Abstract
Setting: The Democratic Republic of Congo suffers from an amalgam of disease outbreaks and other medical emergencies. An efficient response to these relies strongly on the national surveillance system. The Pool d'Urgence Congo (PUC, Congo Emergency Team) of Médecins Sans Frontières is a project that responds to emergencies in highly remote areas through short-term vertical interventions, during which it uses the opportunity of its presence to reinforce the local surveillance system.
Objective: To investigate whether the ancillary strengthening of the peripheral surveillance system during short-term interventions leads to improved disease notification.
Design: A descriptive paired study measuring disease notification before and after 12 PUC interventions in 2013–2014.
Results: A significant increase in disease notification was observed after seven mass-vaccination campaigns and was sustained over 6 months. For the remaining five smaller-scaled interventions, no significant effects were observed.
Conclusion: The observed improvements after even short-term interventions underline, on the one hand, how external emergency actors can positively affect the system through their punctuated actions, and, on the other hand, the dire need for investment in surveillance at peripheral level.
Keywords: Integrated Disease Surveillance and Response, disease notification, emergency response, Médecins Sans Frontières, Pool d'Urgence Congo
Abstract
Contexte : La République Démocratique du Congo souffre d'un amalgame de flambées épidémiques et d'autres urgences médicales. Une réponse efficace à ces problèmes est basée sur le système national de surveillance. Le Pool d'Urgence Congo (PUC) de Médecins Sans Frontières est un projet répondant aux urgences dans les zones très reculées grâce à des interventions verticales à court terme, pendant lesquelles le projet met à profit l'opportunité de sa présence pour renforcer le système de surveillance local.
Objectif : Vérifier si le renforcement complémentaire du système de surveillance périphérique pendant des interventions à court terme amène une amélioration de la notification des maladies.
Schéma : Une étude descriptive par paires mesurant la notification des maladies avant et après 12 interventions PUC en 2013–2014.
Résultats : Une augmentation significative de la notification des maladies a été observée après sept campagnes de vaccination de masse et elle s'est maintenue pendant 6 mois. En ce qui concerne les cinq interventions restantes à plus petite échelle, aucun effet significatif n'a été observé.
Conclusion : Les améliorations observées, même après des interventions à court terme, soulignent d'un côté comment des acteurs externes de l'urgence peuvent affecter positivement le système à travers leurs actions ponctuelles et, d'un autre côté, le besoin pressant d'investir dans la surveillance au niveau périphérique.
Abstract
Marco de referencia: La República Democrática del Congo adolece de una amalgama de brotes epidémicos y otras urgencias médicas y la eficiencia de la respuesta a esta situación depende en gran medida del sistema nacional de vigilancia. El proyecto ‘Pool d'Urgence Congo’ (PUC, en francés) de Médecins Sans Frontières responde a las situaciones de urgencia en zonas muy remotas, mediante intervenciones verticales a corto plazo, durante las cuales se aprovecha la presencia en el terreno con el fin de reforzar el sistema local de vigilancia sanitaria.
Objetivo: Investigar si el fortalecimiento complementario del sistema periférico de vigilancia sanitaria durante las intervenciones de corta duración contribuye a mejorar la notificación de las enfermedades.
Método: Un estudio descriptivo emparejado, en el cual se midió la notificación de las enfermedades antes y después de 12 intervenciones del PUC del 2013 al 2014.
Resultados: Se observó un aumento estadísticamente significativo de la notificación de las enfermedades después de siete campañas de vacunación colectiva, el cual se mantuvo durante 6 meses. En las cinco intervenciones restantes de menor escala no se observaron efectos considerables.
Conclusión: El progreso observado incluso después de intervenciones a corto plazo, por una parte, pone de manifiesto que los actores externos en situaciones de emergencia pueden inducir modificaciones positivas del sistema mediante sus actividades puntuales y, en segundo lugar, destaca la necesidad urgente de invertir en el sistema de vigilancia sanitaria a nivel periférico.
