Abstract
Setting: A non-governmental organisation-supported clinic offering health services including antiretroviral therapy (ART).
Objective: To compare ART retention between younger (age 10–14 years) vs. older (age 15–19 years) adolescents and younger (age 20–29 years) vs. older (age ⩾30 years) adults and determine adolescent- and adult-specific attrition-associated factors among those initiated on ART between 2010 and 2011.
Design: Retrospective cohort study.
Results: Of 110 (7%) adolescents and 1484 (93%) adults included in the study, no differences in retention were observed between younger vs. older adolescents at 6, 12 and 24 months. More younger adolescents were initiated with body mass index <16 kg/m2 compared with older adolescents (64% vs. 47%; P = 0.04). There were more females (74% vs. 52%, P < 0.001) and fewer patients initiating ART with CD4 count ⩽350 cells/mm3 (77% vs. 81%, P = 0.007) among younger vs. older adults. Younger adults demonstrated more attrition than older adults at all time-points. No attrition risk factors were observed among adolescents. Attrition-associated factors among adults included being younger, having a lower CD4 count and advanced human immunodeficiency virus disease at initiation, and initiation on a stavudine-based regimen.
Conclusion: Younger adults demonstrated greater attrition and may require more attention. We were unable to demonstrate differences in attrition among younger vs. older adolescents. Loss to follow-up was the main reason for attrition across all age groups. Overall, earlier presentation for ART care appears important for improved ART retention among adults.
Keywords: retention, attrition, SORT IT, operational research, HIV/AIDS
Abstract
Contexte : Un centre de santé soutenu par une organisation non gouvernementale offrant des services de santé, notamment les services de traitement antirétroviral (TAR).
Objectif : Comparer la rétention du TAR entre des adolescents plus jeunes (10–14 ans) et plus âgés (15–19 ans) et des adultes plus jeunes (20–29 ans) et plus âgés (⩾30 ans) et déterminer les facteurs associés à l'attrition et spécifiques des adolescents et des adultes parmi ceux qui ont mis en route du TAR en 2010–2011.
Schéma : Etude rétrospective de cohorte.
Résultats : L'étude a inclus 110 (7%) adolescents et 1484 (93%) adultes. Aucune différence en termes de rétention n'a été observée entre les adolescents plus jeunes et plus âgés à 6, 12 et 24 mois. Davantage des plus jeunes adolescents ont été initiés au traitement avec un index de masse corporelle <16 kg/m2 comparé aux adolescents plus âgés (64% contre 47% ; P = 0,04). Il y avait plus de femmes (74% contre 52% ; P < 0,001) et moins de patients démarrant le TAR avec un comptage de CD4 ⩽ 350 cellules/mm3 (77% contre 81% ; P = 0,007) parmi les adultes plus jeunes comparés aux plus âgés. Les adultes plus jeunes ont eu davantage d'attrition à tout moment que les plus âgés. Aucun facteur de risque d'attrition n'a été observé parmi les adolescents. Chez les adultes, les facteurs associés à l'attrition ont inclus l'âge plus jeune, un comptage de CD4 plus faible et une infection au virus de l'immunodéficience humaine plus avancée lors de la mise en route du traitement et son initiation dans le cadre d'un protocole basé sur la stavudine.
Conclusion : Les adultes plus jeunes ont eu davantage d'attrition et devraient susciter davantage d'attention. Nous n'avons pas pu démontrer de différences d'attrition entre les adolescents plus jeunes et plus âgés. La perte de vue a été la cause principale d'attrition dans tous les groupes d'âge. Dans l'ensemble, un démarrage plus précoce du TAR parait important pour améliorer la rétention du TAR chez les adultes.
Abstract
Marco de referencia: Un consultorio administrado por una organización no gubernamental que presta servicios de salud, como la administración del tratamiento antirretrovírico (TAR).
Objetivo: Comparar la fidelización al TAR entre los jóvenes (de 10 a 14 años) y los adolescentes mayores (de 15 a 19 años) y entre los adultos jóvenes (de 20 a 29 años) y los adultos de más edad (a partir de los 30 años) y determinar los factores específicos de los adolescentes y los adultos que se asocian con la tasa de abandono, en las personas que iniciaron el tratamiento del 2010 al 2011.
Método: Fue este un estudio de cohortes retrospectivo.
