Abstract
Background
The prevalence and prognostic importance of atrial fibrillation (AF) on survival in nonsmall breed dogs with myxomatous mitral valvular disease (MMVD) and congestive heart failure (CHF) remain unknown.
Aim
To identify the prevalence of AF in nonsmall breed dogs with CHF because of MMVD and to characterize the impact of AF on survival outcome.
Animal
Sixty‐four client‐owned dogs (>15 kg) with MMVD and CHF.
Methods
Retrospective review of medical records for dogs weighing >15 kg with MMVD treated for CHF.
Results
Thirty‐three dogs presented with AF or developed AF during follow‐up examinations, and 31 dogs were free of AF until cardiac‐related death. For dogs with AF, median survival time (MST) was 142 days (range: 9–478) while dogs without AF lived 234 days (range: 13–879 days). AF increased risk of cardiac‐related death (HR = 2.544; 95% CI = 1.41–4.59; P = .0019) when compared to dogs without AF. MST was significantly prolonged for dogs with AF whose rates were adequately controlled (<160 bpm; 171 days; n = 13) when compared to dogs that failed to respond to negative chronotropic agents (61 days; n = 20; P = .032). The administration of combination treatment (diltiazem and digoxin) significantly decreased median HR to 144 bpm (range: 84–218 bpm) in dogs with AF and significantly prolonged MST (diltiazem+digoxin: 130 days versus diltiazem: 35 days, P = .0241) when compared to diltiazem alone.
Conclusions and Clinical Importance
Inadequately controlled AF is associated with a higher rate of mortality. Optimization of therapeutic strategies for the rate control of AF remains determined.
Keywords: Atrial fibrillation, Congestive heart failure, Myxomatous valvular disease, Prognosis
Abbreviations
- AF
atrial fibrillation
- CHF
congestive heart failure
- MMVD
myxomatous valvular disease
Myxomatous valvular degeneration (MMVD) is the most common heart disease in dogs, and is characterized by valvular leaflet thickening, prolapse and regurgitation, leading to secondary changes in cardiac structure and function. The prevalence of the disease is correlated with the age and the breed.1 Only a subpopulation of dogs with MMVD undergoes progression to congestive heart failure (CHF).2 Predictors of poor survival outcome of small dogs with CHF because of MMVD have been well‐documented, and include chordae tendineae rupture, left atrial wall tear and cardiac arrhythmia.2, 3, 4, 5
MMVD also occurs in medium‐sized and large breed dogs, although the disease characteristics are somewhat different from small breed dogs. MMVD in large breed dogs often presents with a lesser degree of AV valvular thickening, and echocardiographic evidence of mild to moderate left ventricular systolic dysfunction (e.g., increased LV end‐systolic diameter, LVESD) is usually present at the time CHF is diagnosed.5, 6 Furthermore, atrial fibrillation (AF) is more frequent in large breed dogs with MMVD.5
AF is the most common supraventricular tachyarrhythmia in dogs and is especially prevalent in those with severe cardiac disease. Cardiac structural changes that increase atrial wall stress predispose to AF.7 Electrical remodeling in the atria (shortening of refractory period, slowing of conduction velocity, alteration in expression level of ion channels) also plays a role in the development of AF.8 AF has several detrimental effects. In experimental models, chronic tachycardia (rate > 180 bpm) results in left ventricular systolic dysfunction and secondary chamber enlargement.9 Loss of atrial contraction and shortened diastolic filling time because of irregular and typically rapid ventricular response rate in AF may decrease cardiac output and elevate atrial filling pressure, both of which ultimately can contribute to the worsening of CHF and decreased quality and quantity of life.10
CHF is the biggest risk factor for development of AF and shortened survival time in humans.11, 12, 13 The combination of AF and CHF carries a worse prognosis than either alone. To the authors' knowledge, the prognostic significance of AF on survival time in medium‐sized to large breed dogs with MMVD and CHF, as well as its prevalence, are unknown. The primary objectives of the present retrospective study were (1) to identify the prevalence of AF in medium‐sized to large breed dogs with CHF due to MMVD, (2) to characterize the impact of AF on survival outcome in these dogs, and (3) to determine if ventricular rate control improves survival.