The Integrated Disease Surveillance and Response (IDSR) strategy is a pillar of the surveillance system of many low- and middle-income countries (LMICs).1 It focuses on the detection of and response to public health threats such as infectious disease outbreaks. A recent systematic review of the IDSR strategy in LMICs highlighted several fundamental constraints that still hamper integrated surveillance, such as complex logistics, lack of financial resources and poor professional skills, motivation and supervision.2
The Democratic Republic of Congo (DRC) has implemented the IDSR strategy through its national surveillance system since 2000.3,4 While it has achieved considerable success, such as controlling wild poliovirus, challenges remain in the most remote and deprived areas of the country.5 Unfortunately, no further evidence on the performance of the surveillance system in the DRC has been published to date.
The medical humanitarian organisation Médecins Sans Frontières (MSF) has a unique opportunity to access the most remote areas of the DRC through its emergency intervention project, the Pool d'Urgence Congo (PUC, Congo Emergency Team). For 20 years, the PUC has aimed at detecting epidemics and responding to medical emergencies occurring in man-made and natural disasters. The PUC intervenes in more than 10 emergencies per year, primarily providing curative and preventive care for vulnerable (mainly paediatric) and difficult to access (geographically) populations. Outbreak responses such as those of the PUC rely to an extent on the good functioning of the IDSR strategy. Although not the first objective in an outbreak response, the PUC uses the opportunity of such interventions to strengthen the national notifiable disease surveillance system (NNDSS) at service and community level in the affected health zone. It is unknown, however, to what extent such emergency, short-term interventions can positively affect the NNDSS and how sustained this putative effect is.
This study aims to investigate whether this ancillary activity of strengthening the NNDSS during short-term PUC interventions results in a change in disease notification in the areas of intervention.
METHODS
Study design
This is a descriptive ‘before and after’ paired study assessing the effects of PUC interventions on the NNDSS in the DRC. The 26 weeks following a PUC intervention were compared to the identical period one year earlier for all sites of intervention.
General setting
The DRC is an equatorial African country of almost 2.5 million km2, with a population of over 70 million, of whom 70% live in rural areas. The country has 515 health zones, and the national health system is decentralised at provincial level and funded by public and private mechanisms with peripheries lacking human and material resources.6–9 DRC is continuously challenged by an amalgam of endemic diseases and severe outbreaks. For example, 11% of Plasmodium falciparum-related deaths in sub-Saharan Africa occur in the DRC,10 and the country experienced its seventh Ebola outbreak in August 2014.11
Disease notification system
In the IDSR strategy implemented by the DRC, the weekly notification of diseases remains a pillar of its functioning.4 Weekly notification forms are compiled at the health zone level once data from the registers of the health posts have been collected. These are transmitted to the provincial Ministry of Health, and are supposed to reach the national level by Thursday of the following epidemiological week. The completeness and timeliness of the notifications are monitored weekly.
Pool d'Urgence Congo
The PUC responds to different epidemic outbreaks, such as measles, typhoid fever, cholera, malaria, bloody diarrhoea, meningitis, yellow fever, haemorrhagic fever, and plague; nutritional emergencies; and man-made and natural disasters. The functional components of the PUC are 1) a coordination unit in Kinshasa; 2) two antennas, in Kinshasa and Kisangani, and three sentinels, in Mbandaka, Mbuji Mayi and Kindu; and 3) a Mobile Intervention Team (Figure 1).
FIGURE 1.