Resultados: Se incluyeron en el estudio 110 adolescentes (7%) y 1484 adultos (93%). No se observaron diferencias en la fidelización de los jóvenes y los adolescentes mayores a los 6, 12 y 24 meses. En el grupo de adolescentes jóvenes, recibió TAR una proporción mayor de los que presentaban un índice de masa corporal <16 kg/m2 que en el grupo de adolescentes mayores (64% contra 47%; P = 0,04). En comparación con los adultos de más edad, los adultos jóvenes que iniciaron el tratamiento fueron con mayor frecuencia de sexo femenino (74% contra 52%; P < 0,001), pero una proporción menor presentaba un recuento de linfocitos CD4 ⩽ 350 células/mm3 (77% contra 81%; P = 0,007). El abandono del tratamiento fue más frecuente en los adultos jóvenes que en los adultos mayores en todos los momentos examinados. No se observaron factores de riesgo de abandono en los adolescentes. En los adultos, los factores asociados con el abandono fueron una menor edad, el recuento más bajo de linfocitos CD4 y la enfermedad avanzada por el virus de la inmunodeficiencia humana en el momento de iniciar el tratamiento y el comienzo de un régimen basado en estavudina.
Conclusión: En los adultos más jóvenes la tasa de abandono del TAR fue más alta y precisan una mayor atención. No fue posible poner en evidencia diferencias en la tasa de abandono de los adolescentes más jóvenes y los adolescentes de mayor edad. La principal causa del abandono en todos los grupos de edad fue la pérdida durante el seguimiento. En general, la búsqueda temprana de TAR es un factor importante en la fidelización al tratamiento de los pacientes adultos.
Human immunodeficiency virus infection and the acquired immune-deficiency syndrome (HIV/AIDS) remain a significant public health burden in Africa, and Zimbabwe has not been spared. Zimbabwe is among 15 countries that account for nearly 75% of people living with HIV/AIDS globally; current HIV prevalence in Zimbabwe is 17% (2014 estimate).1,2 From the advent of antiretroviral therapy (ART), Zimbabwe scaled up treatment from 8000 patients in 2004 to 788 000 by 2014.2,3 As the cohort of ART patients has increased, retaining patients in care has become a challenge. Retention is vital for viral suppression, which leads to better survival and lower HIV transmission.4,5
Retention of people living with HIV (PLHIV) in treatment has been well studied, with a systematic review and meta-analysis of studies in sub-Saharan Africa showing a reduction in mean retention rates over time.6 Most studies in the meta-analysis concerned adult patients, although some included paediatric patients.
The World Health Organization's (WHO's) definition for adolescents is age 10–19 years.7 Most studies on ART retention have reported on adults, with adolescents included within the adult age group, making it difficult to extract information specific to adolescents.8–11
Emerging evidence suggests that adolescents are falling behind in HIV care compared to adults as a result of not receiving specialised attention and services.12,13 A study performed in seven African countries, excluding Zimbabwe, showed that adolescents and young adults combined (age 15–24 years) had higher rates of loss to follow-up (LTFU) and higher mortality compared with older adults (age ⩾50 years).14 What is unknown is whether there are differences in attrition rates between younger vs. older adolescents or between younger vs. older adults.
A systematic search for publications on ART outcome studies in PubMed, Cochrane Database, Google Scholar and ClinicalTrial.gov, comparing younger vs. older adolescents and younger vs. older adults, showed that with respect to ART retention, adolescents (age 10–19 years) were often misclassified as adults aged ⩾15 years,8,9 ⩾16 years11 or ⩾18 years.10 No studies reported attrition rates within the adolescent age group or between younger and older adults.
The Epworth Polyclinic in Epworth, Zimbabwe, has good records on ART care for all age groups; this presented an opportunity to compare the retention and attrition rates in younger and older adolescents as well as in younger and older adults during a time period when adolescent services were the same as for adults in Zimbabwe.
The aim of this study was to describe and compare demographic and clinical characteristics and attrition from ART care between HIV-infected younger (age 10–14 years) vs. older adolescents (age 15–19 years) and between younger (age 20–29 years) vs. older adults (age ⩾30 years) started on ART between January 2010 and December 2011 at Epworth Polyclinic in Zimbabwe.
METHODS
Design
This was a retrospective cohort study.
Setting
General setting
Zimbabwe, located in southern Africa, is a low-income country.15 The public health system is the largest provider of health care services, complemented by mission hospitals and non-governmental organisations (NGOs). Based on 2011 data, HIV/AIDS was among the top five causes of mortality for all age groups in Zimbabwe.16
Study site
Epworth is an informal high-density settlement located 15 km outside Harare. Over 70% of the population resides in plots that were not officially allocated.17 Based on the 2012 census, Epworth represented 8% of the population of Harare.18 Health services in Epworth are provided by two clinics, the Epworth Polyclinic and Epworth Mission clinic.
The Epworth Polyclinic offers general health services integrated with HIV and tuberculosis (TB) services. Since 2006, Médecins Sans Frontières-Holland (MSF-H) has supported the clinic by providing integrated basic health care services, including antenatal care (ANC), general primary health care, HIV and TB services and therapeutic feeding. The clinic was recently handed over to the Ministry of Health. As of December 2014, 9290 patients had been registered in the ART programme.
