Abstract
Modification of tissue engineering scaffolds with bioactive molecules is a potential strategy for modulating cell behavior and guiding tissue regeneration. While adhesion to RGD peptides has been shown to inhibit in vitro chondrogenesis, the effects of extracellular matrix (ECM)-mimetic ligands with complex secondary and tertiary structures are unknown. This study aimed to determine whether collagen- and fibronectin-mimetic ligands would retain biologic functionality in three-dimensional (3D) hydrogels, whether different ECM-mimetic ligands differentially influence in vitro chondrogenesis, and if effects of ligands on differentiation depend on soluble biochemical stimuli. A linear RGD peptide, a recombinant fibronectin fragment containing the seven to 10 Type III repeats (FnIII7-10) and a triple helical, collagen mimetic peptide with the GFOGER motif were covalently coupled to agarose gels using the sulfo-SANPAH crosslinker, and bone marrow stromal cells (BMSCs) were cultured within the 3D hydrogels.. The ligands retained biologic functionality within the agarose gels and promoted density-dependent BMSC spreading. Interactions with all adhesive ligands inhibited stimulation by chondrogenic factors of collagen Type II and aggrecanmRNA levels and deposition of sulfated glycosaminoglycans. In medium containing fetal bovine serum, interactions with the GFOGER peptide enhanced mRNA expression of the osteogenic gene osteocalcin whereas FnIII7-10 inhibited osteocalcin expression. In conclusion, modification of agarose hydrogels with ECM-mimetic ligands can influence the differentiation of BMSCs in a manner that depends strongly on the presence and nature of soluble biochemical stimuli.
Keywords: chondrogenesis, mesenchymal progenitors, extracellular matrix, fibronectin, collagen, tissue engineering, microenvironment
Introduction
Mesenchymal stem cells (MSCs) are an attractive cell source for tissue engineering and cell-based therapies given their multilineage differentiation potential and ability to be expanded in culture (Caplan, 1991; Pittenger et al., 1999). The repair of damaged articular cartilage is one particular application that would be well suited for MSCs as a result of the limited capacity for cartilage regeneration and the rapid dedifferentiation of chondrocytes during monolayer expansion (Benya and Shaffer, 1982). Many populations of mesenchymal progenitors such as bone marrow stromal cells (BMSCs) display chondrogenic-like differentiation in three-dimensional (3D) culture environments and in the presence of transforming growth factor-β (TGF-β) family growth factors (Bosnakovski et al., 2004; Caterson et al., 2001; Johnstone et al., 1998). However, recent studies have shown that even over extended culture periods, engineered cartilage constructs derived from BMSCs have a distinct composition and inferior mechanical properties compared with those derived from native articular chondrocytes (Connelly et al., 2008b; Mauck et al., 2006). It is therefore likely that additional signals are required for complete chondrogenic differentiation and the development of a functional tissue replacement.
Interactions between cells and the extracellular matrix (ECM) provide important cues for the differentiation and development of many tissues, including cartilage. For example, integrin-mediated adhesion to fibronectin is required for precartilage condensation of limb bud cells (Bang et al., 2000), and the presence of specific fibronectin isoforms influences the extent of condensation (White et al., 2003). Interactions with Type II collagen can also enhance the in vitro chondrogenesis of BMSCs (Bosnakovski et al., 2006). Although much work is still required to fully understand the complex roles of cell-matrix interactions in chondrogenesis, it is clear that the ECM provides key signals for guiding this process.
A potential strategy for controlling cell-matrix interactions in vitro is to engineer synthetic matrices topresent specific ligands in a precise manner. The incorporation of peptides containing the integrin adhesive sequence Arginine-Glycine-Aspartic acid (RGD) into nonadhesive hydrogels was reported to enhance MSC survival and osteogenic differentiation (Shin et al., 2005). However, studies in our laboratory suggest that alginate and agarose hydrogels functionalized with RGD peptides inhibit BMSC chondrogenesis(Connelly et al., 2007; Connelly et al., 2008a). In addition to modulating integrin-mediated adhesion, engineered matrices also provide a means for introducing small molecules (Benoit et al., 2008) or larger moieties such as chondroitin sulfate (Varghese et al., 2008)that can enhance in vitro chondrogenesis. Although interactions with the RGD motif inhibit BMSC chondrogenesis, interactions with adhesive sequences that mimic other ECM proteins and target a distinct set of integrin receptors may induce different responses. For example, α5β1 binding to fibronectin requires a synergy site containing the “PHSRN” sequence in addition to RGD(Aota et al., 1994), and α2β1 binds to the “GFOGER” motif within fibrillar collagens(Reyes and Garcia, 2003). However, the effects of extracellular matrix (ECM)-mimetic ligands with complex secondary and tertiary structures on chondrogenesis are unknown. The goals of this study were to determine whether fibronectin- and collagen-mimetic ligands, targeting the α5β1 integrins and α2β1 integrins, respectively, retain biologic functionality when conjugated to 3D agarose hydrogels, whether interactions with different ECM-mimetic ligands differentially influence BMSC chondrogenesis in 3D hydrogels, and whether the responses to such engineered matrices depend on the nature of soluble biochemical stimuli presented through the culture medium.
