Abstract
Cardiac troponins are sensitive and specific markers of myocardial injury. The troponin concentration can be thought of as a quantitative measure of the degree of injury sustained by the heart, however, it provides no information on the cause of injury or the mechanism of troponin release. Conventionally, the cardiac troponins have been used for diagnosis of acute myocardial infarction in humans and have become the gold standard biomarkers for this indication. They have become increasingly recognized as an objective measure of cardiomyocyte status in both cardiac and noncardiac disease, supplying additional information to that provided by echocardiography and ECG. Injury to cardiomyocytes can occur through a variety of mechanisms with subsequent release of troponins. Independent of the underlying disease or the mechanism of troponin release, the presence of myocardial injury is associated with an increased risk of death. As increasingly sensitive assays are introduced, the frequent occurrence of myocardial injury is becoming apparent, and our understanding of its causes and importance is constantly evolving. Presently troponins are valuable for detecting a subgroup of patients with higher risk of death. Future research is needed to clarify whether troponins can serve as monitoring tools guiding treatment, whether administering more aggressive treatment to patients with evidence of myocardial injury is beneficial, and whether normalizing of troponin concentrations in patients presenting with evidence of myocardial injury is associated with reduced risk of death.
Keywords: Biomarker, Cardiac troponins, Companion animals, Myocardial injury
Abbreviations
- AMI
acute myocardial infarction
- APPLE
Acute Patient Physiologic and Laboratory Evaluation
- ARVC
arrhythmogenic right ventricular cardiomyopathy
- cTnC
cardiac troponin C
- cTnI
cardiac troponin I
- cTnT
cardiac troponin T
- DCM
dilated cardiomyopathy
- EDTA
ethylenediaminetetraacetic acid
- GDV
gastric dilatation volvulus
- HCM
hypertrophic cardiomyopathy
- HP
heparin plasma
- ICU
intensive care unit
- IL
interleukin
- IMHA
immune‐mediated hemolytic anemia
- MMVD
myxomatous mitral valve disease
- MODS
multiple organ dysfunction syndrome
- PS
pulmonic stenosis
- S
serum
- SAS
subaortic stenosis
- SIRS
systemic inflammatory response syndrome
- TNF‐α
tumor necrosis factor α
A biomarker is “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention”.1 The field of cardiac biomarkers is continuously evolving. No cardiac marker has yet gained its place in the general veterinary biochemical profile along with, for instance, renal and hepatic biomarkers, and the status of the heart is evaluated mainly through auscultation, ECG, and echocardiography. However, the benefit of applying cardiac biomarkers in the clinical work‐up of critically ill patients (with or without heart disease as their primary diagnosis) is being explored and is showing promise. Among such promising biomarkers are the cardiac troponins. The aim of this review is to describe the current knowledge of cardiac troponins as diagnostic and prognostic markers in dogs and cats compared with that in humans.
Cardiomyocyte Physiology
The myocardial muscle cell is known as a cardiomyocyte. Each cardiomyocyte consists of multiple myofibrils arranged in parallel (Fig 1). A myofibril consists of a linear series of sarcomeres, the functional contractile unit of the cell. A sarcomere contains two types of protein filaments. Thin actin filaments, each consisting of a double helix of actin monomers, project from so‐called Z disks at the ends of the sarcomere. Thick myosin filaments cross‐link at the sarcomere center from where they interdigitate with the actin filaments. A myosin filament contains a series of myosin molecules, each with a helical tail and two globular heads.2, 3, 4 During muscle contraction the many globular heads of a myosin filament repetitively interact with actin in a cross‐bridge cycle, thereby pulling the thin filament along the thick filament to shorten the sarcomere. During muscle relaxation the sites of actin and myosin interaction are sterically blocked by the protein tropomyosin residing in the actin helical groove and a ternary cardiac troponin protein complex located at regular intervals along the actin filament.2, 3, 4, 5, 6 Troponin consists of 3 subunits which together function as the molecular switch of cardiomyocyte contraction. Cardiac troponin T (cTnT), the tropomyosin‐binding subunit, secures the complex to the thin filament. The additional subunits are responsible for inhibition and promotion of contraction mediated through calcium and ATP. In the absence of calcium, cardiac troponin I (cTnI), the inhibitory subunit, inhibits the hydrolysis of ATP necessary for actin and myosin interaction. Calcium is the initiator of contraction, removing the steric blockage of filament interaction through binding to the calcium‐binding subunit, cardiac troponin C (cTnC).2, 3, 5, 7, 8
Figure 1.
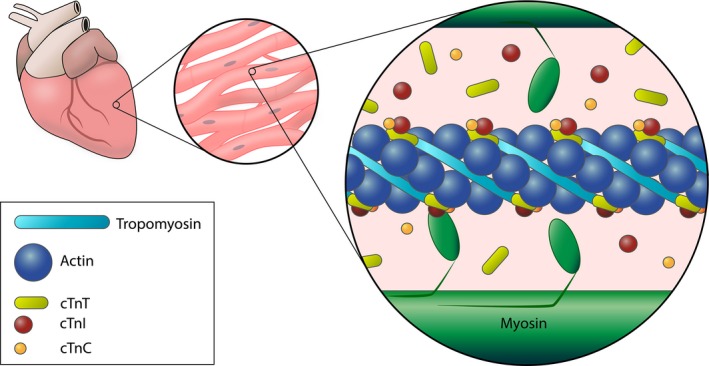
The contractile apparatus of a cardiomyocyte. Interaction of thin (actin) and thick (myosin) filaments is mediated by the troponin complex (troponin I, T, and C) in the presence of calcium. The majority of troponin is structurally bound to the actin filament and its associated protein tropomyosin. A small percentage is found free in the cytosol.
Troponin Characteristics
Troponin I and T subunits have tissue‐specific isoforms for cardiac and skeletal (slow and fast‐twitch) muscle.9 For troponin C the cardiac isoform and one skeletal isoform are completely homologous,9 making the subunit unfit to be used as a cardiac marker. In the remainder of the text the term cardiac troponins will, therefore, refer only to cTnI and cTnT. Cardiac troponin T isoforms share more than 50% homology with skeletal isoforms, but can be separately identified.10, 11 Fetal cardiac isoforms are sometimes expressed in diseased or injured skeletal muscle,12 however, and could, in rare cases, compromise the cardiac specificity of cTnT.13 Adult heart cTnT has a molecular weight of 37 kDa. Cardiac troponin I is a slightly smaller protein of 24 kDa.9 It shares <50% homology with skeletal isoforms and contains a unique N‐terminal peptide.10, 11 It is not expressed in skeletal muscle during disease states and is, thus, uniquely cardiac.14, 15, 16 The full gene sequence of cTnI in dogs and cats has been determined, and the homology between canine/feline and human cTnI genes is 95 and 96%.17
As the troponins are purely intracellular proteins, their presence in circulation reflects intracellular content release from cardiomyocytes.18 The majority of troponin in the cell is structurally bound in the contractile apparatus and is sometimes referred to as the structural pool, while a minor amount of free cytosolic troponin makes up the so‐called cytosolic pool (Fig 1). In humans the cytosolic pool accounts for approximately 6–8% of cTnT19 and 3–4% of cTnI,20 whereas one study comparing humans and dogs found 8% cytosolic cTnT in humans, but only 2% in dogs.21 When destruction of a cardiomyocyte occurs, the cytosolic pool is released quickly with a resultant early rise in circulating troponin. This is followed by the slower release of the structural pool as the contractile apparatus is broken down, resulting in a sustained increase in circulating troponin for days to weeks.19, 20, 22, 23 It is believed that the cytosolic pool alone can also be independently released.22, 24 A blood sample cannot be used to distinguish between the release of only cytosolic or both cytosolic and structural troponin. Release kinetics are complex as the time to peak concentrations and the size of this increase depend on the cause and mechanism of troponin release.22, 25 However, after a cardiac insult, a rise can be seen within 2–3 h,26 and peak concentration is frequently reached in 18–24 h.23
Six pathobiological mechanisms are believed to be responsible for cardiac troponin release either individually or in combination (Fig 2).16, 27 Three mechanisms refer to cell death with resultant release of both cytosolic and structural troponin. Although troponin release is well‐described and always occurs with cell necrosis,28 it is currently unknown whether troponin release does, in fact, occur with cell apoptosis29, 30, 31 or with normal cardiomyocyte turnover.32 Three other mechanisms account for the release of only cytosolic troponin without cell death. Troponin molecules can be subjected to intracellular proteolysis with subsequent release of degradation products small enough to pass through the cell membrane.33 Increased membrane permeability has also been documented in some disease states, resulting in membrane gaps large enough to allow release of the intact protein.34, 35 Finally, it is possible that formation and release of membranous vesicles containing cytosolic troponin can occur from viable cells.22, 36
Figure 2.
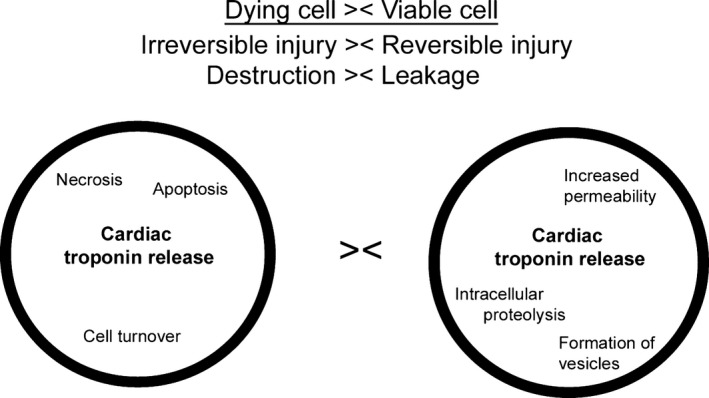
Possible mechanisms of troponin release from dying and from viable cardiomyocytes.
Cardiac troponin I is released in several fold higher concentrations than cTnT after a cardiac insult.8, 37, 38 This might reflect the smaller molecular size of cTnI, but it has also been suggested that cTnT is more tightly bound to the contractile apparatus.38, 39 Accordingly, presence of increased cTnT and cTnI concentrations appears to reveal more severe cardiac injury than increased cTnI alone.
The half‐life of cTnI and cTnT in humans is approximately 2 h when only the cytosolic pool is released, whereas a considerably longer half‐life is seen with release of the structural pool caused by a slow breakdown of the contractile apparatus.22, 40 In the dog, the half‐life of free (experimentally injected, corresponding to cytosolic) cTnI is 1.85 h,41 and release kinetics of cTnT have also been found similar to those of humans.21 Accordingly, release kinetics in dogs and cats are assumed to mimic those of humans. In humans, cardiac troponin is released as the complete ternary complex, as free subunits, but predominantly as a complex of cTnI‐cTnC.42, 43 Whether the same applies for dogs and cats is currently unknown. Additionally, cTnI is often released in a phosphorylated form,44 and troponins undergo proteolysis, oxidation, and reduction in circulation resulting in a variety of different circulating peptides.19, 43
The pathway of elimination of cardiac troponin has not been clarified. Because of its size it has been thought to be eliminated through the reticulo‐endothelial system.45, 46 However, renal clearance of smaller degradation products might also be involved.47
Summing up the above, circulating cardiac troponins have many characteristics of an ideal biomarker: cardiac specificity, high sensitivity for injury (high myocardial tissue content and early release after a cardiac insult),10, 22 negligible presence in circulation of healthy individuals, a high dynamic range, persistence in circulation for days post injury, and correlation with severity of injury.8 Troponins are heart‐specific, but it is important to keep in mind that they are not disease‐specific. Accordingly, an increased troponin concentration reflects myocardial injury irrespective of its cause.18 Another important fact is that troponins do not replace advanced cardiac diagnostics (ie, echocardiography and ECG) in evaluating the heart. Mild primary cardiac disease does not always result in cardiac injury, and exclusion of cardiac disease should, therefore, only follow a complete cardiac work‐up.48, 49
Measurement of Cardiac Troponins
The first troponin assays were described in 1987 (cTnI)50 and 1989 (cTnT).51 The cTnT assay has only been produced by a single manufacturer, and its sensitivity has increased with newer generations of the assay, the most recent being the 5th generation assay. It has been speculated, however, that some of the assay's cross‐species reactivity with animal cTnT might have been lost in the process.52 The cTnT assay has not been validated for use in dogs and cats, but numerous studies exist in which the various generations of the assay have been used in dogs with satisfactory results (Table 2). Only one study has been published in which the cTnT assay has been applied in cats.48 For cTnI, multiple assays have been developed by a range of manufacturers, and, accordingly, assays apply antibodies targeting different amino acid sequences.10 Consequently, results are not easily comparable. Studies comparing different assays have found a reasonable correlation at low concentrations, but a considerable disagreement at high concentrations.53 This disagreement could be due to the release of a higher percentage of modified troponin peptides in severe cardiac injury, with assays having varying ability to detect these forms.53 Two cTnI assays have been validated for use in dogs and one in cats, showing acceptable analytical and overlap performance.54, 55
Table 2.