Management of HIV/AIDS in Zimbabwe
Medical criteria for initiating ART in adults and adolescents
Between 2010 and 2011, ART was provided according to the 2010 national ART guidelines,19 adapted from the 2010 WHO guidelines: treatment was the same for both adolescents (age 10–19 years) and adults (age ⩾20 years).20 ART was offered to all eligible patients with a confirmed HIV diagnosis and a CD4 count of ⩽350 cells/mm3, and those with WHO clinical Stage III and IV, irrespective of CD4 count. ART was initiated regardless of CD4 count among patients with active TB disease and with hepatitis B virus (HBV) co-infection with severe chronic liver disease.19
Recommended treatment regimens for adults and adolescents
In 2010–2011, the preferred first-line ART regimen included tenofovir (TFV), lamivudine (or emtricitabine) and nevirapine (NVP). The alternative regimen was zidovudine (ZDV), lamivudine and NVP. Stavudine (d4T) was being phased out, but was still available for those who were already using it. Single-drug substitutions within the same drug class were recommended for drug toxicities and if TB was being concurrently treated, e.g., efavirenz was substituted for NVP.
Patient monitoring and follow-up
Following ART initiation, patients were seen every 2 weeks for the first month, then monthly for the next 3 months. If they were stable after the initial 3 months, they were seen every 3 months thereafter. While the government provided antiretroviral medicines free of charge, patients were expected to pay a minimal consultation fee and laboratory-associated costs, such as CD4 tests, full blood count and radiography. Recommended clinical monitoring indices included patient weight, WHO clinical stage, development of opportunistic infections and CD4 count.19
Patient population
All HIV-infected adolescents (age 10–19 years) and adults (age ⩾20 years) registered and initiated in the ART register at the Epworth clinic from January 2010 to December 2011 were included in the study.
Data variables, data collection and sources of data
Variables collected included demographics, baseline clinical and immunological characteristics, date of last clinic attendance, date of last scheduled visit, initial and latest ART regimen and date of ART outcome. ART outcomes at date of last clinic attendance were defined as still in care, defaulted (absent from next scheduled clinic visit for ⩽90 days), LTFU (absent for >90 days after next scheduled clinic/pharmacy appointment), died, stopped ART or transferred out. Thinness in adolescents was graded as body mass index (BMI) <16 kg/m2 (Grade 3), 16–16.9 kg/m2 (Grade 2) and 17–18.4 kg/m2 (Grade 1).20
For this study, ‘retention’ referred to patients who were alive and receiving ART at Epworth Polyclinic, while ‘attrition’ referred to patients documented as having died, stopped ART, defaulted or LTFU. Transferred-out patients were excluded from calculations of attrition and retention, as we could not verify their status.
Sources of data were individual patient ART clinic booklets and registers from the Epworth Polyclinic. Immunological characteristics were based on laboratory test results. Baseline clinical characteristics and outcomes were validated using individual patient charts. A data abstraction sheet was used to collect data from medical records between September and October 2015. Outcomes were assessed in October 2015. Each patient was followed up for a maximum of 24 months.
Analysis
EpiData was used for data entry and analysis (version 3.1 for entry and version 2.2.2.182 for analysis, EpiData Association, Odense, Denmark) and Stata/IC 13.1 (StataCorp, College Station, TX, USA) was used for multivariate analysis. Differences in baseline clinical characteristics and outcomes were compared within the two adolescent and adult groups using proportions. Associations were calculated using the χ2 test for associations. Cox proportional hazards models were used to identify factors associated with attrition using univariate and multivariate-adjusted hazard ratios and their respective 95% confidence intervals (CI). Variables excluded in the multivariate Cox proportional hazards models had a P value >0.25 in the univariate analysis (i.e., switching to second-line ART treatment). ‘TB at ART initiation’ was excluded because of collinearity with ‘WHO clinical staging at ART initiation’. Proportional hazards assumptions were met by plotting similar shapes for graphs of the survival function vs. survival time. P values <0.05 were considered statistically significant.
Ethics
Permission to carry out the study was obtained from the Zimbabwe Ministry of Health and Child Care, Harare. Local ethics approval was obtained from the Zimbabwe Medical Research Council, Harare. The study met the MSF Ethics Review Board (Geneva, Switzerland) approved criteria for studies of routinely collected data, and was approved by the Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease, Paris, France. As this was a record review study with anonymised data, informed patient consent was not required.