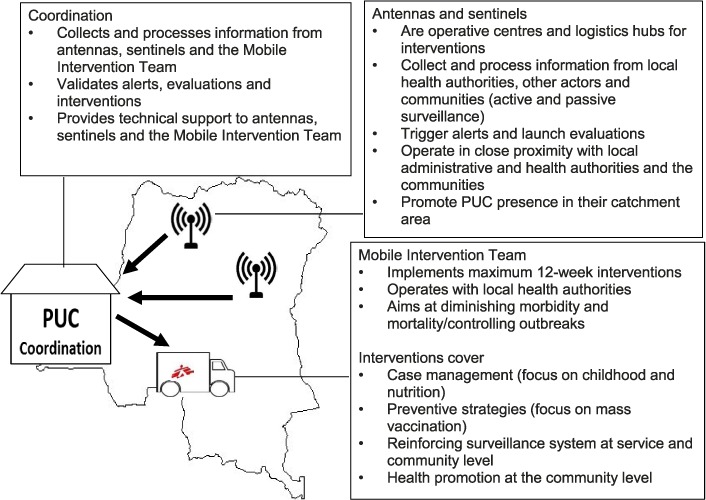
Components and functioning of the PUC. PUC = Pool d'Urgence Congo (Congo Emergency Team).
After a reported alert, the PUC investigates the situation on site, which varies in time according to needs. The actual interventions are supposed to last no longer than 12 weeks.
Strengthening the surveillance system
Despite being an ancillary activity, the strengthening of the local surveillance system is part of each intervention, and consists of 1) on-the-job training of health staff in case definitions (community, clinical and biological), outbreak investigations, data collection, analysis and reporting; 2) implementing case notification registers in health facilities; 3) empowering community surveillance with training and incentives through existing ‘relais communautaires’ (a network of community health workers); 4) ensuring data reporting and transmission; and 5) strengthening biological surveillance according to the local capacity for material and human resources. These activities, which include the use of standardised tools such as notification forms, case definition forms and registers, start at once and last for the duration of the PUC presence at the site.
Included interventions
All health zones where the PUC implemented an intervention in 2013–2014, and for which surveillance data were available from 1 year before to 6 months after the PUC presence, were included in this study. Major confounding events that could influence disease notification, such as a recurring MSF presence in the area due to subsequent emergencies, were reasons for exclusion of the intervention from this analysis.
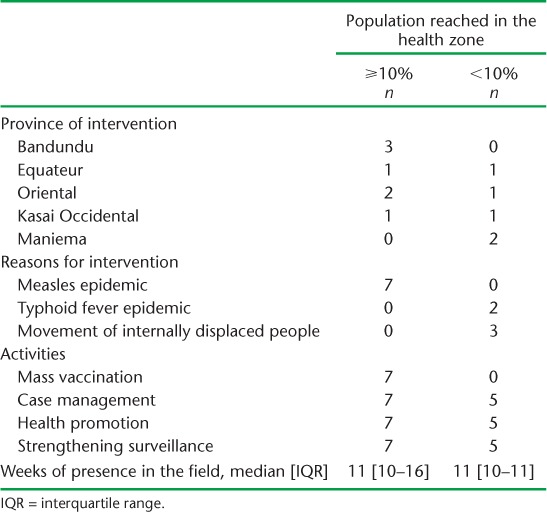
Interventions were categorised by type: those including a mass vaccination component were larger in magnitude (reaching >10% of the population as beneficiaries in the health zone), and represented an extensive presence of the PUC in the majority of health facilities; interventions without a mass vaccination component reached fewer beneficiaries and the PUC presence was generally more localised within the health zone.
Data source
Available weekly disease notifications and estimated populations of each included health zone were sourced from the IDSR database at the Ministry of Health. Data were transferred into a dedicated Excel electronic worksheet (Excel 2010, Microsoft Corp, Redmond, WA, USA).
Analysis and statistics
The study observation period covered the 26 weeks immediately following the PUC intervention and the corresponding 26 weeks of the previous year, to avoid bias resulting from seasonal variations in disease (e.g., a period from January to June following an intervention was compared to the period from January to June before the intervention). The notification of diseases was compared between the paired ‘before’ and ‘after’ periods. Notified cases of malaria, measles, bloody diarrhoea, meningitis, respiratory tract infections and typhoid fever were considered for this analysis; the index disease for intervention was excluded from the analysis of that intervention (e.g., typhoid fever for a typhoid fever intervention).
