Abstract
Background/Aims
Inflammation has been implicated in age-related macular degeneration (AMD). This study investigates the association of mast cells (MCs), a resident choroidal inflammatory cell, with pathological changes in AMD.
Methods
Human donor eyes included aged controls (n=10), clinically diagnosed with early AMD (n=8), geographic atrophy (GA, n=4), and exudative AMD (n=11). The choroids were excised and incubated alkaline phosphatase (APase; blood vessels) and nonspecific esterase activities (MCs). Degranulated (DG) and nondegranulated (NDG) MCs in four areas of posterior choroid (nasal, nonmacular, paramacular, and submacular) were counted in flat mounts (4∼6 fields/area). Choroids were subsequently embedded in JB-4 and sectioned for histological analyses.
Results
The number of MCs was significantly increased in all choroidal areas in early AMD (p=0.0006) and in paramacular area in exudative AMD (139.44±55.3 cells/mm2; p=0.0091) and GA (199.08±82.0 cells/mm2; p=0.0019) compared to the aged controls. DG MCs was also increased in paramacular (p=0.001) and submacula choroid (p=0.02) in all forms of AMD. Areas with the greatest numbers of DG MC had loss of choriocapillaris (CC). Sections revealed that the MCs were widely distributed in Sattler's and Haller's layer in the choroidal stroma in aged controls, whereas MCs were frequently found in close proximity to CC in GA and exudative AMD and in choroidal neovascularization (CNV).
Conclusion
Increased MC numbers and degranulation were observed in all AMD choroids. These results suggest that MC degranulation may contribute to the pathogenesis of AMD: death of CC and RPE and CNV formation. The proteolytic enzymes released from MC granules may result in thinning of AMD choroid.
Keywords: Age-related macular degeneration, choriocapillaris, choroid, degranulation, mast cells
Introduction
Age-related macular degeneration (AMD) is a complex, multifactorial disease characterized by progressive degeneration of the photoreceptor/retinal pigment epithelium/choriocapillaris complex primarily in the macular region of the eye, resulting in irreversible central vision loss among the elderly population.[1] Although the etiology for AMD remains unclear, accumulating evidence suggests that inflammation plays an important role in its pathogenesis. Histopathological studies of human choroids confirm the presence of inflammatory changes, but reveal little regarding the origins of the inflammation. Polymorphisms in complement factor H and other complement factors have been found to be a risk factor in AMD, adding to the evidence that inflammation is involved in AMD.[2]
Mast cells (MCs) are key effector cells of inflammation and play an important role in innate and acquired immunity, as well as autoimmunity.[3] These cells are derived from multilineage hematopoietic progenitors that migrate to vascularized tissues and organs, where they mature and ultimately reside.[4] Mature MCs have high-affinity IgE receptors (FcεRI) on their cell membrane surface, and the cross-linking of these receptors appears to act as a trigger for MC degranulation in IgE-mediated hypersensitivity reactions. Complement 3a and 5a have anaphylatoxin activity and contribute to degranulation of MCs.[5] Activation triggers degranulation of MCs, releasing histamine, cytokines, chemokines, and proteases into the milieu.[6 7] The proteases include chymase and tryptase that are responsible for the immediate hypersensitivity response to allergens and they activate matrix metalloproteinases, which can degrade stroma and basement membranes.
MCs are widely distributed in connective tissue and mucosa and are frequently found in close proximity to blood vessels. MCs are abundant in the human choroid where they are the connective tissue type MC.[8 9] Since MCs play a key role in inflammation in other tissues like skin and conjunctiva, MCs may play a role in choroidal inflammation as well.[10] MCs can interact with endothelial cells (ECs) and induce their proliferation through release of heparin, metalloproteinases, and VEGF.[11-13] MCs have the ability to induce angiogenesis and have been demonstrated to be present around Bruch's membrane during both the early and late stages of choroidal neovascularization (CNV) in AMD.[14] Interestingly, oral tranilast, an antiallergic drug that inhibits the release of chemical mediators from MCs has been shown to suppress laser-induced CNV in the rat.[15] In order to understand the role of MCs in AMD pathology, the total number of choroidal MCs [degranulated (DG) and nondegranulated (NDG)] was determined in aged control and AMD eyes. This study compared MC populations in submacular, paramacular, nonmacular, and nasal choroid and the relationship of MCs to the AMD-associated pathology.
