Abstract
Objective: To allocate resources for household contact investigations, tuberculosis (TB) programs need estimates of the numbers of child contacts requiring care.
Design: We developed two methods to estimate annual numbers of child contacts aged 0–14 years requiring evaluation and treatment. Method 1 combines local data using simple formulas. Using publicly available data, Method 2 uses a linear regression model based on Demographic and Health Survey and World Bank data to estimate the number of children per household, then combines these results with case notifications and risk estimates of disease and infection.
Results: Applying Method 1 to data from Malawi indicated that every year ~21 000 child contacts require evaluation and ~1900 should be diagnosed with TB. Applying Method 2 to all countries suggested that, globally, 2.41 million (95% uncertainty interval [UI] 2.36–2.46) children aged <5 years, and 5.07 million (95%UI 4.81–5.34) children aged 5–14 years live in households of adult patients with known TB. Of these, 239 014 (95%UI 118 649–478581) and 419 816 (95%UI 140600–1 268805), respectively, will have TB. An additional 848 453 (95%UI 705838–1 017551) and 2660 885 (95%UI 2080517–3 413 189), respectively, will be infected.
Conclusion: It is feasible to use available data to set programmatic evaluation and treatment targets to improve care for child contacts of patients with TB.
Keywords: contact tracing, chemoprophylaxis, epidemiology, households
Abstract
Objectif : Pour allouer des ressources aux recherches de contacts domiciliaires, les programmes de lutte contre la tuberculose (TB) ont besoin d'estimations du nombre d'enfants contacts nécessitant une prise en charge.
Schéma : Nous avons élaboré deux méthodes afin d'estimer les nombres annuels d'enfants contacts âgés de 0–14 ans requérant une évaluation et un traitement. La Méthode 1 combine des données locales utilisant des formules simples. En utilisant les données disponibles publiquement, la Méthode 2 se sert d'un modèle de régression linéaire basé sur les données de l'Enquête Démographie et Santé et celles de la Banque Mondiale afin d'estimer le nombre d'enfants dans chaque famille, puis de combiner ces résultats avec ceux de la déclaration des cas et des estimations de risque de maladie et d'infection.
Résultats : En appliquant la Méthode 1 aux données du Malawi, nous avons abouti à ce que ~21 000 enfants contacts par an requéraient une évaluation et ~1900 devraient avoir un diagnostic de TB. Appliquer la Méthode 2 à tous les pays a suggéré que, dans le monde, 2,41 millions d'enfants âgés de <5 ans (intervalle d'incertitude [II] à 95% 2,36–2,46 millions) et 5,07 millions (II95% 4,81–5,34 millions) d'enfants âgés de 5–14 ans vivent dans des foyers comprenant un patient adulte atteint de TB chaque année. Parmi eux, 239 014 (II95% 118 649–478 581) et 419 816 (II95% 140 600–1 268 805), respectivement, auront la TB et 848 453 autres enfants (II95% 705838–1017 551) et 2660 885 (II95% 2080 517–3413 189) seront infectés.
Conclusion : Il est possible d'utiliser les données disponibles pour établir des objectifs d'évaluation programmatique et de traitement afin d'améliorer la prise en charge des enfants contacts de patients tuberculeux.
Abstract
Objetivo: Para designar los recursos necesarios para la evaluación de contactos de pacientes con tuberculosis (TB), los programas necesitan estimados de cuántos contactos niños requieren atención.
Diseño: Desarrollamos dos métodos de estimar cuántos contactos que tienen 0–14 años requieren evaluación y tratamiento cada año. Método 1 usa información local y fórmulas sencillos. Usando información pública, Método 2 usa un modelo de regresión lineal basado en datos de las Encuestas Demográficas y de Salud y del Banco Mundial para estimar el número de niños en cada domicilio, y luego combina estos resultados con números reportados de casos de TB y con estimados del riesgo de enfermedad e infección con TB.
Resultados: Aplicando el Método 1 a datos de Malawi indica que cada año, ~21 000 contactos niños deben ser evaluados y ~1900 deben ser diagnosticados con TB. Aplicando el Método 2 a datos de todos los países del mundo indica que cada año, al nivel mundial, hay 2,41 millón (intervalo de incertidumbre [II] de 95% 2,36–2,46 millón) de niños de edad <5 años, y 5,07 millón (II95% 4,81–5,34 millón) de niños que tienen 5–14 años, quienes viven en domicilios de adultos que se sabe son pacientes con TB. De estos niños, 239 014 (II95% 118649–478 581) y 419816 (II95% 140600–1 268 805), respectivamente, estarán enfermos con TB. Además, 848 453 (II95% 705838–1 017551) y 2 660 885 (II95% 2080 517–3 413189) estarán infectados con TB pero no enfermos.
Conclusión: Es factible usar datos disponibles para generar metas programáticas para la evaluación y el tratamiento, con el fin de mejorar la atención a los contactos niños de pacientes con TB.
Although around 1 million children fall sick with tuberculosis (TB) each year, only 359 000 pediatric cases were notified in 2014.1,2 Household contact investigations are an important method of finding children with TB and identifying contacts who would benefit from preventive therapy. Children living in the households of adult patients with TB are at high risk for tuberculous infection, TB disease, and death.3–5 These children are accessible because the adult patients are already accessing care for TB. The World Health Organization (WHO) recommends an evaluation of household contacts of patients with TB in all settings,6 and many country guidelines contain this recommendation, but routine implementation is variable.7 In addition, many countries recommend preventive therapy for child contacts, although specific guidelines vary by country. To facilitate planning and resource allocation, national TB programs (NTPs) would benefit from estimates of the number of children they should expect to evaluate and treat for disease or infection if they were to perform household contact investigations routinely around adult patients with known TB.
We present an approach that countries, districts, or cities can use to set annual TB care targets based on the number of children to be evaluated via household contact investigations, the number of children expected to have TB disease, and the number who will require preventive therapy.
METHODS
We propose two methods, a preferred one that uses local data that are available in some settings, and a cruder method using publicly available data, which is applicable to any country. The general principle behind these methods is that multiplying the number of infectious adult cases with TB by the number of children likely to be found in each adult patient's household yields the number of children who require evaluation for TB.8 Multiplying this number by appropriate risk estimates yields the number of children expected to have TB disease and tuberculous infection on contact investigation. All of these estimates are restricted to children in the households of adults who have been diagnosed with TB.
All statistical analyses were performed using SAS 9.3 (Statistical Analysis Software Institute, Cary, NC, USA) or R for Mac 3.0.2.
Method 1: Setting programmatic targets using locally available data
Potential data sources
Many countries conduct censuses that enumerate population counts by age group and number of households. Demographic and Health Surveys (DHS) (https://www.dhsprogram.com), which are population-representative surveys carried out periodically in certain countries, also provide household size data.
NTPs collect reports of all diagnosed TB cases in the country. Subnational data are usually available locally, as reports are collected at the health facility level and aggregated up to the national level for reporting to the WHO.2 Furthermore, reporting forms in many countries include disaggregation of cases by age group and type of TB (e.g., pulmonary vs. extra-pulmonary). This makes it possible to determine the number of reported adult pulmonary TB cases, which represent the most infectious cases that have been diagnosed.
Country-specific estimates of TB disease and tuberculous infection risks among child contacts are usually unknown unless routine high-quality household contact investigations are being performed, or a population representative study has been carried out to determine this risk. If neither is the case, then generalized risk estimates can be used.3,9
Child contacts requiring evaluation
The average number of children per household is estimated by dividing the number of children by the number of households. Multiplying the number of annually reported adult pulmonary TB cases by the average number of children per household estimates the number of child household contacts requiring evaluation in one year. This calculation assumes that all adult patients with TB live in households, and that there is only one adult patient per household. For a given region, this calculation is summarized as follows:
where E = the estimated number of child contacts in a specific age group requiring evaluation in one year, C = the number of children in the age group, H = the number of households, and A = the number of adult pulmonary TB cases reported in the previous year.