Myocardial injury in cardiac and noncardiac diseases in dogs
| Species | Cardiac disease | Noncardiac disease* | cTnI measured | cTnT measured |
|---|---|---|---|---|
| Dog | SAS | 71 | ||
| PS | 107 | |||
| MMVD | 49, 58, 71, 72, 73, 74, 108, 109, 110, 111 | 109 | ||
| DCM | 49, 71, 75, 83, 108, 112, 113 | 112, 114, 115 | ||
| ARVC | 71, 79, 116 | |||
| Myocarditis | 117 | |||
| Dirofilariasis | 118, 119, 120 | 118 | ||
| Cardiac hemangiosarcoma | 121 | |||
| Pericardial effusion | 39, 49, 122 | 39 | ||
| Pancreatitis | 52 | |||
| Pyometra | 123, 124 | |||
| Parvoviral enteritis | 125, 126 | |||
| Leptospirosis | 127 | |||
| Leishmaniasis | 128, 129 | |||
| Babesiosis | 130, 131 | 130 | ||
| Erhlichiosis | 132 | |||
| Systemic inflammation | 63 | 63 | ||
| SIRS | 133 | |||
| Meningitis‐arteritis | 134, 135 | |||
| IMHA | 136 | |||
| Anemia | 52 | |||
| Neoplasia (mixed) | 52, 121, 137 | 115 | ||
| Lymphoma | 52 | |||
| Meningioma | 138 | |||
| Hemangiosarcoma | 121 | |||
| Respiratory disease | 52, 139 | |||
| Brachycephalic syndrome | 140 | |||
| Hypoadrenocorticism | 52 | |||
| Hyperadrenocorticism | 52 | |||
| Snake envenomation | 141, 142, 143 | 143 | ||
| Heatstroke | 144 | |||
| GDV | 145, 146 | 146, 147 |
cTnI, Cardiac troponin I; cTnT, Cardiac troponin T; SAS, Subaortic stenosis; PS, Pulmonic stenosis; MMVD, Myxomatous mitral valve disease; DCM, Dilated cardiomyopathy; ARVC, Arrhythmogenic right ventricular cardiomyopathy; HCM, Hypertrophic cardiomyopathy; IMHA, Immune‐mediated hemolytic anemia; GDV, Gastric dilatation‐volvulus; SIRS, Systemic inflammatory response syndrome.
*NB: Not all studies in the noncardiac disease group have ruled out primary cardiac disease as a cause of myocardial injury.
In recent years increasingly sensitive assays have been introduced, and the term “high‐sensitivity” has often been used indiscriminately to include all these assays with a higher sensitivity than the so‐called conventional assays. With conventional assays troponin concentrations in healthy individuals were below the detection limit. This meant that the true upper reference limit could not be determined and led to many studies assessing the biomarker qualitatively, with patients classified as “troponin positive” (detectable) and “troponin negative” (undetectable).56, 57 With the sensitivity of current assays, the actual concentrations of cardiac troponins in healthy individuals are becoming apparent, and a quantitative interpretation of troponin concentrations is strongly recommended as troponin concentrations correlate well with both clinical disease severity58, 59 and with the degree of cardiac injury seen histopathologically.60, 61, 62 A definition of the term “high‐sensitivity cardiac troponin assay” has recently been published to help navigate in the terminology.10 Applying the published definition of the term “high‐sensitivity”, it should be reserved for assays that 1) have an imprecision below 10% at the 99th percentile of a healthy population and 2) are able to measure concentrations below the 99th percentile, but above the detection limit in more than 50% (ideally more than 95%) of healthy individuals. Recent studies using the Siemens ADVIA Centaur CP TnI‐ultra assay indicate that this assay might be a true high‐sensitivity assay in dogs and cats as it detected cTnI in more than 95% of healthy dogs and cats examined and had a low imprecision at both high and low concentrations.48, 55, 63 The true upper reference limits for dogs and cats (the 99th percentile of a large healthy population) using this or any other assay have, however, not been reported.
Several factors can influence the results of cardiac troponin analysis. Serum and plasma values are significantly correlated, but a tendency toward slightly lower serum concentrations has been documented in dogs,54 whereas the opposite has been found in humans.54, 64 Separate reference intervals might, therefore, be needed. Troponin reportedly has long‐term stability at −70 to −80°C,8, 65 but is not stable at room temperature,54 refrigerator temperature,60 or −20°C.60 Short‐term storage (up to 24 h at 4°C and up to 3 months at −20°C) before analysis might be acceptable according to studies in alpacas and in cats.48, 66 The effect of freeze–thaw cycles has varied with different studies, but might also affect troponin concentrations.60 Interfering substances in the blood such as seen with hemolysis, lipemia, fibrin, increased alkaline phosphatase, rheumatoid factor, heterophilic antibodies, or immune complexes can falsely increase troponin concentrations.18, 67 Circulating troponin autoantibodies can cause negative interference.68
Troponin T has not been measurable in healthy dogs and cats in published studies. Reported cTnI concentrations of healthy dogs and cats are shown in Table 1.
Table 1.
Reported cardiac troponin I (cTnI) concentrations from studies examining at least 20 healthy dogs or cats
| Species | n | cTnI (ng/mL) | Sample | % screened |
|---|---|---|---|---|
| Dog | 54 | <0.05–0.12 | EP | 10069 |
| 41 | <0.03–0.07 | HP | 2570 | |
| 176 | <0.02–0.15 | HP | 10071 | |
| 24 | <0.006–0.128 | S | 100*, 72 | |
| 22 | 0.004–0.095 | S | 100*, 55 | |
| 30 | <0.006–0.136 | S | 10073 | |
| 26 | <0.1–0.17 | S | 10074 | |
| 58 | <0.01–0.05 | EP | 100*, 75 | |
| Cat | 58 | <0.05 | EP | 10069 |
| 21 | <0.03–0.16 | HP | 2570 | |
| 23 | <0.003–0.09 | S | 100*, 48 | |
| 20 | 0.004–0.091 | S | 100*, 55 | |
| 37 | <0.02–0.17 | HP | 10076 | |
| 33 | <0.03–0.16 | HP | 1677 |
cTnI, Cardiac troponin I; % screened, Percentage of dogs and cats screened free of cardiac disease with echocardiography; EP, EDTA plasma; HP, Heparin plasma; S, Serum.
*The study included hematological and biochemical profiles in the health screening protocol.
Greyhounds and Boxers might have inherently higher cTnI concentrations than other breeds.78, 79 The minute amounts of troponin present in circulation in healthy individuals can likely be attributed to normal cardiomyocyte turnover.18, 80 In humans, male sex has been found to be correlated with increased troponin concentration,81 but similar findings have not been reported in dogs or cats. In humans,82 dogs,52, 58, 83 and cats,52 however, mildly increased concentrations have been documented in older individuals, possibly reflecting increased myocardial remodeling with cardiomyocyte loss.18 Biological variation of both cTnI and cTnT should also be taken into account when interpreting small increases in seemingly healthy individuals.84, 85 Finally, it is note‐worthy that extreme exercise can cause transient myocardial injury in both humans and dogs.86, 87, 88, 89
Primary Myocardial Injury
Acute Myocardial Infarction
Cardiac troponins are the biomarkers of choice for diagnosis of acute myocardial infarction (AMI) in humans.28, 90 Atherosclerosis of the coronary arteries is generally the underlying cause, and spontaneous rupture of an atherosclerotic plaque leads to platelet aggregation, clotting, vessel stenosis or occlusion, ischemia, and ultimately cardiomyocyte necrosis and myocardial infarction.28, 67 A diagnosis of AMI is made in the presence of a dynamic pattern in cardiac biomarker concentrations (preferably troponins) over 3–6 h with at least one measurement above the upper reference limit (with acceptable precision, that is, a coefficient of variation (CV) <10% at this cut‐off) together with clinical, electrocardiographic, or imaging findings consistent with myocardial ischemia.28 Troponins can be regarded as the clinical pathological correlate of myocardial lesions as evidenced by histopathology, as the size of the infarcted area correlates with cTnT concentration at 72 h postinfarction and with peak cTnI concentration.61, 62 In parallel with the increase in circulating cardiac troponin, a decrease in myocardial tissue troponin content occurs, reflecting release of the myocardial contractile apparatus.21, 91 Interestingly, AMI occurs very rarely in dogs and cats, most likely due to the infrequent occurrence of atherosclerosis in these species,92, 93 and possibly to a well‐developed coronary lateral circulation62, 94 which could provide a certain protection against infarction.
There is an independent association between cardiac troponin concentration and case fatality in AMI, and risk stratification according to level of troponin concentration has revealed that even small elevations result in an increased risk of death both short‐term and long‐term.95, 96, 97, 98 With the increasing use of high‐sensitivity assays, it has become apparent that even mild elevations, within the range undetected by conventional assays, are associated with an increased risk of death.99 Therefore, cardiac troponins are prognostic markers not only in patients with AMI, but also in patients with stable coronary artery disease.
Cardiac Trauma
Cardiac trauma resulting from penetrating chest trauma with cardiac involvement, blunt chest trauma causing myocardial contusions, cardiac surgery, catheterization procedures, or cardiopulmonary resuscitation causes direct mechanical damage to the heart.100, 101, 102, 103 In the veterinary clinic, direct cardiac trauma occurs frequently in conditions such as hit‐by‐car trauma, high‐rise syndrome, and thoracic bite injuries.38, 104, 105 The diagnosis of traumatic injury to the heart is important as it can lead to cardiogenic shock, acute heart failure, life‐threatening arrhythmias, or structural damage.38, 67 Troponin measurement is of value in detecting or ruling out significant blunt cardiac injury.106
Primary Cardiac Disease
When evaluating cardiac disease, cardiac injury is not limited to cases with overt myocardial ischemia, but is also very common in those with primary structural cardiac disease. Most human studies in this area have focused on patients in heart failure regardless of cause, whereas studies in dogs and cats have focused more on the individual heart disease, revealing that increased troponin concentrations occur in both congenital and acquired heart diseases (Tables 2 and 3).
Table 3.
Myocardial injury in cardiac and noncardiac diseases in cats
| Species | Cardiac disease | Noncardiac disease* | cTnI measured | cTnT measured |
|---|---|---|---|---|
| Cat | HCM | 48, 77, 148, 149, 150 | 48 | |
| Anemia | 52, 151 | |||
| Neoplasia (mixed) | 52 | |||
| Respiratory disease | 52, 76, 152 | |||
| Hyperthyroidism | 153, 154 |
cTnI, Cardiac troponin I; cTnT, Cardiac troponin T, HCM: Hypertrophic cardiomyopathy.
*NB: Not all studies in the noncardiac disease group have ruled out primary cardiac disease as a cause of myocardial injury.