Subjects and Methods
Donor eyes
All procedures in this study adhered to the tenets of the Declaration of Helsinki regarding research involving human tissue and were approved by the institutional review boards of the Johns Hopkins School of Medicine and Tufts Medical Center. Human donor eyes were obtained from the ongoing genetic and epidemiologic studies of Dr. Johanna Seddon (n=19 from New England Eye Center, Tufts Medical Center, Boston, MA, USA) and patient population of Dr. Janet Sunness (Greater Baltimore Medical Center, Baltimore, MD, USA) and from the National Disease Research Interchange (NDRI; Philadelphia, PA, USA) within 6–30 hours of death. Choroids from 23 subjects clinically diagnosed with AMD (age range 73–102 years; mean age 84.8±10.1 years) and from 10 aged control donors (age range 53–93 years; mean age 77.7±13.3 years) with no evidence of macular disease were studied. All donors were Caucasian. Table 1 summarizes the characteristics of each subject. The severity and phenotype of AMD was categorized according to our previously reported grading system,[16] using clinical records and ocular images, by reviewing ocular medical history on the eye bank transmittal sheets and by the post mortem gross examination of posterior eyecup, using transmitted and reflected illumination with a dissecting microscope (Stemi 2000; Carl Zeiss Meditec, Inc., Thornwood, New York, USA). During gross examination, AMD eyes were classified as follows: no AMD (Grade 1, without drusen or with fewer than 10 small drusen and without pigment abnormalities)(n=10); early AMD (Grades 2 and 3 combined for the purpose of these analyses, grade 2 had several small drusen with or without pigmentary change and grade 3 had large drusen)(n=8); dry or non-exudative AMD with geographical atrophy (GA, Grade 4, atrophy ≥350 μm characterized by distinct areas of retinal pigment epithelial (RPE) loss (n=4); or wet (exudative) AMD (Grade 5, with evidence of RPE detachment and/or CNV)(n=11).[16]
Table 1. Characteristics of human subjects.
| Subjects | Time (hours) | Age/race/sex | Primary cause of death | Medical history | |
|---|---|---|---|---|---|
|
| |||||
| DET | PMT | ||||
| Aged Control | |||||
|
| |||||
| 1 | 3.5 | 25 | 53/C/F | Breast cancer | Mets to brain & liver, pleural effusion, Crohn's disease |
| 2 | 4.2 | 31 | 78/C/M | Prostate cancer | COPD, HTN, CAD, MI, emphysema |
| 3 | 3 | 29 | 93/C/F | COPD | CAD, CHF, COPD, HTN |
| 4 | 6.5 | 28 | 77/C/M | Respiratory failure | Alzheimer's disease, dementia, CAD, DM, HTN |
| 5 | 3.8 | 26 | 59/C/M | Respiratory failure | Pneumonia, MI, schizophrenia, anemia |
| 6 | 5 | 29 | 88/C/F | CHF | CVA, CHF, MI, renal insufficiency |
| 7 | 4 | 24 | 84/C/M | Pancreatic cancer | AFIB, CVA, CHF, CAD, PVD |
| 8 | 4.5 | 32 | 80/C/F | Coronary artery disease | Dementia, CVD, gastroesophageal reflux |
| 9 | 3.3 | 23 | 86/C/M | MI | Osteoarthritis, CVA, arthritis |
| 10 | 4 | 24 | 79/C/F | Unknown | COPD, dementia |
| Early AMD | |||||
|
| |||||
| 11 | 6.5 | 25 | 79/C/M | COPD | Mild NIDDM, CHF, CVA, CAD, COPD, arthritis |
| 12 | 5 | 31 | 73/C/F | Respiratory failure | Anemia, arthritis, HTN, gastroesophageal reflux |
| 13 | 4 | 36 | 81/C/M | CAD | AFIB, CAD, peptic ulcer |
| 14 | 16 | 35 | 86/C/M | COPD & enlarged heart | COPD, smoker |
| 15 | 8 | 31 | 92/C/M | CHF | CHF, PVD, arthritis, eczema, renal disease, skin cancer |
| 16 | 18 | 37 | 91/C/M | Heart failure | PVD, arthritis |
| 17 | 10 | 48 | 86/C/F | Dementia/Alzheimer's | Arthritis, colitis |
| 18 | 10 | 50 | 94/C/M | Heart attack | HTN, DM, arthritis, prostate cancer |
| Late AMD (Wet) | |||||
|
| |||||
| 19 | 6 | 26 | 91/C/F | Heart failure | HTN |
| 20 | 8 | 24 | 91/C/M | Dementia, heart failure | Heart attack, colitis |
| 21 | 8 | 28 | 90/C/F | CHF, kidney transplant complications | DM, HTN, CAD, PVD |
| 22 | 13 | 35 | 100/C/F | Heart failure | HTN, stroke, arthritis |
| 23 | 6 | 23 | 97/C/F | Alzheimer's disease | DM, HTN, stroke, arthritis, |
| 24 | 4.5 | 24 | 97/C/F | Lung cancer | osteoporosis, eczema |
| 25 | 5.5 | 24 | 102/C/F | CHF | HTN, CHF, reflux |
| 26 | 2 | 23 | 86/C/M | Acute respiratory failure | HTN, skin cancer, angina |
| 27 | 28 | 48 | 96/C/F | Respiratory | AFIB, DM, Parkinson's, |
| 28 | 14 | 35 | 96/C/F | failure/pneumonia | esophageal reflux |
| 29 | 5.3 | 17 | 85/C/M | Unknown Esophageal cancer | Breast cancer DM, HTN, arthritis, heart attack, osteoporosis HTN, stroke, shingles |
|
| |||||
| Late AMD (GA) | |||||
| 30 | 6 | 9 | 88/C/M | CHF | CAD, HTN, MI, GI bleed |
| 31 | 3 | 24 | 81/C/F | Liver disease | Asthma, DM, HTN, arthritis, COPD, osteoporosis, liver cancer |
| 32 | 17 | 40 | 87/C/M | CHF | HTN, DM, myocardial infarction, |
| 33 | 12 | 38 | 97/C/F | Spinal stenosis & CHF | arthritis, prostate cancer CHF, PVD, arthritis, HTN, stroke, osteoporosis, angina |
DET, death to enucleation time; PMT, postmortem time (death to fixation); C, Caucasian; M, male; F, female; AMD, age-related macular degeneration; CVA, cerebrovascular accident; DM, diabetes mellitus; NIDDM, non-insulin dependent diabetes mellitus; HTN, hypertension; COPD, chronic obstructive pulmonary disorder; GA, geographic atrophy; MI, myocardial infarction; CAD, coronary artery disease; CHF, congestive heart failure; AFIB, arterial fibrillation; PVD, Pulmonary vascular disease; GI, gastro-intestinal.
