Abstract
Setting: All health facilities providing tuberculosis (TB) care in Swaziland.
Objective: To describe the impact of human immunodeficiency virus (HIV) interventions on the trend of TB treatment outcomes during 2010–2013 in Swaziland; and to describe the evolution in TB case notification, the uptake of HIV testing, antiretroviral therapy (ART) and cotrimoxazole preventive therapy (CPT), and the proportion of TB-HIV co-infected patients with adverse treatment outcomes, including mortality, loss to follow-up and treatment failure.
Design: A retrospective descriptive study using aggregated national TB programme data.
Results: Between 2010 and 2013, TB case notifications in Swaziland decreased by 40%, HIV testing increased from 86% to 96%, CPT uptake increased from 93% to 99% and ART uptake among TB patients increased from 35% to 75%. The TB-HIV co-infection rate remained around 70% and the proportion of TB-HIV cases with adverse outcomes decreased from 36% to 30%. Mortality remained high, at 14–16%, over the study period, and anti-tuberculosis treatment failure rates were stable over time (<5%).
Conclusion: Despite high CPT and ART uptake in TB-HIV patients, mortality remained high. Further studies are required to better define high-risk patient groups, understand the reasons for death and design appropriate interventions.
Keywords: operational research, tuberculosis, mortality, Swaziland, antiretroviral treatment
Abstract
Contexte : Toutes les structures de santé offrant une prise en charge de la tuberculose (TB) au Swaziland.
Objectif : Décrire l'impact des interventions pour le virus de l'immunodéficience humaine (VIH) sur les tendances des résultats du traitement de la TB en 2010–2013, au Swaziland. Décrire l'évolution de la notification des cas de TB, la couverture du test VIH, de le traitement antirétroviral (TAR) et du traitement préventif au cotrimoxazole (CPT) et la proportion de patients coinfectées par TB-VIH avec les mauvais résultats du traitement incluant la mortalité, les abandons et les échecs du traitement.
Schéma : Etude descriptive rétrospective basée sur les données agrégées du programme national TB.
Résultats : Entre 2010 et 2013, les notifications de cas de TB au
Swaziland ont diminué de 40%, le test VIH a augmenté de 86% à 96%, la couverture du CPT a augmenté de 93% à 99% et la couverture du TAR parmi les patients tuberculeux est passée de 35% à 75%. Le taux de coinfection TB-VIH est resté autour de 70% et la proportion de cas de TB-VIH avec des résultats médiocres a diminué de 36% à 30% entre 2010 et 2013. La mortalité est restée élevée entre 14% et 16% pendant la période d'étude et les taux d'échec du traitement TB ont été stables dans le temps (<5%).
Conclusion : En dépit d'une couverture élevée du CPT et du TAR parmi les patients TB-VIH, la mortalité est restée élevée. D'autres études sont nécessaires pour mieux définir les groupes de patients à haut risque, pour mieux comprendre les causes de décès et pour concevoir des interventions appropriées.
Abstract
Marco de referencia: Todos los establecimientos de salud que prestan atención antituberculosa en Swasilandia.
Objetivo: Describir la repercusión de las intervenciones contra el virus de la inmunodeficiencia humana (VIH) sobre la evolución de los desenlaces terapéuticos de la tuberculosis (TB) del 2010 al 2013 en Swasilandia. Describir la evolución de la notificación de casos de TB, la aceptación de la prueba diagnóstica del VIH, el tratamiento antirretrovírico (TAR) y del tratamiento preventivo con cotrimoxazol (CPT) y la proporción de pacientes coinfectados por el VIH y el bacilo de la TB que presenta desenlaces terapéuticos desfavorables como la mortalidad, la pérdida durante el seguimiento y el fracaso del tratamiento.
Método: Fue este un estudio descriptivo retrospectivo a partir de los datos agregados del Programa Nacional contra la TB.