Longitudinal studies have revealed that humans as well as animals with cardiac disease have chronically increased troponin concentrations signifying ongoing myocardial injury.30, 48 Generally, although influenced by the chosen assay, the concentrations of cardiac troponins in primary cardiac disease are only mildly increased (<1 ng/mL) in dogs and cats, and even in those with severe congestive heart failure concentrations rarely increase above 1–2 ng/mL.48, 58, 110 Nevertheless, this limited, persistent, and often subclinical cardiac injury seems to play a significant role. Importantly, unlike AMI in which dynamic changes in a patient's troponin concentrations can be used for a diagnostic purpose, troponins have limited value in diagnosing primary heart diseases. This is not only true because noncardiac diseases can cause cardiac injury, but also because an overlap between troponin concentration in healthy individuals and those with cardiac disease has been repeatedly shown.48, 49, 71 Those with only mild disease might not have any evidence of cardiac injury, and, consequently, troponins should not be used to either confirm or exclude primary cardiac disease without the simultaneous use of echocardiography and ECG. They might, however, have a diagnostic purpose in distinguishing between cardiac and noncardiac dyspnea in the emergency setting although conflicting evidence exists with more promising results in cats.76, 139, 152, 155
The pathogenesis of myocardial injury in primary structural heart disease is believed to be multifactorial, resulting from complex interactions of mechanical, neuro‐humoral, inflammatory, and ischemic alterations in the myocardium.67 These factors reflect underlying disease (eg, coronary artery disease or sarcomeric gene mutations associated with development of hypertrophic cardiomyopathy (HCM)), initiating cause of injury (eg, arrhythmia or treatment with cardiotoxic drugs), and possible amplifying factors (eg, renal dysfunction).90 Specifically for HCM, mild chronic ischemia has often been suggested as a cause, mediated through an oxygen demand‐supply mismatch due to the hypertrophied left ventricle.156, 157 An association between troponin concentration and left ventricular free wall thickness has been reported in both humans and cats,77, 148, 158, 159 but a recent study failed to show an association between changes in myocardial wall thickness and in troponin concentrations over time.48 Ongoing myocardial injury in HCM is, therefore, not simply explained by the degree of hypertrophy. Table 4 lists known or suspected factors initiating or contributing to myocardial injury in structural cardiac disease.
Table 4.
Possible causes of myocardial injury in primary cardiac disease
| Initiating or contributing cause |
|---|
| Genetically abnormal myocyte function160, 161 |
| Ventricular hypertrophy (subendocardial ischemia)156, 162 |
| Fibrosis111, 158 |
| Hemodynamic overload (altered calcium‐handling)33 |
| Increased myocardial wall stretch34, 163 |
| Endothelial/microvascular dysfunction157, 164 |
| Activation of the renin–angiotensin–aldosterone system165 |
| Activation of the sympathetic nervous system (norepinephrine toxicity)30, 166, 167 |
| Toxic effects of inflammatory cytokines168, 169 |
| Oxidative stress170 |
| Troponin autoimmunity171, 172 |
| Systemic hypotension46, 173, 174 |
| Anemia175 |
| Arrhythmia90, 175, 176 |
| Inotropic drugs46, 173, 174 |
Rather than simply being a result of cardiac disease, myocardial injury itself might also be a possible cause of disease progression.16 The gradual development of heart failure is accompanied by cardiac remodeling as a result of cardiomyocyte death, hypertrophy, and replacement fibrosis.111, 158 As the remodeling progresses, a concurrent reduction in tissue content of troponin occurs.177 It has been hypothesized that myocardial remodeling increases susceptibility to further cardiac injury,18 and, in support of this, higher circulating troponin concentrations have been found in humans as well as dogs with myocardial fibrosis.111, 158 Therefore, the consequence of myocardial injury in cardiac disease, independent of its causes and mechanisms, is thought to be a worsening of cardiac function.16
In addition to structural heart disease, other diseases directly involving the heart have been associated with myocardial injury. These include infiltrative cardiac disease, cardiac neoplasia, inflammatory cardiac disease, pericardial effusion, and parasitic cardiac disease (Table 2 lists those described in dogs).
Prognostic capacity of troponins has been detected in humans with HCM,178 dilated cardiomyopathy (DCM),179 and heart failure,180 cats with HCM,48, 150 and dogs with myxomatous mitral valve disease (MMVD),110, 181 cardiomyopathies,71, 75 and a combined group of congenital and acquired heart diseases.59 Long survival times are generally seen with low troponin concentrations, whereas even patients who are clinically stable but have evidence of myocardial injury are at risk of poorer outcome.182 Interestingly, not all human studies have found an association between degree of cardiac injury and risk of death,180 and it is possible that presence rather than degree of injury is the actual prognostic indicator. A recent study of cats with HCM, however, found a prognostically significant increase in cTnT concentrations in nonsurvivors over the course of the study.48 Similarly, a study in dogs with various heart diseases revealed a significantly higher risk of death with increasing concentrations of cardiac troponins.59 Accordingly, the value of measuring cardiac troponin longitudinally in the individual is a matter of great interest. Research in humans with chronic heart disease has indicated that for the individual patient an increase over time could be associated with a higher risk of death,182, 183 and conversely, that outcome tends to improve in patients with decreasing concentrations.182, 183 This is supported by a recent study in dogs which showed a decrease in cTnI in dogs with severe MMVD during the first two weeks after initiation of treatment.73 However, it has been reported in both human and feline research that longitudinal changes in troponin concentrations in a population only slightly improve the discriminative power of the baseline measurements for fatal outcomes.48, 184 Thus, while longitudinal measurements might be of value in monitoring the individual, it appears that a single measurement of cardiac troponin at any time during disease progression provides strong and independent prognostic information.184
Secondary Myocardial Injury
Noncardiac Disease
Myocardial injury has been documented in a large number of noncardiac diseases, most of them involving critically ill patients, and especially those with inflammatory diseases and shock56, 185, 186, 187 (Tables 2 and 3 lists those described in dogs and cats). Many dogs and cats have mildly increased cTnI concentrations (<1 ng/mL), however, severe myocardial injury is rather common in critically ill dogs and cats with noncardiac disease in the experience of the authors (concentrations >10 ng/mL are relatively common and even concentrations >100 ng/mL have been reported in several cases).63, 143, 146 Accordingly, most human studies and an increasing number of veterinary studies focus on populations hospitalized in the intensive care unit (ICU). In most human ICU patients increased troponin concentrations are found already at or within 24 h of admission,188, 189 and a similar tendency has recently been reported in dogs.190 This fact has led to speculation that in‐hospital complications follow rather than precede development of myocardial injury.189 This supports a theory of myocardial injury as a partial cause rather than purely a result of the patient's critical status.189
The pathogenesis of myocardial injury in noncardiac disease is still being investigated, and possible causes are listed in Table 5.
Table 5.
Possible causes of myocardial injury in noncardiac critical disease
| Initiating or contributing cause |
|---|
| Hypotension186, 188, 189 |
| Hypoxemia36, 191 |
| Anemia136, 191, 192 |
| Fever193, 194 |
| Tachycardia188, 191 |
| Increased myocardial wall stress195, 196 |
| Arrhythmia188, 189 |
| Endothelial/microvascular dysfunction194, 195, 197 |
| Microthrombosis198, 199 |
| Pulmonary thromboembolism200 |
| Toxic effects of endotoxin194, 195, 201 |
| Toxic effects of inflammatory cytokines35, 56 |
| Oxidative stress188, 195 |
| Epi‐ and endocardial hemorrhage130 |
| Reperfusion injury associated with resuscitation procedures194, 195 |
| Inotropic/vasopressor drugs187, 188, 202 |
| Cardiotoxic drugs (eg, doxorubicin)137 |
| Envenomation (eg, snake venom)203 |
Overall, with the use of high‐sensitivity assays, myocardial injury has been detected in a large percentage of the critically ill.63, 204 Importantly, those with and without myocardial injury generally have similar clinical characteristics.189, 205 Measurement of cardiac troponin is, therefore, necessary to discover the involvement of myocardial injury in the individual's critical status.125
Myocardial dysfunction is a serious complication of critical disease, most frequently of sepsis, in both humans and animals. It is characterized by ventricular dilatation, hypocontractility, and diminished relaxation.56, 206, 207 Myocardial dysfunction is frequently associated with troponin elevations,56, 187, 191, 195whereas it remains unclear whether the dysfunction results from, accompanies, or causes myocardial injury.187, 195 Myocardial dysfunction as visualized on echocardiography is reversible with recovery from sepsis.208, 209 This argues against major cardiomyocyte death, and it is believed that cardiomyocyte injury can thus also occur reversibly with release of mainly the cytosolic troponin pool.198 In human medicine, a necropsy case study of deceased septic patients failed to show irreversible cardiomyocyte necrosis in half of those that had increased cardiac troponin concentrations antemortem,195 which supports the theory of reversible cardiac injury in some of these patients.
Cytokines are thought to play a very important role in causing myocardial injury in inflammatory disease. Increased troponin concentrations in critically ill patients can be associated with significantly higher tumor necrosis factor‐α (TNF‐α) and interleukin‐ (IL‐) 6 concentrations,56 and improvement of echocardiographically visible myocardial dysfunction in one study occurred in parallel with decreases in cTnI, TNF‐α, IL‐8, and IL‐10.210 Additionally, TNF‐α and IL‐1 cause reduced cardiomyocyte contractility in in vitro studies.211 In critically ill dogs with systemic inflammation, several cytokines, especially IL‐10 and IL‐15, are thought to play a role in the events leading to myocardial injury.63 The mechanism underlying cytokine‐mediated injury is believed to be a toxic effect on the cardiomyocyte membrane leading to increased permeability.35, 56 With resolution of this effect, the injury might be reversible.
Intoxication is another cause of increased troponin concentrations. In dogs receiving doxorubicin, a directly cardiotoxic drug, an increased troponin concentration is the first indicator of impending cardiac failure.137 Envenomation such as seen with snake bite is also a cause of cardiac injury.141, 142, 143 Some venoms contain directly cardiotoxic substances, but systemic inflammation induced by envenomation could also be a possible cause of myocardial injury in these cases.141, 142
Interestingly, critically ill patients with noncardiac disease often have higher troponin concentrations than patients with severe primary cardiac disease. The cause of this has not been established, but it could be speculated that systemic critical disease that affects the heart most likely affects all cardiomyocytes, whereas primary cardiac disease is more likely to chronically overburden the heart, causing death of consecutive cells over time as part of the ongoing remodeling process.
Even today the case fatality rate of the critically ill (dogs and cats as well as humans) admitted to ICUs is high despite increasingly sophisticated diagnostic and therapeutic management. Though, clinically, myocardial injury is often unrecognized, its presence is associated with prolonged morbidity and increased risk of death: Humans and dogs with evidence of myocardial injury have an up to 4 times higher case fatality rate than those with normal troponin concentrations,56, 63, 189, 191, 205 and increased troponins have also been associated with prolonged ICU hospitalization189, 212 (only found in humans, likely because of euthanasia of many dogs with poor prognosis). Cardiac troponins have been shown to contribute independently to established prognostic composite scores in both humans and dogs.56, 63, 191 In dogs cTnI provided additional prognostic specificity to the Acute Patient Physiologic and Laboratory Evaluation (APPLE) score without compromising its prognostic sensitivity.63 It seems that myocardial injury, thus not accounted for by the scores, supplies additional prognostic information to already powerful prognostic scoring systems, a fact that reveals the prognostic strength of the troponins and identifies a possible need for a general inclusion of the status of the myocardium in patient evaluation and prognostic scoring.197
Development of multiple organ dysfunction syndrome (MODS) is a frequent complication and cause of death in critical illness.213 Troponin is not a dysfunction marker, but it correlates well with echocardiographic evidence of myocardial dysfunction56, 187, 191, 195 and has also been significantly associated with other organ failure in human ICU patients.191 Inflammatory or hypoxic stimuli that affect the heart will likely affect other organs simultaneously. Thus, the association between increased cardiac troponins and case fatality could be attributable to an independent progression to MODS occurring along with myocardial injury, but a myocardial injury‐related increased risk of MODS is also a possibility if impaired organ perfusion follows dysfunction of the myocardium.191 Increased cardiac troponin in critically ill individuals might, therefore, indicate a critical state of a noncardiac condition, and troponin has been referred to as a marker of multiorgan failure.214
In veterinary studies an association between cardiac troponin concentrations and short‐term case fatality has been found in dogs with gastric dilatation volvulus (GDV)145, parvoviral enteritis125, babesiosis130, systemic inflammatory response syndrome (SIRS)133, and systemic inflammation of any cause in dogs without primary structural cardiac disease.63 Two studies looked into temporal changes of circulating troponin in hospitalized dogs, and these changes did not distinguish short‐term nonsurvivors from survivors.133, 190 Larger studies are necessary in order to examine the value of serial troponin measurements in monitoring the individual.