Child contacts expected to have tuberculosis disease
To estimate the number of child contacts requiring treatment, E is multiplied by the estimated proportion of child contacts with TB disease at the time of the contact investigation (D). If possible, this should be done separately for the 0–4 and 5–14 year age groups, as disease risk varies between younger and older children.3 Therefore, in one year, the number of children expected to require treatment for TB disease following household contact investigation (T) is calculated as follows:
Child contacts expected to require preventive therapy
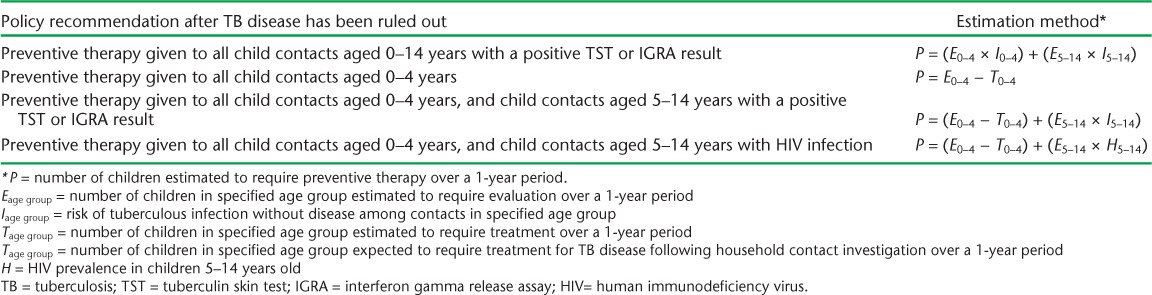
Multiplying E by the estimated proportion of child contacts who have tuberculous infection without disease at the time of contact investigation (I) yields the annual number of infected child contacts. However, national policies differ as regards the eligibility of child household contacts for preventive therapy, and not all require testing for tuberculous infection. Table 1 presents methods of estimating the number of children expected to require preventive therapy (P) in a 1-year period, according to four different policies.
TABLE 1.
Formulas for estimating the number of children in households of adult patients with pulmonary TB who will require preventive therapy during a 1-year period, based on four policies
Method 2: Crude national programmatic targets based on publicly available data
Children per household
We used DHS data to estimate the average number of children per household. For each country, we used the most recent survey that included the household survey component. We calculated point estimates and 95% confidence intervals (95%CI) for the number of children aged 0–4 and 5–14 years (SAS 9.3 SURVEYMEANS procedure).
To estimate the average number of children per household in countries where DHS data were not available, we constructed linear regression models for the two age groups. We included countries with DHS data in the models, with point estimates for the average number of children per household as the response variable. Observations were weighted by the inverse of the standard error around these point estimates. Candidate predictor variables were demographic and socio-economic indicators that were available for all countries for 2012 from the World Bank (http://data.worldbank.org/indicator). We considered: fertility (births per woman), life expectancy, per cent of the population aged <15 years, per cent of the population that is female, per cent of the population living in rural areas, deaths among children aged <5 years per 100 live births, and gross national income per capita based on purchasing power parity. We used manual backward elimination with assessment for confounding to produce the final multivariable models, and used the final model to produce point estimates and 95% prediction intervals for the average number of children per household in countries without a DHS.
Child contacts requiring evaluation
We used the numbers of TB cases notified in 201410 to calculate the number of adult pulmonary TB cases diagnosed in each country. We calculated the proportion of all TB cases that were pulmonary, and applied this proportion to the total number of adult cases. For countries without age-disaggregated data, we assumed all notified pulmonary cases to be adult. For countries lacking 2014 case notification data, we used 2013 data.
For each country, we multiplied the assumed number of adult pulmonary TB cases by the estimated average number of children per household, by age group (0–4 and 5–14 years), to estimate the number of child contacts requiring evaluation in a 1-year period. To produce point estimates and their 95% uncertainty intervals (95%UI) for each country, we first drew 1000 different potential values for the average number of children per household based on the point estimate and its prediction interval (or confidence interval for DHS-based estimates), assuming that this variable was normally distributed. We then multiplied each of the 1000 potential values for each country and age group by the assumed number of adult pulmonary cases in that country (with no uncertainty). Hence, we produced 1000 estimates of the number of child contacts requiring evaluation, taking the median of these estimates as the point estimate and the 2.5th and 97.5th percentiles as the lower and upper uncertainty bounds.
Child contacts expected to have tuberculosis disease and tuberculous infection
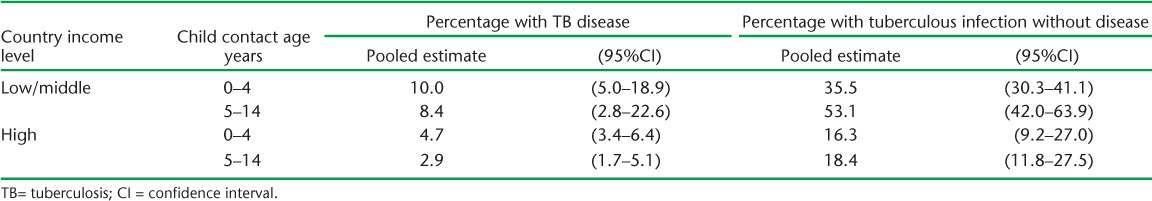
To estimate the numbers of children expected to have TB disease and tuberculous infection on contact investigations, we applied risk estimates from a 2013 meta-analysis3 (Table 2) to the estimates of children requiring evaluation. This meta-analysis reported pooled estimates of the proportion of child contacts diagnosed with TB disease and the proportion of child contacts diagnosed with tuberculous infection without disease (using tuberculin skin testing) at the time of contact investigation, stratified by country income level. We used risk estimates for high-income or low-/middle-income countries based on the 2015 World Bank classification of country income level. We applied separate risk estimates for the 0–4 and 5–14 year age groups, and estimated children with disease and infection separately.
TABLE 2.
Pooled risks of TB disease and tuberculous infection without disease among child contacts at the time of contact evaluation, adapted from a 2013 meta-analysis3
To produce point estimates and uncertainty intervals, we estimated the standard error of the natural logarithm of the risk estimates from their confidence intervals, assuming that they were normally distributed. We then produced 1000 estimates of the log-transformed risk estimate for each age group and country income level, assuming they were normally distributed. For each country and age group, we multiplied the 1000 estimates of the number of child contacts requiring evaluation by the 1000 exponentiated risk estimates, matching sequentially. We took the median of the resulting 1000 estimates as the point estimate and the 2.5th and 97.5th percentiles as the lower and upper uncertainty bounds.
RESULTS
Method 1: Example of care targets based on locally available data
In 2014, a total of 10 508 adult pulmonary TB cases (age ⩾15 years) were reported to the Malawi NTP. According to the 2008 census, Malawi had 2 869 933 households,11 2370011 children aged 0–4 years, and 3 638 690 children aged 5–14 years.12 Thus, each household had an average of 0.8 children aged 0–4 years and 1.2 children aged 5–14 years. Assuming minimal annual change in the number of diagnosed adult pulmonary cases and in average children per household, approximately 8414 child household contacts aged 0–4 years and 12 918 child household contacts aged 5–14 years require an evaluation each year.
Applying estimated risks of TB disease for low- and middle-income countries (Table 2) to all child contacts in the country suggests that, annually, around 841 children aged 0–4 years and 1085 children aged 5–14 years would have TB at the time of the contact investigation. Malawi's NTP guidelines indicate that once TB disease has been ruled out, all child contacts aged 0–60 months, as well as older child contacts with the human immunodeficiency virus (HIV), should receive isoniazid preventive therapy.13 Therefore, around 7572 child contacts aged 0–4 years are eligible for preventive therapy annually. The prevalence of HIV infection among children aged 5–14 years is unknown, but estimating a 2.7% prevalence (the prevalence reported among 15–17 year olds in the 2010 Malawi DHS,14 which is likely to be higher than in the younger age group) would suggest an additional 319 child contacts requiring preventive therapy because of HIV infection.
The same data sources allowed us to make district-level estimates for the 28 districts of Malawi (data not shown), which the Malawi NTP is using to plan contact investigation activities.