Resultados: Del 2010 al 2013, la notificación de casos de TB en Swasilandia disminuyó un 40%, la aceptación de la prueba diagnóstica del VIH aumentó de 86% a 96%, la utilización del CPT aumentó del 93% al 99% y en los pacientes con TB, y la aceptación del TAR aumentó del 35% al 75%. La tasa de coinfección permaneció alrededor del 70% y la proporción de estos pacientes que presentaba desenlaces desfavorables disminuyó del 36% en el 2010 al 30% en el 2013. Durante el período del estudio la mortalidad permaneció alta, entre el 14% y 16%, y las tasas de fracaso del tratamiento antituberculoso permanecieron estables con el transcurso del tiempo (menos del 5%).
Conclusión: Pese a una alta aceptación del CPT y el TAR por parte de los pacientes coinfectados por el VIH y la TB, la mortalidad sigue siendo alta. Se precisan nuevos estudios que definan con mayor precisión los grupos de pacientes con alto riesgo de desenlaces desfavorables y que contribuyan a comprender las causas de las defunciones y a diseñar intervenciones apropiadas.
Tuberculosis (TB) remains a major global public health problem, annually affecting approximately 9 million people and resulting in 1.5 million deaths. Most of the notified cases and reported deaths are from sub-Saharan Africa.1 Swaziland, a small southern African country, has one of the highest TB incidence rates globally and one of the highest generalised prevalences of human immunodeficiency virus (HIV) in the world, at 31% among persons aged 18–49 years.2 According to the 2014 National TB Control Programme (NTCP) report, 97% of TB patients were tested for HIV, of whom 73% were co-infected with HIV.3
HIV has been shown to be the single most important risk factor both for developing TB and for the association with poor TB outcomes, including high fatality rates.4,5 The use of highly active antiretroviral therapy (ART) has been shown to reduce the risk of acquiring TB by up to 70–90%.6 Furthermore, the combined use of cotrimoxazole preventive therapy (CPT) and ART has been shown to reduce mortality among TB-HIV co-infected individuals.7,8 CPT and ART have been proposed as part of the strategy to reduce the adverse outcomes among TB patients co-infected with HIV.6,7,9
Several studies have shown the impact of the initial scaling up of ART on TB case notification in southern Africa.10,11 Clear reductions in mortality among HIV co-infected patients have also been reported in association with ART scale-up.7 However, in a recent study in Malawi, little additional progress in terms of mortality and other adverse outcomes had been seen over the previous 4–5 years.7 One of the reasons could be that, despite substantial progress, complete national coverage of HIV services might be difficult to achieve in resource-constrained countries with relatively weak health systems. As such, the full impact of HIV service provision in national TB programmes might not have been realised in these settings.
In this respect, a country such as Swaziland is unusual. Swaziland has a high prevalence of TB and HIV,3 it is a relatively small country and it is better resourced than many others, with a reasonably well functioning health care system. Universal ART coverage was reportedly achieved for the general HIV population in 2011.12 However, this was not the case for the TB patients in our study.
The aim of this study was to describe the impact of HIV interventions on the trend of TB treatment outcomes during 2010–2013 in Swaziland. Using aggregate NTCP data, we describe the evolution in TB case notifications, the uptake of HIV testing and ART and CPT initiation and the proportion of TB-HIV co-infected patients with adverse treatment outcomes, including mortality, loss to follow-up and treatment failure.
METHODS
Study design
This was a retrospective descriptive study using routinely collected aggregated NTCP data.