It is still debated whether cardiac troponins have prognostic significance for long‐term outcome. Some studies have failed to show an association,215 whereas other studies indicate that myocardial injury might be a predictor of long‐term negative outcome,196 perhaps even being a partial cause of eventual clinical deterioration. In dogs, an association of admission (cTnT) and peak (cTnI) troponin concentrations with 1‐year case fatality has been shown, although considerably weaker than that with short‐term case fatality.190 The study suggested that cTnI was a better short‐term predictor, whereas cTnT appeared to predict long‐term outcome with greater certainty. Troponins might thus complement each other as prognostic markers. Because of the possible association of long‐term outcome with cardiac troponin concentrations, critically ill individuals with evidence of myocardial injury might have a need for close follow‐up after hospital discharge. Cardiac troponins could, therefore, play a role in identification of long‐term risk patients (animals as well as humans) in the ICU.
Renal Disease
Renal disease poses a dilemma for interpretation of cardiac troponins because it is presently unknown whether the markers are reliable when renal function is compromised. Many human studies have shown an increase in circulating cardiac troponins, especially cTnT, in patients with renal disease.57, 216 The troponin detected is definitely of cardiac origin,90 but it is an ongoing debate whether its rise is caused by a reduced renal clearance, concurrent cardiac disease, or a deleterious effect on the myocardium caused by uremic toxins.27, 216, 217 Troponins have been considered too large for renal elimination in total, but fragments of these molecules also occur in circulation at a size that could possibly be cleared by the kidneys.45, 47 Interestingly, cTnT is often increased in humans with renal insufficiency, whereas cTnI does not appear to be as frequently affected by renal disease.57, 217, 218 In dogs and cats, however, two studies found frequent elevations of cTnI in azotemic animals.219, 220 These studies, unfortunately, did not include echocardiographic examination in their protocols, but one reported histopathological findings of cardiac pathology in three of four necropsies, suggesting a concurrent cardiac disease as a cause of troponin release.219 A high risk of death or cardiac events is known to exist in humans with end‐stage renal disease and increased concentrations of troponins,57, 90 a fact which further supports this theory. Importantly, troponins have been shown to retain their prognostic ability in humans even after adjustment for renal function,221 but, as a rule, it is still recommended to interpret an increased troponin concentration cautiously in the presence of renal disease.
Perspectives
Cardiac troponins are quantitative markers of myocardial injury which can be reliably measured in dogs and cats and which provide prognostic information, seemingly irrespective of clinical presentation (acute or chronic), suspected type of myocardial injury (reversible or irreversible), and underlying disease (cardiac or noncardiac). Clinically, the greatest strength of troponins can be summed up in their exceptional negative predictive value in both cardiac and noncardiac disease with low troponin concentrations generally associated with improved chances of survival. Increased concentrations, on the other hand, identify individuals at increased risk of death.
In veterinary medicine, cTnI has generally been the chosen marker. Cardiac troponin T is less sensitive than cTnI, being released only with more severe cardiac injury. As dogs and cats rarely develop AMI, and primary cardiac disease is often associated with low‐grade myocardial injury, cTnI became the obvious choice in the initial studies of troponins in animals which involved mainly this disease category. Today more sensitive cTnT assays are available as well, and the marker is becoming increasingly available for veterinary research. Cardiac troponin I and cTnT might differ slightly in their prognostic potential, but overall the two markers are highly correlated sources of similar information, and clinically, it is considered sufficient to measure one or the other.60 With the prognostic importance of even minimal myocardial injury, the authors recommend cTnI as the cardiac injury marker of choice in dogs and cats. Publication of upper reference limits for relevant assays based on large healthy populations is warranted for optimal use.
There are many possible causes of cardiac injury, each of which leads to a rise in circulating cardiac troponins through one or more mechanisms of troponin release. However, questions still in need of answering include whether reversible and irreversible injury occur as two separate entities in different disease processes or occur simultaneously; whether cardiac disease and noncardiac disease each might be most likely to cause either reversible or irreversible injury; and whether one is “worse” than the other from a prognostic point of view. Shedding light on the pathophysiology behind myocardial injury in renal disease is also crucial in order to be able to apply the marker to all disease categories.
Researchers increasingly recommend using a multi‐marker approach in the evaluation and prognostication of any patient.181 The combined potential of troponins with markers of hemodynamic stress on the heart (eg, natriuretic peptides), other markers of cardiomyocyte injury (eg, fatty acid binding protein 3), and markers of cardiac remodelling (eg, matrix metalloproteinases) is of high importance in cardiology and requires further research.18 In noncardiac disease, the contribution of cardiac troponins to prognostic scoring systems shows great promise, and it is believed that inclusion of troponins in future scores will have both clinical and research benefits. As assays hopefully become increasingly available, and costs are reduced, it is also considered worthwhile to include measurements of cTnI among the routine biochemical variables examined in the clinical work‐up of dogs and cats, just as biochemical variables reflecting renal and hepatic status are routinely measured.
Because of its possible reversible nature, it has been discussed whether patients with evidence of myocardial injury might benefit from more aggressive treatment, in which case normalizing of troponin concentrations might be associated with an improved outcome.189, 222 At this point in time, whether troponins are useful in monitoring effects of intervention, and whether administering more aggressive treatment to individuals with evidence of myocardial injury is beneficial, are still unanswered questions. Further studies, for example, using troponins as surrogate endpoints for clinical trials, are necessary to examine whether normalizing of troponin concentrations in cases presenting with evidence of myocardial injury is associated with improvement in outcome.18 It is hoped that treatment strategies will be developed which have the ability to reduce the risk associated with myocardial injury.
Acknowledgments
The authors thank Karen Riiber Mandrup, DVM, PhD, for creation of Fig 1.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
References
- 1. Biomarkers definitions Working Group . Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 2001;69:89–95. [DOI] [PubMed] [Google Scholar]
- 2. Li MX, Wang X, Sykes BD. Structural based insights into the role of troponin in cardiac muscle pathophysiology. J Muscle Res Cell Motil 2004;25:559–579. [DOI] [PubMed] [Google Scholar]
- 3. Cunningham JG, Klein BG. Textbook of Veterinary Physiology, 4th edn Philadelphia, PA, USA: Saunders; 2007:81–88; 193‐200. [Google Scholar]
- 4. Pagani ED, Silver PJ. Physiological and pharmacological modulation of cardiac contractile proteins. Drug Dev Res 1989;18:279–293. [Google Scholar]
- 5. Gomes A, Potter J. Cellular and molecular aspects of familial hypertrophic cardiomyopathy caused by mutations in the cardiac troponin I gene. Mol Cell Biochem 2004;263:99–114. [DOI] [PubMed] [Google Scholar]
- 6. Filatov VL, Katrukha AG, Bulargina TV, Gusev NB. Troponin: structure, properties, and mechanism of functioning. Biochemistry (Mosc) 1999;64:969–985. [PubMed] [Google Scholar]
- 7. Metzger JM, Westfall MV. Covalent and noncovalent modification of thin filament action ‐ the essential role of troponin in cardiac muscle regulation. Circ Res 2004;94:146–158. [DOI] [PubMed] [Google Scholar]
- 8. O'Brien PJ. Cardiac troponin is the most effective translational safety biomarker for myocardial injury in cardiotoxicity. Toxicology 2008;245:206–218. [DOI] [PubMed] [Google Scholar]
- 9. Missov ED, De Marco T. Clinical insights on the use of highly sensitive cardiac troponin assays. Clin Chim Acta 1999;284:175–185. [DOI] [PubMed] [Google Scholar]
- 10. Apple FS, Collinson PO. Analytical characteristics of high‐sensitivity cardiac troponin assays. Clin Chem 2012;58:54–61. [DOI] [PubMed] [Google Scholar]
- 11. Gaze DC, Collinson PO. Multiple molecular forms of circulating cardiac troponin: analytical and clinical significance. Ann Clin Biochem 2008;45:349–355. [DOI] [PubMed] [Google Scholar]
- 12. Bodor GS, Survant L, Voss EM, et al. Cardiac troponin T composition in normal and regenerating human skeletal muscle. Clin Chem 1997;43:476–484. [PubMed] [Google Scholar]
- 13. Jaffe AS, Vasile VC, Milone M, et al. Diseased skeletal muscle: a noncardiac source of increased circulating concentrations of cardiac troponin T. J Am Coll Cardiol 2011;58:1819–1824. [DOI] [PubMed] [Google Scholar]
- 14. Adams JE, Bodor GS, Davilaroman VG, et al. Cardiac Troponin‐I ‐ a marker with high specificity for cardiac injury. Circulation 1993;88:101–106. [DOI] [PubMed] [Google Scholar]
- 15. Bodor GS, Porterfield D, Voss EM, et al. Cardiac troponin‐I is not expressed in fetal and healthy or diseased adult human skeletal‐muscle tissue. Clin Chem 1995;41:1710–1715. [PubMed] [Google Scholar]
- 16. Kociol RD, Pang PS, Gheorghiade M, et al. Troponin elevation in heart failure prevalence, mechanisms, and clinical implications. J Am Coll Cardiol 2010;56:1071–1078. [DOI] [PubMed] [Google Scholar]
- 17. Rishniw M, Barr S, Simpson K, et al. Cloning and sequencing of the canine and feline cardiac troponin I genes. Am J Vet Res 2004;65:53–58. [DOI] [PubMed] [Google Scholar]
- 18. Barison A, Pastormerlo LE, Giannoni A. Troponin in non‐ischaemic dilated cardiomyopathy. Eur Cardiol 2011;7:220–224. [Google Scholar]