RESULTS
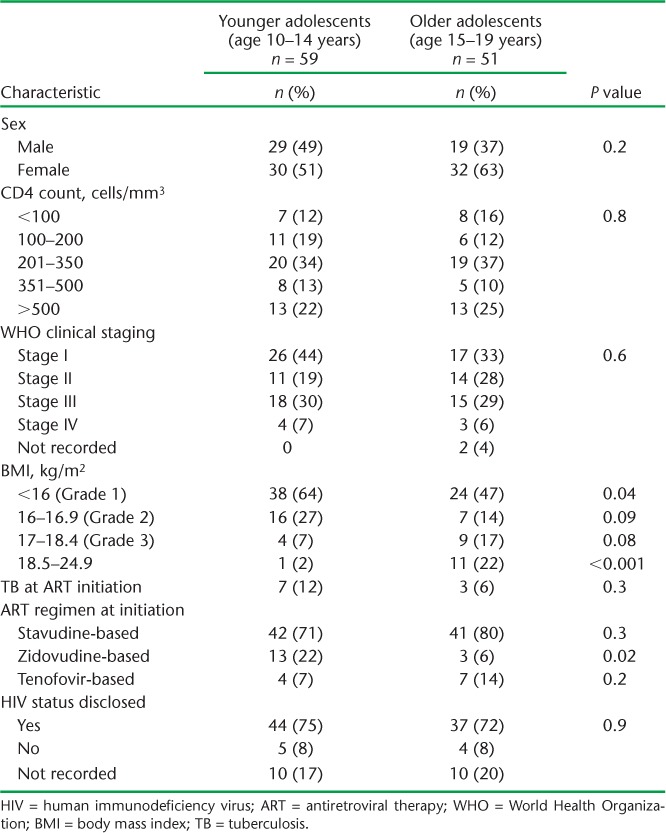
A total of 1594 HIV-infected patients were enrolled on ART at Epworth Polyclinic, of whom 110 (7%) were adolescents and 1484 (93%) were adults. A larger proportion of younger adolescents had Grade 3 BMI compared with older adolescents, while fewer had BMI of between 18.5 kg/m2 and 24.9 kg/m2. A higher proportion of younger adolescents started on ZDV-based ART (Table 1).
TABLE 1.
Comparison of demographic and baseline clinical characteristics between younger and older HIV-infected adolescents initiating ART between 2010 and 2011 at Epworth Polyclinic, Epworth, Zimbabwe
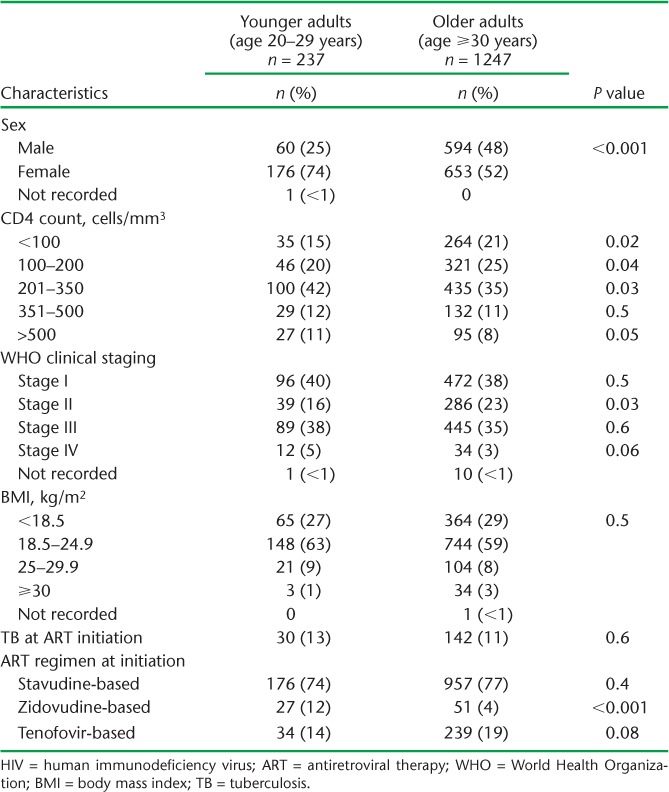
More younger adults than older adults were females and had started ART at WHO Stage IV, although fewer had a CD4 count ⩽350 cells/mm3 (Table 2). In a sub-analysis of baseline CD4 count by sex, a higher proportion of older adult males vs. older adult females were initiated on ART with CD4 counts <350 cells/mm3; no differences were noted among younger adults.
TABLE 2.
Comparison of demographic and baseline clinical characteristics between younger and older HIV-infected adults initiating ART between 2010 and 2011 at Epworth Polyclinic, Epworth, Zimbabwe
There was no difference in retention or attrition at 6, 12 and 24 months between younger and older adolescents, although overall retention declined over the three time periods in both groups (Table 3). Older adults tended to be in care longer, with greater retention (less attrition) at 6, 12 and 24 months compared to younger adults (Table 4).
TABLE 3.