Tissue preparation
The globes were opened at the limbus and then the anterior segment of the eye was removed. The posterior eyecup was grossly examined and digital images were captured as previously published.[17] After removal of vitreous, the choroid with sclera was placed in 1% ethylenediaminetetraacetic acid (EDTA; J. T. Baker Chemical Co., Center Valley, PA, USA) in Ca2++/Mg2++ free phosphate-buffered saline, pH 7.4, at room temperature for 2 h to remove RPE. Gross digital images of choroid were again captured with and then without RPE. The choroid was then dissected from the sclera at the lamina fusca and fixed in 2% paraformaldehyde in 0.1 M cacodylate at 4°C for 1 h, washed in several changes of 0.1 M cacodylate buffer at 4°C, and then incubated for the alkaline phosphatase (APase) activity as previously described.[18] After APase staining, nonspecific esterase enzyme (NSE) staining was performed using a naphthol AS-D chloroacetate kit (Sigma Diagnostics, St. Louis, MO, USA).[17] This method preferentially stains granulocytes and MCs bright red. The two cell types can be easily distinguished since MCs are 4 times larger than granulocytes. The APase and NSE stained choroid was post-fixed in 2% paraformaldehyde for 24 h, then washed and melanin was bleached with 30% hydrogen peroxide (Sigma-Aldrich Co.) at 4°C as previously reported.[18]
Mast Cell Counts
A total of 23 AMD and 10 aged control choroids were counted for the number of MCs. After flat-mounting the wet choroid, the posterior pole choroid (7mm × 5mm) was excised and analyzed for MC numbers. The tissue included was from 1 disc diameter (DD) nasal to 6 DD temporal to the disc and 2.5 DD inferior and superior to the disc (supplemental figure 1). Within this piece of choroid, four areas were evaluated (nasal, nonmacular, paramacular, and submacular). Using a Zeiss Photomic 2 microscope at 16× magnification (field area = 1 mm2), total number of MCs (DG and NDG) in each choroid was counted manually in four to six random microscopic fields and then expressed as number/mm2 in each area. More than 20 fields were counted and documented per choroid. Areas of viable capillaries were determined by the presence of APase+ microvasculature. MCs were stained intensely red after NSE incubation and they appeared rounded in choroid stroma under brightfield illumination. MC numbers were compared in pathological and non-pathological areas of choriocapillaris (CC). Pathological areas were defined as areas that had CC dropout (loss in APase activity) and/or apparent CNV (irregular vascular patterns with abnormally high APase activity). The density of the choroidal vasculature was determined by image analysis of images from the APase/NSE flat mounts as previously published.[19]
Figure 1.
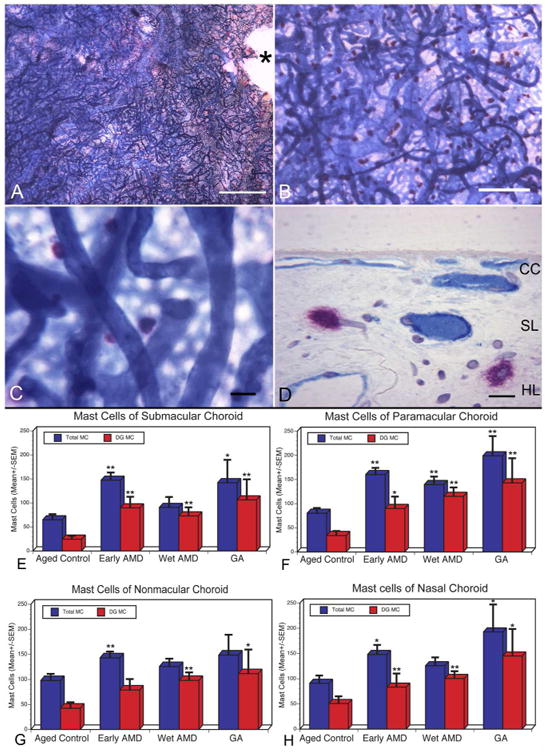
Flat mount of submacular choroid from an aged control subject stained with alkaline phosphatase and nonspecific esterase (APase/NSE) (Subject#4). (A and B) APase+ choroidal vessels are stained blue and NSE-positive MCs are stained red with this preparation. (C) MCs are largely distributed in the intermediate and deep choroid so the choriocapillaris (CC) is out of focus. (D) Histological section of the choroid shown in (A) demonstrates red MCs are associated with blood vessels in Sattler's layer (SL) and Haller's layer (HL), which are APase+ and therefore have viable endothelial cells.(*, optic nerve; Bar=1mm in A, 200μm in B, 50μm in C, and 20mm in D). (E-H) Comparison of the number of MCs (total MCs, blue bar; degranulated or DG MCs, red bar) present per mm2 of choroid counted in the flat perspective in aged control and AMD subjects. MC numbers/mm2 are presented from four areas of posterior choroid [submacular (E), paramacular (F), nonmacular (G), and nasal (H)] and compared to the aged control in those regions. Total numbers of MCs as well as degranulated MCs were statistically significantly increased in almost all areas examined in AMD choroids compared to the aged control. The significance of the difference between aged control and AMD (p< 0.05) is indicated using the Students t-test (*) and Wilcoxon rank-sum test (**).