- 19. Katus HA, Remppis A, Scheffold T, et al. Intracellular compartmentation of cardiac troponin‐T and its release kinetics in patients with reperfused and nonreperfused myocardial infarction. Am J Cardiol 1991;67:1360–1367. [DOI] [PubMed] [Google Scholar]
- 20. Adams JE, Schechtman KB, Landt Y, et al. Comparable detection of acute myocardial‐infarction by Creatine‐Kinase Mb isoenzyme and cardiac troponin‐I. Clin Chem 1994;40:1291–1295. [PubMed] [Google Scholar]
- 21. Voss EM, Sharkey SW, Gernert AE, et al. Human and canine cardiac troponin‐T and Creatine Kinase‐Mb distribution in normal and diseased myocardium ‐ infarct sizing using serum profiles. Arch Pathol Lab Med 1995;119:799–806. [PubMed] [Google Scholar]
- 22. Hickman PE, Potter JM, Aroney C, et al. Cardiac troponin may be released by ischemia alone, without necrosis. Clin Chim Acta 2010;411:318–323. [DOI] [PubMed] [Google Scholar]
- 23. Babuin L, Jaffe AS. Troponin: the biomarker of choice for the detection of cardiac injury. Can Med Assoc J 2005;173:1191–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu AHB, Ford L. Release of cardiac troponin in acute coronary syndromes: ischemia or necrosis? Clin Chim Acta 1999;284:161–174. [DOI] [PubMed] [Google Scholar]
- 25. Mikaelian I, Buness A, de Vera‐Mudry M, et al. Primary endothelial damage is the mechanism of cardiotoxicity of tubulin‐binding drugs. Toxicol Sci 2010;117:144–151. [DOI] [PubMed] [Google Scholar]
- 26. MacRae AR, Kavsak PA, Lustig V, et al. Assessing the requirement for the 6‐hour interval between specimens in the american heart association classification of myocardial infarction in epidemiology and clinical research studies. Clin Chem 2006;52:812–818. [DOI] [PubMed] [Google Scholar]
- 27. White HD. Pathobiology of troponin elevations: do elevations occur with myocardial ischemia as well as necrosis? J Am Coll Cardiol 2011;57:2406–2408. [DOI] [PubMed] [Google Scholar]
- 28. Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol 2012;60:1581–1598. [DOI] [PubMed] [Google Scholar]
- 29. Sobel BE, LeWinter MM. Ingenuous interpretation of elevated blood levels of macromolecular markers of myocardial injury: a recipe for confusion. J Am Coll Cardiol 2000;35:1355–1358. [DOI] [PubMed] [Google Scholar]
- 30. Latini R, Masson S, Anand IS, et al. Prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation 2007;116:1242–1249. [DOI] [PubMed] [Google Scholar]
- 31. Shih H, Lee B, Lee RJ, Boyle AJ. The aging heart and post‐infarction left ventricular remodeling. J Am Coll Cardiol 2011;57:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bergmann O, Bhardwaj RD, Bernard S, et al. Evidence for cardiomyocyte renewal in humans. Science 2009;324:98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Feng J, Schaus BJ, Fallavollita JA, et al. Preload induces troponin I degradation independently of myocardial ischemia. Circulation 2001;103:2035–2037. [DOI] [PubMed] [Google Scholar]
- 34. Hessel MHM, Atsma DE, van der Valk EJM, et al. Release of cardiac troponin I from viable cardiomyocytes is mediated by integrin stimulation. Pflugers Archiv 2008;455:979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu AHB. Increased troponin in patients with sepsis and septic shock: myocardial necrosis or reversible myocardial depression? Intensive Care Med 2001;27:959–961. [DOI] [PubMed] [Google Scholar]
- 36. Piper HM, Schwartz P, Spahr R, et al. Early enzyme‐release from myocardial‐cells is not due to irreversible cell‐damage. J Mol Cell Cardiol 1984;16:385–388. [DOI] [PubMed] [Google Scholar]
- 37. Apple FS, Murakami MM, Ler R, et al. Analytical characteristics of commercial cardiac troponin I and T immunoassays, in serum from rats, dogs, and monkeys with induced acute myocardial injury. Clin Chem 2008;54:1982–1989. [DOI] [PubMed] [Google Scholar]
- 38. Schober KE, Kirbach B, Oechtering G. Noninvasive assessment of myocardial cell injury in dogs with suspected cardiac contusion. J Vet Cardiol 1999;1:17–25. [DOI] [PubMed] [Google Scholar]
- 39. Shaw SP, Rozanski EA, Rush JE. Cardiac troponins I and T in dogs with pericardial effusion. J Vet Intern Med 2004;18:322–324. [DOI] [PubMed] [Google Scholar]
- 40. Gerhardt W, Katus H, Ravkilde J, et al. S‐troponin‐T in suspected ischemic myocardial injury compared with mass and catalytic concentrations of s‐creatine kinase isoenzyme‐Mb. Clin Chem 1991;37:1405–1411. [PubMed] [Google Scholar]
- 41. Dunn ME, Coluccio D, Hirkaler G, et al. The complete pharmacokinetic profile of serum cardiac troponin I in the rat and the dog. Toxicol Sci 2011;123:368–373. [DOI] [PubMed] [Google Scholar]
- 42. Katrukha AG, Bereznikova AV, Esakova TV, et al. Troponin I is released in bloodstream of patients with acute myocardial infarction not in free form but as complex. Clin Chem 1997;43:1379–1385. [PubMed] [Google Scholar]
- 43. Wu A, Feng Y, Moore R, et al. Characterization of cardiac troponin subunit release into serum after acute myocardial infarction and comparison of assays for troponin T and I. Clin Chem 1998;44:1198–1208. [PubMed] [Google Scholar]
- 44. Katrukha A, Bereznikova A, Filatov V, Esakova T. Biochemical factors influencing measurement of cardiac troponin I in serum. Clin Chem Lab Med 1999;37:1091–1095. [DOI] [PubMed] [Google Scholar]
- 45. Freda BJ, Tang WHW, Van Lente F, et al. Cardiac troponins in renal insufficiency ‐ review and clinical implications. J Am Coll Cardiol 2002;40:2065–2071. [DOI] [PubMed] [Google Scholar]
- 46. Sato Y, Fujiwara H, Takatsu Y. Cardiac troponin and heart failure in the era of high‐sensitivity assays. J Cardiol 2012;60:160–167. [DOI] [PubMed] [Google Scholar]
- 47. Diris JHC, Hackeng CM, Kooman JP, et al. Impaired renal clearance explains elevated troponin T fragments in hemodialysis patients. Circulation 2004;109:23–25. [DOI] [PubMed] [Google Scholar]
- 48. Langhorn R, Tarnow I, Willesen JL, et al. Cardiac troponin I and T as prognostic markers in cats with hypertrophic cardiomyopathy. J Vet Intern Med 2014;28:1485–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Spratt D, Mellanby R, Drury N, Archer J. Cardiac troponin I: evaluation of a biomarker for the diagnosis of heart disease in the dog. J Small Anim Pract 2005;46:139–145. [DOI] [PubMed] [Google Scholar]
- 50. Cummins B, Auckland ML, Cummins P. Cardiac‐specific troponin‐I radioimmunoassay in the diagnosis of acute myocardial‐infarction. Am Heart J 1987;113:1333–1344. [DOI] [PubMed] [Google Scholar]
- 51. Katus HA, Remppis A, Looser S, et al. Enzyme linked immuno assay of cardiac troponin‐T for the detection of acute myocardial‐infarction in patients. J Mol Cell Cardiol 1989;21:1349–1353. [DOI] [PubMed] [Google Scholar]
- 52. Serra M, Papakonstantinou S, Adamcova M, O'Brien PJ. Veterinary and toxicological applications for the detection of cardiac injury using cardiac troponin. Vet J 2010;185:50–57. [DOI] [PubMed] [Google Scholar]
- 53. Adin DB, Oyama MA, Sleeper MM, Milner RJ. Comparison of canine cardiac troponin I concentrations as determined by 3 analyzers. J Vet Intern Med 2006;20:1136–1142. [DOI] [PubMed] [Google Scholar]
- 54. Oyama MA, Solter PF. Validation of an immunoassay for measurement of canine cardiac troponin‐I. J Vet Cardiol 2004;6:17–24. [DOI] [PubMed] [Google Scholar]
- 55. Langhorn R, Willesen JL, Tarnow I, Kjelgaard‐Hansen M. Evaluation of a high‐sensitivity assay for measurement of canine and feline serum cardiac troponin I. Vet Clin Pathol 2013;42:490–498. [DOI] [PubMed] [Google Scholar]
- 56. Ammann P, Maggiorini M, Bertel O, et al. Troponin as a risk factor for mortality in critically ill patients without acute coronary syndromes. J Am Coll Cardiol 2003;41:2004–2009. [DOI] [PubMed] [Google Scholar]
- 57. Apple FS, Murakami MM, Pearce LA, Herzog CA. Predictive value of cardiac troponin I and T for subsequent death in end‐stage renal disease. Circulation 2002;106:2941–2945. [DOI] [PubMed] [Google Scholar]
- 58. Ljungvall I, Hoglund K, Tidholm A, et al. Cardiac troponin I is associated with severity of myxomatous mitral valve disease, age, and C‐reactive protein in dogs. J Vet Intern Med 2010;24:153–159. [DOI] [PubMed] [Google Scholar]
- 59. Fonfara S, Loureiro J, Swift S, et al. Cardiac troponin I as a marker for severity and prognosis of cardiac disease in dogs. Vet J 2010;184:334–339. [DOI] [PubMed] [Google Scholar]
- 60. O'Brien PJ, Smith DEC, Knechtel TJ, et al. Cardiac troponin I is a sensitive, specific biomarker of cardiac injury in laboratory animals. Lab Anim 2006;40:153–171. [DOI] [PubMed] [Google Scholar]
- 61. Licka M, Zimmermann R, Zehelein J, et al. Troponin T concentrations 72 hours after myocardial infarction as a serological estimate of infarct size. Heart 2002;87:520–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gallegos R, Swingen C, Xu X, et al. Infarct Extent by MRI Correlates with peak serum troponin level in the canine model RID F‐2496‐2010. J Surg Res 2004;120:266–271. [DOI] [PubMed] [Google Scholar]
- 63. Langhorn R, Oyama MA, King LG, et al. Prognostic importance of myocardial injury in critically ill dogs with systemic inflammation. J Vet Intern Med 2013;27:895–903. [DOI] [PubMed] [Google Scholar]
- 64. Stiegler H, Fischer Y, Vazquez‐Jimenez JF, et al. Lower cardiac troponin T and I results in heparin‐plasma than in serum. Clin Chem 2000;46:1338–1344. [PubMed] [Google Scholar]
- 65. Basit M, Bakshi N, Hashem M, et al. The effect of freezing and long‐term storage on the stability of cardiac troponin T. Am J Clin Pathol 2007;128:164–167. [DOI] [PubMed] [Google Scholar]
- 66. Blass KA, Kraus MS, Rishniw M, et al. Measurement of cardiac troponin I utilizing a point of care analyzer in healthy alpacas. J Vet Cardiol 2011;13:261–266. [DOI] [PubMed] [Google Scholar]
- 67. Wells SM, Sleeper M. Cardiac troponins. J Vet Emerg Crit Care 2008;18:235–245. [Google Scholar]
- 68. Eriksson S, Halenius H, Pulkki K, et al. Negative interference in cardiac troponin I immunoassays by circulating troponin autoantibodies. Clin Chem 2005;51:839–847. [DOI] [PubMed] [Google Scholar]
- 69. Adin DB, Milner RJ, Berger KD, et al. Cardiac troponin I concentrations in normal dogs and cats using a bedside analyzer. J Vet Cardiol 2005;7:27–32. [DOI] [PubMed] [Google Scholar]
- 70. Sleeper M, Clifford C, Laster L. Cardiac troponin I in the normal dog and cat. J Vet Intern Med 2001;15:501–503. [DOI] [PubMed] [Google Scholar]
- 71. Oyama MA, Sisson DD. Cardiac troponin‐I concentration in dogs with cardiac disease. J Vet Intern Med 2004;18:831–839. [DOI] [PubMed] [Google Scholar]
- 72. Winter RL, Saunders AB, Gordon SG, et al. Analytical validation and clinical evaluation of a commercially available high‐sensitivity immunoassay for the measurement of troponin I in humans for use in dogs. J Vet Cardiol 2014;16:81–89. [DOI] [PubMed] [Google Scholar]