Study setting
Swaziland, located in southern Africa, has a population of approximately 1.1 million, of whom 70% live in rural areas.13 Although Swaziland is a low to middle-income country, more than 60% of the population lives below the poverty line.14 In 2014, the prevalence of TB was estimated to be 605 per 100 000 population, with an incidence that is among the highest in the world, at 733/100 000.15
Swaziland has a DOTS-based programme that applies all procedures, including case finding, registration, diagnosis, treatment and treatment outcomes, in line with international guidelines.16 Xpert® MTB/RIF (Cepheid, Sunnyvale, CA, USA) was introduced for TB diagnosis in 2011 and adopted nationwide as an initial diagnostic test for all presumptive TB cases in 2012. Culture and drug susceptibility testing (DST) were performed for specific indications, such as treatment failure, during the study period. The NTCP has provided treatment for multidrug-resistant TB (MDR-TB) since 2006.17
The ART programme in Swaziland was launched in 2003. Indications for ART follow World Health Organization (WHO) guidelines, and since 2010 a CD4 count threshold of 350 cells/μl has been used. As mentioned, universal ART coverage was achieved in 2011.12 In 2006, the country adopted a partial TB-HIV services integrated model by which all TB patients are offered HIV testing and all HIV patients are screened for TB. All TB-HIV co-infected patients are initiated on CPT, and since 2008 all HIV-positive TB patients have been initiated on ART, regardless of their CD4 cell count, within 8 weeks of initiating anti-tuberculosis treatment.18 During the study period, first-line ART for TB patients consisted of the combination of stavudine, lamivudine and efavirenz. Isoniazid preventive therapy (IPT) has been recommended for HIV patients since 2007, but this has not been systematically and consistently implemented. At the primary health care level, TB and HIV services are co-localised under the same roof, whereas at higher levels these services are located on the same premises but in different departments.
Study population
The study population included all TB patients registered by the NTCP between 2010 and 2013. Cases with documented drug resistance were excluded, as notification and reporting on treatment outcomes is performed separately.
Data variables, source of data and analysis
The case finding and treatment outcome data for all TB patients are recorded with a unique identifier in the TB register. In terms of data collection and collation, the NTCP has a robust data verification system in which the regional levels validate and collate the health facility data, which are in turn transmitted to the central unit of the NTCP. The central unit conducts quarterly onsite data verification to ascertain data quality assurance, performs analyses and transmits the results to the WHO global reporting system annually. Quarterly review meetings are organised to provide a forum for feedback, peer review on findings of data quality assessments and discussions on corrective actions for addressing gaps in data quality. In addition, Regional TB Coordinators provide quarterly support supervision to health facility staff.
For this study, we used aggregated data sourced from the NTCP reports. The following information was retrieved from the case finding report: year of enrolment, number of patients enrolled nationally for anti-tuberculosis treatment, HIV testing and results, initiation of CPT and ART, and sex. Data variables for anti-tuberculosis treatment outcomes among those registered with TB-HIV included the number of TB-HIV patients evaluated for final treatment outcomes among new and retreatment cases for all TB treatment categories. Adverse outcomes included death, loss to follow-up, treatment failure and not evaluated. (Loss to follow-up refers to what was previously reported as treatment default.19) Patients who miss appointments are contacted by phone and/or visited at home by adherence officers, although the system was not universally functional at all treatment centres during the study period. Family treatment supporters, where available, provide directly observed treatment (DOT) for patients on anti-tuberculosis medications.
We used EpiData software for data entry (version 3.1, EpiData Association, Odense, Denmark). All data were independently validated by a second person. Data were then transferred to Excel software (Microsoft Corp, Redmond, WA, USA) for a descriptive analysis, presenting absolute numbers and proportions of HIV coinfection, uptake of CPT and ART, and adverse treatment outcomes per year.
Ethics approval
Permission to carry out the study was obtained from the Swaziland Ministry of Health Scientific Research and Ethics Committee, Mbabane, Swaziland. The Médecins Sans Frontières Ethics Review Board (Geneva, Switzerland) approved the criteria for studies of routinely collected data, and approval was also granted by the Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease, Paris, France. As this was a record review study with aggregated anonymised data, the issue of informed patient consent did not apply.
RESULTS
The number of TB cases notified between 2010 and 2013, along with the numbers of patients who were tested for HIV, tested HIV-positive, tested HIV-negative, started on CPT and on ART, are shown in Figure 1. There was a decrease of 40% in the number of patients enrolled for anti-tuberculosis treatment, from 11 057 cases in 2010 to 6665 in 2013. The same trend was observed among HIV-positive patients with TB. HIV testing uptake increased from 86% in 2010 to 96% in 2013. The coinfection rate remained stable, at around 70%. Over 90% of the patients were prescribed CPT over the time period, from 93% in 2010 to 99% in 2013. ART uptake among TB-HIV co-infected patients increased from 35% to 75% over the study period.