Flat-embedding of Choroid
After flat perspective analysis, two choroids from each group were post-fixed flat in 25% Karnovsky's glutaraldehyde/paraformaldehyde, washed, dehydrated as described previously,[18] and infiltrated and embedded in freshly catalyzed glycol methacrylate (JB-4; Polysciences, Warrington, PA, USA), as recommended by the manufacturer. Sections (2.5 μm) were cut with a dry glass knife on a Sorvall NTB2 microtome. Morphometric analysis was done with a Zeiss Photomic II microscope. Choroidal thickness (Bruchs membrane to lamina fusca) was measured in 5 fields of each image and 5 measurements/field in submacular choroid in sections.
Statistical analysis
The number of MCs/mm2 for all fields in each area of each group of subjects was averaged and expressed as the means ± SEM/mm2 of choroid. The p values were determined by comparing mean numbers from the aged control choroids with numbers from choroids with either early AMD, CNV or GA using the Welch's t-test, which assumes unequal variance and the Wilcoxon rank sum (WRS) test. A p value <0.05 was considered statistically significant.
Results
Double labeling with APase and NSE resulted in choroids with blue-stained blood vessels and bright red-stained MCs (figure 1B-C). Neutrophils were also stained red but were 3-4 times smaller than mast cells and mostly confined to blood vessel lumens. NDG MCs were large round or oval, bright red cells. We have previously determined that loss in APase activity represents loss of viable endothelial cells and, therefore, CC dropout.[18] No or very small areas of CC dropout were observed in choroids from aged control subjects (figure 1A). MCs were not present at the level of CC but were rather obvious around blood vessels in Haller's and Sattler's layer deeper in the flat choroid (figure 1B-D). In aged control choroids, the total number of MCs (DG and NDG; mean±SEM) in nasal, nonmacular, paramacular, and submacular areas was 90.97±48.8 cells/mm2, 97.69±42.3 cells/mm2, 80.08±34.8 cells/mm2 and 64.87±37.04 cells/mm2 respectively (figure 1E-H). Most MCs were not degranulated in aged control choroids. In aged control choroids, histologic sections confirmed that the MCs were widely distributed in Sattler's and Haller's layers in the choroidal stroma but rarely at the level of CC (figure 1D).
In choroids from early AMD, some CC channels were not viable (APase-) in the submacular area. In Subject #15 who was graded 2C, having drusen and pigmentary changes (figure 2A), the area of CC loss was predominantly nasal to the optic nerve and submacula. In areas of capillary loss in early AMD, we have often observed apparent buds of intrachoroidal neovascularization (figure 2B). Many MCs in early AMD were degranulated having a larger irregular shape and free NSE+ granules surrounding the cell soma (figure 2C). The total number of MCs was significantly increased in all areas in early AMD compared to controls (p values from p=0.032 to p=0.0006) and DG MCs were also increased significantly except in nonmacular areas (figure 1E-H). Degranulation was also apparent in the sections from early AMD choroids and MCs appeared to be located more anteriorly, near CC or in open spaces created after CC degeneration (figure 2D-F).
Figure 2.
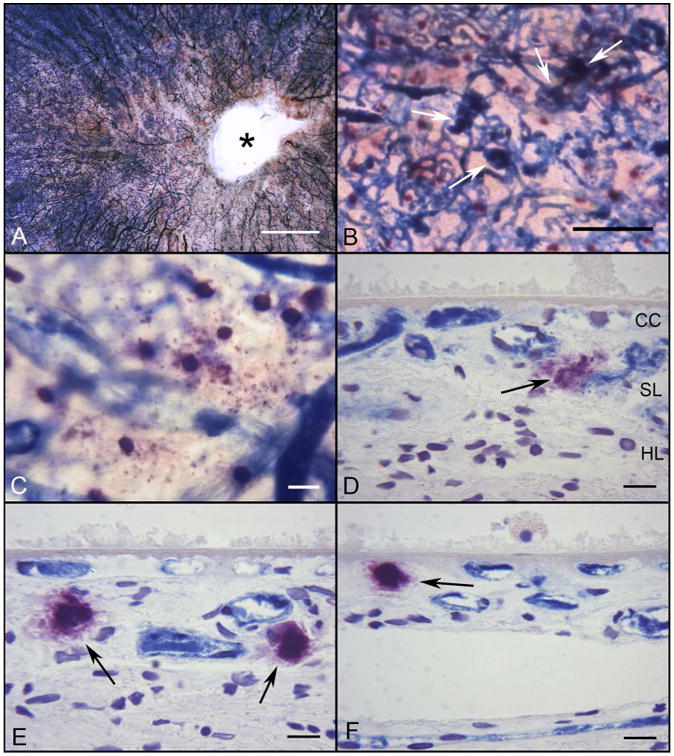
Flat mount of APase/NSE stained choroid from a subject with early AMD (Subject#15). A succinct area of CC degeneration (lacking APase staining) is evident in the submacular region and around the optic disc(*)(A). Several neovascular buds have dark blue APase activity (B, white arrows). (C) At higher magnification, some degranulated MCs are apparent at the level of CC, which has reduced APase activity in this area. (D, E, F) Histological sections from same choroid demonstrate the DG MCs (black arrows) present near degenerative CC channels, which are APase− and around the dark blue APase+ neovascular buds. A thin basal laminar deposit is present internal to Bruchs membrane.(Bar=1mm in A, 200μm in B, 50μm in C, and 20μm in D-F)
High levels of APase blue reaction product were observed in the endothelial cells of viable CNV (figure 3A) and CC loss was observed at the borders of CNV in exudative AMD (figure 3A-B). Large numbers of MCs were degranulated in the exudative AMD choroids (115.1±61.3; p=0.0032 [WSR]) and the numbers of DG MCs were significantly increased in all areas compared to control subjects (p values from p=0.0032 to 0.024)(figure 1E-H). In sections, MCs were observed adjacent to CNV feeder vessels where they traversed breaks in Bruchs membrane (figure 3D-E) and in the basal laminar deposit overlying CNV or even within the CNV itself (figure 3F-G).