Disease notification was normalised as follows: for each observed disease, the weekly notified cases were divided by the median number of notified cases over the period of observation; a normalised notification curve was therefore created for each disease. The weekly normalised values were summed into a cumulative weekly normalised value, thus defining a unique normalised curve of notification. Subsequently, modelled notification values at 1, 5, 13 and 26 weeks were calculated by linear regression over these paired periods (Figure 2).
FIGURE 2.
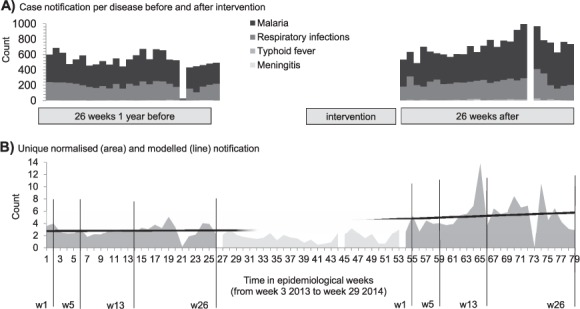
A) Case notification per disease before and after one intervention of the PUC. B) Unique normalised notification and modelled notification values over the periods of observation before and after one intervention of the PUC; modelled notification observed at 1, 5, 13 and 26 weeks per period of observation. Demonstration for the response in the health zone of Idiofa, 2013–2014. Similar analyses were performed for each intervention (not shown). PUC = Pool d'Urgence Congo (Congo Emergency Team).
Considering the hyper-endemicity of malaria in the DRC,10 as a disease reported in the framework of the IDSR strategy, the incidence of malaria per semester (number of cases/10 000/week) was calculated as a proxy for disease notification, and the number of weeks without any malaria notifications was considered as a proxy for incompleteness of notification.
Median modelled notification values, malaria incidence, and number of weeks with zero malaria notification were compared before and after the PUC interventions using the Wilcoxon matched-pairs signed-rank test; the level of significance was set at 5%. Analyses were performed using Stata version 11.1 (StataCorp, College Station, TX, USA).
Ethics
This study used routinely collected and aggregated surveillance data. Permission to carry out the study was obtained from the Ethics Committee of the Ecole de Santé Publique of Kinshasa University, Kinshasa, DRC (n° d'approbation: ESP/CE/055/2015). The study met the MSF Ethics Review Board (Geneva, Switzerland) approved criteria for studies of routinely collected data, and was also approved by the Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease (Paris, France).
RESULTS
Of 22 interventions implemented by the PUC in 2013–2014, 10 were excluded: 6 due to the recurrent presence of MSF in the area over the period of observation of this analysis, thus possibly affecting disease notification; 1 was the emergency response to the 2014 Ebola outbreak in the DRC, which lasted for more than 3 months and most likely influenced the entire reporting system in the areas; and 1 occurred in a closed refugee camp with no PUC activity in the health zone. Two other interventions were excluded because the surveillance data were not available over the periods of this analysis.
Of the 12 interventions included, seven implemented a mass vaccination campaign against measles, reaching >10% of the population in the health zone. The characteristics of the interventions are given in the Table.
TABLE.
Characteristics of included interventions conducted by the Pool d'Urgence Congo, 2013–2014
Modelled notification at 1, 5, 13 and 26 weeks per observation period and for the two types of intervention is indicated in Figure 3. A significant increase in the median modelled notification value was observed after mass vaccination campaigns (interventions reaching ⩾10% of the population in the health zone) over the post-intervention period, at 1, 5, 13 and 26 weeks. At week 26 there was a median increase from 1.8 (interquartile range [IQR] 1.5–2.3) to 3.9 (IQR 3.1–4.8, P = 0.02).
FIGURE 3.