FIGURE 1.
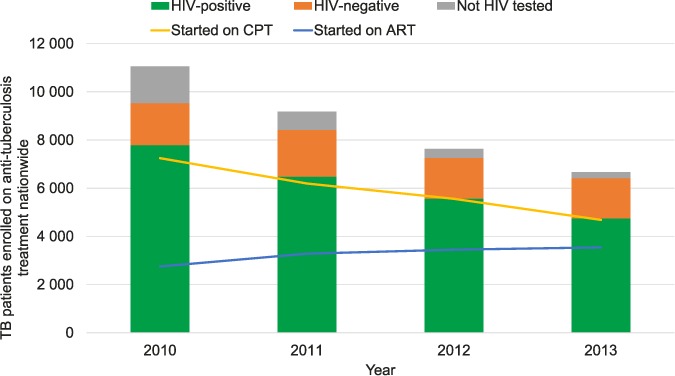
Trends in HIV testing and CPT and ART uptake amongst patients with TB in Swaziland, 2010–2013. HIV = human immunodeficiency virus; CPT = cotrimoxazole preventive therapy; ART = antiretroviral treatment; TB = tuberculosis.
Annual trends in adverse outcomes among TB-HIV patients are shown in Figure 2. The proportion with adverse outcomes amongst all notified TB-HIV patients decreased from 36% in 2010 to 30% in 2013 (Figure 2A). Mortality remained high, at between 14% and 16% over the study period notified. Failure rates were stable over time (<5%). The proportion of loss to follow-up decreased from 11% in 2010 to 9% in 2013. Similarly, the proportion without treatment outcome information declined from 7% in 2010 to 2% in 2013. Failure rates among retreatment patients were higher, ranging from 7% to 10% (Figure 2C). Apart from the higher mortality among TB retreatment cases in 2011, the trends were generally comparable between new and retreatment cases (Figure 2B and 2C).
FIGURE 2.
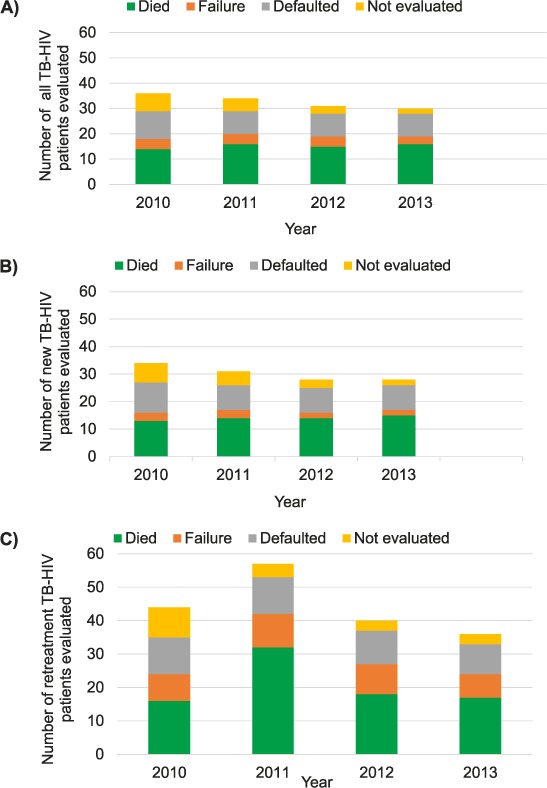
Adverse treatment outcomes amongst HIV-positive patients with TB in Swaziland, 2010–2013. A) All patients with TB; B) New patients with TB; C) Retreatment cases. Adverse treatment outcomes include death, failure, loss to follow-up and not evaluated. Not evaluated refers to patients without outcome data available, including those transferred out. TB = tuberculosis; HIV = human immunodeficiency virus.
