Abstract
Setting: National Tuberculosis (TB) Program sites in northwest Cambodia.
Objective: To evaluate the impact of Xpert® MTB/RIF at point of care (POC) as compared to non-POC sites on the diagnostic evaluation of people living with the human immunodeficiency virus (PLHIV) with TB symptoms and patients with possible multidrug-resistant (MDR) TB.
Design: Observational cohort of patients undergoing routine diagnostic evaluation for TB following the rollout of Xpert.
Results: Between October 2011 and June 2013, 431 of 822 (52%) PLHIV with TB symptoms and 240/493 (49%) patients with possible MDR-TB underwent Xpert. Xpert was more likely to be performed when available as POC. A smaller proportion of PLHIV at POC sites were diagnosed with TB than at non-POC sites; however, at POC sites, a higher proportion of those diagnosed with TB were bacteriologically positive. There was poor agreement between Xpert and other tests such as smear microscopy and culture. Overall, the evaluation of patients with possible MDR-TB increased following Xpert rollout, yet for patients confirmed as having drug resistance on drug susceptibility testing, only 46% had rifampin resistance that would be identified with Xpert.
Conclusion: Although utilization of Xpert was low, it may have contributed to an increase in evaluations for possible MDR-TB and a decline in empiric treatment for PLHIV when available as POC.
Keywords: HIV, drug resistance, MDR-TB, point-of-care testing
Abstract
Contexte : Sites du Programme National contre la Tuberculose (TB) dans le nord-ouest du Cambodge.
Objectif : Evaluer l'impact du Xpert® MTB/RIF dans des sites où il est réalisé sur place (POC) comparés aux autres sites sur le diagnostic des personnes vivant avec le VIH (PVVIH) et ayant des symptômes de TB ainsi que des patients présumées de TB multirésistante (MDR).
Schéma : Cohorte d'observation de patients bénéficiant d'une évaluation diagnostique de routine pour la TB après le lancement de l'Xpert.
Résultats : Entre octobre 2011 et juin 2013, 431/822 (52%) PVVIH ayant des symptômes de TB et 240/493 (49%) patients avec suspicion de TB-MDR ont eu un test Xpert. L'Xpert a été réalisé plus souvent lorsqu'il était disponible en POC. Une plus faible proportion de PVVIH a eu un diagnostic de TB dans les sites POC que dans les sites non-POC ; cependant, dans les sites POC, une proportion plus élevée des patients ayant eu un diagnostic de TB a eu une bactériologie positive. L'accord entre l'Xpert et les autres tests (par exemple la microscopie de frottis ou la culture) a été médiocre. Dans l'ensemble, l'évaluation des patients présumées de TB-MDR a augmenté après le lancement de l'Xpert, mais parmi les patients ayant eu une pharmacorésistance confirmée par test de pharmacosensibilité, seulement 46% ont eu une résistance à la rifampicine qui aurait été identifiée par Xpert.
Conclusion : Même si l'utilisation de l'Xpert a été faible, l'Xpert pourrait avoir contribué à une augmentation de l'évaluation des suspicions de TB-MDR et à un déclin du traitement empirique des PVVIH quand il est disponible sur place.
Abstract
Marco de referencia: Los centros del Programa Nacional contra la Tuberculosis en el noroeste de Camboya.
Objetivo: Evaluar la repercusión de la práctica de la prueba Xpert® MTB/RIF en el lugar de la consulta, en comparación con la realización de la prueba en otro centro, sobre la evaluación diagnóstica de las personas aquejadas de infección por el virus de la inmunodeficiencia humana (PVVIH) que presentan síntomas de tuberculosis (TB) y de los pacientes con presunción de TB multidrogorresistente (TB-MDR).
Método: Fue este un estudio observacional de cohortes de pacientes en curso de evaluación diagnóstica corriente de la TB, después de la introducción de la prueba Xpert.
Resultados: De octubre del 2011 a junio del 2013 se practicó la prueba Xpert a 431 de los 822 PVVIH que presentaban síntomas de TB (52%) y a 240 de los 493 pacientes con presunción de TB-MDR (49%). La probabilidad de realizar la prueba Xpert fue mayor cuando esta se podía practicar en el lugar de la consulta. La proporción de PVVIH en quienes se diagnosticó TB en los centros que practicaban localmente la prueba Xpert fue menor que en los demás centros; sin embargo, en los centros que contaban con la prueba fue más alta la proporción de casos de TB confirmados bacteriológicamente. Se observó una baja concordancia entre los resultados de la prueba Xpert y las otras pruebas (la baciloscopia y el cultivo). En general, tras el despliegue de la prueba molecular se investigó un mayor número de pacientes con presunción de TB-MDR; sin embargo, de los pacientes en quienes se confirmó la farmacorresistencia mediante pruebas de sensibilidad solo un 46% presentaba resistencia a rifampicina, que podía detectar la prueba Xpert.