- 73. Polizopoulou ZS, Koutinas CK, Dasopoulou A, et al. Serial analysis of serum cardiac troponin I changes and correlation with clinical findings in 46 dogs with mitral valve disease. Vet Clin Pathol 2014;43:218–225. [DOI] [PubMed] [Google Scholar]
- 74. Guglielmini C, Civitella C, Diana A, et al. Serum cardiac troponin I concentration in dogs with precapillary and postcapillary pulmonary hypertension. J Vet Intern Med 2010;24:145–152. [DOI] [PubMed] [Google Scholar]
- 75. Noszczyk‐Nowak A. NT‐Pro‐BNP and troponin I as predictors of mortality in dogs with heart failure. Pol J Vet Sci 2011;14:551–556. [DOI] [PubMed] [Google Scholar]
- 76. Wells SM, Shofer FS, Walters PC, et al. Evaluation of blood cardiac troponin I concentrations obtained with a cage‐side analyzer to differentiate cats with cardiac and noncardiac causes of dyspnea. J Am Vet Med Assoc 2014;244:425–430. [DOI] [PubMed] [Google Scholar]
- 77. Herndon W, Kittleson M, Sanderson K, et al. Cardiac troponin I in feline hypertrophic cardiomyopathy. J Vet Intern Med 2002;16:558–564. [DOI] [PubMed] [Google Scholar]
- 78. LaVecchio D, Marin LM, Baumwart R, et al. Serum cardiac troponin I concentration in retired racing greyhounds. J Vet Intern Med 2009;23:87–90. [DOI] [PubMed] [Google Scholar]
- 79. Baumwart RD, Orvalho J, Meurs KM. Evaluation of serum cardiac troponin I concentration in boxers with arrhythmogenic right ventricular cardiomyopathy. Am J Vet Res 2007;68:524–528. [DOI] [PubMed] [Google Scholar]
- 80. Giannoni A, Giovannini S, Clerico A. Measurement of circulating concentrations of cardiac troponin I and T in healthy subjects: a tool for monitoring myocardial tissue renewal? Clin Chem Lab Med 2009;47:1167–1177. [DOI] [PubMed] [Google Scholar]
- 81. Kubo T, Kitaoka H, Okawa M, et al. Serum cardiac troponin I is related to increased left ventricular wall thickness, left ventricular dysfunction, and male gender in hypertrophic cardiomyopathy. Clin Cardiol 2010;33:E1–E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Reiter M, Twerenbold R, Reichlin T, et al. Early diagnosis of acute myocardial infarction in the elderly using more sensitive cardiac troponin assays. Eur Heart J 2011;32:1379–1389. [DOI] [PubMed] [Google Scholar]
- 83. Wess G, Simak J, Mahling M, Hartmann K. Cardiac troponin I in doberman pinschers with cardiomyopathy. J Vet Intern Med 2010;24:843–849. [DOI] [PubMed] [Google Scholar]
- 84. Wu AHB, Lu QA, Todd J, et al. Short‐ and long‐term biological variation in cardiac troponin I measured with a high‐sensitivity assay: implications for clinical practice. Clin Chem 2009;55:52–58. [DOI] [PubMed] [Google Scholar]
- 85. Vasile VC, Saenger AK, Kroning JM, Jaffe AS. Biological and analytical variability of a novel high‐sensitivity cardiac troponin T assay. Clin Chem 2010;56:1086–1090. [DOI] [PubMed] [Google Scholar]
- 86. Laslett L, Eisenbud E, Lind R. Evidence of myocardial injury during prolonged strenuous exercise. Am J Cardiol 1996;78:488–490. [DOI] [PubMed] [Google Scholar]
- 87. McKenzie EC, Jose‐Cunilleras E, HinchCliff KW, et al. Serum chemistry alterations in Alaskan sled dogs during five successive days of prolonged endurance exercise. J Am Vet Med Assoc 2007;230:1486–1492. [DOI] [PubMed] [Google Scholar]
- 88. Tharwat M, Al‐Sobayil F, Buczinski S. Influence of racing on the serum concentrations of the cardiac biomarkers troponin I and creatine kinase myocardial band (CK‐MB) in racing greyhounds. Vet J 2013;197:900–902. [DOI] [PubMed] [Google Scholar]
- 89. Wakshlag JJ, Kraus MS, Gelzer AR, et al. The influence of high‐intensity moderate duration exercise on cardiac troponin I and C‐reactive protein in sled dogs. J Vet Intern Med 2010;24:1388–1392. [DOI] [PubMed] [Google Scholar]
- 90. Januzzi JL Jr, Filippatos G, Nieminen M, Gheorghiade M. Troponin elevation in patients with heart failure: on behalf of the Third Universal Definition of Myocardial Infarction Global Task Force: heart Failure Section. Eur Heart J 2012;33:2265–2271. [DOI] [PubMed] [Google Scholar]
- 91. Ricchiuti V, Sharkey SW, Murakami MM, et al. Cardiac troponin I and T alterations in dog hearts with myocardial infarction ‐ correlation with infarct size. Am J Clin Pathol 1998;110:241–247. [DOI] [PubMed] [Google Scholar]
- 92. Kidd L, Stepien RL, Amrheiw DP. Clinical findings and coronary artery disease in dogs and cats with acute and subacute myocardial necrosis: 28 cases. J Am Anim Hosp Assoc 2000;36:199–208. [DOI] [PubMed] [Google Scholar]
- 93. Driehuys S, Van Winkle TJ, Sammarco CD, Drobatz KJ. Myocardial infarction in dogs and cats: 37 cases (1985‐1994). J Am Vet Med Assoc 1998;213:1444–1448. [PubMed] [Google Scholar]
- 94. Verdouw PD, van den Doel MA, de Zeeuw S, Duncker DJ. Animal models in the study of myocardial ischaemia and ischaemic syndromes. Cardiovasc Res 1998;39:121–135. [DOI] [PubMed] [Google Scholar]
- 95. Antman EM, Tanasijevic MJ, Thompson B, et al. Cardiac‐specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes. N Engl J Med 1996;335:1342–1349. [DOI] [PubMed] [Google Scholar]
- 96. Aldous SJ, Florkowski CM, Crozier IG, et al. High sensitivity troponin outperforms contemporary assays in predicting major adverse cardiac events up to two years in patients with chest pain. Ann Clin Biochem 2011;48:249–255. [DOI] [PubMed] [Google Scholar]
- 97. Hochholzer W, Reichlin T, Twerenbold R, et al. Incremental value of high‐sensitivity cardiac troponin T for risk prediction in patients with suspected acute myocardial infarction. Clin Chem 2011;57:1318–1326. [DOI] [PubMed] [Google Scholar]
- 98. Newby LK, Christenson RH, Ohman EM, et al. Value of serial troponin T measures for early and late risk stratification in patients with acute coronary syndromes. Circulation 1998;98:1853–1859. [DOI] [PubMed] [Google Scholar]
- 99. Omland T, de Lemos JA, Sabatine MS, et al. A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med 2009;361:2538–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Shih AC, Maisenbacher HW, Barreirinha A, et al. Effect of routine cardiovascular catheterization on cardiac troponin I concentration in dogs. J Vet Cardiol 2009;11(Suppl 1):S87–S92. [DOI] [PubMed] [Google Scholar]
- 101. Bussadori R, Tamborini A, Locatelli C, et al. Troponin I perioperative trend in dogs undergoing the correction of patent ductus arteriosus: preliminary investigations. Vet Res Commun 2008;32:S255–S258. [DOI] [PubMed] [Google Scholar]
- 102. Adams JE, DavilaRoman VG, Bessey PQ, et al. Improved detection of cardiac contusion with cardiac troponin l. Am Heart J 1996;131:308–312. [DOI] [PubMed] [Google Scholar]
- 103. Allan JJ, Feld RD, Russell AA, et al. Cardiac troponin I levels are normal or minimally elevated after transthoracic cardioversion. J Am Coll Cardiol 1997;30:1052–1056. [DOI] [PubMed] [Google Scholar]
- 104. Kirbach B, Schober K, Oechtering G, Aupperle H. Diagnostic of myocardial cell injury in cats with blunt thoracic trauma by circulating biochemical markers. Tierarztliche Praxis Ausgabe Kleintiere Heimtiere 2000;28:25–33. [Google Scholar]
- 105. Diniz PPVP, Schwartz DS, Collicchio‐Zuanaze RC. Cardiac trauma confirmed by cardiac markers in dogs: two case reports. Arq Bras Med Vet Zootec 2007;59:85–89. [Google Scholar]
- 106. Velmahos GC, Karaiskakis M, Salim A, et al. Normal electrocardiography and serum troponin I levels preclude the presence of clinically significant blunt cardiac injury. J Trauma 2003;54:45–51. [DOI] [PubMed] [Google Scholar]
- 107. Saunders AB, Smith BE, Fosgate GT, et al. Cardiac troponin I and C‐reactive protein concentrations in dogs with severe pulmonic stenosis before and after balloon valvuloplasty. J Vet Cardiol 2009;11:9–16. [DOI] [PubMed] [Google Scholar]
- 108. Pelander L, Häggström J, Jones B. Troponin I ‐ a possible marker of myocardial cell damage in the dog? Eur J Comp Anim Pract 2002;12:66–71. [Google Scholar]
- 109. Bakirel U, Gunes S. Value of cardiac markers in dogs with chronic mitral valve disease. Acta Vet (Beogr) 2009;59:223–229. [Google Scholar]
- 110. Linklater AKJ, Lichtenberger MK, Thamm DH, et al. Serum concentrations of cardiac troponin I and cardiac troponin T in dogs with class IV congestive heart failure due to mitral valve disease. J Vet Emerg Crit Care 2007;17:243–249. [Google Scholar]
- 111. Falk T, Ljungvall I, Zois NE, et al. Cardiac troponin‐I concentration, myocardial arteriosclerosis, and fibrosis in dogs with congestive heart failure because of myxomatous mitral valve disease. J Vet Intern Med 2013;27:500–506. [DOI] [PubMed] [Google Scholar]
- 112. Cakiroglu D, Meral Y, Bakirel U, Kazanci D. Cardiac troponin levels in dogs with dilate cardiomyopathy. Kafkas Universitesi Veteriner Fakultesi Dergisi 2009;15:13–17. [Google Scholar]
- 113. Oyama MA, Sisson DD, Solter PF. Prospective screening for occult cardiomyopathy in dogs by measurement of plasma atrial natriuretic peptide, B‐type natriuretic peptide, and cardiac troponin‐I concentrations. Am J Vet Res 2007;68:42–47. [DOI] [PubMed] [Google Scholar]
- 114. Tarducci A, Abate O, Borgarelli M, et al. Serum values of cardiac troponin‐T in normal and cardiomyopathic dogs. Vet Res Commun 2004;28:385–388. [DOI] [PubMed] [Google Scholar]
- 115. DeFrancesco TC, Atkins CE, Keene BW, et al. Prospective clinical evaluation of serum cardiac troponin T in dogs admitted to a veterinary teaching hospital. J Vet Intern Med 2002;16:553–557. [DOI] [PubMed] [Google Scholar]
- 116. Noszczyk‐Nowak A, Paslawska U, Cepiel A, et al. 24‐hour holter monitoring and troponin I level in boxers with arrhythmogenic right ventricular cardiomyopathy. Kafkas Universitesi Veteriner Fakultesi Dergisi 2013;19:A99–A104. [Google Scholar]
- 117. Church WM, Sisson DD, Oyama MA, Zachary JF. Third degree atrioventricular block and sudden death secondary to acute myocarditis in a dog. J Vet Cardiol 2007;9:53–57. [DOI] [PubMed] [Google Scholar]
- 118. Carreton E, Corbera JA, Juste MC, et al. Dirofilaria Immitis infection in dogs: cardiopulmonary biomarker levels. Vet Parasitol 2011;176:313–316. [DOI] [PubMed] [Google Scholar]
- 119. Carreton E, Grandi G, Morchon R, et al. Myocardial damage in dogs affected by heartworm disease (Dirofilaria Immitis): immunohistochemical study of cardiac myoglobin and troponin I in naturally infected dogs. Vet Parasitol 2012;189:390–393. [DOI] [PubMed] [Google Scholar]
- 120. Carreton E, Morchon R, Gonzalez‐Miguel J, et al. Utility of cardiac biomarkers during adulticide treatment of heartworm disease (Dirofilaria Immitis) in dogs. Vet Parasitol 2013;197:244–250. [DOI] [PubMed] [Google Scholar]