ART outcomes at 6, 12 and 24 months among HIV-infected adolescents enrolled at Epworth Polyclinic, Epworth, Zimbabwe between 2010 and 2011
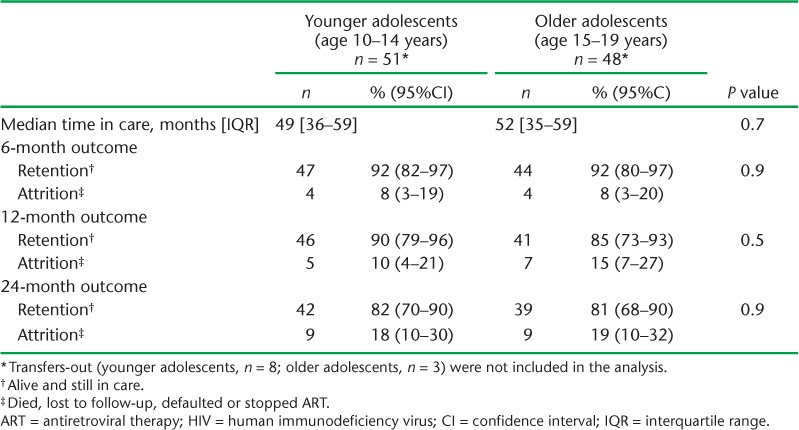
TABLE 4.
ART outcomes at 6, 12 and 24 months among HIV-infected adults enrolled at Epworth Polyclinic, Epworth, Zimbabwe between 2010 and 2011

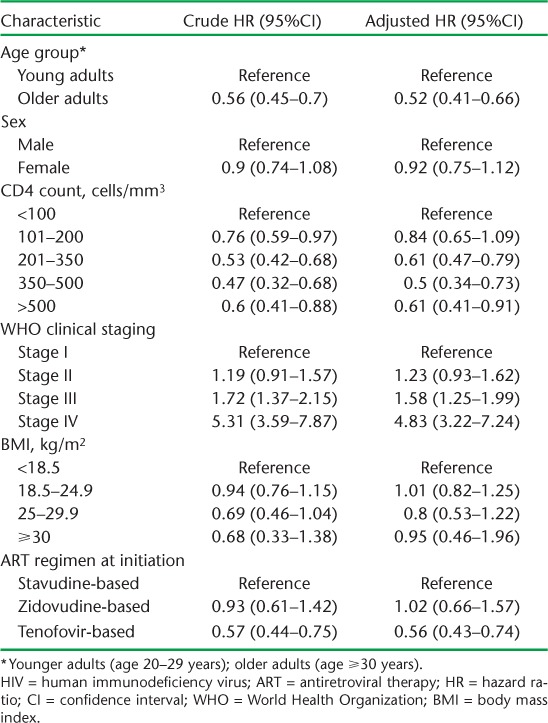
Among adolescents, initiation on ART with WHO Stage IV (compared to Stage I) was associated with more attrition (Table 5). Factors associated with lower attrition among adults included being older, having a higher CD4 count at initiation and initiation on a TFV-based regimen. Having a high WHO clinical stage at baseline was associated with higher risk of attrition (Table 6).
TABLE 5.
Factors associated with attrition among HIV-infected adolescents initiating ART between 2010 and 2011 at Epworth Polyclinic, Epworth, Zimbabwe
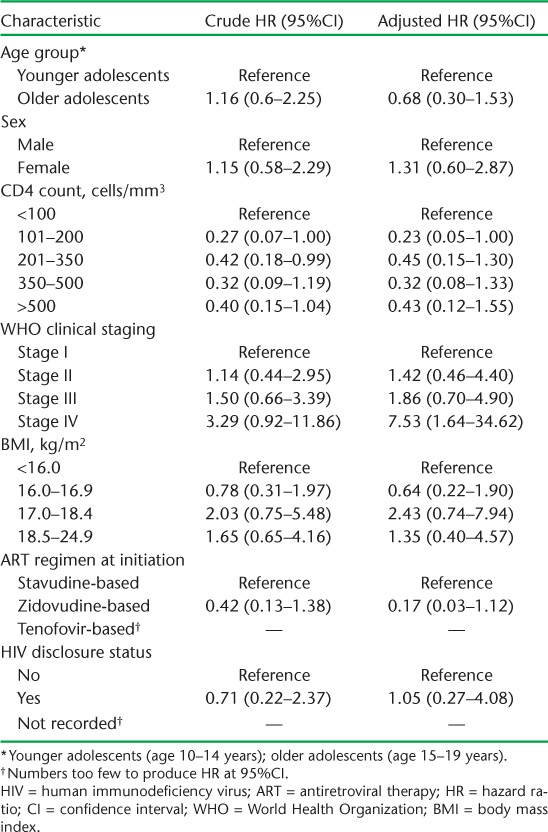
TABLE 6.