Conclusión: Si bien la utilización de la prueba Xpert fue muy limitada, su disponibilidad contribuyó a la investigación de más casos con presunción de TB-MDR y a una disminución del tratamiento empírico de las PPVIH, cuando la prueba Xpert se practicaba en el lugar de la consulta.
In recognition of the pressing need for improved diagnostics for tuberculosis (TB), the World Health Organization (WHO) endorsed the Xpert® MTB/RIF assay (Cepheid, Sunnyvale, CA, USA) in December 2010.1 Since that endorsement, there has been rapid global scale-up, with nearly 18 000 GeneXpert modules and 10 million Xpert cartridges procured by high-burden countries eligible for discounted, concessional pricing.2
Following the WHO recommendation to use Xpert as an initial test for people living with the human immunodeficiency virus (PLHIV) and people with possible drug-resistant TB, multiple research groups have sought to assess the impact of Xpert on patient outcomes.1 Several reports from southern Africa and Brazil have indicated that Xpert implementation results in shorter times to anti-tuberculosis treatment initiation, particularly when located at point of care (POC).3–6 However, other studies in South Africa and Zimbabwe reported no difference in time to treatment.7,8 In Cambodia, Xpert was found to increase the yield of active case finding for patients with a cough of >2 weeks.9,10 We are unaware, however, of any reports from South-East Asia evaluating the impact of Xpert when introduced for routine programmatic use for the diagnosis of TB among priority populations, as recommended by the WHO.
Cambodia is one of the world's 22 high-burden countries for TB, with an estimated incidence of 400 cases per 100 000 population.11 Rates of multidrug-resistant TB (MDR-TB) remain low, nevertheless, and are estimated at 1.4% among newly diagnosed patients and 11% among patients retreated for TB. Among adults, HIV prevalence is 0.6%, while 4% of persons with TB are infected with HIV.12–14
The Cambodia National TB Program (CENAT) placed two GeneXpert machines at provincial referral hospitals in northwest Cambodia in February and June 2012. These machines were designated for the diagnosis of TB among PLHIV with TB symptoms and patients with possible MDR-TB.15 We reported previously on the programmatic challenges of incorporating Xpert into routine diagnostic algorithms.16 We now report on Xpert utilization, turnaround times (TATs) for Xpert results, impact on TB diagnosis, and time to initiation of anti-tuberculosis treatment following Xpert rollout, and compare these data for sites with and those without access to POC Xpert.
METHODS
Study setting
This study was a prospective cohort evaluation conducted from October 2011 through June 2013 in four rural Cambodian provinces, Battambang, Bantay Meanchey, Pursat, and Pailin, with eight district TB facilities and nine HIV clinics. Further details regarding these sites, including the populations served and the process and challenges of implementing Xpert, have been described elsewhere.16
Evaluation of people living with HIV
National guidelines stipulate that PLHIV be screened for TB symptoms of fever, cough, weight loss, or drenching night sweats at monthly HIV clinic visits. PLHIV reporting any of these symptoms (i.e., a positive symptom screen) should be referred to the TB program for further evaluation. Prior to Xpert rollout, PLHIV were evaluated with a clinical examination and smear microscopy, followed by chest X-ray (CXR) at the discretion of the clinician and both solid and liquid culture for patients with a sputum smear-negative result for acid-fast bacilli. In preparation for Xpert rollout, CENAT introduced new algorithms whereby Xpert replaced smear microscopy as the initial diagnostic test for PLHIV. If rifampin (RMP) resistance was detected by Xpert, culture and drug susceptibility testing (DST) were performed at the National Reference Laboratory.
Evaluation of patients with possible multidrug-resistant TB
The following groups were classified as patients with possible MDR-TB: 1) all previously treated patients with pulmonary TB (retreatment cases); 2) patients newly diagnosed with pulmonary TB who remained sputum smear-positive 2 or 3 months after initiating first-line anti-tuberculosis treatment (non-converters); and 3) patients with TB symptoms with known contact with someone with drug-resistant TB. Prior to Xpert rollout, patients with possible MDR-TB were evaluated with smear microscopy, culture, and DST. After Xpert rollout, these patients were to have separate sputum specimens sent simultaneously for Xpert, culture, and DST.
Data collection and analysis
Details of the data collection for this study have been published previously.16 Briefly, data were collected from four paper-based registers: 1) PLHIV-TB screening registers, 2) TB laboratory registers located at the two GeneXpert sites, 3) district TB treatment registers, and 4) MDR-TB treatment registers.
Patients evaluated at one of the two sites with a GeneXpert machine located at the same facility were classified as receiving POC testing; otherwise they were classified as off-site for Xpert. We divided the study period into 3-month quarters, beginning on 1 March 2012, when the first GeneXpert machine was introduced. Pre-Xpert data were available for patients with possible MDR-TB from October 2011 through March 2012. Proportions were compared using Pearson's χ2 or the exact test, and data were analyzed using SAS 9.3 (SAS Institute, Cary, NC, USA).