Figure 3.
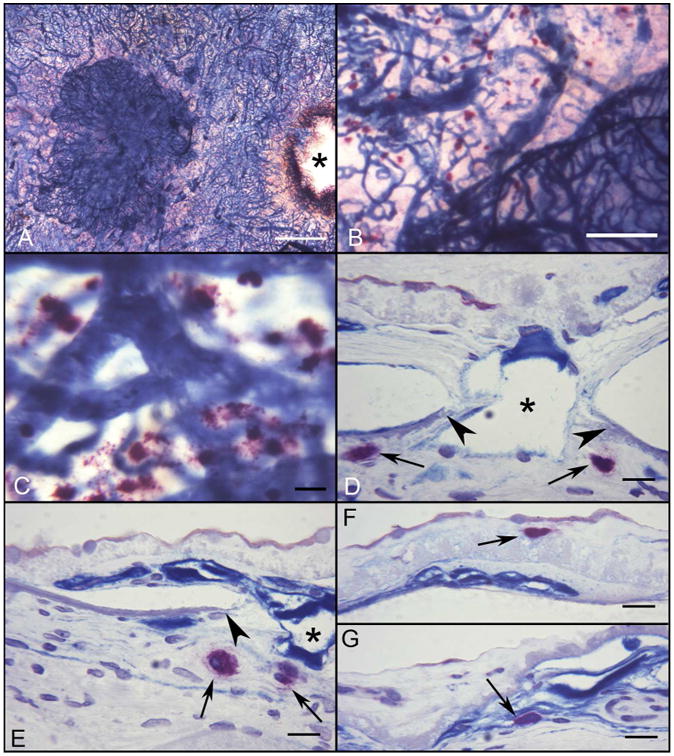
Flat mount of APase/NSE choroid from a subject with wet AMD (Subject#20). A large APase stained choroidal neovascular formation (CNV) is present in submacular region of the choroid (A)(*, optic nerve). (B) Numerous degranulated MCs are adjacent to the edge of CNV, where the CC is greatly attenuated. (C) At higher magnification, degranulated MCs are present at the edge of CNV. (D-G) Histological sections from same choroid demonstrate the degranulated MCs (black arrows) adjacent to the CNV feeder vessel. An MC is present in a basal laminar deposit (F) and in the CNV itself (G). Breaks in Bruch's membrane are indicated by arrowheads. (*, CNV breaching Bruchs membrane; Bar=1mm in A, 200μm in B, 50μm in C, and 20μm in D-G)
The most severe areas of CC dropout were noted in GA subjects in regions of RPE atrophy (figure 4A-B). The largest total numbers of MCs overall in the study were counted in GA paramacular regions (199.08±82.0 cells/mm2; p=0.0019) and nasal (192.84+108.6 cells/mm2; p=0.028) areas (figures 1E-H and 4). In addition all areas of GA choroids had significantly elevated numbers of DG MCs compared to controls (p values from p=0.0073-0.012). In border regions and areas lacking RPE atrophy, MCs were at the level of Sattler's layer (figure 4F) but also at the level of CC in regions with complete RPE atrophy (figure 4D-E).
Figure 4.