Method 2: Crude national-level care targets
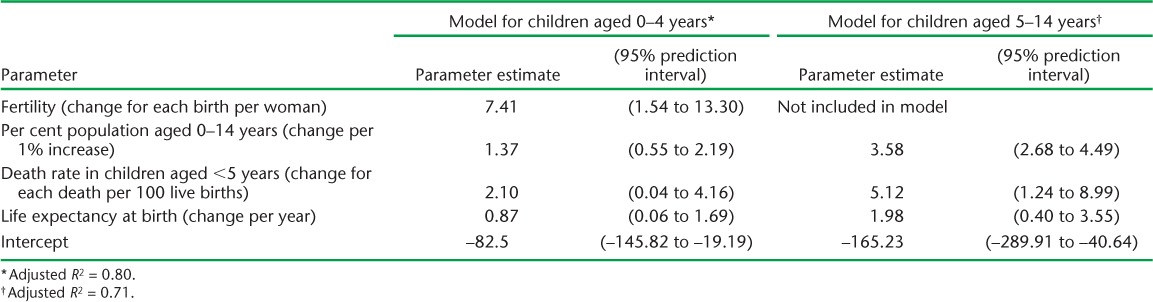
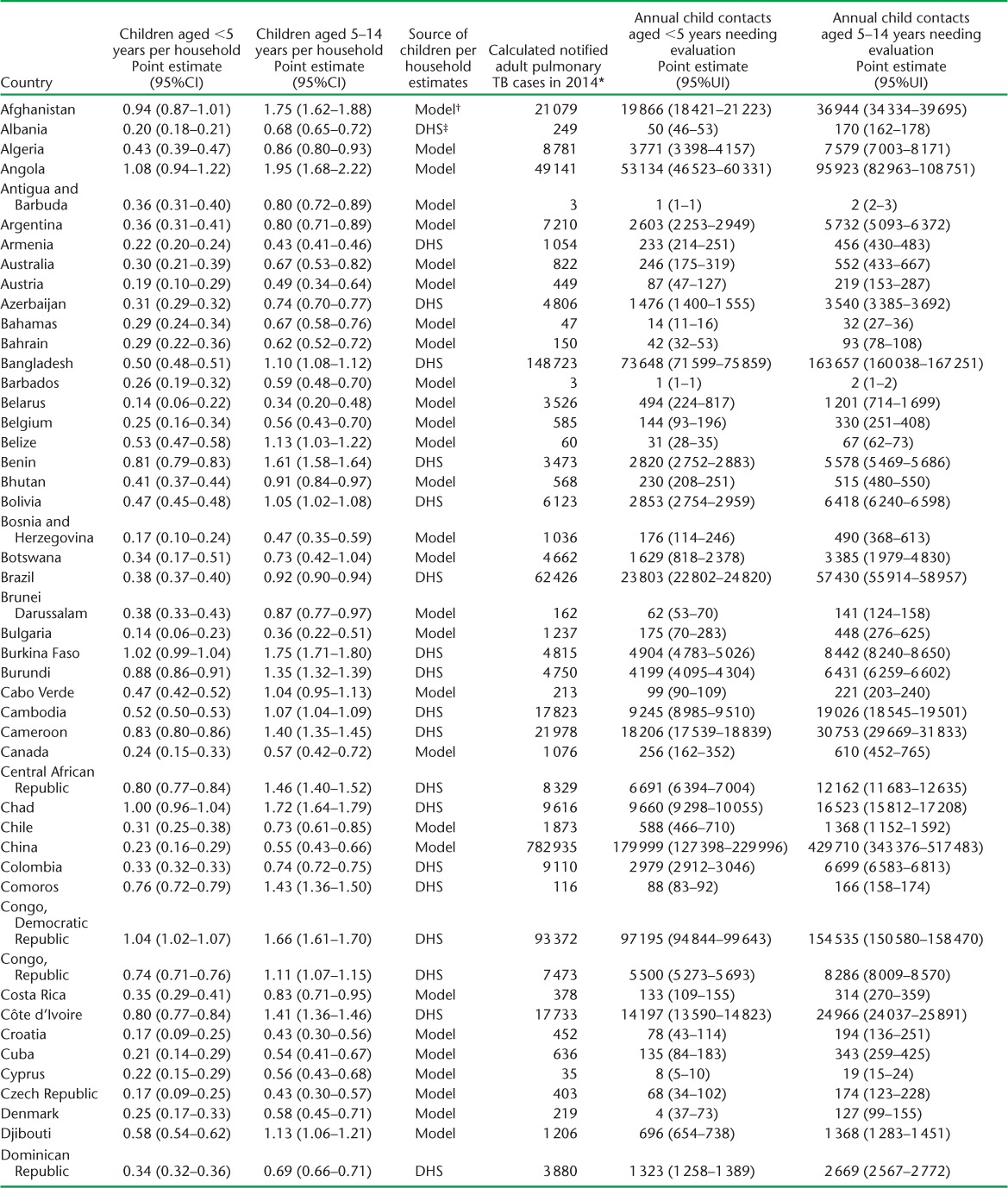
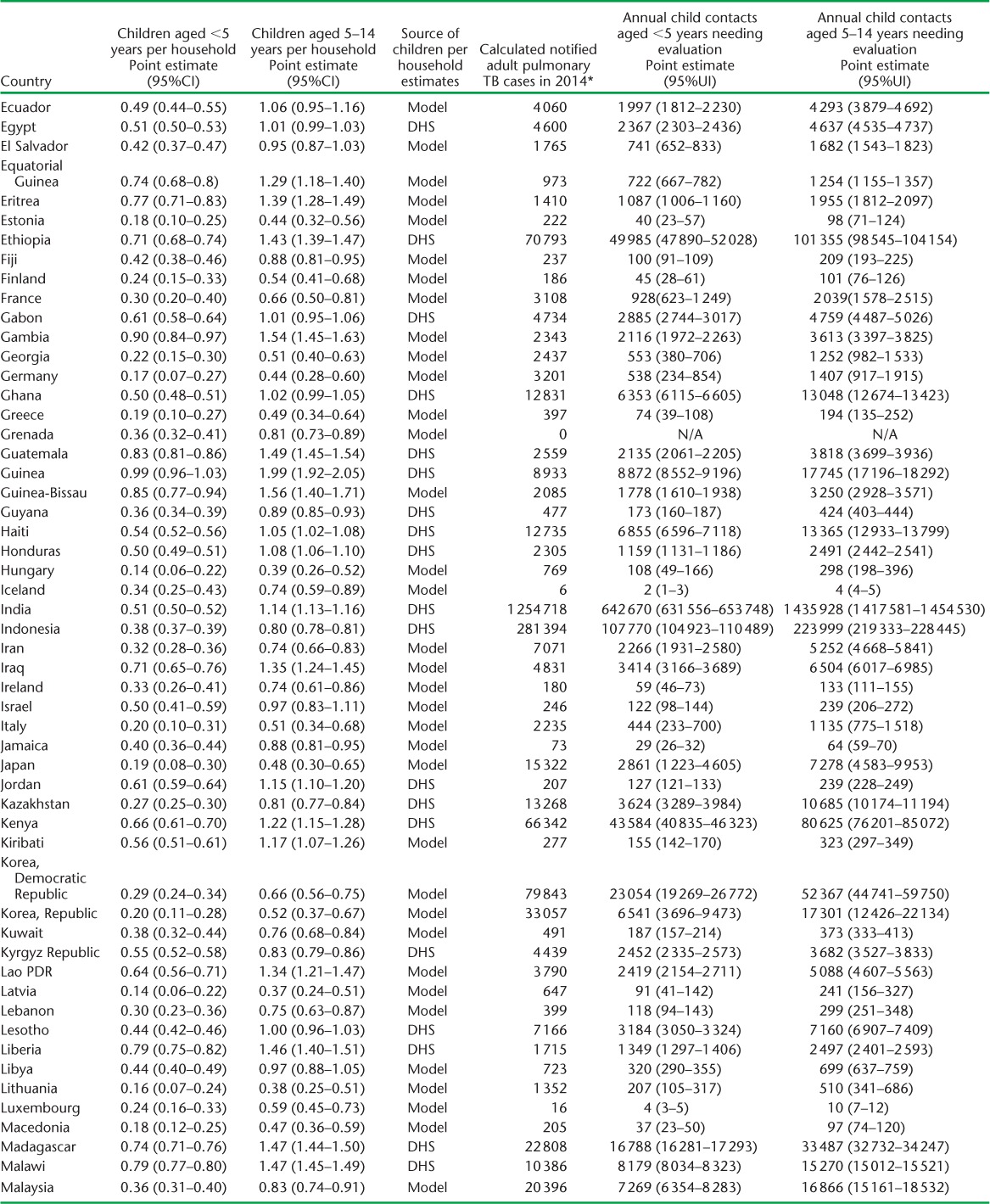
We used DHS data to estimate the average number of children per household for 69 countries. Multivariable models for the association between the number of children per household and demographic indicators in these countries (Table 3) indicated that the number of children aged 0–4 years per household was predictable based on fertility, percentage of the population aged 0–14 years, mortality rate in children aged <5 years, and life expectancy at birth (adjusted R2 = 0.80). The number of children aged 5–14 years per household was predicted based on the percentage of the population aged 0–14 years, mortality rate in children aged <5 years, and life expectancy at birth (adjusted R2 = 0.71). Estimates of the average number of children per household for all countries are provided in Appendix Table A.1.