Ethics approval
The Centers for Disease Control and Prevention (CDC), Atlanta, GA, USA, and the CENAT determined the study to be a program evaluation and not research involving human participants; institutional review board approval was therefore not required.
RESULTS
People living with HIV
Study population
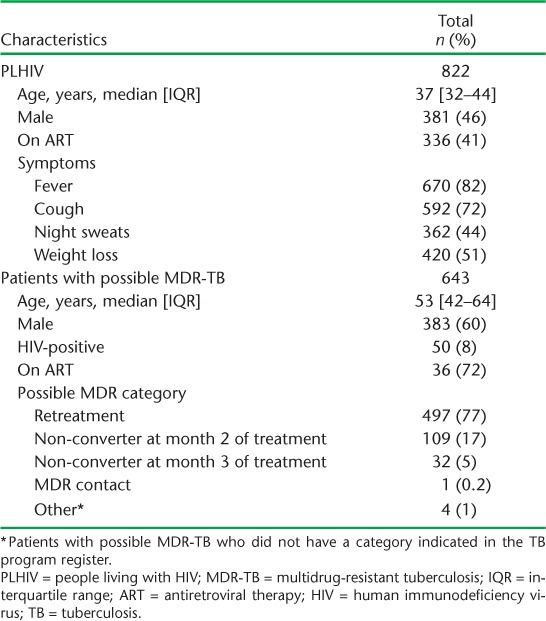
From March 2012 to June 2013, 822 PLHIV had a positive symptom screen and were eligible to receive Xpert. Of these, 381 (46%) were male, and the median age was 37 years (interquartile range [IQR] 32–44) (Table 1; see Appendix Table A.1 for patient characteristics by POC vs. off-site Xpert). At the time of their TB evaluation, 336 (41%) PLHIV were on antiretroviral therapy (ART). Fever (n = 670, 82%) and cough (n = 592, 72%) were the most commonly reported symptoms.
TABLE 1.
Patient characteristics for PLHIV with a positive symptom screen and patients with possible MDR-TB
Diagnostic testing
Overall, 431 (52%) PLHIV with a positive symptom screen were tested using Xpert, of whom 48 (11%) were positive for Mycobacterium tuberculosis and one (2%) had resistance to RMP (Table 2). A greater proportion of patients underwent smear microscopy (72%) or CXR (72%) than Xpert testing (P < 0.01). PLHIV were significantly more likely to have undergone any diagnostic testing if they were evaluated at a site with POC Xpert as compared to a site with off-site Xpert (61% vs. 43% for Xpert, 76% vs. 66% for smear microscopy, 19% vs. 6% for culture, 79% vs. 63% for CXR; P < 0.01 for all). After the first quarter, the proportion of patients tested with Xpert did not change appreciably over the evaluation period (Appendix Table A.2).
TABLE 2.
Diagnostic testing and results for PLHIV
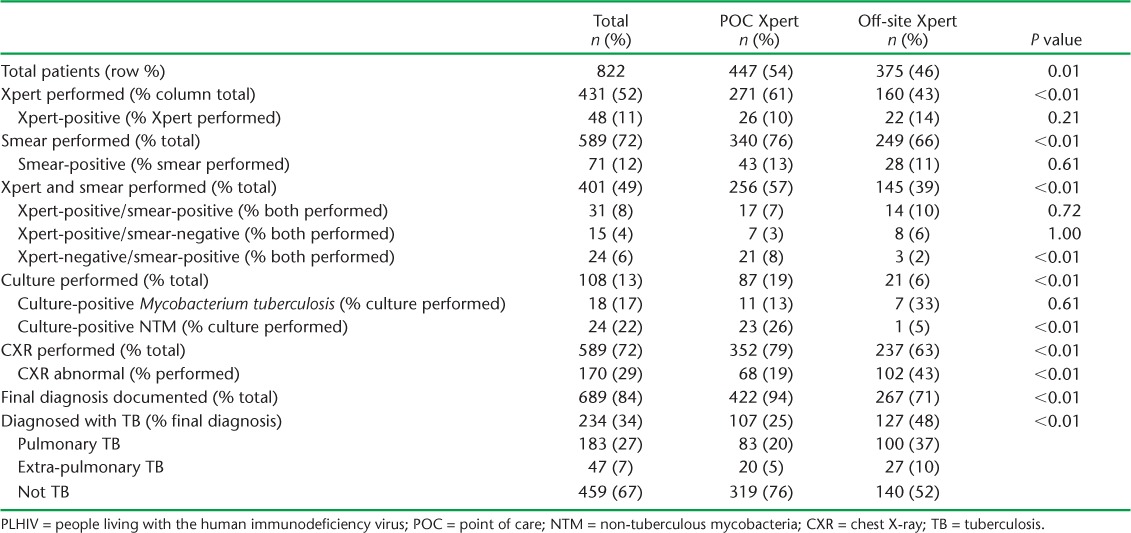
Both smear microscopy and Xpert testing were performed for 401 (49%) patients. Of 46 PLHIV with a positive Xpert and a smear result, 31 (67%) had a positive smear and 15 (33%) had a negative smear. Conversely, among 55 PLHIV with a positive smear and an Xpert result, 24 (44%) were Xpert-negative.