Materials and Methods
Animals and Criteria
The University of California‐Davis William R. Pritchard Veterinary Medical Teaching Hospital (UCD‐VMTH) database was examined retrospectively (January 1, 2005 to December 31, 2010) to identify medium‐sized to large (>15 kg) dogs with CHF (current or history of) due to MMVD. A total of 435 medical records were identified, using the search terms of “congestive”, “valve”, and “degeneration”. Information collected from the records included signalment, body weight (BW), serum biochemical profiles, dosage of cardiac medications, echocardiographic measurements, and electrocardiographic findings. Due to a wide range of variation in biochemical values and drug dosages throughout the management period of individual patient, data at the last examination close to death were subjected to maximum likelihood analysis.
Differentiating severe MMVD from dilated cardiomyopathy in large breed dogs can be problematic. To be diagnosed with severe MMVD as the cause of CHF, each dog had to have a shortening fraction >22%, color flow Doppler evidence of severe mitral regurgitation (MR), normal to hyperdynamic interventricular septal motion, and a normal to only mildly increased E‐point to septal separation (EPSS). Severe MR was defined as large eccentric regurgitant jets by color flow Doppler mapping and a severely enlarged left atrium. Dogs with sustained AF had to have an average resting heart rate (HR) >160 bpm on the surface electrocardiogram (ECG) at baseline. Entry time into the study was defined as the date of CHF diagnosis, and its diagnosis was reviewed and confirmed by board‐certified veterinary cardiologists. Exclusion criteria were as follows: (1) infective endocarditis, (2) congenital cardiac anomalies, (3) dilated cardiomyopathy (large LVESD, FS < 20%), (4) Boxers and Doberman Pinchers, (5) dogs without the aforementioned echocardiographic criteria or that were never in CHF, (6) presence of a pacemaker, (7) lone AF, (8) concomitant ventricular arrhythmias, (9) concurrent systemic diseases (i.e., endocrinopathy, cancer, primary renal failure), (10) noncardiac‐related death, or (11) still alive or lost to follow‐up at the time of study.
Definitions
CHF was defined as pulmonary edema, ascites and/or pleural effusion coupled with severe cardiac disease (severe left, right or both atrial enlargement) that required furosemide administration. Pulmonary edema was diagnosed based on clinical signs (cough, tachypnea, dyspnea, orthopnea), radiographic appearance and/or an elevated sleeping respiratory rate (SRR) that decreased following institution of furosemide treatment all in a dog with a severely enlarged left atrium.
Electrocardiographic evaluation was done by recording a surface ECG in an exam area. Average resting HR was defined as a mean value of 3 independent HR calculations from 3 different areas on the ECG tracings. The number of QRS complexes were counted over 6 seconds and multiplied by 10 to calculate the HR per minute. Antiarrhythmic agents utilized for the rate control of AF included diltiazem, extended release diltiazem (e.g., Dilacor XR), digoxin, and atenolol. Drugs were used alone or in combination. Adequate HR control of AF was defined as an average resting HR ≤ 160 bpm in an exam area and was determined by records at UCD‐VMTH or referring hospitals throughout the management period of each individual patient. When modifications were made in the dose and type of negative chronotropic agents, ECGs were re‐evaluated in 5–7 days to determine the adequacy of HR control of AF by either a clinician at the UCD‐VMTH or by a referring veterinarian.
Follow‐up on Outcome Events
The primary endpoint was cardiac‐related death. Cardiac‐related death was defined as a composite of death that resulted from sudden death or euthanasia related with worsening or refractory CHF. All dogs enrolled in the study reached the primary endpoint. When documented in the record, the survival time intervals from the diagnosis of CHF to cardiac‐related death were retrieved. For dogs for which survival time was not retrievable from the medical record, follow‐up information (survival status, final HR for dogs with AF, cause and date of death) was obtained by a phone interviews with the owner or the referring veterinarian.