TABLE 3.
Association between number of children per 100 households and demographic indicators among 69 countries with Demographic and Health Surveys
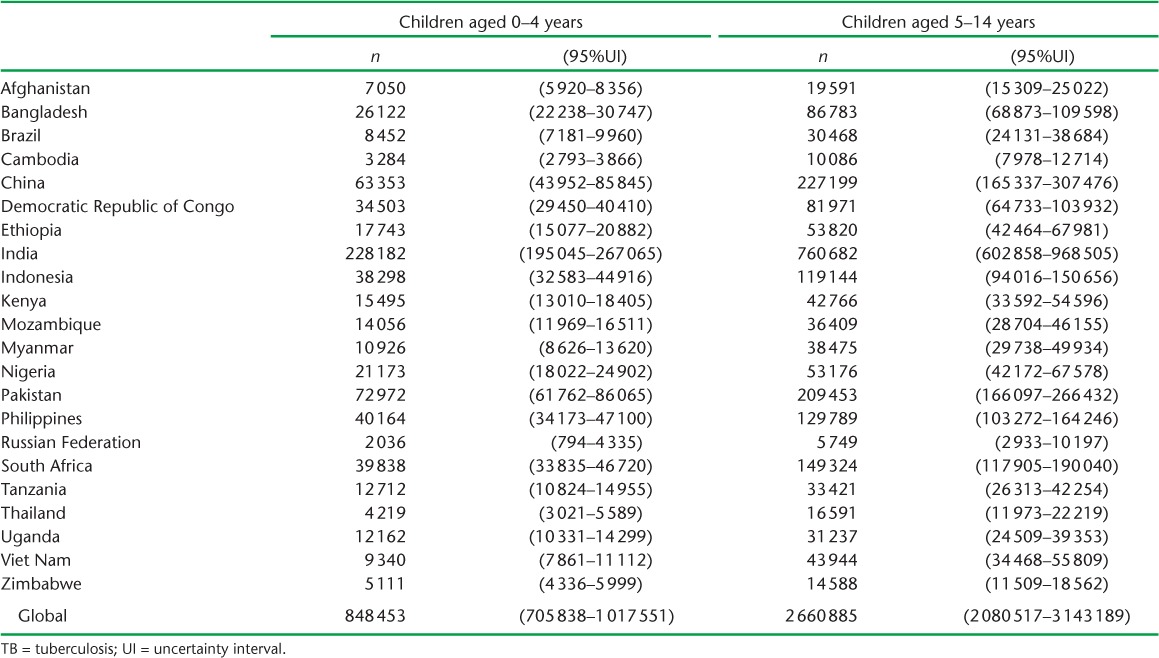
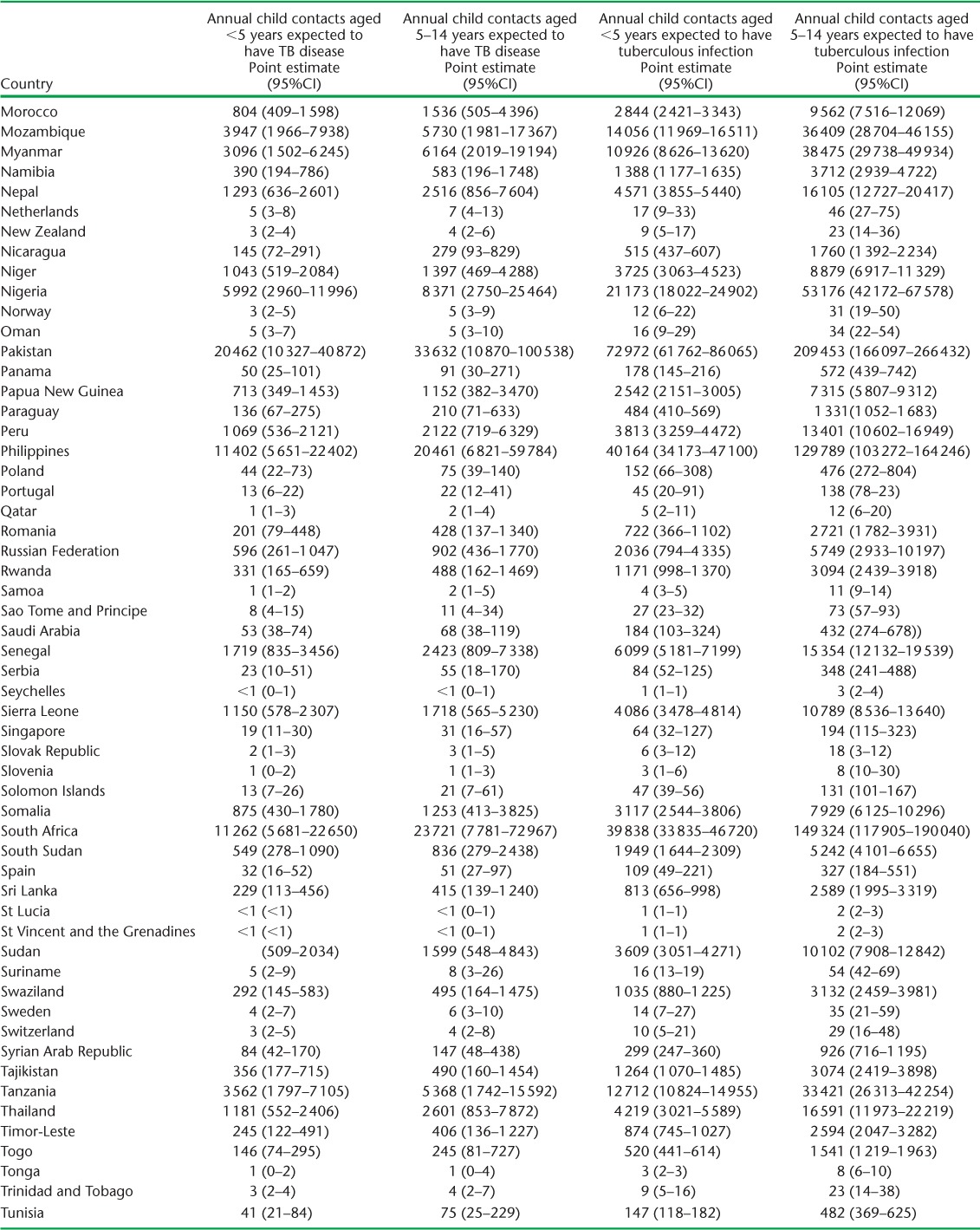
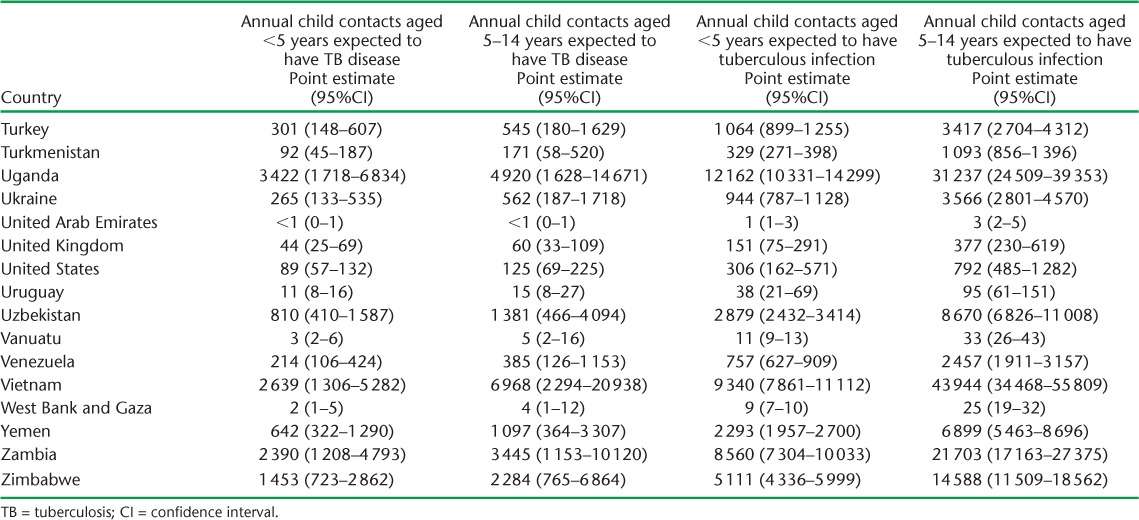
Based on 2014 adult case notifications, we estimated that, globally, 2.41 million (95%UI 2.36–2.46) children aged <5 years, and 5.07 million (95%UI 4.81–5.34) children aged 5–14 years require evaluation annually because they live in households with a known adult patient with pulmonary TB (Table 4). Of these, 239 014 (95%UI 118 649–478 581) child contacts aged <5 years and 419 816 (95% UI 140 600–1 268 805) child contacts aged 5–14 years are expected to have TB disease at the time of the contact investigation (Table 5). An additional 848 453 (95%UI 705838–1017 551) child contacts aged <5 years and 2 660 885 (95%UI 2 080 517–3 413 189) child contacts aged 5–14 years are expected to have tuberculous infection without disease (Table 6). Estimates for all 184 countries and territories where data were available are provided in Appendix Table A.2.