DISCUSSION
We report on the impact of HIV interventions, which included high uptake of CPT and ART, on anti-tuberculosis treatment outcomes in all TB-HIV co-infected patients in an African country with a reasonably well-functioning health care system. Between 2010 and 2013, ART coverage among patients co-infected with TB-HIV increased substantially, reaching 75%. Across the same time period, a clear reduction in total and HIV-associated TB case notifications was observed, in line with other studies.7,10 Contrary to our expectations, the rate of mortality amongst TB-HIV patients did not decline but remained stable, at 14–16%, despite the high coverage of CPT and increasing ART uptake.
Our findings on the stable mortality rate are in line with a recent study from Malawi where, despite an ART uptake of up to 88%, no reduction in mortality among notified TB cases was seen between 2008 and 2012,7 but rather a trend towards higher mortality, which reached 13–15%. The WHO and other international stakeholders have recently engaged to reduce TB mortality by 90% by 2030.15 With 70% of the TB burden occurring in HIV-infected patients in countries such as Swaziland, the high case fatality rates in TB-HIV coinfection seriously compromise the chances of achieving these targets.
Why do high numbers of TB-HIV patients still die despite increasing ART coverage, and what can be done about it? Why was universal ART coverage not achieved in TB patients? We note a number of challenges in the NTP that could explain why universal ART coverage was not achieved for TB patients. First, the responsibility for ART initiation was confined to doctors during the study period. In addition, there was only partial integration of TB-HIV services and decentralisation was slow, which could have contributed to the high rates of loss to follow-up among TB-HIV co-infected patients. The separate monitoring and evaluation systems for the TB and HIV programmes possibly led to loss of data. Finally, while meetings of multidisciplinary teams were foreseen to discuss and address common challenges in TB and HIV service delivery, these meetings were inconsistent. In response to this, task shifting of ART initiation to nurses has been undertaken, the decentralisation and integration of TB services has been enhanced, and mechanisms for better coordination of TB-HIV collaborative activities at all levels have been put in place.
The Table presents a number of key operational research questions that should follow this study. Who is missing out on ART in Swaziland, and when exactly is ART being initiated? A recent study from South Africa revealed that ART uptake was particularly low in individuals with very low CD4 counts, and that this group also had the highest mortality.20 Targeting this patient group would thus be a potential strategy to further reduce case fatality. This approach, however, requires CD4 testing to be available, and in the current climate where ‘HIV test and treat’ is now being recommended, widespread coverage with CD4 technology might be difficult. What is the cause of death in TB-HIV patients? In South Africa, it was recently found that only 34% of deaths at autopsy in TB-HIV patients could be attributed to TB.21 What is the role of non-communicable diseases, an emerging problem linked to TB?22,23 How good is adherence to TB and HIV treatment, and is quality assurance of anti-tuberculosis medications strictly maintained?
TABLE.
Priority operational research questions to address mortality in TB-HIV co-infected patients
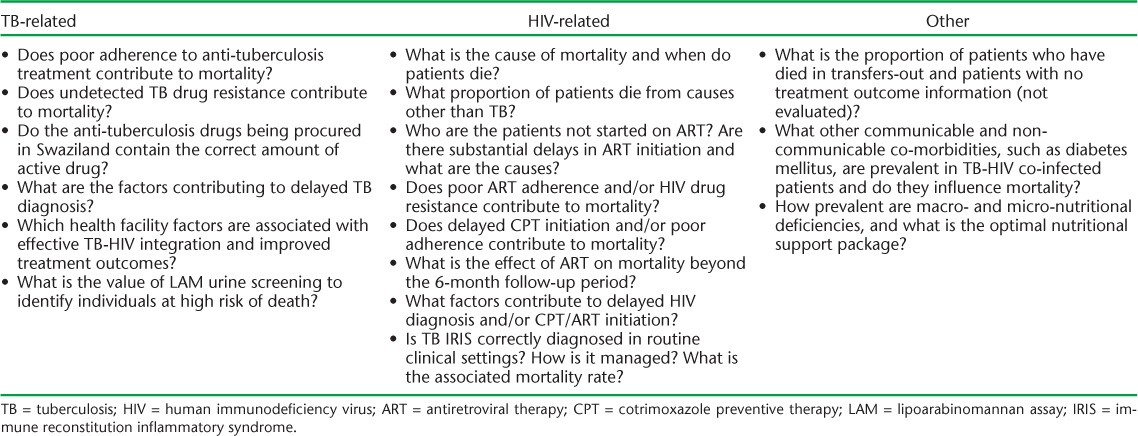
Ultimately, what is needed are effective tools for the identification of TB-HIV patients at high risk for death that are linked to appropriate operational and clinical management strategies addressing co-morbidities beyond TB and HIV.