Statistical Analysis
A Shapiro–Wilk normality test was used to determine the normality of data distribution. Normally distributed data were expressed as mean ± standard deviation (SD). Data with non‐normal distribution were expressed as median and range. Demographic and clinical variables were evaluated for difference between 2 groups (AF versus No AF). Homogeneity of the continuous variables such as age was compared by a Wilcoxon's signed‐rank procedure. Categorical data such as the prevalence of AF between males and females were analyzed by a Fisher's exact test. Cox proportional hazards regression analysis was performed to determine whether a significant relationship existed between clinical variables and survival endpoint. The hazard ratio (HR) and 95% confidence intervals (CI) were calculated. Results of analysis were considered significant when P values were <.05 and with the hazard ratio when the 95% CI was different from 1. The Kaplan–Meier (KM) method was used to compare median survival time (the time at which 50% of dogs in each group were dead) and 95% CI between the groups. A log‐rank test was used to determine whether a significant difference in KM survival curves existed between the groups. A simple linear regression analysis was performed to examine the relationship between the dosage of each antiarrhythmic agent and the degree of HR reduction. For all analyses,1 statistical significance was set at P < .05.
Results
Overall Demographic Data and Clinical Outcome
A total of sixty‐four dogs met the inclusion criteria. The most common breeds were Australian Shepherd (n = 16), German Shepherd (n = 9) and Labrador Retriever (n = 7). Twenty‐one other breeds with 1–3 dogs each were also identified. Median age at the time of diagnosis of CHF was 11 years (range: 6–15 years). Median body weight (BW) was 24 kg (range: 16–64 kg). Thirty‐eight dogs were male, and 26 female. The majority (n = 50) exhibited left‐sided CHF, and 14 dogs were in both left‐ and right‐sided CHF. All dogs (n = 64) were administered furosemide, and their median daily dose was 5.6 mg/kg/day (range: 3–15 mg/kg/day). The majority of dogs (n = 59) were administered enalapril, and their median dose was 0.45 mg/kg q12h (range: 0.43–0.56 mg/kg q12h). Half (n = 32) of the dogs were administered pimobendan, and their median dose was 0.23 mg/kg q12h (range: 0.21–0.27 mg/kg q12h). Other medications for heart failure concurrently utilized were hydrochlorothiazide (n = 3) and spironolactone (n = 3). Median LA/Ao ratio measured at the end of T‐wave was 2.5 (range: 1.7–3.7). Median FS% and indexed LVESD (LVESD/BW1/3) were 36% (range: 22–55%) and 2.9 (range: 2.7–3.4), respectively (Table 1).
Table 1.
Demographic data of 64 dogs with MMMVD and CHF separated by AF status
| Parameter | AF (n = 33) | No AF (n = 31) | P‐value |
|---|---|---|---|
| Age (year) | 11 (6–15) | 11 (6–14) | .78 |
| Sex (M/F) | 20/13 | 18/13 | .99 |
| BW (kg) | 28 (16–64) | 22 (16–45) | .016a |
| Na (mmol/L) | 146 (124–151) | 148 (126–154) | .19 |
| K (mmol/L) | 4.3 (3.1–4.9) | 4.1 (3.2–4.3) | .27 |
| BUN (mg/dL) | 34 (11–128) | 27 (14–99) | .33 |
| Crea (mg/dL) | 1.4 (0.6–2.9) | 1.3 (0.8–2.6) | .30 |
| CHF (L/R) | 25/8 | 25/6 | .76 |
| Pimobendan (Y/N) | 16/17 | 16/15 | .99 |
| Furosemide (mg/kg/day) | 6 (3–12) | 5.1 (3–15) | .62 |
| Enalapril (Y/N) | 30/3 | 29/2 | .91 |
| LA/Ao | 2.6 (1.7–3.7) | 2.3 (1.8–3.5) | .21 |
| FS% | 36 (23–48) | 39 (22–55) | .21 |
| Indexed LVESD | 3 (2.8–3.3) | 2.9 (2.7–3.4) | .13 |
| Survival time (days) | 142 (9–478) | 234 (13–879) | .002b |
AF = atrial fibrillation; CHF = congestive heart failure; MMMVD = myxomatous mitral valve degeneration; LA = left atrium; Ao = aorta; FS = fractional shortening; LVESD = left ventricular end‐systolic diameter; BUN = blood urea nitrogen; BW = body weight; M = male; F = female; BUN = blood urea nitrogen; Crea = creatinine; Na = sodium; K = potassium. Median (range) or proportion is reported.