TABLE 4.
Number of children in households of notified adult pulmonary TB patients estimated to have required evaluation in 2014, in the 22 high-burden countries and globally
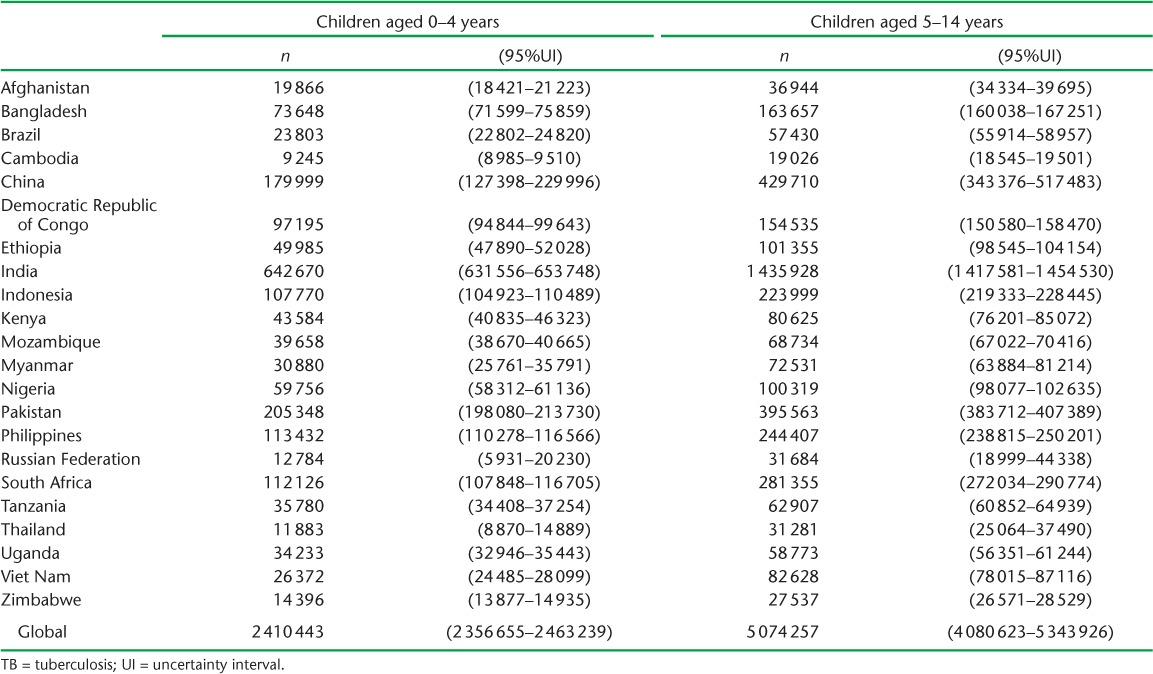
TABLE 5.
Estimated number of children in households of notified adult pulmonary TB patients who would have had TB disease upon contact investigation in 2014, in the 22 high-burden countries and globally, and child cases reported by national governments in the same year
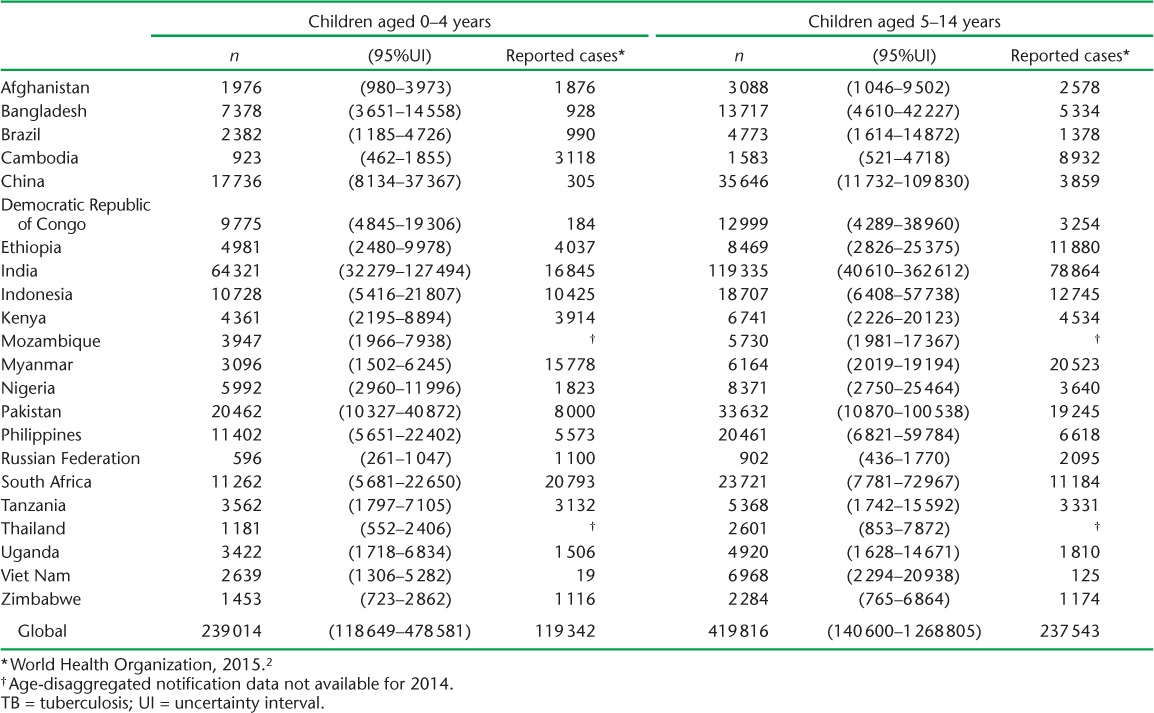
TABLE 6.
Estimated number of children in households of notified adult pulmonary TB patients who would have had tuberculous infection without TB disease upon contact investigation in 2014, in the 22 high-burden countries and globally
In 30 (17%) of the 174 countries and territories that reported age-disaggregated TB data in 2014, the number of notified pediatric TB cases was less than 20% of the number expected. In 20 (11%) countries, the number of pediatric cases reported exceeded the upper bound of the estimated number of cases expected; 12 (60%) of these countries are classified as having low TB incidence (⩽10 cases per 100 000).2
DISCUSSION
Health systems currently collect abundant data on patients with TB, but these data are rarely used for any purpose other than reporting to an NTP or to the WHO. However, existing data can enable NTPs and their partners to plan interventions and monitor impact at local or national level.15 The simple methods proposed are intended to support efforts to expand and improve contact investigations, which, if implementation is incomplete, represent millions of missed opportunities to find, treat, and prevent TB in children. Care targets can help to quantify the staffing, drug supply, and health system capacity required to treat the children who are consequently diagnosed with TB disease and tuberculous infection. They can also help those NTPs already performing contact investigations to assess performance.