- 121. Chun R, Kellihan HB, Henik RA, Stepien RL. Comparison of plasma cardiac troponin I concentrations among dogs with cardiac hemangiosarcoma, noncardiac hemangiosarcoma, other neoplasms, and pericardial effusion of nonhemangiosarcoma origin. J Am Vet Med Assoc 2010;237:806–811. [DOI] [PubMed] [Google Scholar]
- 122. Linde A, Summerfield NJ, Sleeper MM, et al. Pilot study on cardiac troponin I levels in dogs with pericardial effusion. J Vet Cardiol 2006;8:19–23. [DOI] [PubMed] [Google Scholar]
- 123. Pelander L, Hagman R, Haggstrom J. Concentrations of cardiac troponin I before and after ovariohysterectomy in 46 female dogs with pyometra. Acta Vet Scand 2008;50:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Hagman R, Lagerstedt A, Fransson BA, et al. Cardiac troponin I levels in canine pyometra. Acta Vet Scand 2007;49:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Kocaturk M, Martinez S, Eralp O, et al. Tei index (myocardial performance index) and cardiac biomarkers in dogs with parvoviral enteritis. Res Vet Sci 2012;92:24–29. [DOI] [PubMed] [Google Scholar]
- 126. Bastan I, Kurtdede A, Sel T, et al. Serum cardiac troponin‐I in dogs with CPV‐2 infection. Ankara Universitesi Veteriner Fakultesi Dergisi 2013;60:251–255. [Google Scholar]
- 127. Mastrorilli C, Dondi F, Agnoll C, et al. Clinicopathologic features and outcome predictors of Leptospira Interrogans Australis serogroup infection in dogs: a retrospective study of 20 cases (2001‐2004). J Vet Intern Med 2007;21:3–10. [DOI] [PubMed] [Google Scholar]
- 128. Silvestrini P, Piviani M, Alberola J, et al. Serum cardiac troponin I concentrations in dogs with Leishmaniasis: correlation with age and clinicopathologic abnormalities. Vet Clin Pathol 2012;41:568–574. [DOI] [PubMed] [Google Scholar]
- 129. Luciani A, Sconza S, Civitella C, Guglielmini C. Evaluation of the cardiac toxicity of n‐methyl‐glucamine antimoniate in dogs with naturally occurring leishmaniasis. Vet J 2013;196:119–121. [DOI] [PubMed] [Google Scholar]
- 130. Lobetti R, Dvir E, Pearson JC. Cardiac troponins in canine babesiosis. J Vet Intern Med 2002;16:63–68. [DOI] [PubMed] [Google Scholar]
- 131. Lobetti R, Kirberger R, Keller N, et al. NT‐proBNP and cardiac Troponin I in virulent canine babesiosis. Vet Parasitol 2012;190:333–339. [DOI] [PubMed] [Google Scholar]
- 132. Koutinas CK, Mylonakis ME, O'Brien PJ, et al. Serum cardiac troponin I concentrations in naturally occurring myelosuppressive and non‐myelosuppressive canine monocytic ehrlichiosis. Vet J 2012;194:259–261. [DOI] [PubMed] [Google Scholar]
- 133. Hamacher L, Doerfelt R, Mueller M, Wess G. Serum cardiac troponin I concentrations in dogs with systemic inflammatory response syndrome. J Vet Intern Med 2015;29:164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Navarro‐Cubas J, Bell R, Wotton PR, et al. Steroid‐responsive meningitis‐arteritis with spontaneous echocardiographic contrast and elevated cardiac troponin I in a dog. Vet Rec 2011;169:527. [DOI] [PubMed] [Google Scholar]
- 135. Snyder K, Saunders AB, Levine JM, Clubb FJ. Arrhythmias and elevated troponin I in a dog with steroid‐responsive meningitis‐arteritis. J Am Anim Hosp Assoc 2010;46:61–65. [DOI] [PubMed] [Google Scholar]
- 136. Gow DJ, Gow AG, Bell R, et al. Serum cardiac troponin I in dogs with primary immune‐mediated haemolytic anaemia. J Small Anim Pract 2011;52:259–264. [DOI] [PubMed] [Google Scholar]
- 137. Selting KA, Lana SE, Ogilvie GK, et al. Cardiac troponin I in canine patients with lymphoma and osteosarcoma receiving doxorubicin: comparison with clinical heart disease in a retrospective analysis. Vet Comp Oncol 2004;2:142–156. [DOI] [PubMed] [Google Scholar]
- 138. Kent M, Reiss C, Blas‐Machado U. Elevated cardiac troponin I in a dog with an intracranial meningioma and evidence of myocardial necrosis. J Am Anim Hosp Assoc 2010;46:48–55. [DOI] [PubMed] [Google Scholar]
- 139. Prosek R, Sisson DD, Oyama MA, Solter PF. Distinguishing cardiac and noncardiac dyspnea in 48 dogs using plasma atrial natriuretic factor, B‐type natriuretic factor, endothelin, and cardiac troponin‐I. J Vet Intern Med 2007;21:238–242. [DOI] [PubMed] [Google Scholar]
- 140. Planellas M, Cuenca R, Tabar M, et al. Evaluation of C‐reactive protein, haptoglobin and cardiac troponin 1 levels in brachycephalic dogs with upper airway obstructive syndrome. BMC Vet Res 2012;8:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Pelander L, Ljungvall I, Haggstrom J. Myocardial cell damage in 24 dogs bitten by the common European viper (Vipera Berus). Vet Rec 2010;166:687–690. [DOI] [PubMed] [Google Scholar]
- 142. Langhorn R, Persson F, Ablad B, et al. Myocardial injury in dogs with snake envenomation and its relation to systemic inflammation. J Vet Emerg Crit Care 2014;24:174–181. [DOI] [PubMed] [Google Scholar]
- 143. Segev G, Ohad DG, Shipov A, et al. Cardiac arrhythmias and serum cardiac troponins in Vipera Palaestinae envenomation in dogs. J Vet Intern Med 2008;22:106–113. [DOI] [PubMed] [Google Scholar]
- 144. Mellor PJ, Mellanby RJ, Baines EA, et al. High serum troponin I concentration as a marker of severe myocardial damage in a case of suspected exertional heatstroke in a dog. J Vet Cardiol 2006;8:55–62. [DOI] [PubMed] [Google Scholar]
- 145. Schober KE, Cornand C, Kirbach B, et al. Serum cardiac troponin I and cardiac troponin T concentrations in dogs with gastric dilatation‐volvulus. J Am Vet Med Assoc 2002;221:381–388. [DOI] [PubMed] [Google Scholar]
- 146. Burgener IA, Kovacevic A, Mauldin GN, Lombard CW. Cardiac troponins as indicators of acute myocardial damage in dogs. J Vet Intern Med 2006;20:277–283. [DOI] [PubMed] [Google Scholar]
- 147. Kovacevic A, Burgener IA, Doherr MG, et al. Longterm electrocardiograms and serum levels of cardiac troponin T as prognostic factors in dogs with gastric torsion. Kleintierpraxis 2005;50:355–364. [Google Scholar]
- 148. Connolly D, Cannata J, Boswood A, et al. Cardiac troponin I in Cats with hypertrophic cardiomyopathy. J Feline Med Surg 2003;5:209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Jung SW, Kittleson MD. The effect of atenolol on NT‐proBNP and troponin in asymptomatic cats with severe left ventricular hypertrophy because of hypertrophic cardiomyopathy: a pilot study. J Vet Intern Med 2011;25:1044–1049. [DOI] [PubMed] [Google Scholar]
- 150. Borgeat K, Sherwood K, Payne JR, et al. Plasma cardiac troponin I concentration and cardiac death in cats with hypertrophic cardiomyopathy. J Vet Intern Med 2014;28:1731–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Lalor SM, Gunn‐Moore DA, Cash R, et al. Serum cardiac troponin I concentrations in cats with anaemia ‐ a preliminary, single‐centre observational study. J Small Anim Pract 2014;55:320–322. [DOI] [PubMed] [Google Scholar]
- 152. Herndon WE, Rishniw M, Schrope D, et al. Assessment of plasma cardiac troponin I concentration as a means to differentiate cardiac and noncardiac causes of dyspnea in cats. J Am Vet Med Assoc 2008;233:1261–1264. [DOI] [PubMed] [Google Scholar]
- 153. Connolly D, Guitian J, Boswood A, Neiger R. Serum troponin I levels in hyperthyroid cats before and after treatment with radioactive iodine. J Feline Med Surg 2005;7:289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Sangster JK, Panciera DL, Abbott JA, et al. Cardiac biomarkers in hyperthyroid cats. J Vet Intern Med 2014;28:465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Payne EE, Roberts BK, Schroeder N, et al. Assessment of a point‐of‐care cardiac troponin I test to differentiate cardiac from noncardiac causes of respiratory distress in dogs. J Vet Emerg Crit Care 2011;21:217–225. [DOI] [PubMed] [Google Scholar]
- 156. Krams R, Kofflard MJM, Duncker DJ, et al. Decreased coronary flow reserve in hypertrophic cardiomyopathy is related to remodeling of the coronary microcirculation. Circulation 1998;97:230–233. [DOI] [PubMed] [Google Scholar]
- 157. Maron BJ, Wolfson JK, Epstein SE, Roberts WC. Intramural (small vessel) coronary‐artery disease in hypertrophic cardiomyopathy. J Am Coll Cardiol 1986;8:545–557. [DOI] [PubMed] [Google Scholar]
- 158. Moreno V, Hernandez Romero D, Antonio Vilchez J, et al. Serum levels of high‐sensitivity troponin T: a novel marker for cardiac remodeling in hypertrophic cardiomyopathy. J Card Fail 2010;16:950–956. [DOI] [PubMed] [Google Scholar]
- 159. Sato Y, Taniguchi R, Nagai K, et al. Measurements of cardiac troponin T in patients with hypertrophic cardiomyopathy. Heart 2003;89:659–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Cambronero F, Marin F, Roldan V, et al. Biomarkers of pathophysiology in hypertrophic cardiomyopathy: implications for clinical management and prognosis. Eur Heart J 2009;30:139–151. [DOI] [PubMed] [Google Scholar]
- 161. Harada K, Morimoto S. Inherited cardiomyopathies as a troponin disease. Jpn J Physiol 2004;54:307–318. [DOI] [PubMed] [Google Scholar]
- 162. Hamwi SM, Sharma AK, Weissman NJ, et al. Troponin‐I elevation in patients with increased left ventricular mass. Am J Cardiol 2003;92:88–90. [DOI] [PubMed] [Google Scholar]
- 163. Cheng W, Li BS, Kajstura J, et al. Stretch‐induced programmed myocyte cell‐death. J Clin Invest 1995;96:2247–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Bugiardini R, Merz CNB. Angina with “normal” coronary arteries: a changing philosophy. J Am Med Assoc 2005;293:477–484. [DOI] [PubMed] [Google Scholar]
- 165. Tan LB, Jalil JE, Pick R, et al. Cardiac myocyte necrosis induced by angiotensin‐II. Circ Res 1991;69:1185–1195. [DOI] [PubMed] [Google Scholar]
- 166. Mann DL, Kent RL, Parsons B, Cooper G. Adrenergic effects on the biology of the adult mammalian cardiocyte. Circulation 1992;85:790–804. [DOI] [PubMed] [Google Scholar]
- 167. Todd GL, Baroldi G, Pieper GM, et al. Experimental catecholamine‐induced myocardial necrosis. Morphology, quantification and regional distribution of acute contraction band lesions. J Mol Cell Cardiol 1985;17:317–338. [DOI] [PubMed] [Google Scholar]
- 168. Levine B, Kalman J, Mayer L, et al. Elevated circulating levels of tumor‐necrosis‐factor in severe chronic heart‐failure. N Engl J Med 1990;323:236–241. [DOI] [PubMed] [Google Scholar]
- 169. Krown KA, Page MT, Nguyen C, et al. Tumor necrosis factor alpha‐induced apoptosis in cardiac myocytes ‐ involvement of the sphingolipid signaling cascade in cardiac cell death. J Clin Invest 1996;98:2854–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Grieve DJ, Shah AM. Oxidative stress in heart failure ‐ more than just damage. Eur Heart J 2003;24:2161–2163. [DOI] [PubMed] [Google Scholar]
- 171. Goeser S, Andrassy M, Buss SJ, et al. Cardiac troponin I but not cardiac troponin T induces severe autoimmune inflammation in the myocardium. Circulation 2006;114:1693–1702. [DOI] [PubMed] [Google Scholar]
