2008;154:205–213. [DOI] [PubMed] [Google Scholar]
- 25. Goddard A, Wiinberg B, Schoeman JP, et al. Mortality in virulent canine babesiosis is associated with a consumptive coagulopathy. Vet J 2013;196:213–217. [DOI] [PubMed] [Google Scholar]
- 26. Mrljak V, Kucer N, Kules J, et al. Serum concentrations of eicosanoids and lipids in dogs naturally infected with Babesia canis . Vet Parasitol 2014;201:24–30. [DOI] [PubMed] [Google Scholar]
- 27. Hilpertshauser H, Deplazes P, Schnyder M, et al. Babesia spp. identified by PCR in ticks collected from domestic and wild ruminants in southern Switzerland. Appl Environ Microbiol 2006;72:6503–6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thorneloe C, Bedard C, Boysen S. Evaluation of a hand‐held lactate analyzer in dogs. Can Vet J 2007;48:283–288. [PMC free article] [PubMed] [Google Scholar]
- 29. Cohen TA, Nelson RW, Kass PH, et al. Evaluation of six portable blood glucose meters for measuring blood glucose concentration in dogs. J Am Vet Med Assoc 2009;235:276–280. [DOI] [PubMed] [Google Scholar]
- 30. Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32–35. [DOI] [PubMed] [Google Scholar]
- 31. Greiner M, Sohr D, Gobel P. A modified ROC analysis for the selection of cut‐off values and the definition of intermediate results of serodiagnostic tests. J Immunol Methods 1995;185:123–132. [DOI] [PubMed] [Google Scholar]
- 32. Button C. Metabolic and electrolyte disturbances in acute canine babesiosis. J Am Vet Med Assoc 1979;175:475–479. [PubMed] [Google Scholar]
- 33. Leisewitz AL, Jacobson LS, de Morais HS, et al. The mixed acid‐base disturbances of severe canine babesiosis. J Vet Intern Med 2001;15:445–452. [DOI] [PubMed] [Google Scholar]
- 34. Jacobson LS, Lobetti RG. Glucose, lactate, and pyruvate concentrations in dogs with babesiosis. Am J Vet Res 2005;66:244–250. [DOI] [PubMed] [Google Scholar]
- 35. Zygner W, Gojska‐Zygner O. Association between decreased blood pressure and azotaemia in canine babesiosis. Pol J Vet Sci 2014;17:173–175. [DOI] [PubMed] [Google Scholar]
- 36. Cortellini S, Seth M, Kellett‐Gregory LM. Plasma lactate concentrations in septic peritonitis: a retrospective study of 83 dogs (2007–2012). J Vet Emerg Crit Care 2015;25:388–395. [DOI] [PubMed] [Google Scholar]
- 37. Giunti M, Troia R, Bergamini PF, et al. Prospective evaluation of the acute patient physiologic and laboratory evaluation score and an extended clinicopathological profile in dogs with systemic inflammatory response syndrome. J Vet Emerg Crit Care 2015;25:226–233. [DOI] [PubMed] [Google Scholar]
- 38. Scheepers E, Leisewitz AL, Thompson PN, et al. Serial haematology results in transfused and non‐transfused dogs naturally infected with Babesia rossi . J S Afr Vet Assoc 2011;82:136–143. [DOI] [PubMed] [Google Scholar]
- 39. Kassa D, Petros B, Mesele T, et al. Characterization of peripheral blood lymphocyte subsets in patients with acute Plasmodium falciparum and P. vivax malaria infections at Wonji Sugar Estate, Ethiopia. Clin Vaccine Immunol 2006;13:376–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wykes MN, Horne‐Debets JM, Leow CY, et al. Malaria drives T cells to exhaustion. Front Microbiol 2014;5:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Onishi T, Ueda K, Horie M, et al. Serum hemolytic activity in dogs infected with Babesia gibsoni . J Parasitol 1990;76:564–567. [PubMed] [Google Scholar]
- 42. Fabisiak M, Sapierzyński R, Kluciński W. Analyis of haematological abnormalities observed in dogs infected by a large Babesia. Bull Vet Inst Pulawy 2010;54:167–170. [Google Scholar]
- 43. Žvorc Z, Baric Rafaj R, Kules J, et al. Erythrocyte and platelet indices in babesiosis of dogs. Vet Arhiv 2010;80:259–267. [Google Scholar]