The methods proposed are meant to help programs reach the most accessible children at high risk for TB, i.e., those living in the homes of adult patients already in care for TB. They do not generate estimates of the true burden of childhood disease (separate methods exist for this1,2,16), as the true burden is likely to be much higher in places with incomplete detection of adult TB cases. We estimated that, in 2014, in the 22 high-burden countries, approximately 6.1 million children were living in households of adult patients who had been diagnosed with TB. In contrast, using a mechanistic model that simulated risks of household and community exposure to all prevalent TB cases, Dodd et al. estimated that 15 million children in these same countries lived in a household with a TB patient in 2010.16 Focusing on children in households of known patients with TB allows us to generate realistic programmatic targets for contact investigations, but one should not forget that there are large numbers of children with TB outside these households.
Our estimates of child household contacts expected to have tuberculous infection without TB disease suggest how many children would benefit from preventive therapy. However, as country policies regarding eligibility for preventive therapy differ, these estimates do not correspond to the number of eligible children in a given setting under local guidelines.
While we aimed to present a simple approach that can be used easily, several of our simplifying assumptions limit its accuracy. We assumed that all adult patients with TB live in households. However, in some settings, a large proportion of patients with TB are in prisons,17 where children are unlikely to be exposed; our method would tend to overestimate child contacts in such situations. We also assumed that each adult patient corresponds to a single household. However, some households have multiple patients with TB, and some patients move between different households, so the effect of this assumption is unclear and will vary by setting. Our assumption that the average number of children per household in a particular geographic region applies to TB patient households is another limitation, as TB cases are not randomly distributed through the population. For example, in many of the countries where the actual number of notified childhood TB cases exceeded the upper bound of our estimate, large proportions of patients with TB are immigrants,18–21 who may live in households with more children than the national average. Local estimates could avoid this limitation if data are routinely collected on the household size of TB patients through contact registers or rosters.
Finally, for simplicity, we proposed using generalized risk estimates for disease and infection, although this risk varies across settings. While the risk estimates we used do not take into account the differential infectiousness of index cases (e.g., smear-positive vs. smear-negative) or factors other than age that could affect the susceptibility of child contacts (e.g., HIV infection, malnutrition), it is possible to make more refined estimates using stratified risk estimates,3 if data are available on the prevalence of these factors among patients with TB and their child contacts. In addition, more refined estimates could be produced if robust estimates of infection and disease risk were available for narrower pediatric age bands, as disease risk is known to be quite variable across infancy, childhood, and adolescence.22
Household contact investigations represent an efficient, targeted strategy to diagnose some of the most easily accessible of the roughly 640 000 children with TB disease who are currently missed by health systems every year. NTPs need adequate resources and staffing to effectively implement household contact investigations and ensure that child contacts are treated for TB disease and infection. Transparent methods of estimating performance targets can be used to quantify resource gaps, engage partners, and advocate for the resources required to screen and treat this high-risk population. To begin this process, it is currently feasible, in many locations, to use available data to set programmatic evaluation and treatment targets focused on improving care for these vulnerable children.
Acknowledgments
We are grateful to Carly Rodriguez for research assistance.
This work was supported by a grant from Janssen to Harvard Medical School and by the National Institutes of Health [K01AI102944 to HEJ]. The funders had no role in the study design or conduct; in collection, management, analysis, or interpretation of the data; or in preparation, review, approval, or submission of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health. Funded by the National Institutes of Health (NIH).
APPENDIX
APPENDIX TABLE A.1.
Estimated children per household and child contacts of notified tuberculosis patients needing evaluation in 2014
APPENDIX TABLE A.1.
(continued)
APPENDIX TABLE A.1.
(continued)
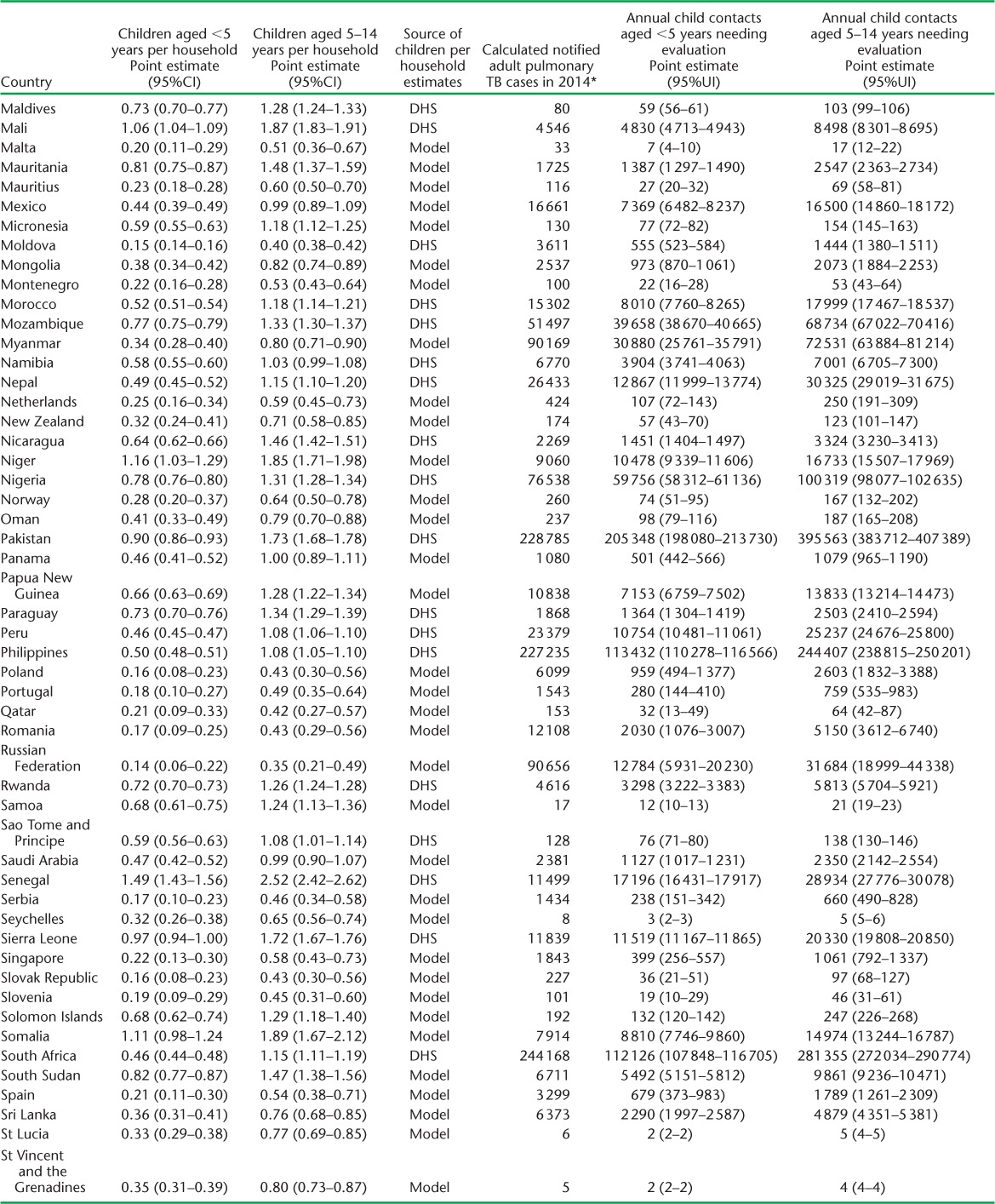
APPENDIX TABLE A.1.