Time to Xpert result and treatment
For PLHIV with POC Xpert, the median TAT to Xpert result was zero days (IQR 0–3), which was shorter than the median TAT of 9.5 days (IQR 5–16) for those with off-site Xpert. However, PLHIV with POC Xpert had a median time to treatment initiation of 10.5 days (IQR 4.5–5), which was longer than the median time to treatment initiation of 2 days (IQR 1–4) for those with off-site Xpert.
Tuberculosis diagnosis
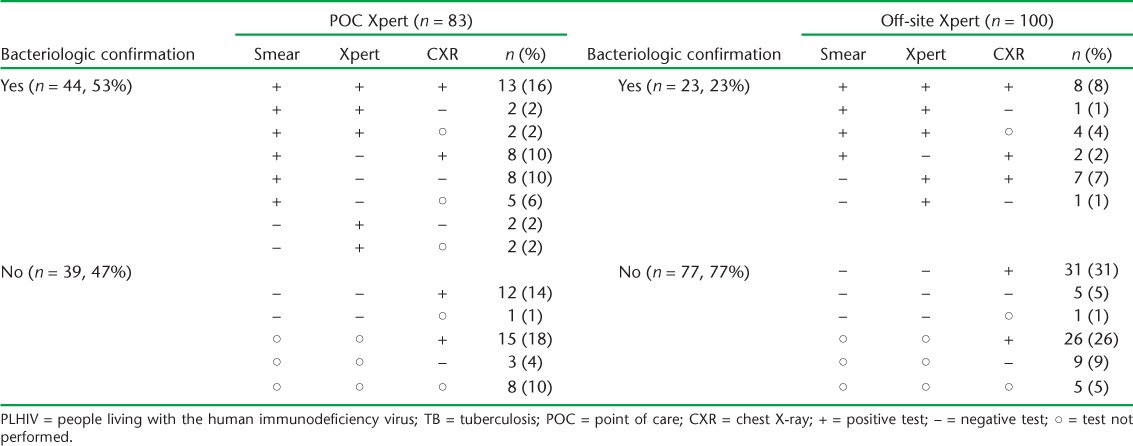
Among 689 PLHIV with documentation of a final diagnosis, 183 (27%) were diagnosed with pulmonary TB, 47 (7%) with extra-pulmonary TB, and for 459 (67%) TB was ruled out (Table 2). PLHIV evaluated at a POC Xpert site were significantly less likely to be diagnosed with any form of TB than PLHIV evaluated at a site with off-site Xpert (P < 0.01). Furthermore, among 83 PLHIV diagnosed with pulmonary TB at a POC Xpert site, 44 (53%) had bacteriologic confirmation (i.e., a positive smear or Xpert) (Table 3). In contrast, only 23 (23%) of 100 PLHIV diagnosed with pulmonary TB at a site with off-site Xpert had bacteriologic confirmation.
TABLE 3.
Diagnostic testing and results for PLHIV diagnosed with pulmonary TB
Patients with possible MDR-TB
Study population
From October 2011 to June 2013, we identified 643 patients with possible MDR-TB, the majority of whom were retreatment cases (n = 497, 77%), followed by non-converters at 2 months (n = 109, 17%) and non-converters at 3 months (n = 32, 5%) (Table 1). The median age was 53 years (IQR 42–64) and the majority were male (n = 383, 60%). HIV co-infection was present in 50 (8%) patients, of whom 36 (72%) were on ART.
Diagnostic testing
Among 493 patients with possible MDR-TB evaluated following Xpert rollout, 240 (49%) were tested using Xpert, of whom 84 (35%) were positive for M. tuberculosis (Table 4). A similar proportion of patients were tested with Xpert, whether POC (n = 86/169, 51%) or off-site (n = 154/324, 48%); however, patients were more likely to have a positive Xpert result when available as POC (n = 42, 49%) than off-site (n = 42, 27%). As with PLHIV, the quarterly proportion tested with Xpert did not increase during the evaluation period (Appendix Table A.2).
TABLE 4.