We also need more evidence on whether the effect on mortality of early ART initiation observed in clinical trials is confirmed in routine clinical settings. For such interventions, the 6-month follow-up period might be too short-term to reveal efficacy. In the CAMELIA trial in Cambodia, for example, the effect of early ART initiation was only apparent beyond 6 months after TB diagnosis.8,24
Pending further research, we need to strengthen a number of strategies that are likely to impact TB and HIV-related mortality and morbidity. The 90–90–90 treatment targets for HIV that have recently been proposed imply that, by 2020, 90% of individuals living with HIV have to be diagnosed and know their HIV status, 90% of HIV-infected people have to be on ART, and 90% of these should have viral suppression.12,25 Effective implementation of this strategy would contribute to TB prevention in people living with HIV infection.4,25 In parallel, 100% ART uptake in TB patients should be prioritised. In addition, the introduction of culture and DST for all bacteriologically confirmed TB cases will facilitate early diagnosis of drug-resistant strains. This particular intervention was not implemented during the study period.
Systematic screening for TB with Xpert in HIV patients, combined with the use of the urine lipoarabinomannan (LAM) assay, which shows higher sensitivity with lower CD4 counts, would enhance early TB diagnosis and might identify patients at higher risk for death.26–28 Increased uptake of IPT would further contribute to TB prevention in people living with HIV, and ultimately reduce TB-related mortality.29
MDR-TB is also an increasing problem in Swaziland and, particularly if not detected or detected late, is associated with adverse outcomes.30 A recent study in Swaziland found that, due to the prevalence of specific mutations in the rifampicin (RMP) gene, the sensitivity of Xpert to detect RMP resistance is reduced.31 In response to this, routine culture and DST have now been introduced. The last MDR-TB prevalence survey dates from 2009; the planned drug resistance survey will give more insight into the current situation.
One of the strengths of this study is the use of national data that are representative of what is happening in the country. A fairly robust system for data validation was in place during the entire study period. Limitations include the fact that mortality is likely to be underestimated as deaths might have occurred in those lost to follow-up, transferred out or not evaluated.7 Consequently, we cannot exclude the possibility that the improvements in outcome reporting over time—with decreasing proportions of patients lost to follow-up or who lacked outcome data—might have concealed a modest decline in mortality in our data. The use of aggregated data precluded more in-depth evaluation, for example, on the timing of ART. Data on CD4 cell counts were also not available. One important limitation of the study is that TB-HIV data prior to 2010 were not available, hence the short proposed study period. In addition, we only had treatment outcomes for patients with HIV-associated TB; we did not have treatment outcomes for patients with HIV-negative TB. TB treatment outcomes for HIV-positive and HIV-negative patients could not therefore be compared.
In conclusion, this study revealed that, despite increasing ART coverage in TB patients, mortality remained high in TB-HIV co-infected patients. Further studies are required to better define patient groups with a high risk for death, to understand the reasons for death and failure to start ART, and to design appropriate interventions. Reducing TB case fatality rates in HIV-infected patients will be vital to achieve the targeted 90% reduction in TB mortality within 15 years.