(continued)
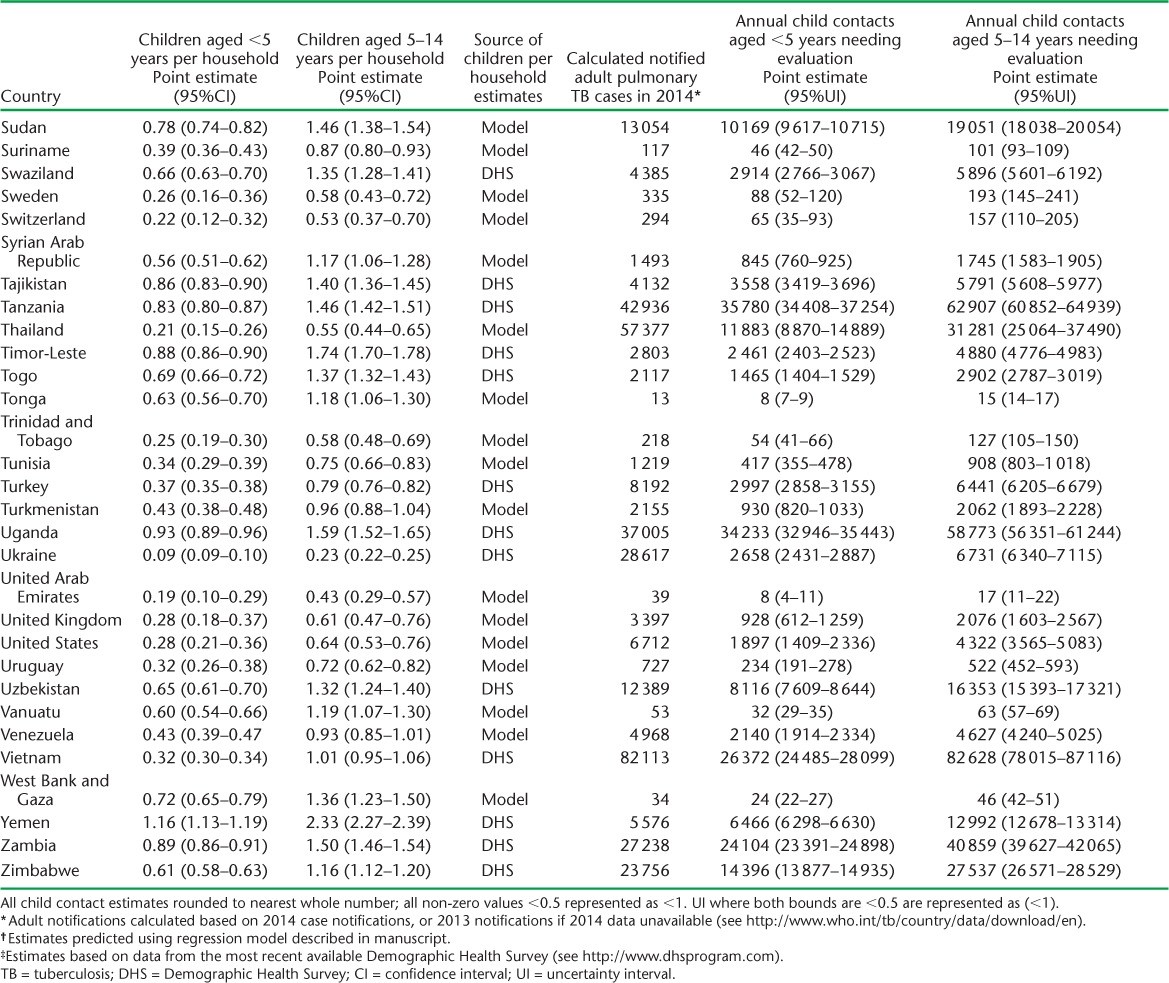
APPENDIX TABLE A.2.
Child contacts of notified TB patients estimated to have had tuberculosis disease and infection in 2014
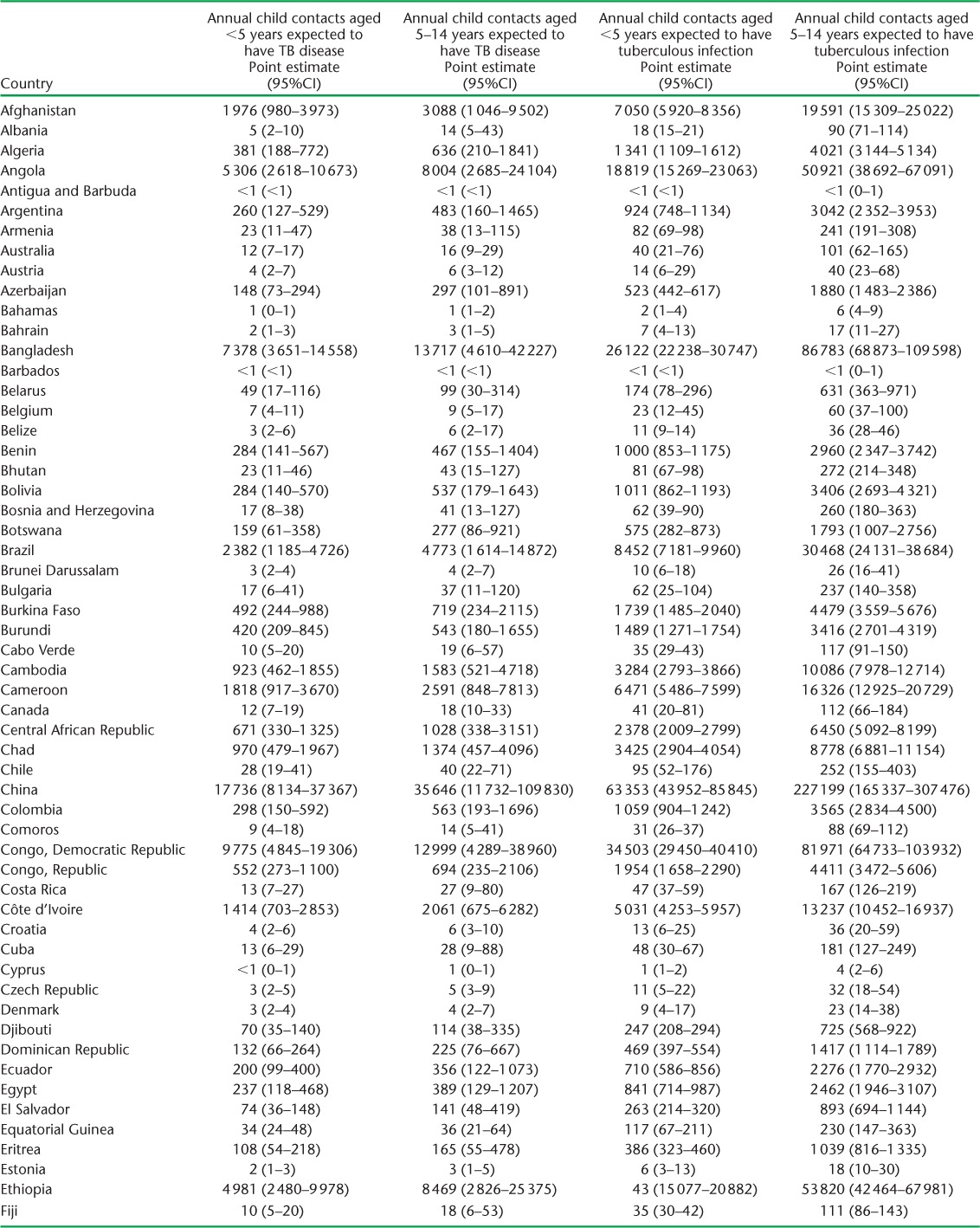
APPENDIX TABLE A.2.