P < .05.
P < .01.
Median survival time (MST: time from the diagnosis of CHF to cardiac‐related death) in all 64 dogs was 172 days (range: 9–879 days). Thirty‐three of the dogs (52%) were diagnosed with AF. AF was documented in 21 dogs at the time CHF was diagnosed. Twelve dogs developed AF during the course of follow‐up examinations, and the median interval from CHF diagnosis to AF was 63 days (range: 5–267 days). Median period from the documentation of AF to cardiac‐related death was 75 days (range: 3–470 days). Thirty‐one dogs (48%) were in sinus rhythm from the onset until cardiac‐related death. The distribution of the above mentioned clinical variables was not significantly different between dogs in AF and dogs not in AF except for 2 variables, BW, and survival time (Table 1). Dogs in AF (median: 28 kg, range: 16–64 kg) weighed more than dogs without AF (median: 22, range: 16–45; P = .0155). For dogs with AF, MST was 142 days (range: 9–478) while dogs without AF had a MST of 234 days (range: 13–879 days; P = .002).
Cox Proportional Hazard Analyses of the Effect of Clinical Variables on Survival Time
The univariate analysis of 12 covariables (AF, LA/Ao, FS%, furosemide, pimobendan, serum sodium concentration, serum potassium concentration, BUN, serum creatinine concentration, age, sex, BW) was performed to identify risk factors that significantly impacted the survival time. AF had a significant effect on the risk of reaching the primary endpoint (cardiac‐related death) (HR = 2.544; 95% CI = 1.41–4.59; P = .0019) when compared to dogs without AF. No other variable was associated with a positive or a negative clinical outcome (Table 2). Since AF was the only significant clinical variable identified in the univariate analysis, a subsequent multivariate analysis was not indicated. Consistent with the univariate logistic regression, the Log‐Rank test for the Kaplan–Meier (KM) survival curves confirmed that there was a significant difference in survival time between dogs with AF and dogs without AF (P = .002, Fig 1).
Table 2.
Univariate analysis of maximum likelihood estimates
| Predictors | HR (95% CI) | P‐value |
|---|---|---|
| AF | 2.544 (1.410–4.590) | .0019a |
| LA/Ao | 1.475 (0.844–2.577) | .17 |
| FS | 1.020 (0.979–1.062) | .34 |
| Furosemide | 0.953 (0.881–1.031) | .22 |
| Pimobendan | 0.724 (0.413–1.270) | .26 |
| Na | 1.012 (0.953–1.074) | .70 |
| K | 1.158 (0.891–1.135) | .39 |
| BUN | 0.994 (0.972–1.016) | .56 |
| Crea | 1.998 (0.859–4.696) | .11 |
| Age | 0.907 (0.785–1.048) | .18 |
| Sex | 1.727 (0.909–3.279) | .09 |
| BW | 1.005 (0.963–1.049) | .83 |
HR = hazard ratio; CI = confidence interval; AF = atrial fibrillation; LA = left atrium; Ao = aorta; FS = fractional shortening; BUN = blood urea nitrogen; Crea = creatinine; Na = sodium; K = potassium; BW = body weight. HR (95% CI) is presented.
P < .01.
Figure 1.
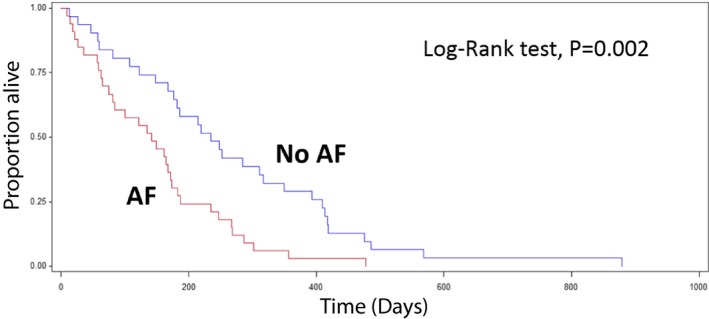
Kaplan–Meier survival statistics of dogs with or without AF. Dogs without AF had a significantly longer median survival time (MST) compared with dogs with AF (MST of No AF = 234 days versus AF = 142 days, P = .002).