Factors associated with attrition among HIV-infected adults initiating ART between 2010 and 2011 at Epworth Polyclinic, Epworth, Zimbabwe
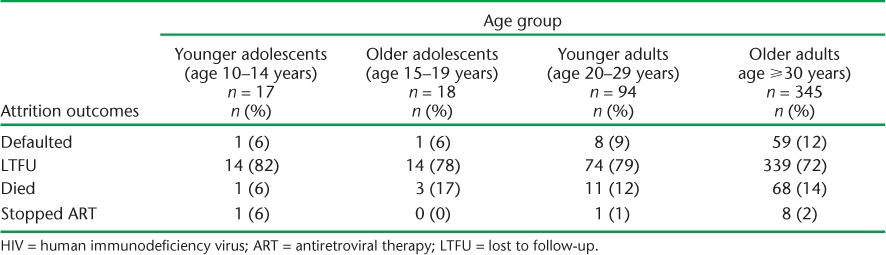
Most patients who were not retained in care across all age groups were recorded as LTFU (Table 7).
TABLE 7.
Attrition outcomes stratified by age group among HIV-infected patients enrolled on ART at Epworth Polyclinic, Epworth, Zimbabwe, 2010–2011
DISCUSSION
This is the first study to examine ART retention in care and attrition among younger vs. older adolescents and younger vs. older adults within the same cohort at a time when ART care was the same for both groups.19 Overall, there were no differences in ART retention within the adolescent group. Younger adults suffered more attrition from ART care compared to older adults; this was associated with late presentation for ART initiation, lower CD4 counts and presentation at WHO clinical Stages III or IV. Across all age groups, LTFU was the main reason for attrition. Other findings include higher proportions of young adolescents with Grade 3 thinness compared to older adolescents. Among adults, being male and presenting later for ART care were more common among older vs. younger adults.
The strengths of the study included the large cohort of adult patients and standardised ART care within a well-resourced, NGO-supported clinic. Study data management quality was good, with validation at data collection and double-entry into EpiData. The study also adhered to the Strengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines.22 Limitations included a small cohort of adolescent patients, implying that these data must be interpreted with caution, and the use of routine clinic data, which may contain some inaccuracies.
In our clinic, where services were similar for adults and adolescents, we did not demonstrate any differences in retention between younger vs. older adolescents up to 24 months of follow-up. This lack of difference may be attributed to better ART care in an NGO-supported clinic with more than average resources permitting efficient and timely management of all ART patients, whether adolescents or adults. Alternatively, the lack of difference could simply imply that adolescents within the 10–19 year age group do not require differentiated care.
Our documentation of Grade 3 thinness among more younger adolescents on ART initiation may reflect residual stunting/wasting associated with HIV infection in children.23 Infection with HIV may cause chronic diarrhoea in children, contributing to HIV-associated malnutrition. Other factors include late presentation for medical services and food insecurity in households with HIV-positive members, especially in limited-resource settings.24 Policy guidelines should provide adequate nutritional care and support for younger adolescents in HIV care. More older adolescents presented with normal BMI at initiation; this could be because, in this setting, older adolescents had their own income and were not relying on parental support.25
Among adults, there were more women in the younger age group. This can be attributed to fertility rates, which are highest in the 20–24 years age group,25 coupled with the well-established routine of HIV testing in ANC settings and subsequent ART initiation among those who are HIV-infected.19,25
Older adults presented later in their disease, as reflected by lower CD4 counts, and were mostly men. These findings are in line with previous studies from Zimbabwe and elsewhere, where men tend to present late for HIV care.26–28
Attrition from HIV care was associated with late presentation, advanced HIV disease and low CD4 counts, similar to previous studies.8 We also found greater attrition amongst younger adults. This may be because younger adults are mobile and often migrate for work,29 or, as most of the young adults were females identified in ANC, they may have been lost to care in the postpartum period, as shown in one South African study.30 Among adults, we found that initiation with d4T was associated with greater attrition in comparison to TFV, probably because TFV has been shown to be safer and more effective than both d4T21 and ZDV.31 Both d4T and ZDV have been associated with greater mortality compared to TFV.32 Zimbabwe has adapted recommendations to switch patients to a TFV-based regimen.19
After 24 months of follow-up, LTFU accounted for at least 70% of attrition across all age groups. The literature also shows that LTFU is the major cause of attrition in sub-Saharan Africa.6 There is a need to improve tracing of patients and document reasons why patients drop out of ART care.
This study has several implications. First, there is a need to focus more on younger adults to improve their retention in ART care. Our findings do not, however, support the need for differential care among adolescents, although further studies may be needed to validate this conclusion. Second, there is a clear need for innovative HIV-testing strategies that target men, especially younger men, for early identification and diagnosis and improved linkage to HIV treatment and care services. Third, there is a need to improve patient tracing procedures across all age groups to better retain patients in care. Fourth, the high proportion of malnourished younger adolescents highlights the importance of routinely assessing nutritional status, particularly in this age group, followed by provision of therapeutic feeding.