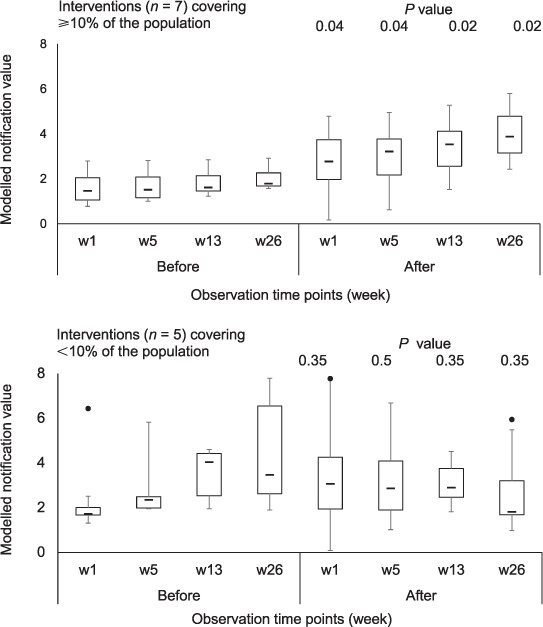
Modelled notification values at 1, 5, 13 and 26 weeks per observation period, before and after intervention by the PUC, 2013–2014; Tukey boxplots for different interventions according to the proportion of population reached in the health zone; median values at different time points compared using the Wilcoxon matched-pairs signed-rank test. PUC = Pool d'Urgence Congo (Congo Emergency Team).
When analysing malaria incidence as a proxy for surveillance system performance, the median incidence (number of cases per 10 000 population per week) over the 26 weeks before and after the PUC presence increased from 22.9 (IQR 18.5–27.5) to 29.8 (IQR 20.3–31.9) for mass vaccination interventions (P = 0.24) and from 30.7 (IQR 29.5–37.2) to 32.4 (IQR 29.1–33.6) for the other interventions (P = 0.69). The median number of weeks with zero malaria notification (data incompleteness) remained at 1 for before (IQR 0–3) and after (IQR 0.5–2) the mass vaccination interventions (P = 0.80); it decreased from 4 weeks (IQR 3–5) to 2 weeks (IQR 1–2) for the other interventions (P = 0.10). These differences were not statistically significant.
DISCUSSION
To the best of our knowledge, this is the first study to report on a pragmatic approach to strengthening a national IDSR system, in which emergency interventions in remote areas of the DRC are used as an opportunity to improve surveillance. Strengthening of the surveillance system remains a side activity for the PUC, and is performed as well as possible under time- and resource-constrained conditions. The results nevertheless suggest that even a short-term emergency intervention can be associated with an increase, albeit modest, in disease notification up to at least 6 months after leaving the area.
The main strength of this study was the availability of consistent data sets spanning the full study period across all the interventions, which allowed the seasonal variation of disease to be taken into account by paired analysis before and after the interventions. Furthermore, all activities aiming at surveillance strengthening were systematically implemented in a standardised manner; the sole difference amongst the interventions was in the area covered and the total population covered, according to the specific objective of each response.
Limitations of this study were the small number of included interventions, affecting its representativeness. In addition, a geographic bias may exist: the three interventions for internally displaced people (with no mass vaccination) typically took place in the most deprived areas of the country, possibly associated with poorer baseline surveillance system performance. In areas with a higher innate capacity of the surveillance system, efforts to strengthen the IDSR may be more successful. Furthermore, based on observational data, we cannot exclude that the increased notification was due to the nature of the emergency itself, which could result in modified health-seeking behaviour and/or better transmission of information. The absence of increased notification after the non-vaccination interventions suggests, however, that an emergency by itself is insufficient to lead to sustained improvements in the surveillance system.
A final limitation was the lack of information on structured training on the NNDSS at the peripheral level in the included intervention locations, which could have biased our findings. The general remoteness and poor access to the included locations, however, likely precluded sufficient training coverage to achieve a noticeable effect.