Effect of Negative Chronotropic Treatment for AF on Survival Outcome
Reduction in the ventricular response to AF is the primary goal of rate treatment and the survival benefit of achieving an average HR < 160 bpm with antiarrhythmic treatment was examined (Fig 2). Adequate heart rate control was defined as a ventricular rate <160 bpm, and so inadequate control was defined as a ventricular rate >160 bpm. MST was significantly longer for dogs whose HR was adequately controlled than for dogs that failed to adequately respond to negative chronotropic agents. MST from the diagnosis of CHF to cardiac‐related death of dogs with an average HR < 160 bpm (n = 13) was 171 days, whereas dogs with an average HR > 160 bpm (n = 20) lived for 61 days on average (P = .032, Fig 2). Nine dogs of 13 with an adequate rate control received diltiazem and digoxin. For dogs with AF, median FS% and LVESD of dogs with HR < 160 bpm were not different from dogs with HR > 160 bpm. Median HR before the administration of any negative chronotropic agents for dogs with AF was 220 bpm (range: 160–270 bpm). Diltiazem alone was the most commonly used method of treatment. The median dose was 1 mg/kg q8h (range: 0.5–2 mg/kg q8h). Median HR after diltiazem alone was 180 bpm (range: 120–240 bpm). There was a statistically significant reduction in median HR after diltiazem alone (P = .006). Cox proportional hazards analysis revealed there was no relationship between the use of diltiazem and MST (P = .669). For dogs administered diltiazem plus digoxin, the average HR was better controlled (median: 144 bpm; range: 84–218 bpm) as compared to dogs with diltiazem alone (median: 180 bpm; range: 120–240 bpm), and the KM analysis demonstrated that the administration of digoxin along with diltiazem significantly improved survival. Thirteen dogs received digoxin in combination with diltiazem and had a longer MST than dogs without digoxin administration (diltiazem plus digoxin: 130 days versus diltiazem alone: 35 days, P = .0241, Fig 3). Median dose of digoxin was 0.004 mg/kg q12h (range: 0.003–0.006 mg/kg q12h). Two dogs received sustained release diltiazem (Dilacor XR, 3 mg/kg q12h) in combination with digoxin (0.003 mg/kg q12h). Both exhibited adequate HR reduction (range: 140–160 bpm). Resting average HR did not improve with atenolol alone in 3 dogs at a dose of 0.3–1.5 mg/kg q12h (HR before: 220–250 bpm; HR after 200–240 bpm).
Figure 2.
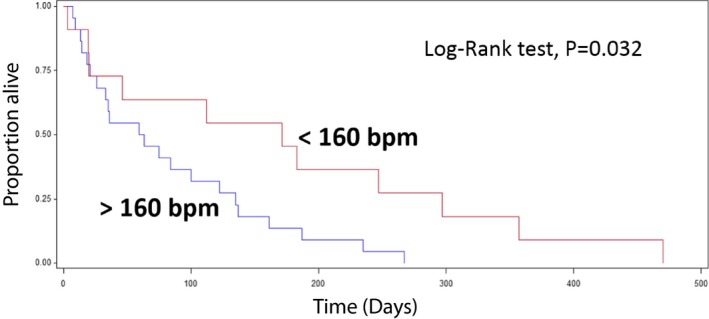
Kaplan–Meier survival curve of dogs with (<160 bpm) and without (>160 bpm) adequate rate control of AF. Dogs with adequate rate control showed a significantly longer median survival time compared to dogs with inadequate rate control (<160 bpm: 171 days versus >160 bpm: 61 days, P = .032).
Figure 3.