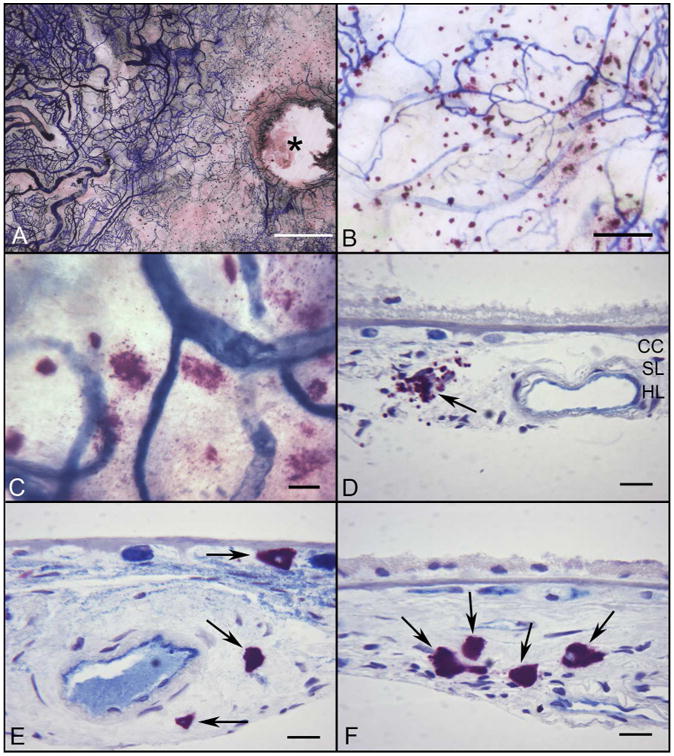
Flat mount preparation of APase/NSE incubated choroid from a subject with GA (Subject#30) (A) There is a large area of CC loss (APase−) around the optic disc (*) and in the submacular region of the choroid. (B) Many degranulated MCs are associated with areas of RPE atrophy and CC degeneration. (C) At higher magnification, most MCs are degranulated. (D-F) Histological sections from same choroid demonstrate the degranulated MCs (black arrows) aggregating in choroidal stroma in a region of RPE atrophy with a thin basal laminar deposit (D) and in the wall of an artery with atherosclerotic changes (E). MCs are increased in number but in their normal position in Sattler's and Haller's layers in a region without RPE atrophy (F). (Bar=1mm in A, 200μm in B, 50μm in C, and 20μm in D-F)
In summary, degranulation of MCs was increased in submacular (WRS test p=0.02) and in paramacular choroid (WSR test, p=0.001) and in all forms of AMD (figure 1E-H). Areas with the greatest numbers of DG MC had a significant reduction in vascular area, i.e. had loss of CC (data not shown). Also, in AMD choroids, DG MCs were frequently found in close proximity to CC and adjacent to degenerated CC (lacked APase activity)(figures 2D-F and 3D-E) and were present in abundance in exudative AMD choroid (figure 4).
Discussion
A central role for inflammation in AMD has been proposed. This is based on the findings that there are many genes in the alternative complement pathway that are risk factors for AMD.[20] The resident inflammatory cells of choroid are tissue macrophages and mast cells.[9] This is the first report that mast cell numbers and activation are increased in AMD. This increase occurred in all forms of AMD, including early AMD. Furthermore, the numbers presented are probably underestimations of the increase because the numbers were counted in flat mounts by area of choroid (mm2) but all forms of AMD have thinning of choroid [21]. Therefore, counting MCs per volume of choroid, i.e. mm3, would likely result in more significant increases in numbers in AMD.
MC activation results in degranulation, the release of a myriad of factors and enzymes into the milieu. MCs are chemotactic to and activated by C3a and C5a,[5] by- products of complement activation, which are present in drusen in AMD.[22] MCs can be also be activated by a wide range of stimuli, including immunoglobulins via Fc receptors (FcεRI, FcγR), microbial pattern-recognition receptors (PRRs, such as TLRs), neuropeptides, cytokines, growth factors, toxins, venoms or venom components and physical stimuli.[23] These stimuli trigger MCs to release a diverse array of biologically active products via granular release and simple secretion, many of which can potentially mediate pro-inflammatory, anti-inflammatory and/or immunosuppressive functions.[24] This would contribute to the already proinflammatory choroidal environment in aging and AMD, downregulation of CFH and upregulation of CRP,[25] which can also activate MCs.[26] MCs can participate in multiple cycles of activation for mediator release and can be differentially activated to release distinct patterns of mediators or cytokines, depending on the type and strength of the activating stimuli.[24]
The consequences of activation and degranulation are myriad. Smith, as early as 1966, found that inducing choroidal MC degranulation resulted in increased blood flow probably as a result of histamine release.[27] More recently, Bousquet and associates observed ocular inflammation and increased vascular dilation and permeability in choroidal vessels after inducing degranulation.[10] This is accomplished by the release of chemokines and cytokines that attract and activate macrophages and T-cells [23], which is well documented in cardiovascular disease, atherosclerosis, and aortic aneurysms.[5]
MCs populate the connective tissue near blood vessels in many tissues, positioned as key elements in processes like wound healing, tissue regeneration, and remodeling after injury, fibrosis, and angiogenesis. These observations suggest an interaction between MCs and ECs; indeed, many MC-derived mediators are angiogenic. MCs in the human intima and adventitia express basic fibroblast growth factor and produce angiogenic histamine, leptin, VEGF, and angiopoietin-1.[23] MC tryptase stimulates microvessel tube formation and enhances the growth of microvessel EC, whereas chymase promotes angiogenesis through its effects of angiopoietin-II.[28 29] In this study, MCs appeared within and adjacent to CNV in exudative AMD choroids (figure 4). Tryptase and chymase activate matrix metalloproteinases (MMPs), which degrade stroma and basement membranes,[30] and in doing so may be responsible for the death of smooth muscle and endothelial cells and RPE, cells dependent on an intact basement membrane.[5] Sohn and associates have observed disruption of the stromal ultrastructure in thin choroids of AMD subjects, suggesting degradation of choroidal stroma in AMD.[31] Therefore, MMP activation and increased proteolysis after degranulation may cause choroidal thinning and loss of CC and RPE, all of which occur in GA. Godfrey hypothesized that choroidal mast cells may also be involved in fibrosis and scarring, as they are in skin, and as occurs in end stage exudative AMD.[32]
In conclusion, MCs contain an abundance of vasoactive, proinflammatory, anticoagulant, and proteolytic mediators that are released upon activation and degranulation, which was increased in all AMD groups.[23] The proteolytic enzymes released by MCs may lead to thinning of the choroid in AMD as well as degradation of vascular basement membranes and Bruch's membrane, which in turn could result in RPE death and choriocapillaris degeneration that we have documented in GA and exudative AMD.[1 19] In addition, MC degranulation releases angiogenic factors that may contribute to formation of CNV. Therefore, the increase in MCs and MC degranulation observed and the release of various soluble and granular factors may contribute to the pathogenesis of AMD, suggesting that MC stabilization may be a therapeutic goal in controlling progression of AMD.