The observed improvement to the surveillance system after mass vaccination-type interventions may be explained by a number of factors. Such interventions include the extensive and systematic presence of the PUC in a large range of health facilities; all health structures and villages are accessed for the planning and implementation of the vaccination and health promotion activities. The PUC staff engages in the reinforcement of surveillance with local staff operating in the most peripheral settings, e.g., infirmiers titulaires (nurses at health posts) and the relais communautaires. Phalkey et al. documented the role of local human resources in the performance of the IDSR system across Africa.2 Sow et al. reported on the association of IDSR training coverage at district level with increased completeness and timeliness of reporting.12 Other studies also noted the effect of providing feedback, supervision and on-the-job training to local staff as a means of improving motivation and performance and consequently strengthening the IDSR system.13,14 Our observations on such improvements over a period of at least 6 months add to this body of evidence. In general, establishing contact and sharing knowledge with staff at local service level may represent the sole opportunity to reinforce their commitment and performance; a qualitative analysis of perception of the surveillance system among local staff may help to seize opportunities for improvement and identify missed opportunities for surveillance.
Findings of this study relate to areas where barely any organisation except the PUC supports local Ministry of Health services; it may thus be of relevance to other settings that are equally remote and deprived. Clearly, as even modest short-term support to the surveillance system offered during the PUC's vertical interventions can result in an increase in disease notification, the baseline performance of the system in such settings is likely to be extremely weak, rendering organisations working in emergency interventions functionally blind. A broad investment in strengthening surveillance systems primarily at the peripheral level is urgently needed. Our results also suggest that, as a stopgap measure, no opportunity should be missed by emergency units—even if they are present for only a limited period of time—to reinforce existing peripheral systems. In addition, for such emergency units, more reliable surveillance in the period after intervention may represent the only opportunity to assess their own performance.
An understanding of the mechanisms underpinning the surveillance system at peripheral level is key to its improvement, and represents a means to hear the voices of those most in need. In the words of Briceño-León,15 this study reminds us that it is the populations that are neglected more than the diseases.
A spark may create only a moment of light, but it can be enough to show us the needs of those around us.
Acknowledgments
The authors would like to thank the women and men of the Pool d'Urgence Congo for their professionalism, commitment and endurance in the fulfilment of the mission of Médecins Sans Frontières (MSF).
This research was made possible with the kind support of the Ministère de la Santé Publique, Secrétariat Général à la Santé, Direction de Lutte contre la Maladie, Kinshasa, the Democratic Republic of Congo.
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Programme for Research and Training in Tropical Diseases at the World Health Organization (WHO/TDR), Geneva, Switzerland. The model is based on a course developed jointly by the International Union Against Tuberculosis and Lung Disease (The Union), Paris, France, and MSF, Centre for Operational Research, Brussels Operational Centre, Luxembourg. The specific SORT IT programme that resulted in this publication was jointly developed and implemented by the Operational Research Unit (LUXOR), MSF; the Centre for Operational Research, The Union; the Centre for International Health, University of Bergen, Bergen, Norway; the Institute of Tropical Medicine, Antwerp, Belgium; and Partners in Health, Boston, MA, USA. The programme was funded by The Union, MSF, and the Department for International Development, London, UK. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. La Fondation Veuve Emile Metz-Tesch, Luxembourg, also supported the open access publication costs.
Footnotes
Conflicts of interest: none declared.
In accordance with the WHO's open-access publication policy for all work funded by the WHO or authored/co-authored by WHO staff members, the WHO retains the copyright of this publication through a Creative Commons Attribution IGO licence (http://creativecommons.org/licenses/by/3.0/igo/legalcode), which permits unrestricted use, distribution and reproduction in any medium provided the original work is properly cited.