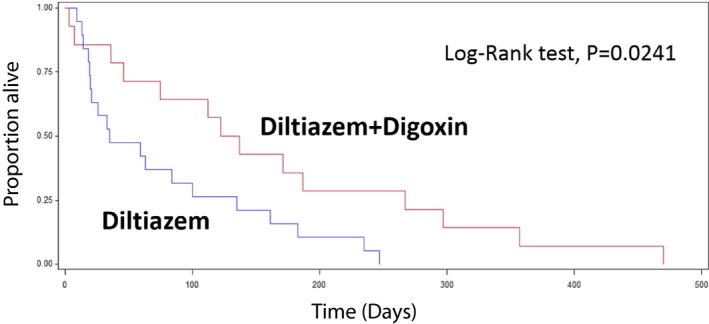
Kaplan–Meier survival curve of dogs with and without digoxin for AF heart rate control. Dogs administered diltiazem plus digoxin had a significantly longer median survival time compared with dogs not administered digoxin (diltiazem+digoxin: 130 days versus diltiazem: 35 days, P = .024).
Discussion
Atrial fibrillation has a high prevalence in dogs with CHF due to MMVD and is associated with a shorter survival time in medium to large‐sized dogs with MMVD and CHF. The left atrial size of all 64 dogs that met the inclusion criteria was severely enlarged (median LA/Ao ratio of 2.5), and approximately 52% of them (n = 33) developed AF. Atrial pathology or increased atrial size predisposes to the development of AF, since atrial stretch is associated with increased dispersion of refractoriness and altered electrical propagation.14, 15
MST of the dogs in this study with severe MR because of MMVD but no AF was approximately 8 months. That is comparable to the previously reported MST of 9 months in small dogs with severe MMVD and CHF when only cardiac‐related death was considered as an endpoint.2 MST of dogs with AF in this study, however, was significantly shorter (4.7 months).
Pharmacological rate control remains central to long‐term management of AF in dogs. In this study less than half of dogs (42%) in the AF group achieved adequate rate control on an ECG in the exam area (HR < 160 bpm) with negative chronotropic treatment. Median survival was shorter in dogs where HR of 160 bpm or less was not achieved. Chronic tachycardia (HR > 180 bpm) for over a 2–4 week period in dogs leads to tachycardia‐induced myocardial failure.16, 17 A primary therapeutic goal of AF is thought to be prevention of tachycardia‐induced myocardial failure either by controlling ventricular response rate (rate control) or by converting AF to sinus rhythm (rhythm control).15 Human studies have established that morbidity and mortality are comparable between rate and rhythm control treatment.18 AF management with the rate control approach is at least as effective as rhythm‐based management for outcomes from human studies.19
Although FS% is not the most accurate or consistent echocardiographic variable for evaluating left ventricular (LV) function, it is commonly used as a reasonable surrogate of LV systolic pump function.20 It was previously reported that small breed dogs (<15 kg) with MMVD and severe CHF exhibited hyperdynamic LV function (mean FS% approximately 52%) because of a marked increase in LVEDD and a normal to mildly increased LVESD.5 In this study, median FS% was much lower but within reference range (36%) due to a marked increase in LVEDD and most commonly a moderate increase in LVESD, which are characteristic features of severe MR in medium to large‐sized dogs with MMVD and CHF.5, 6 Since FS% and indexed LVESD were not different between dogs with AF and dogs without AF, decreased survival time in the dogs with AF may have more to do with lack of atrial contribution to cardiac function than to left ventricular systolic dysfunction.
The available pharmacological interventions for AF rate control can broadly be divided into nondihydropyridine calcium channel blockers (e.g., diltiazem), digoxin, and beta‐adrenergic receptor blockers (e.g., atenolol, sotalol). While Dilacor XR was used only in 2 dogs in the study, its antiarrhythmic potency and extended release property make it an attractive option for AF rate control. These established pharmacological therapies exert their beneficial effect via modulation of AV nodal conduction by prolonging the refractory period of the node and slowing conduction through it. A resting average HR of less than 160 bpm on an ECG in the hospital has been suggested as the target HR for AF rate control.21 The rationale behind this cutoff is that tachycardia‐induced myocardial failure consistently occurs at a heart rate of 180 bpm or above.16, 17 The fact that a cutoff of 160 bpm yielded a significant difference in survival in this study strengthens the argument that this cutoff is potentially valid. However, there was no attempt to identify the optimal cutoff in this study because of the small dataset so it is possible that an even lower limit could be more beneficial. The optimal level of rate control with respect to morbidity and mortality rates remains to be determined.