Diagnostic testing for people with possible MDR-TB
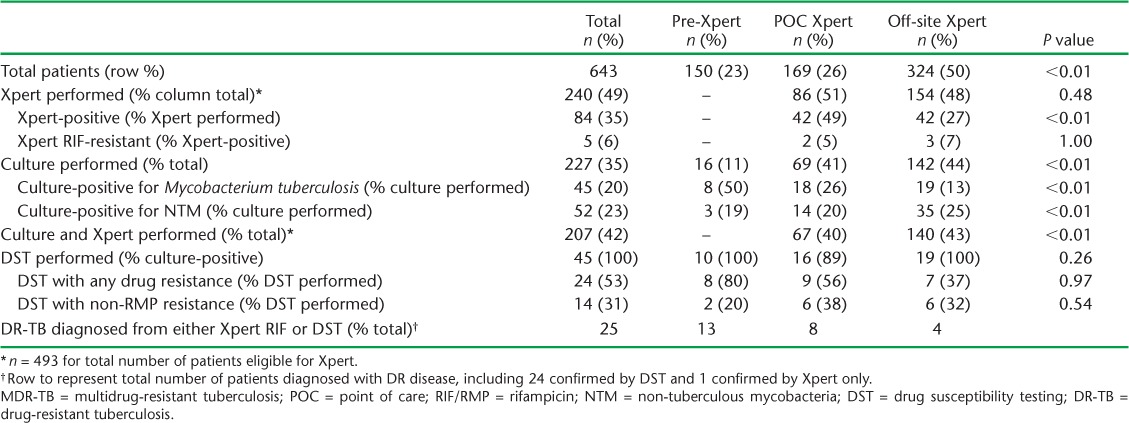
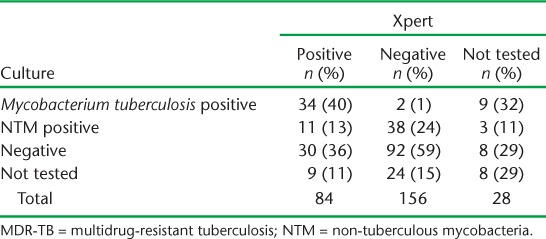
Prior to Xpert rollout, 11% of patients with possible MDR-TB had a sputum specimen sent for culture, compared to 43% who underwent culture after Xpert rollout. Of 227 patients with a culture result, 45 (20%) were positive for M. tuberculosis and 52 (23%) were positive for non-tuberculous mycobacteria (NTM). Among 84 patients with a positive Xpert result, 34 (40%) were culture-positive for M. tuberculosis, 11 (13%) were culture-positive for NTM, 30 (36%) were culture-negative and 9 (11%) did not have a culture result (Table 5). Conversely, among 156 patients with a negative Xpert result, 2 (1%) were culture-positive for M. tuberculosis, 38 (24%) were culture-positive for NTM, 92 (59%) were culture-negative, and 24 (15%) did not have a culture result. A greater proportion of non-converters were Xpert-positive and culture-negative as compared to retreatment cases (Appendix Table A.3).
TABLE 5.
Xpert and culture results for patients with possible MDR-TB
Drug resistance
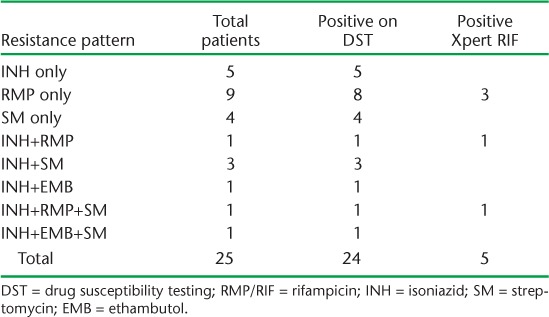
Among 84 patients with a positive Xpert, five (6%) were resistant to RMP by Xpert. Overall, DST was performed for 45 patients with possible MDR-TB, of whom 24 (53%) had resistance to at least one of the four drugs tested, i.e., isoniazid, RMP, streptomycin, or ethambutol (Table 4 and Appendix Table A.4). Of note, 14 (58%) of the 24 patients with drug resistance on DST had non-RMP drug resistance.
Turnaround time
The median TAT to an Xpert result for patients with possible MDR-TB was 9 days (IQR 4–30.5) with POC Xpert compared to 22 days (IQR 8–67) for those with off-site Xpert. The median TAT to culture results was also shorter for those with POC Xpert than off-site Xpert, at 18 days (IQR 12–34) and 32 days (IQR 15–71), respectively.
DISCUSSION
Several themes emerged from this evaluation of Xpert rollout in Cambodia: utilization of Xpert for both priority populations was generally low, agreement between Xpert and other diagnostic testing (i.e., smear microscopy and culture) was often low, and TAT for Xpert was faster when available as POC. We found that PLHIV with POC Xpert were less likely to be diagnosed with TB, yet more likely to have bacteriologic confirmation of a TB diagnosis as compared to off-site Xpert. Overall, Xpert increased the number of bacteriologically confirmed pulmonary TB cases by 26%. Also, more patients with possible MDR-TB were evaluated for drug resistance, whether with culture or Xpert, following Xpert rollout. One additional case of drug-resistant TB was diagnosed using Xpert, for an incremental yield of 4%.