Acknowledgments
The authors are grateful to the eye donors and their relatives for their generosity, Mercedes B. Villalonga, B.A., and Rachel E. Silver, M.P.H., from the Seddon Lab, Tufts University, and Carol Applegate from the Sunness Lab, Greater Baltimore Medical Center, for assistance in acquiring tissue. This work was supported by NIH grants EY-01765 (Wilmer), R01-EY016151 (GAL), and RO1 EY08552 (JSS), unrestricted funds from Research to Prevent Blindness (Wilmer), the Arnold and Mabel Beckman Foundation (GAL and JMS), the Foundation Fighting Blindness (GAL and JMS), Bright Focus Foundation (IAB), Age-Related Macular Degeneration Research Fund Tufts Medical Center, Boston, MA (JMS), and the Altsheler Durell Foundation. GAL received an RPB Senior Scientific Investigator Award.
Footnotes
Competing interests: None.
Ethics approval: This study was conducted with the approval for the use of post mortem tissue from the Johns Hopkins JCCI and Tufts Medical Center. The protocol of the study adhered to the tenets of the Declaration of Helsinki regarding research involving human tissue.
Contributors: IAB and DSM carried out experiments. IAB, TJ and GAL analyzed data. DSM captured the images and prepared the graphs. IAB, DSM, and GAL conceived experiments, JMS and JSS provided eyes and clinical histories from subjects, and all authors were involved in writing the paper and had final approval of the submitted and published versions.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Bhutto I, Lutty G. Understanding age-related macular degeneration (AMD): relationships between the photoreceptor/retinal pigment epithelium/Bruch's membrane/choriocapillaris complex. Molecular aspects of medicine. 2012;33(4):295–317. doi: 10.1016/j.mam.2012.04.005. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schramm EC, Clark SJ, Triebwasser MP, Raychaudhuri S, Seddon JM, Atkinson JP. Genetic variants in the complement system predisposing to age-related macular degeneration: a review. Molecular immunology. 2014;61(2):118–25. doi: 10.1016/j.molimm.2014.06.032. Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costa JJ, Weller PF, Galli SJ. The cells of the allergic response: mast cells, basophils, and eosinophils. JAMA. 1997;278(22):1815–22. [PubMed] [Google Scholar]
- 4.Galli SJ. Mast cells and basophils. Current opinion in hematology. 2000;7(1):32–9. doi: 10.1097/00062752-200001000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Xu JM, Shi GP. Emerging role of mast cells and macrophages in cardiovascular and metabolic diseases. Endocr Rev. 2012;33(1):71–108. doi: 10.1210/er.2011-0013. doi:er.2011-0013[pii]10.1210/er.2011-0013. Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dvorak AM. New aspects of mast cell biology. International archives of allergy and immunology. 1997;114(1):1–9. doi: 10.1159/000237635. [DOI] [PubMed] [Google Scholar]
- 7.Galli SJ. New concepts about the mast cell. N Engl J Med. 1993;328(4):257–65. doi: 10.1056/NEJM199301283280408. Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 8.May CA. Mast cell heterogeneity in the human uvea. Histochemistry and cell biology. 1999;112(5):381–6. doi: 10.1007/s004180050420. [DOI] [PubMed] [Google Scholar]
- 9.McMenamin PG. The distribution of immune cells in the uveal tract of the normal eye. Eye (Lond) 1997;11(Pt 2):183–93. doi: 10.1038/eye.1997.49. Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 10.Bousquet E, Zhao M, Thillaye-Goldenberg B, et al. Choroidal mast cells in retinal pathology: a potential target for intervention. Am J Pathol. 2015;185(8):2083–95. doi: 10.1016/j.ajpath.2015.04.002. Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 11.Azizkhan RG, Azizkhan JC, Zetter BR, Folkman J. Mast cell heparin stimulates migration of capillary endothelial cells in vitro. J Exp Med. 1980;152(4):931–44. doi: 10.1084/jem.152.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tharp MD. The interaction between mast cells and endothelial cells. J Invest Dermatol. 1989;93(2 Suppl):107S–12S. doi: 10.1111/1523-1747.ep12581221. [DOI] [PubMed] [Google Scholar]
- 13.Tonnesen MG, Feng X, Clark RA. Angiogenesis in wound healing. The journal of investigative dermatology. Symposium proceedings/the Society for Investigative Dermatology, Inc [and] European Society for Dermatological Research. 2000;5(1):40–6. doi: 10.1046/j.1087-0024.2000.00014.x. Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 14.Penfold P, Killingsworth M, Sarks S. An ultrastructural study of the role of leucocytes and fibroblasts in the breakdown of Bruch's membrane. Australian journal of ophthalmology. 1984;12(1):23–31. [PubMed] [Google Scholar]