In conclusion, we found that younger adults demonstrated greater attrition from ART than older adults and may require more attention to improve retention. We were unable to find differences in attrition among younger vs. older adolescents. A more focused approach is needed to improve retention in ART care amongst young adults. We also found that loss to follow-up was the main reason for attrition across all age groups; tracing procedures should be improved in all patients.
Acknowledgments
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Programme for Research and Training in Tropical Diseases at the World Health Organization (WHO/TDR). The model is based on a course developed jointly by the International Union Against Tuberculosis and Lung Disease (The Union) and Medécins Sans Frontières (MSF). The specific SORT IT programme that resulted in this publication was jointly developed and implemented by the Operational Research Unit (LUXOR), MSF, Brussels Operational Centre, Luxembourg; the Centre for Operational Research, The Union, Paris, France; the Centre for International Health, University of Bergen, Bergen, Norway; the Institute of Tropical Medicine, Antwerp, Belgium; and Partners in Health, Boston, MA, USA.
The programme was funded by The Union, MSF, and the Department for International Development, London, UK. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. La Fondation Veuve Emile Metz-Tesch, Luxembourg, also supported the open access publication costs.
Footnotes
Conflicts of interest: none declared.
In accordance with the WHO's open-access publication policy for all work funded by the WHO or authored/co-authored by WHO staff members, the WHO retains the copyright of this publication through a Creative Commons Attribution IGO licence (http://creativecommons.org/licenses/by/3.0/igo/legalcode) which permits unrestricted use, distribution and reproduction in any medium, provided the original work is properly cited.
References
- 1.Zimbabwe Ministry of Health and Child Care. Zimbabwe national and sub-national HIV and AIDS estimates 2014. Harare, Zimbabwe: Ministry of Health and Child Care; 2014. [Google Scholar]
- 2.UNAIDS. Gap Report. Geneva, Switzerland: UNAIDS; 2014. http://www.unaids.org/sites/default/files/media_asset/UNAIDS_Gap_report_en.pdf Accessed March 2016. [Google Scholar]
- 3.Zimbabwe Ministry of Health and Child Care. 2014 AIDS and TB programme report. Harare, Zimbabwe: Ministry of Health and Child Care; 2014. [Google Scholar]
- 4.Hosseinipour M C, Kumarasamy N, Hakim J G et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Granich R M, Gilks C F, Dye C, De Cock K M, Williams B G. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 6.Rosen S, Fox M P, Gill C J. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLOS Med. 2007;4:e298. doi: 10.1371/journal.pmed.0040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Adolescent development. A critical transition. Geneva, Switzerland: WHO; 2016. http://www.who.int/maternal_child_adolescent/topics/adolescence/dev/en/. Accessed March 2016. [Google Scholar]
- 8.Mutasa-Apollo T, Shiraishi R W, Takarinda K C et al. Patient retention, clinical outcomes and attrition-associated factors of HIV-infected patients enrolled in Zimbabwe's National Antiretroviral Therapy Programme, 2007–2010. PLOS ONE. 2014;9:e86305. doi: 10.1371/journal.pone.0086305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eduardo E, Lamb M R, Kandula S et al. Characteristics and outcomes among older HIV-positive adults enrolled in HIV programs in four sub-Saharan African countries. PLOS ONE. 2014;9:e103864. doi: 10.1371/journal.pone.0103864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nuwagaba-Biribonwoha H, Jakubowski A, Mugisha V et al. Low risk of attrition among adults on antiretroviral therapy in the Rwandan national program: a retrospective cohort analysis of 6, 12, and 18 month outcomes. BMC Public Health. 2014;14:889. doi: 10.1186/1471-2458-14-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mutevedzi P C, Lessells R J, Rodger A J, Newell M L. Association of age with mortality and virological and immunological response to antiretroviral therapy in rural South African adults. PLOS ONE. 2011;6:e21795. doi: 10.1371/journal.pone.0021795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans D, Menezes C, Mahomed K et al. Treatment outcomes of HIV-infected adolescents attending public-sector HIV clinics across Gauteng and Mpumalanga, South Africa. AIDS Res Hum Retroviruses. 2013;29:892–900. doi: 10.1089/aid.2012.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nglazi M D, Kranzer K, Holele P et al. Treatment outcomes in HIV-infected adolescents attending a community-based antiretroviral therapy clinic in South Africa. BMC Infect Dis. 2012;12:21. doi: 10.1186/1471-2334-12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Auld A F, Agolory S G, Shiraishi RW et al. Antiretroviral therapy enrollment characteristics and outcomes among HIV-infected adolescents and young adults compared with older adults — seven African countries, 2004–2013. MMWR Morb Mortal Wkly Rep. 2014;63:1097–1103. [PMC free article] [PubMed] [Google Scholar]