Only 52% of PLHIV and 49% of patients with possible MDR-TB underwent Xpert testing, and a greater proportion of patients were evaluated when Xpert was available as POC. This relatively low utilization of Xpert is not unexpected given the novelty of the test and the logistical challenges in rollout that we and others have previously described.16,17 Our concerns about the underutilization of Xpert are further compounded by the results of a survey in the evaluation area, in which only 37% of clinical providers believed Xpert to be more accurate than smear microscopy, while only 43% responded that a patient always had TB if Xpert was positive.18
The poor agreement between Xpert, smear microscopy, and culture may have several explanations and raises several concerns. Among PLHIV tested with both Xpert and smear, 44% of those with a positive smear had a negative Xpert, which is higher than expected given the high specificity (>99%) of Xpert.19,20 Likewise, among patients with possible MDR-TB with a positive Xpert result, 13% were culture-positive for NTM and 36% were culture-negative. One possible explanation for these discrepancies is that Xpert might identify dead M. tuberculosis bacilli that would not grow on culture. However, the high rates of NTM in Cambodia could also lead to false-positive smears and complicate culture confirmation.21 In addition, as Xpert, smear microscopy, and culture were often performed on different sputum samples, mixed infection with both M. tuberculosis and NTM may have contributed. Finally, additional quality control measures may be needed to guard against contamination and ensure optimal performance of both Xpert and culture.
We believe the higher rates of bacteriologic confirmation among PLHIV observed at POC Xpert sites were a function of more direct access to Xpert, enabling clinicians to make fewer empiric diagnoses. In contrast, PLHIV at sites with off-site Xpert experienced a time to treatment initiation that was actually shorter than the time to Xpert result. Based on this seemingly counterintuitive finding, we hypothesize that clinicians with off-site Xpert were not utilizing Xpert results in their diagnostic decision-making and rather were initiating empiric treatment without regard for the Xpert result. Considering the high rates of NTM in Cambodia, this ongoing reliance on smear microscopy and clinical impression may lead to over-diagnosis of TB and inappropriate administration of anti-tuberculosis treatment. Our finding that POC Xpert reduced empiric treatment among PLHIV is in agreement with findings from investigators in Nepal and India.22,23 Given the potential for empiric treatment to undermine the benefits of more sensitive assays such as Xpert,24 ongoing monitoring of such tests will be critical as their utility will differ based on local epidemiology and clinical practice norms.
While 4% of patients with possible MDR-TB were documented as having drug-resistant disease, only 49% of eligible patients received Xpert and only 7% underwent DST (although this represented 100% of those with a positive culture). Thus, within the small subset of patients with DST results, an alarming 53% were found to have some degree of drug resistance. Furthermore, 14 of 25 patients with drug resistance on DST had non-RMP resistance that would not have been detected using Xpert. However, an unexpectedly high number of isolates were RMP-monoresistant, the reasons for which are unclear and merit further investigation. These results highlight the ongoing need for culture and DST in addition to Xpert for patients with possible MDR-TB. We believe the increase in culture for patients with possible MDR-TB was an unanticipated benefit of the intensive training and renewed programmatic support for identification of MDR-TB with Xpert rollout.
This evaluation is limited by its reliance on retrospective programmatic data without the ability to distinguish incomplete data recording from truly missing data. We were also unable to determine the impact of Xpert rollout on time to initiation of second-line treatment for those diagnosed with drug-resistant TB, given the small number of patients.
CONCLUSIONS
Our evaluation provides a comprehensive picture of the early experiences, successes, and challenges of introducing Xpert for routine programmatic use among PLHIV and patients with possible MDR-TB in rural Cambodia. Given Cambodia's high TB burden, with historically low rates of drug resistance and HIV infection, the findings from this rollout will be useful for other countries with a similar epidemiology. Continuing education for clinicians focusing on the benefits of Xpert over smear microscopy, and ongoing support for high-quality laboratory performance of culture and DST, may reduce empiric diagnosis of TB and potential misdiagnosis of NTM.
POC diagnostics such as Xpert remain an important advance in clinicians' ability to rapidly diagnose TB in priority populations; however, continued monitoring remains important to realize the full potential of Xpert. Lessons learned from this rollout underscore the need for careful planning and consideration to maximize the benefits of novel diagnostics in diverse clinical settings.
Acknowledgments
This work has been supported in part by the President's Emergency Plan for AIDS Relief through the US Centers for Disease Control and Prevention (CDC), Atlanta, GA, USA. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
APPENDIX
TABLE A.1.