(continued)
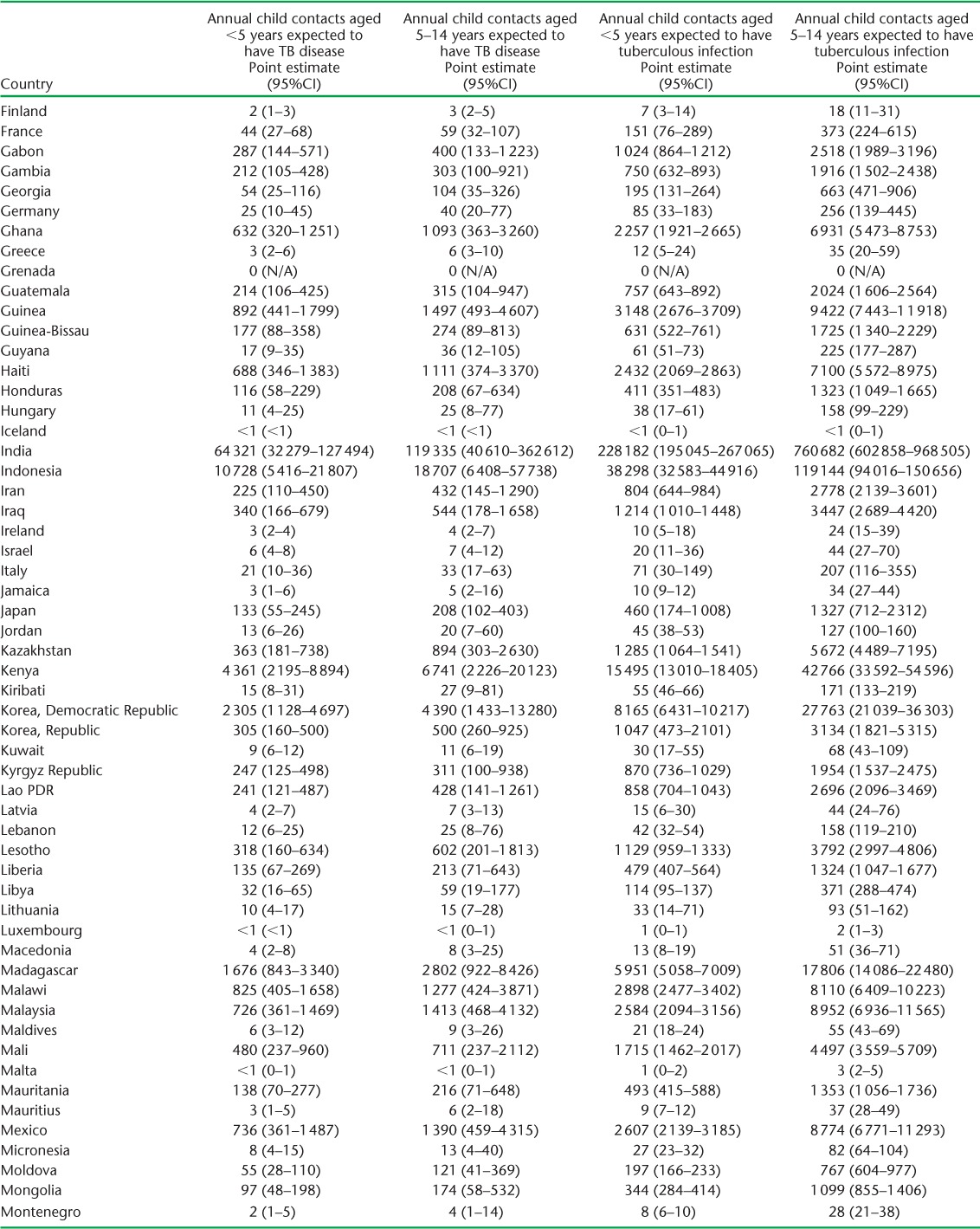
APPENDIX TABLE A.2.
(continued)
APPENDIX TABLE A.2.
(continued)
Footnotes
Conflicts of interest: none declared.
References
- 1.Jenkins H E, Tolman A W, Yuen C M et al. Incidence of multidrug-resistant tuberculosis disease in children: systematic review and global estimates. Lancet. 2014;383:1572–1579. doi: 10.1016/S0140-6736(14)60195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Global tuberculosis report, 2015. Geneva, Switzerland: WHO; 2015. WHO/HTM/TB/2015.22. [Google Scholar]
- 3.Fox G J, Barry S E, Britton W J, Marks G B. Contact investigation for tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2013;41:140–156. doi: 10.1183/09031936.00070812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marais B J, Gie R P, Schaaf H S et al. The clinical epidemiology of childhood pulmonary tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis. 2004;8:278–285. [PubMed] [Google Scholar]
- 5.Gomes V F, Andersen A, Wejse C et al. Impact of tuberculosis exposure at home on mortality in children under 5 years of age in Guinea-Bissau. Thorax. 2011;66:163–167. doi: 10.1136/thx.2010.141309. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Recommendations for investigating contacts of persons with infectious tuberculosis in low- and middle-income countries. Geneva, Switzerland: WHO; 2013. [PubMed] [Google Scholar]
- 7.Hill P C, Rutherford M E, Audas R, van Crevel R, Graham S M. Closing the policy-practice gap in the management of child contacts of tuberculosis cases in developing countries. PLOS Med. 2011;8:e1001105. doi: 10.1371/journal.pmed.1001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becerra M C, Swaminathan S. A targets framework: dismantling the invisibility trap for children with drug-resistant tuberculosis. J Public Health Policy. 2014;35:425–454. doi: 10.1057/jphp.2014.35. [DOI] [PubMed] [Google Scholar]
- 9.Blok L, Sahu S, Creswell J, Alba S, Stevens R, Bakker M I. Comparative meta-analysis of tuberculosis contact investigation interventions in eleven high burden countries. PLOS ONE. 2015;10:e0119822. doi: 10.1371/journal.pone.0119822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Case notifications. Geneva, Switzerland: WHO; 2016. http://www.who.int/tb/country/data/download/en Accessed May 2016. [Google Scholar]
- 11.Malawi National Statistical Office. 2008 Population and Housing Census Main Report. Zomba, Malawi: National Statistical Office; 2009. http://www.nsomalawi.mw/2008-population-and-housing-census/107-2008-population-and-housing-census-results.html Accessed May 2016. [Google Scholar]
- 12.Malawi National Statistical Office. 2008 Population and Housing Census Results Population Characteristics. Zomba, Malawi: National Statistical Office; 2009. http://www.nsomalawi.mw/2008-population-and-housing-census/107-2008-population-and-housing-census-results.html Accessed May 2016. [Google Scholar]
- 13.Malawi Ministry of Health. Malawi National Tuberculosis Control Programme Manual; 2012. Lilongwe, Malawi: Ministry of Health; 2012. [Google Scholar]
- 14.Malawi National Statistical Office (NSO), ICF Macro. Malawi Demographic and Health Survey 2010. Zomba, Malawi and Calverton, Maryland, USA: NSO and ICF Macro; 2011. [Google Scholar]
- 15.Theron G, Jenkins H E, Cobelens F et al. Data for action: collection and use of local data to end tuberculosis. Lancet. 2015;386:2324–2333. doi: 10.1016/S0140-6736(15)00321-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dodd P J, Gardiner E, Coghlan R, Seddon J A. Burden of childhood tuberculosis in 22 high-burden countries: a mathematical modelling study. Lancet Glob Health. 2014;2:e453–e459. doi: 10.1016/S2214-109X(14)70245-1. [DOI] [PubMed] [Google Scholar]
- 17.O'Grady J, Maeurer M, Atun R et al. Tuberculosis in prisons: anatomy of global neglect. Eur Respir J. 2011;38:752–754. doi: 10.1183/09031936.00041211. [DOI] [PubMed] [Google Scholar]
- 18.Falzon D, Ait-Belghiti F. What is tuberculosis surveillance in the European Union telling us? Clin Infect Dis. 2007;44:1261–1267. doi: 10.1086/514343. [DOI] [PubMed] [Google Scholar]
- 19.Denholm J T, McBryde E S. Can Australia eliminate TB? Modelling immigration strategies for reaching MDG targets in a low-transmission setting. Aust N Z J Public Health. 2014;38:78–82. doi: 10.1111/1753-6405.12161. [DOI] [PubMed] [Google Scholar]
- 20.Mor Z, Pinsker G, Cedar N, Lidji M, Grotto I. Adult tuberculosis in Israel and migration: trends and challenges between 1999 and 2010. Int J Tuberc Lung Dis. 2012;16:1613–1618. doi: 10.5588/ijtld.12.0296. [DOI] [PubMed] [Google Scholar]
- 21.Scott C, Kirking H L, Jeffries C, Price S F, Pratt R, Centers for Disease Control and Prevention Tuberculosis trends—United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:265–269. [PMC free article] [PubMed] [Google Scholar]
- 22.Marais B J, Gie R P, Schaaf H S et al. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis. 2004;8:392–402. [PubMed] [Google Scholar]