References
- 1.World Health Organization. International Health Regulations. 2nd ed. Geneva, Switzerland: WHO; 2008. [Google Scholar]
- 2.Phalkey R K, Yamamoto S, Awate P, Marx M. Challenges with the implementation of an Integrated Disease Surveillance and Response (IDSR) system: systematic review of the lessons learned. Health Policy Plan. 2015;30:131–143. doi: 10.1093/heapol/czt097. [DOI] [PubMed] [Google Scholar]
- 3.Kasolo F, Yoti Z, Bakyaita N et al. IDSR as a platform for implementing IHR in African countries. Biosecur Bioterror. 2013;11:163–169. doi: 10.1089/bsp.2013.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Democratic Republic of Congo, Health Ministry. Guide technique pour la surveillance intégrée de la maladie et riposte: SIMR. 2nd ed. Kinshasa, DRC: Health Ministry; 2011. [French] [Google Scholar]
- 5.Alleman M M, Meyer S A, Mulumba A et al. Improved acute flaccid paralysis surveillance performance in the Democratic Republic of the Congo, 2010–2012. J Inf Disease. 2014;210(Suppl 1):50–61. doi: 10.1093/infdis/jit670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Democratic Republic of Congo, Health Ministry. Programme élargi de vaccination, plan de contrôle de la rougeole, 2013–2015. Kinshasa, DRC: Health Ministry; 2014. [French] [Google Scholar]
- 7.Democratic Republic of Congo, Health Ministry. Bulletin n°16. En collaboration avec les Ministère de l'Agriculture/INS Bulletin SNSAP mai–julliet. Kinshasa, DRC: Health Ministry; 2014. [French] [Google Scholar]
- 8.Tshikuka J G, Okenge L, Lukuka A et al. Severity of outcomes associated to illnesses funded by GFATM initiative and socio demographic and economic factors associated with HIV/AIDS, TB and malaria mortality in Kinshasa hospitals, DRC. Ethiop J Health Sci. 2014;24:299–306. doi: 10.4314/ejhs.v24i4.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mbeva J B, Schirvel C, Godelet E, Wodon A, Porignon D, Bonami M. Reorganization of the provincial health system in the Democratic Republic of the Congo. Santé Publique. 2014;26:849–858. [French] [PubMed] [Google Scholar]
- 10.Taylor S M, Messina J P, Hand C C et al. Molecular malaria epidemiology: mapping and burden estimates for the Democratic Republic of the Congo, 2007. PLOS ONE. 6:e16420. doi: 10.1371/journal.pone.0016420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maganga G D, Kapetshi J, Berthet N et al. Ebola virus disease in the Democratic Republic of Congo. N Engl J Med. 2014;371:2083–2091. doi: 10.1056/NEJMoa1411099. [DOI] [PubMed] [Google Scholar]
- 12.Sow I, Alemu W, Nanyunja M, Duale S, Perry H N, Gaturuku P. Trained district health personnel and the performance of integrated disease surveillance in the WHO African region. East Afr J Public Health. 2010;7:16–19. doi: 10.4314/eajph.v7i1.64671. [DOI] [PubMed] [Google Scholar]
- 13.Adokiya M N, Awoonor-Williams J K, Beiersmann C, Müller O. The integrated disease surveillance and response system in northern Ghana: challenges to the core and support functions. BMC Health Serv Res. 2015;15:288. doi: 10.1186/s12913-015-0960-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nnebue C C, Onwasigwe C N, Ibeh C C, Adogu P O. Effectiveness of data collection and information transmission process for disease notification in Anambra State, Nigeria. Niger J Clin Pract. 2013;16:483–489. doi: 10.4103/1119-3077.116894. [DOI] [PubMed] [Google Scholar]
- 15.Briceño-León R, Méndez Galván J. The social determinants of Chagas disease and the transformation of Latin America. Mem Inst Oswaldo Cruz. 2007;102(Suppl 1):109–112. doi: 10.1590/s0074-02762007005000095. [DOI] [PubMed] [Google Scholar]