The study results show that survival outcome was significantly better in dogs with adequate HR control (<160 bpm) in comparison to those with poor rate control (Fig 2). Strict rate control was apparently difficult to achieve with a single agent like diltiazem. Whether a higher dose of diltiazem in any given dog would have been beneficial or would have been tolerated is unknown. For dogs concurrently administered digoxin and diltiazem, the target HR was more frequently accomplished than with diltiazem alone, and the KM analysis supports the conclusion that the administration of digoxin along with diltiazem significantly improved the survival outcome (Fig 3). This observation is consistent with a previous report that the combination of diltiazem and digoxin provides better rate control than either drug alone in dogs with AF.22
The question remains whether stricter rate control (target HR < 140 bpm) improves prognosis even further. The question also remains whether or not higher drug doses or combinational treatment of multiple drugs could lead to increased incidence of drug‐related adverse effects. A recent human study demonstrated that lenient rate control is not inferior to strict rate control when it comes to survival benefit, and it is easier to achieve.23
The study limitations are the retrospective study design and so its potential selection bias. Longitudinal data were limited in some dogs. Weak statistical power related with nonrandomized study groups (AF versus No AF) limits our ability to expand the study results to a larger population. It is also important to emphasize that enrolled dogs were managed by different clinicians spanning 6 years. Although the primary study interest was to determine the prognostic value of AF in dogs with MMVD and CHF, successful management of CHF and different types of treatment regimens as confounding variables could have influenced the survival outcome. Adequacy of HR control for AF antiarrhythmic treatment was only assessed by ECG. A 24‐hour ambulatory ECG (Holter monitor) before and after the medical treatment was not performed in the assessment of daily average HR. Some dates for establishing CHF diagnosis and cardiac‐related death were solely based on client's memory, but the majority of information was collected on the basis of the medical records.
Conclusion
The prevalence of AF was high in medium‐sized to large dogs with MMVD and CHF, and AF significantly increased the risk of cardiac‐related death in these dogs. A lower MST in dogs with AF and suboptimal rate control suggests that adequate rate control is a critical element in determining long‐term survival. Adequate ventricular rate control brought about by the administration of digoxin and diltiazem produced favorable effects on MST. A prospective clinical trial is warranted to determine effective treatment methods and prognostic benefits of optimal HR control in the management of AF.
Acknowledgments
Authors thank all clinicians who contributed to clinical management of dogs enrolled in the study.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Footnote
SAS v.9.2, Cary, NC
References
- 1. Haggstrom J, Hansson K, Kvart C, et al. Chronic valvular disease in the cavalier King Charles spaniel in Sweden. Vet Rec 1992;131:549–553. [PubMed] [Google Scholar]
- 2. Borgarelli M, Savarino P, Crosara S, et al. Survival characteristics and prognostic variables of dogs with mitral regurgitation attributable to myxomatous valve disease. J Vet Intern Med 2008;22:120–128. [DOI] [PubMed] [Google Scholar]
- 3. BENazepril in Canine Heart disease Study Group . The effect of benazepril on survival times and clinical signs of dogs with congestive heart failure: results of a multicenter, prospective, randomized, double‐blinded, placebo‐controlled, long‐term clinical trial. J Vet Cardiol 1999;1:7–18. [DOI] [PubMed] [Google Scholar]