Patient characteristics for PLHIV and patients with possible MDR-TB, by POC vs. off-site Xpert®
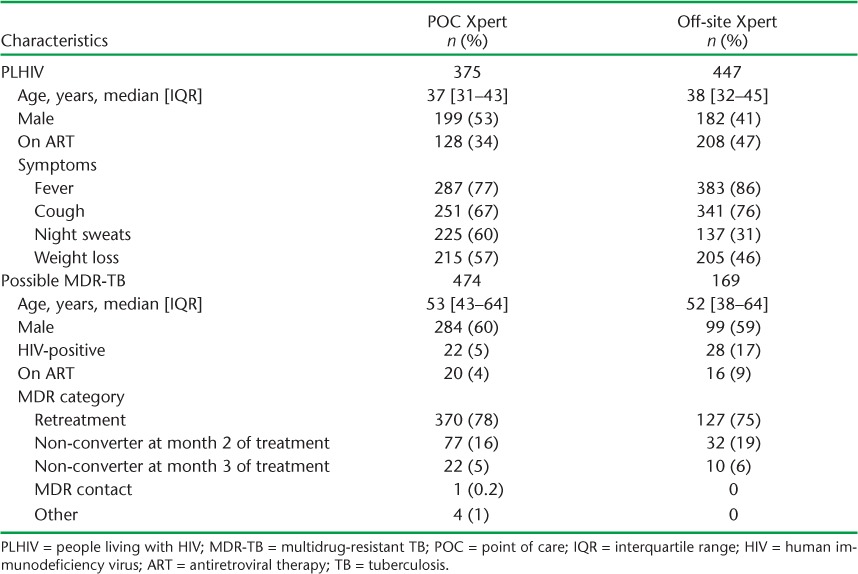
TABLE A.2.
Test utilization over time for PLHIV and patients with possible MDR-TB
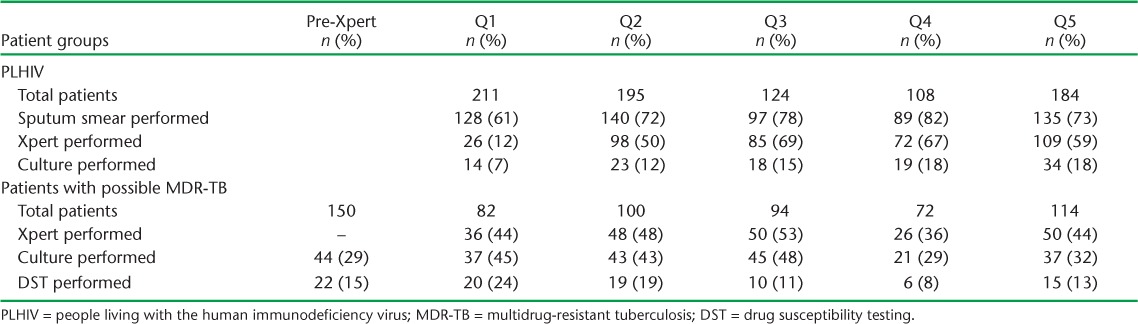
TABLE A.3.
Xpert® MTB/RIF and culture results for patients with possible MDR-TB by MDR-TB risk category
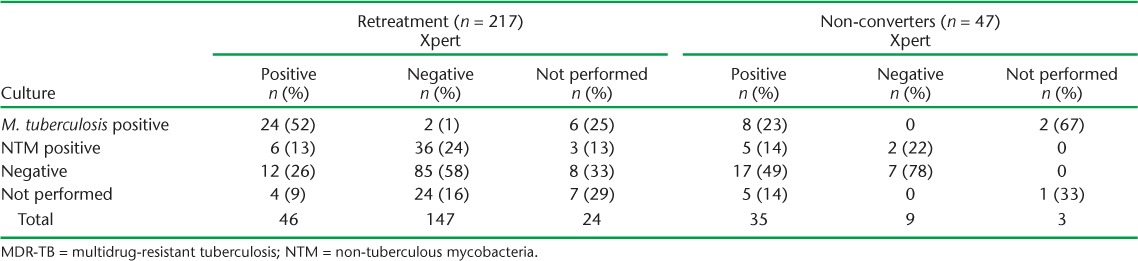
TABLE A.4.
Drug resistance by Xpert® MTB/RIF and DST
Footnotes
Conflicts of interest: none declared.