- 172. Okazaki T, Tanaka Y, Nishio R, et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD‐1‐deficient mice. Nat Med 2003;9:1477–1483. [DOI] [PubMed] [Google Scholar]
- 173. Peacock WF, De Marco T, Fonarow GC, et al. Cardiac troponin and outcome in acute heart failure. N Engl J Med 2008;358:2117–2126. [DOI] [PubMed] [Google Scholar]
- 174. Kuwabara Y, Sato Y, Miyamoto T, et al. Persistently increased serum concentrations of cardiac troponin in patients with acutely decompensated heart failure are predictive of adverse outcomes. Circ J 2007;71:1047–1051. [DOI] [PubMed] [Google Scholar]
- 175. de Lemos JA. Increasingly sensitive assays for cardiac troponins. A review. J Am Med Assoc 2013;309:2262–2269. [DOI] [PubMed] [Google Scholar]
- 176. Wallace T, Abdullah S, Drazner M, et al. Prevalence and determinants of troponin T elevation in the general population. Circulation 2006;113:1958–1965. [DOI] [PubMed] [Google Scholar]
- 177. OBrien PJ. Deficiencies of myocardial troponin‐T and creatine kinase MB isoenzyme in dogs with idiopathic dilated cardiomyopathy. Am J Vet Res 1997;58:11–16. [PubMed] [Google Scholar]
- 178. Kubo T, Kitaoka H, Okawa M, et al. Combined measurements of cardiac troponin I and brain natriuretic peptide are useful for predicting adverse outcomes in hypertrophic cardiomyopathy. Circ J 2011;75:919–926. [DOI] [PubMed] [Google Scholar]
- 179. Sato Y, Yamada T, Taniguchi R, et al. Persistently increased serum concentrations of cardiac troponin T in patients with idiopathic dilated cardiomyopathy are predictive of adverse outcomes. Circulation 2001;103:369–374. [DOI] [PubMed] [Google Scholar]
- 180. Horwich TB, Patel J, MacLellan WR, Fonarow GC. Cardiac troponin I is associated with impaired hemodynamics, progressive left ventricular dysfunction, and increased mortality rates in advanced heart failure. Circulation 2003;108:833–838. [DOI] [PubMed] [Google Scholar]
- 181. Hezzell MJ, Boswood A, Chang Y, et al. The combined prognostic potential of serum high‐sensitivity cardiac troponin I and N‐terminal pro‐B‐type natriuretic peptide concentrations in dogs with degenerative mitral valve disease. J Vet Intern Med 2012;26:302–311. [DOI] [PubMed] [Google Scholar]
- 182. Miller WL, Hartman KA, Burritt MF, et al. Profiles of serial changes in cardiac troponin T concentrations and outcome in ambulatory patients with chronic heart failure. J Am Coll Cardiol 2009;54:1715–1721. [DOI] [PubMed] [Google Scholar]
- 183. Miller WL, Hartman KA, Burritt MF, et al. Serial biomarker measurements in ambulatory patients with chronic heart failure ‐ the importance of change over time. Circulation 2007;116:249–257. [DOI] [PubMed] [Google Scholar]
- 184. Masson S, Anand I, Favero C, et al. Serial measurement of cardiac troponin T using a highly sensitive assay in patients with chronic heart failure data from 2 large randomized clinical trials. Circulation 2012;125:280–U249. [DOI] [PubMed] [Google Scholar]
- 185. Ammann P, Fehr T, Minder EI, et al. Elevation of troponin I in sepsis and septic shock. Intensive Care Med 2001;27:965–969. [DOI] [PubMed] [Google Scholar]
- 186. Arlati S, Brenna S, Prencipe L, et al. Myocardial necrosis in ICU patients with acute non‐cardiac disease: a prospective study. Intensive Care Med 2000;26:31–37. [DOI] [PubMed] [Google Scholar]
- 187. Turner A, Tsamitros M, Bellomo R. Myocardial cell injury in septic shock. Crit Care Med 1999;27:1775–1780. [DOI] [PubMed] [Google Scholar]
- 188. Noble JS, Reid AM, Jordan LVM, et al. Troponin I and myocardial injury in the ICU. Br J Anaesth 1999;82:41–46. [DOI] [PubMed] [Google Scholar]
- 189. Guest T, Ramanathan A, Tuteur P, et al. Myocardial injury in critically ill patients ‐ a frequently unrecognized complication. J Am Med Assoc 1995;273:1945–1949. [PubMed] [Google Scholar]
- 190. Langhorn R, Thawley V, Oyama MA, et al. Prediction of long‐term outcome by measurement of serum concentration of cardiac troponins in critically ill dogs with systemic inflammation. J Vet Intern Med 2014;28:1492–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Wu TT, Yuan A, Chen CY, et al. Cardiac troponin I levels are a risk factor for mortality and multiple organ failure in noncardiac critically ill patients and have an additive effect to the APACHE II score in outcome prediction. Shock 2004;22:95–101. [DOI] [PubMed] [Google Scholar]
- 192. Vasile VC, Babuin L, Perez JAR, et al. Long‐term prognostic significance of elevated cardiac troponin levels in critically ill patients with acute gastrointestinal bleeding. Crit Care Med 2009;37:140–147. [DOI] [PubMed] [Google Scholar]
- 193. Kollef MH, Ladenson JH, Eisenberg PR. Clinically recognized cardiac dysfunction: an independent determinant of mortality among critically ill patients ‐ is there a role for serial measurement of cardiac troponin I? Chest 1997;111:1340–1347. [DOI] [PubMed] [Google Scholar]
- 194. Quenot JP, Le Teuff G, Quantin C, et al. Myocardial injury in critically ill patients ‐ relation to increased cardiac troponin I and hospital mortality. Chest 2005;128:2758–2764. [DOI] [PubMed] [Google Scholar]
- 195. ver Elst KM, Spapen HD, Nguyen DN, et al. Cardiac troponins I and T are biological markers of left ventricular dysfunction in septic shock. Clin Chem 2000;46:650–657. [PubMed] [Google Scholar]
- 196. Babuin L, Vasile VC, Perez JAR, et al. Elevated cardiac troponin is an independent risk factor for short‐ and long‐term mortality in medical intensive care unit patients. Crit Care Med 2008;36:759–765. [DOI] [PubMed] [Google Scholar]
- 197. Bessiere F, Khenifer S, Dubourg J, et al. Prognostic value of troponins in sepsis: a meta‐analysis. Intensive Care Med 2013;39:1181–1189. [DOI] [PubMed] [Google Scholar]
- 198. Markou N, Gregorakos L, Myrianthefs P. Increased blood troponin levels in ICU patients. Curr Opin Crit Care 2011;17:454–463. [DOI] [PubMed] [Google Scholar]
- 199. Maeder M, Fehr T, Rickli H, Ammann P. Sepsis‐associated myocardial dysfunction ‐ diagnostic and prognostic impact of cardiac troponins and natriuretic peptides. Chest 2006;129:1349–1366. [DOI] [PubMed] [Google Scholar]
- 200. Giannitsis E, Muller‐Bardorff M, Kurowski V, et al. Independent prognostic value of cardiac troponin T in patients with confirmed pulmonary embolism. Circulation 2000;102:211–217. [DOI] [PubMed] [Google Scholar]
- 201. Solomon MA, Correa R, Alexander HR, et al. Myocardial energy‐metabolism and morphology in a canine model of sepsis. Am J Physiol 1994;266:H757–H768. [DOI] [PubMed] [Google Scholar]
- 202. Szakacs JE, Mehlman B. Pathologic changes induced by 1‐norepinephrine ‐ quantitative aspects. Am J Cardiol 1960;5:619–627. [DOI] [PubMed] [Google Scholar]
- 203. Cher C, Armugam A, Zhu Y, Jeyaseelan K. Molecular basis of cardiotoxicity upon cobra envenomation. Cell Mol Life Sci 2005;62:105–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Rosjo H, Varpula M, Hagve T, et al. Circulating high sensitivity troponin T in severe sepsis and septic shock: distribution, associated factors, and relation to outcome. Intensive Care Med 2011;37:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Wright RS, Williams BA, Cramner H, et al. Elevations of cardiac troponin I are associated with increased short‐term mortality in noncardiac critically ill emergency department patients. Am J Cardiol 2002;90:634–636. [DOI] [PubMed] [Google Scholar]
- 206. Nelson OL, Thompson PA. Cardiovascular dysfunction in dogs associated with critical illnesses. J Am Anim Hosp Assoc 2006;42:344–349. [DOI] [PubMed] [Google Scholar]
- 207. Dickinson AE, Rozanski EA, Rush JE. Reversible myocardial depression associated with sepsis in a dog. J Vet Intern Med 2007;21:1117–1120. [DOI] [PubMed] [Google Scholar]
- 208. Altmann DR, Korte W, Maeder MT, et al. Elevated cardiac troponin I in sepsis and septic shock: no evidence for thrombus associated myocardial necrosis. PLoS ONE 2010;5:e9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Parker MM. Pathophysiology of cardiovascular dysfunction in septic shock. New Horiz 1998;6:130–138. [PubMed] [Google Scholar]
- 210. Bouhemad B, Nicolas‐Robin A, Arbelot C, et al. Isolated and reversible impairment of ventricular relaxation in patients with septic shock. Crit Care Med 2008;36:766–774. [DOI] [PubMed] [Google Scholar]
- 211. Kumar A, Thota V, Dee L, et al. Tumor necrosis factor alpha and interleukin 1 beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. J Exp Med 1996;183:949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Lim W, Whitlock R, Khera V, et al. Etiology of troponin elevation in critically ill patients. J Crit Care 2010;25:322–328. [DOI] [PubMed] [Google Scholar]
- 213. Johnson V, Gaynor A, Chan DL, Rozanski E. Multiple organ dysfunction syndrome in humans and dogs. J Vet Emerg Crit Care 2004;14:158–166. [Google Scholar]
- 214. Alcalai R, Planer D, Culhaoglu A, et al. Acute coronary syndrome vs nonspecific troponin elevation ‐ clinical predictors and survival analysis. Arch Intern Med 2007;167:276–281. [DOI] [PubMed] [Google Scholar]
- 215. Stein R, Gupta B, Agarwal S, et al. Prognostic implications of normal (< 0.10 ng/ml) and borderline (0.10 to 1.49 ng/ml) troponin elevation levels in critically ill patients without acute coronary syndrome. Am J Cardiol 2008;102:509–512. [DOI] [PubMed] [Google Scholar]
- 216. Kanderian AS, Francis GS. Cardiac troponins and chronic kidney disease. Kidney Int 2006;69:1112–1114. [DOI] [PubMed] [Google Scholar]
- 217. Ziebig R, Lun A, Hocher B, et al. Renal elimination of troponin T and troponin I. Clin Chem 2003;49:1191–1193. [DOI] [PubMed] [Google Scholar]
- 218. Ellis K, Dreisbach AW, Lertora JJL. Plasma elimination of cardiac troponin I in end‐stage renal disease. South Med J 2001;94:993–996. [PubMed] [Google Scholar]
- 219. Sharkey LC, Berzina I, Ferasin L, et al. Evaluation of serum cardiac troponin I concentration in dogs with renal failure. J Am Vet Med Assoc 2009;234:767–770. [DOI] [PubMed] [Google Scholar]
- 220. Porciello F, Rishniw M, Herndon WE, et al. Cardiac troponin I is elevated in dogs and cats with azotaemia renal failure and in dogs with non‐cardiac systemic disease. Aust Vet J 2008;86:390–394. [DOI] [PubMed] [Google Scholar]
- 221. Neeland IJ, Drazner MH, Berry JD, et al. Biomarkers of chronic cardiac injury and hemodynamic stress identify a malignant phenotype of left ventricular hypertrophy in the general population. J Am Coll Cardiol 2013;61:187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Krishnagopalan S, Kumar A, Parrillo JE, Kumar A. Myocardial dysfunction in the patient with sepsis. Curr Opin Crit Care 2002;8:376–388. [DOI] [PubMed] [Google Scholar]