- 15.World Bank. Zimbabwe data. Washington, DC, USA: World Bank; 2016. http://data.worldbank.org/country/zimbabwe. Accessed March 2016. [Google Scholar]
- 16.Zimbabwe Ministry of Health and Child Welfare. Department of Epidemiology and Disease Control. National Health Information and Surveillance Unit in conjunction with the Zimbabwe National Statistics Agency. Zimbabwe National Health Profile 2011. Harare, Zimbabwe: Ministry of Health and Child Welfare; 2011. http://www.zimstat.co.zw/sites/default/files/img/publications/Health/ZNHP_2011.pdf. Accessed May 2016. [Google Scholar]
- 17.Msindo P D, Gutsa I, Choguya N Z. Squatter settlements an urban menace in Zimbabwe? Examining factors behind the continued resurfacing of squatter settlements in Epworth suburb, Harare. JSSP. 2013;4:171–182. [Google Scholar]
- 18.Zimbabwe National Statistics Agency. Census 2012. Provincial report: Harare. Harare, Zimbabwe: National Statistics Agency; 2012. http://www.zimstat.co.zw/sites/default/files/img/publications/Census/CensusResults2012/Harare.pdf. Accessed May 2016. [Google Scholar]
- 19.Zimbabwe National Medicine and Therapeutics Policy Advisory Committee and AIDS and TB Directorate, Ministry of Health and Child Care. Guidelines for antiretroviral therapy for the prevention and treatment of HIV in Zimbabwe. Harare, Zimbabwe: Ministry of Health and Child Care; 2010. http://apps.who.int/medicinedocs/documents/s19254en/s19254en.pdf. Accessed March 2016. [Google Scholar]
- 20.Cole T J, Flegal K M, Nicholls D, Jackson A A. Body mass index cut offs to define thinness in children and adolescents: international survey. BMJ. 2007;335:194. doi: 10.1136/bmj.39238.399444.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents. Recommendations for a public health approach 2010 revision. Geneva, Switzerland: WHO; 2010. http://apps.who.int/iris/bitstream/10665/44379/1/9789241599764_eng.pdf. Accessed March 2016. [PubMed] [Google Scholar]
- 22.von Elm E, Altman D G, Egger M et al. The Strengthening the Reporting of OBservational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 23.McDonald C M, Kupka R, Manji K P et al. Predictors of stunting, wasting and underweight among Tanzanian children born to HIV-infected women. Eur J Clin Nutr. 2012;66:1265–1276. doi: 10.1038/ejcn.2012.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rose A M, Hall C S, Martinez-Alier N. Aetiology and management of malnutrition in HIV-positive children. Arch Dis Child. 2014;99:546–551. doi: 10.1136/archdischild-2012-303348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zimbabwe National Statistics Agency and ICF International. Zimbabwe Demographic and Health Survey 2010–11. Calverton, MD, USA: IMSTAT and ICF International Inc; 2012. http://dhsprogram.com/pubs/pdf/FR254/FR254.pdf. Accessed March 2016. [Google Scholar]
- 26.Takarinda K C, Harries A D, Shiraishi R W, Mutasa-Apollo T, Abdul-Quader A, Mugurungi O. Gender-related differences in outcomes and attrition on antiretroviral treatment among an HIV-infected patient cohort in Zimbabwe: 2007–2010. Int J Infect Dis. 2015;30:98–105. doi: 10.1016/j.ijid.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kipp W, Alibhai A, Saunders L D et al. Gender differences in antiretroviral treatment outcomes of HIV patients in rural Uganda. AIDS Care. 2010;22:271–278. doi: 10.1080/09540120903193625. [DOI] [PubMed] [Google Scholar]
- 28.Taylor-Smith K, Tweya H, Harries A D, Schoutene E, Jahn A. Gender differences in retention and survival on antiretroviral therapy of HIV-1 infected adults in Malawi. Malawi Med J. 2010;22:49–56. doi: 10.4314/mmj.v22i2.58794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fatti G, Mothibi E, Meintjes G, Grimwood A. Antiretroviral treatment outcomes amongst older adults in a large multicentre cohort in South Africa. PLOS ONE. 2014;9:e100273. doi: 10.1371/journal.pone.0100273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phillips T, Thebus E, Bekker L G et al. Disengagement of HIV-positive pregnant and postpartum women from antiretroviral therapy services: a cohort study. J Int AIDS Soc. 2014;17:19242. doi: 10.7448/IAS.17.1.19242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gallant J E, DeJesus E, Arribas J R et al. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med. 2006;354:251–260. doi: 10.1056/NEJMoa051871. [DOI] [PubMed] [Google Scholar]
- 32.Velen K, Lewis J J, Charalambous S, Grant A D, Churchyard G J, Hoffmann CJ. Comparison of tenofovir, zidovudine, or stavudine as part of first-line antiretroviral therapy in a resource-limited-setting: a cohort study. PLOS ONE. 2013;8:e64459. doi: 10.1371/journal.pone.0064459. [DOI] [PMC free article] [PubMed] [Google Scholar]