References
- 1.World Health Organization. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF system: policy statement. Geneva, Switzerland: WHO; 2011. WHO/HTM/TB/2011.4. [PubMed] [Google Scholar]
- 2.World Health Organization. WHO monitoring of Xpert MTB/RIF roll-out. Geneva, Switzerland: WHO; 2016. http://who.int/tb/laboratory/mtbrifrollout/en/ Accessed March 2016. [Google Scholar]
- 3.Cox H S, Mbhele S, Mohess N et al. Impact of Xpert MTB/RIF for TB diagnosis in a primary care clinic with high TB and HIV prevalence in South Africa: a pragmatic randomised trial. PLOS Med. 2014;11:e1001760. doi: 10.1371/journal.pmed.1001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Theron G, Zijenah L, Chanda D et al. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: a multicentre, randomised, controlled trial. Lancet. 2014;383:424–435. doi: 10.1016/S0140-6736(13)62073-5. [DOI] [PubMed] [Google Scholar]
- 5.Durovni B, Saraceni V, van den Hof S et al. Impact of replacing smear microscopy with Xpert MTB/RIF for diagnosing tuberculosis in Brazil: a stepped-wedge cluster-randomized trial. PLOS Med. 2014;11:e1001766. doi: 10.1371/journal.pmed.1001766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanrahan C F, Clouse K, Bassett J et al. The patient impact of point-of-care vs. laboratory placement of Xpert® MTB/RIF. Int J Tuberc Lung Dis. 2015;19:811–816. doi: 10.5588/ijtld.15.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mupfumi L, Makamure B, Chirehwa M et al. Impact of Xpert MTB/RIF on antiretroviral therapy-associated tuberculosis and mortality: a pragmatic randomized controlled trial. Open Forum Infect Dis. 2014;1:ofu038. doi: 10.1093/ofid/ofu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Churchyard G J, Stevens W S, Mametja L D et al. Xpert MTB/RIF versus sputum microscopy as the initial diagnostic test for tuberculosis: a cluster-randomised trial embedded in South African roll-out of Xpert MTB/RIF. The Lancet Glob Health. 2015;3:e450–457. doi: 10.1016/S2214-109X(15)00100-X. [DOI] [PubMed] [Google Scholar]
- 9.Lorent N, Choun K, Thai S et al. Community-based active tuberculosis case finding in poor urban settlements of Phnom Penh, Cambodia: a feasible and effective strategy. PLOS ONE. 2014;9:e92754. doi: 10.1371/journal.pone.0092754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Page A L, Ardizzoni E, Lassovsky M et al. Routine use of Xpert® MTB/RIF in areas with different prevalences of HIV and drug-resistant tuberculosis. Int J Tuberc Lung Dis. 2015;19:1078–1083. doi: 10.5588/ijtld.14.0951. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. Global tuberculosis report 2015. Geneva, Switzerland: WHO; 2015. WHO/HTM/TB/2015.22. [Google Scholar]
- 12.Cambodia Ministry of Health. National Center for HIV/AIDS Dermatology and STD. Estimations and Projections of HIV/AIDS in Cambodia 2010–2015. Phnom Penh, Cambodia: Cambodia Ministry of Health; 2011. [Google Scholar]
- 13.Cambodia Ministry of Health. National HIV seroprevalence survey amongst TB patients in Cambodia. Phnom Penh, Cambodia: Cambodia Ministry of Health; 2009. [Google Scholar]
- 14.UNAIDS. HIV and AIDS estimates. Geneva, Switzerland: UNAIDS; 2014. http://www.unaids.org/en/regionscountries/countries/cambodia/ Accessed March 2016. [Google Scholar]
- 15.World Health Organization. Tuberculosis diagnostics: automated DNA test. Geneva, Switzerland: WHO; 2010. http://www.who.int/tb/features_archive/xpert_factsheet.pdf Accessed April 2016. [Google Scholar]
- 16.Auld S C, Moore B K, Killam W P et al. Rollout of Xpert® MTB/RIF in Northwest Cambodia for the diagnosis of tuberculosis among PLHA. Public Health Action. 2014;4:216–221. doi: 10.5588/pha.14.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Creswell J, Codlin A J, Andre E et al. Results from early programmatic implementation of Xpert MTB/RIF testing in nine countries. BMC Infect Dis. 2014;14:2. doi: 10.1186/1471-2334-14-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eng B LR, Ly V, Hy C Barriers and enablers affecting utilization of Xpert MTB/RIF testing for tuberculosis (TB) in Cambodia. Phnom Penh, Cambodia: National Annual TB Program Conference, 2015.
- 19.Boehme C C, Nabeta P, Hillemann D et al. Rapid molecular detection of tuberculosis and rifampin resistance. New Eng J Med. 2010;363:1005–1015. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boehme C C, Nicol M P, Nabeta P et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet. 2011;377:1495–1505. doi: 10.1016/S0140-6736(11)60438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCarthy K D, Cain K P, Winthrop K L et al. Nontuberculous mycobacterial disease in patients with HIV in Southeast Asia. Am J Respir Crit Care Med. 2012;185:981–988. doi: 10.1164/rccm.201107-1327OC. [DOI] [PubMed] [Google Scholar]
- 22.Creswell J, Rai B, Wali R et al. Introducing new tuberculosis diagnostics: the impact of Xpert® MTB/RIF testing on case notifications in Nepal. Int J Tuberc Lung Dis. 2015;19:545–551. doi: 10.5588/ijtld.14.0775. [DOI] [PubMed] [Google Scholar]
- 23.Sachdeva K S, Raizada N, Sreenivas A et al. Use of Xpert MTB/RIF in decentralized public health settings and its effect on pulmonary TB and DR-TB case finding in India. PLOS ONE. 2015;10:e0126065. doi: 10.1371/journal.pone.0126065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Theron G, Peter J, Dowdy D, Langley I, Squire S B, Dheda K. Do high rates of empirical treatment undermine the potential effect of new diagnostic tests for tuberculosis in high-burden settings? Lancet Infect Dis. 2014;14:527–532. doi: 10.1016/S1473-3099(13)70360-8. [DOI] [PubMed] [Google Scholar]