- 4. Serres F, Chetboul V, Tissier R, et al. Chordae tendineae rupture in dogs with degenerative mitral valve disease: prevalence, survival, and prognostic factors (114 cases, 2001–2006). J Vet Intern Med 2007;21:258–264. [DOI] [PubMed] [Google Scholar]
- 5. Borgarelli M, Zini E, D'Agnolo G, et al. Comparison of primary mitral valve disease in German Shepherd dogs and in small breeds. J Vet Cardiol 2004;6:27–34. [DOI] [PubMed] [Google Scholar]
- 6. Cornell CC, Kittleson MD, Della Torre P, et al. Allometric scaling of M‐mode cardiac measurements in normal adult dogs. J Vet Intern Med 2004;18:311–321. [DOI] [PubMed] [Google Scholar]
- 7. Hunter RJ, Liu Y, Lu Y, et al. Left atrial wall stress distribution and its relationship to electrophysiologic remodeling in persistent atrial fibrillation. Circ Arrhythm Electrophysiol 2012;5:351–360. [DOI] [PubMed] [Google Scholar]
- 8. van den Berg MP, Tjeerdsma G, Jan de Kam P, et al. Longstanding atrial fibrillation causes depletion of atrial natriuretic peptide in patients with advanced congestive heart failure. Eur J Heart Fail 2002;4:255–262. [DOI] [PubMed] [Google Scholar]
- 9. Shinbane JS, Wood MA, Jensen DN, et al. Tachycardia‐induced cardiomyopathy: a review of animal models and clinical studies. J Am Coll Cardiol 1997;29:709–715. [DOI] [PubMed] [Google Scholar]
- 10. Burashnikov A, Antzelevitch C. New developments in atrial antiarrhythmic drug therapy. Nat Rev Cardiol 2010;7:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation 2003;107:2920–2925. [DOI] [PubMed] [Google Scholar]
- 12. Ehrlich JR, Nattel S, Hohnloser SH. Atrial fibrillation and congestive heart failure: specific considerations at the intersection of two common and important cardiac disease sets. J Cardiovasc Electrophysiol 2002;13:399–405. [DOI] [PubMed] [Google Scholar]
- 13. Cha YM, Redfield MM, Shen WK, et al. Atrial fibrillation and ventricular dysfunction: a vicious electromechanical cycle. Circulation 2004;109:2839–2843. [DOI] [PubMed] [Google Scholar]
- 14. Solti F, Vecsey T, Kekesi V, et al. The effect of atrial dilatation on the genesis of atrial arrhythmias. Cardiovasc Res 1989;23:882–886. [DOI] [PubMed] [Google Scholar]
- 15. Wann LS, Curtis AB, Ellenbogen KA, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2011;57:1330–1337. [DOI] [PubMed] [Google Scholar]
- 16. Armstrong PW, Stopps TP, Ford SE, et al. Rapid ventricular pacing in the dog: pathophysiologic studies of heart failure. Circulation 1986;74:1075–1084. [DOI] [PubMed] [Google Scholar]
- 17. Wilson JR, Douglas P, Hickey WF, et al. Experimental congestive heart failure produced by rapid ventricular pacing in the dog: cardiac effects. Circulation 1987;75:857–867. [DOI] [PubMed] [Google Scholar]
- 18. Perez A, Touchette DR, DiDomenico RJ, et al. Comparison of rate control versus rhythm control for management of atrial fibrillation in patients with coexisting heart failure: a cost‐effectiveness analysis. Pharmacotherapy 2011;31:552–565. [DOI] [PubMed] [Google Scholar]
- 19. Roy D, Talajic M, Nattel S, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med 2008;358:2667–2677. [DOI] [PubMed] [Google Scholar]
- 20. Bonagura JD, Schober KE. Can ventricular function be assessed by echocardiography in chronic canine mitral valve disease? J Small Anim Pract 2009;50(Suppl 1):12–24. [DOI] [PubMed] [Google Scholar]
- 21. Kittleson MD, Kienle RD. Small Animal Cardiovascular Medicine. St Louis, MO: Mosby Inc; 1998:473. [Google Scholar]
- 22. Gelzer AR, Kraus MS, Rishniw M, et al. Combination therapy with digoxin and diltiazem controls ventricular rate in chronic atrial fibrillation in dogs better than digoxin or diltiazem monotherapy: a randomized crossover study in 18 dogs. J Vet Intern Med 2009;23:499–508. [DOI] [PubMed] [Google Scholar]
- 23. Van Gelder IC, Groenveld HF, Crijns HJ, et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med 2010;362:1363–1373. [DOI] [PubMed] [Google Scholar]