Acknowledgments
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Programme for Research and Training in Tropical Diseases at the World Health Organization (WHO/TDR). The model is based on a course developed jointly by the International Union Against Tuberculosis and Lung Disease (The Union), Paris, France, and Médecins Sans Frontières (MSF), Brussels Operational Centre, Luxembourg. The specific SORT IT programme that resulted in this publication was jointly developed and implemented by the Operational Research Unit (LUXOR), MSF; the Centre for Operational Research, The Union; the Centre for International Health, University of Bergen, Bergen, Norway; the Institute of Tropical Medicine, Antwerp, Belgium; and Partners in Health, Boston, MA, USA.
The programme was funded by The Union, MSF and the Department for International Development, London, UK. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. La Fondation Veuve Emile Metz-Tesch, Luxembourg, also supported the open access publication costs.
Footnotes
Conflicts of interest: none declared.
In accordance with the WHO's open-access publication policy for all work funded by the WHO or authored/co-authored by WHO staff members, the WHO retains the copyright of this publication through a Creative Commons Attribution IGO licence (http://creativecommons.org/licenses/by/3.0/igo/legalcode) which permits unrestricted use, distribution and reproduction in any medium provided the original work is properly cited.
References
- 1.World Health Organization. Global tuberculosis control, 2014. Geneva, Switzerland: WHO; 2014. WHO/HTM/TB/2014.08. [Google Scholar]
- 2.Swaziland Ministry of Health. Swaziland HIV incidence measurement survey report 2012. Mbabane, Swaziland: Swaziland Ministry of Health; 2012. [Google Scholar]
- 3.Swaziland Ministry of Health. Swaziland National Tuberculosis Control Programme annual report 2014. Mbabane, Swaziland: National Tuberculosis Control Programme, Swaziland Ministry of Health; 2014. [Google Scholar]
- 4.World Health Organization. WHO policy on collaborative TB-HIV activities. Guidelines for national programmes and other stakeholders. Geneva, Switzerland: WHO; 2012. WHO/HTM/TB/2012.1. [PubMed] [Google Scholar]
- 5.Crampin A C, Glynn J R, Fine P E. What has Karonga taught us? Tuberculosis studied over three decades. Int J Tuberc Lung Dis. 2009;13:153–164. [PMC free article] [PubMed] [Google Scholar]
- 6.Middelkoop K, Bekker L G, Myer L et al. Antiretroviral therapy and TB notification rates in a high HIV prevalence South African community. J Acquir Immune Defic Syndr. 2011;56:263–269. doi: 10.1097/QAI.0b013e31820413b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanyerere H, Mganga A, Harries A D et al. Decline in national tuberculosis notifications with national scale-up of antiretroviral therapy in Malawi. Public Health Action. 2015;2:116–118. doi: 10.5588/pha.14.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lessells R, Swaminathan S, Godfrey-Faussett P. HIV treatment cascade in tuberculosis patients. Curr Opin HIV AIDS. 2015;10:439–446. doi: 10.1097/COH.0000000000000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maher D, Harries A D, Getahun H. Tuberculosis and HIV interaction in sub-Saharan Africa: impact on patients and programmes; implications for policies. Trop Med Int Health. 2005;10:734–742. doi: 10.1111/j.1365-3156.2005.01456.x. [DOI] [PubMed] [Google Scholar]
- 10.Haumba S, Dlamini T, Calnan M et al. Declining tuberculosis notification trend associated with strengthened TB and expanded HIV care in Swaziland. Public Health Action. 2015;5:103–105. doi: 10.5588/pha.15.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suthar A B, Lawn S D, del Amo J et al. Antiretroviral therapy for prevention of tuberculosis in adults with HIV: a systematic review and meta-analysis. PLOS Med. 2012;9:e1001270. doi: 10.1371/journal.pmed.1001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swaziland Ministry of Health. Swaziland HIV integrated management guidelines. Mbabane, Swaziland: National AIDS Programme, Swaziland Ministry of Health; 2015. [Google Scholar]