- 44. Kirtz G, Leschnik M, Hooijberg E, et al. In‐clinic laboratory diagnosis of canine babesiosis (Babesia canis canis) for veterinary practitioners in Central Europe. Tierarztl Prax Ausg K 2012;40:87–94. [PubMed] [Google Scholar]
- 45. Reyers F, Leisewitz AL, Lobetti RG, et al. Canine babesiosis in South Africa: more than one disease. Does this serve as a model for falciparum malaria? Ann Trop Med Parasitol 1998;92:503–511. [PubMed] [Google Scholar]
- 46. Brandao LP, Hagiwara MK, Myiashiro SI. Humoral immunity and reinfection resistance in dogs experimentally inoculated with Babesia canis and either treated or untreated with imidocarb dipropionate. Vet Parasitol 2003;114:253–265. [DOI] [PubMed] [Google Scholar]
- 47. Kettner F, Reyers F, Miller D. Thrombocytopaenia in canine babesiosis and its clinical usefulness. J S Afr Vet Assoc 2003;74:63–68. [DOI] [PubMed] [Google Scholar]
- 48. Liebenberg C, Goddard A, Wiinberg B, et al. Hemostatic abnormalities in uncomplicated babesiosis (Babesia rossi) in dogs. J Vet Intern Med 2013;27:150–156. [DOI] [PubMed] [Google Scholar]
- 49. Stockham SL, Scott MA. Fundamentals of Veterinary Clinical Pathology, 2nd ed Ames, Iowa, USA: Blackwell Publishing; 2008. [Google Scholar]
- 50. Möhr AJ, Lobetti RG, van der Lugt JJ. Acute pancreatitis: a newly recognised potential complication of canine babesiosis. J S Afr Vet Assoc 2000;71:232–239. [DOI] [PubMed] [Google Scholar]
- 51. Ceron JJ, Eckersall PD, Martynez‐Subiela S. Acute phase proteins in dogs and cats: current knowledge and future perspectives. Vet Clin Pathol 2005;34:85–99. [DOI] [PubMed] [Google Scholar]
- 52. Eckersall PD, Bell R. Acute phase proteins: Biomarkers of infection and inflammation in veterinary medicine. Vet J 2010;185:23–27. [DOI] [PubMed] [Google Scholar]
- 53. Köster LS, Van Schoor M, Goddard A, et al. C‐reactive protein in canine babesiosis caused by Babesia rossi and its association with outcome. J S Afr Vet Assoc 2009;80:87–91. [DOI] [PubMed] [Google Scholar]
- 54. Rossi G, Kuleš J, Barić Rafaj R, et al. Relationship between paraoxonase 1 activity and high density lipoprotein concentration during naturally occurring babesiosis in dogs. Res Vet Sci 2014;97:318–324. [DOI] [PubMed] [Google Scholar]
- 55. Webster JD, Dennis MM, Dervisis N, et al. Recommended guidelines for the conduct and evaluation of prognostic studies in veterinary oncology. Vet Pathol 2011;48:7–18. [DOI] [PubMed] [Google Scholar]
- 56. Martinod S, Laurent N, Moreau Y. Resistance and immunity of dogs against Babesia canis in an endemic area. Vet Parasitol 1986;19:245–254. [DOI] [PubMed] [Google Scholar]
- 57. Schaarschmidt D, Gilli U, Gottstein B, et al. Questing Dermacentor reticulatus harbouring Babesia canis DNA associated with outbreaks of canine babesiosis in the Swiss Midlands. Ticks Tick Borne Dis 2013;4:334–340. [DOI] [PubMed] [Google Scholar]
- 58. Eichenberger RM, Deplazes P, Mathis A. Ticks on dogs and cats: A pet owner‐based survey in a rural town in northeastern Switzerland. Ticks Tick Borne Dis 2015;6:267–271. [DOI] [PubMed] [Google Scholar]
- 59. Porchet MJ, Sager H, Muggli L, et al. A descriptive epidemiological study on canine babesiosis in the Lake Geneva region. Schweiz Arch Tierheilkd 2007;149:457–465. [DOI] [PubMed] [Google Scholar]
- 60. Hamel D, Rohrig E, Pfister K. Canine vector‐borne disease in travelled dogs in Germany‐a retrospective evaluation of laboratory data from the years 2004–2008. Vet Parasitol 2011;181:31–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental File 1. Characteristics of the individual dogs (animal description, travel history, and clinical signs).
Supplemental File 2. Data on differential WBC count. Differential WBC count in the course of 3 experimentally inoculated dogs, and in dogs that did or did not survive a naturally acquired acute Babesia canis infection.