- 15.Takehana Y, Kurokawa T, Kitamura T, et al. Suppression of laser-induced choroidal neovascularization by oral tranilast in the rat. Invest Ophthalmol Vis Sci. 1999;40(2):459–66. [PubMed] [Google Scholar]
- 16.Seddon JM, Sharma S, Adelman RA. Evaluation of the clinical age-related maculopathy staging system. Ophthalmology. 2006;113(2):260–6. doi: 10.1016/j.ophtha.2005.11.001. Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 17.Lutty GA, Cao J, McLeod DS. Relationship of polymorphonuclear leukocytes (PMNs) to capillary dropout in the human diabetic choroid. Am J Pathol. 1997;151:707–14. [PMC free article] [PubMed] [Google Scholar]
- 18.McLeod DS, Lutty GA. High resolution histologic analysis of the human choroidal vasculature. Invest Ophthalmol Vis Sci. 1994;35:3799–811. [PubMed] [Google Scholar]
- 19.McLeod DS, Grebe R, Bhutto I, Merges C, Baba T, Lutty GA. Relationship between RPE and choriocapillaris in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50(10):4982–91. doi: 10.1167/iovs.09-3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sobrin L, Seddon JM. Nature and nurture-genes and environment-predict onset and progression of macular degeneration. Prog Retin Eye Res. 2014;40:1–15. doi: 10.1016/j.preteyeres.2013.12.004. Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mullins RF, Dewald AD, Streb LM, Wang K, Kuehn MH, Stone EM. Elevated membrane attack complex in human choroid with high risk complement factor H genotypes. Exp Eye Res. 2011;93(4):565–7. doi: 10.1016/j.exer.2011.06.015. doi:S0014-4835(11)00205-3[pii]10.1016/j.exer.2011.06.015. Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nozaki M, Raisler BJ, Sakurai E, et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc Natl Acad Sci U S A. 2006;103(7):2328–33. doi: 10.1073/pnas.0408835103. Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Theoharides TC, Alysandratos KD, Angelidou A, et al. Mast cells and inflammation. Biochim Biophys Acta. 2012;1822(1):21–33. doi: 10.1016/j.bbadis.2010.12.014. Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nature reviews Immunology. 2008;8(6):478–86. doi: 10.1038/nri2327. Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhutto IA, Baba T, Merges C, Juriasinghani V, McLeod DS, Lutty GA. C-reactive protein and complement factor H in aged human eyes and eyes with age-related macular degeneration. Br J Ophthalmol. 2011;95(9):1323–30. doi: 10.1136/bjo.2010.199216. doi:bjo.2010.199216[pii]10.1136/bjo.2010.199216. Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nazarov PG, Pronina AP. The influence of cholinergic agents on histamine release from HMC-1 human mast cell line stimulated with IgG, C-reactive protein and compound 48/80. Life Sci. 2012;91(21-22):1053–7. doi: 10.1016/j.lfs.2012.08.004. Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 27.Smith RS, Trokel S. Effects of mast-cell degranulation on the choroid. Arch Ophthalmol. 1966;75(3):390–4. doi: 10.1001/archopht.1966.00970050392016. [DOI] [PubMed] [Google Scholar]
- 28.Blair RJ, Meng H, Marchese MJ, et al. Human mast cells stimulate vascular tube formation. Tryptase is a novel, potent angiogenic factor. J Clin Invest. 1997;99(11):2691–700. doi: 10.1172/JCI119458. Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muramatsu M, Katada J, Hattori M, Hayashi I, Majima M. Chymase mediates mast cell-induced angiogenesis in hamster sponge granulomas. Eur J Pharmacol. 2000;402(1-2):181–91. doi: 10.1016/s0014-2999(00)00350-2. [DOI] [PubMed] [Google Scholar]
- 30.Iddamalgoda A, Le QT, Ito K, Tanaka K, Kojima H, Kido H. Mast cell tryptase and photoaging: possible involvement in the degradation of extra cellular matrix and basement membrane proteins. Archives of dermatological research. 2008;300(Suppl 1):S69–76. doi: 10.1007/s00403-007-0806-1. Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 31.Sohn EH, Khanna A, Tucker BA, Abramoff MD, Stone EM, Mullins RF. Structural and biochemical analyses of choroidal thickness in human donor eyes. Invest Ophthalmol Vis Sci. 2014;55(3):1352–60. doi: 10.1167/iovs.13-13754. Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Godfrey WA. Characterization of the choroidal mast cell. Trans Am Ophthalmol Soc. 1987;85:557–99. [PMC free article] [PubMed] [Google Scholar]