- 13.Swaziland Central Statistics Office. Swaziland Demographic and Health Survey, 2006–2007. Mbabane, Swaziland: Swaziland Central Statistics Office; 2008. http://dhsprogram.com/pubs/pdf/fr202/fr202.pdf Accessed April 2016. [Google Scholar]
- 14.United Nations Development Programme. United Nations Development Assistance Framework Report for Swaziland 2011–2015. New York, NY, USA: UNDP; 2012. [Google Scholar]
- 15.World Health Organization. Global tuberculosis control, 2015. Geneva, Switzerland: WHO; 2015. WHO/HTM/TB/2015.22. [Google Scholar]
- 16.Swaziland Ministry of Health. Swaziland tuberculosis management guidelines. Mbabane, Swaziland: National Tuberculosis Control Programme, Swaziland Ministry of Health; 2012. [Google Scholar]
- 17.Swaziland Ministry of Health. Swaziland drug resistant tuberculosis management guidelines. Mbabane: Swaziland: National Tuberculosis Control Programme, Swaziland Ministry of Health; 2012. [Google Scholar]
- 18.Swaziland Ministry of Health. Swaziland national TB-HIV collaborative guidelines. Mbabane, Swaziland: Swaziland Ministry of Health; 2015. [Google Scholar]
- 19.World Health Organization. Definitions and reporting framework for tuberculosis. Geneva, Switzerland: WHO; 2013. WHO/HTM/TB/2013.2. [Google Scholar]
- 20.Kaplan R, Caldwell J, Wood R et al. The impact of ART on TB case fatality stratified by CD4 count for HIV-positive TB patients in Cape Town, South Africa (2009–2011) J Acquir Immune Defic Syndr. 2015;66:487–494. doi: 10.1097/QAI.0000000000000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Field N, Lim M, Murray J et al. Timing, rates, and causes of death in a large South African tuberculosis programme. BMC Infect Dis. 2014;14:3858. doi: 10.1186/s12879-014-0679-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dooley K E, Chaisson R E. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis. 2009;9:737–746. doi: 10.1016/S1473-3099(09)70282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dooley K E, Tang T, Golub J E, Dorman S E, Cronin W. Impact of diabetes mellitus on treatment outcomes of patients with active tuberculosis. Am J Trop Med Hyg. 2009;80:634–639. [PMC free article] [PubMed] [Google Scholar]
- 24.Marcy O, Laureillard D, Madec Y et al. Causes and determinants of mortality in HIV-infected adults with tuberculosis: an analysis from the CAMELIA ANRS 1295-CIPRA KH001 randomized trial. Clin Infect Dis. 2014;59:435–445. doi: 10.1093/cid/ciu283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joint United Nations Programme on HIV/AIDS. 90–90–90: an ambitious treatment target to help end the AIDS epidemic by 2020. Geneva, Switzerland: UNAIDS; 2014. UNAIDS/JC 2684/2014. [Google Scholar]
- 26.Shah M, Sengooba W, Armstrong D et al. Comparative performance of urinary lipoarabinomannan assays and Xpert MTB/RIF in HIV-infected individuals. AIDS. 2014;28:1307–1314. doi: 10.1097/QAD.0000000000000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawn S D, Harries A D, Anglaret X et al. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22:1897–1908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawn S D, Meintjes G, McIlleron H, Harries A D, Wood R. Management of HIV-associated tuberculosis in resource-limited settings: a state-of-the-art review. BMC Med. 2013;11:253. doi: 10.1186/1741-7015-11-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harries A D, Lawn S D, Getahun H, Zachariah R, Havlir D V. HIV and tuberculosis science and implementation to turn the tide and reduce deaths. J Int AIDS Soc. 2012;15:17396. doi: 10.7448/IAS.15.2.17396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Falzon D, Gandhi N, Migliori G B et al. Resistance to fluoroquinolones and second-line injectable drugs: impact on multidrug-resistant TB outcomes. Eur Respir J. 2013;42:156–168. doi: 10.1183/09031936.00134712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanchez-Padilla E, Merker M, Beckert P et al. Detection of drug-resistant tuberculosis by Xpert MTB/RIF in Swaziland. N Engl J Med. 2015;372:1181–1182. doi: 10.1056/NEJMc1413930. [DOI] [PubMed] [Google Scholar]