Materials and Methods
Materials
The synthetic peptides GRGDSP (RGD) and the nonadhesive control GRGESP (RGE) were obtained from Bachem (King of Prussia, PA), and the collagen mimetic peptide GGYGGGPC[GPP]5GFOGER[GPP]5GPC (GFOGER) was prepared by the Emory University Microchemical Facility. The sulfo-SANPAH crosslinker, EZ-link Maleimide-PEG-Biotin kit, and Ultralink Immobilized Avidin were from Pierce (Rockford, IL). Seaprepagarose was from Cambrex (Frederick, MD). Immature bovine hind limbs were from Research 87 (Marlborough, MA). Recombinant human TGF-β1 was from R&D Systems (Minneapolis, MN), and basic-fibroblast growth factor (bFGF) was from Peprotech (Rocky Hill, NJ). Dexamethasone, 1,9 dimethyl methylene blue (DMMB), agarase, and Hoechst dye 33258 were from Sigma Aldrich (St Louis, MO). The ITS+ premix and Proteinase K were from BD Biosciences (San Jose, CA). Fetal bovine serum was from Hyclone (Logan, UT). Dulbecco’s modified Eagle medium (DMEM), antibiotic/antimycotic, trypsin, nonessential amino acids (NEAA), and phosphate buffered saline (PBS) were from Invitrogen (Carlsbad, CA). The radiolabel precursor 35S-sodium sulfate was from MP Biomedicals (Irvine, CA). The 3H-proline and ECF substrate were purchased from GE Healthcare (Piscataway, NJ). The antivinculin and antibiotin antibodies were from Sigma Aldrich, and the FITC antimouse antibody was from Abcam (Cambridge, UK). AlexaFluor 594-conjugated phalloidin was from Molecular Probes (Eugene, OR). The RNeasy mini kit was from Qiagen (Valencia, CA), and the AMV reverse transcriptase kit was from Promega (Madison, WI). The Sybr Green master mix was from Applied Biosystems (Foster City, CA), and the primers were from Invitrogen (Carlsbad, CA).
Preparation of ligand-modified agarose
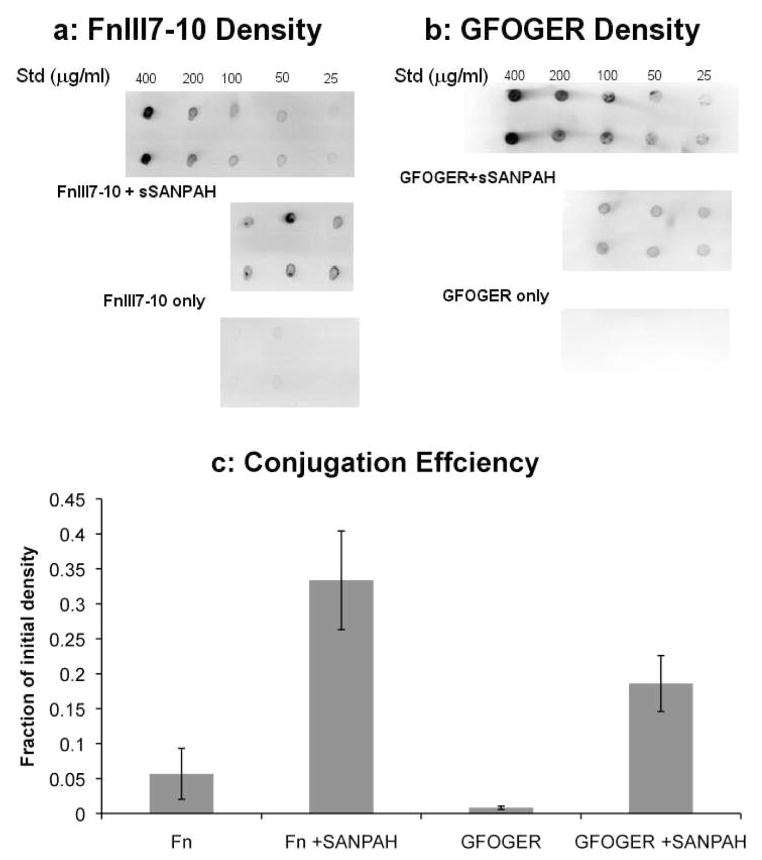
A monobiotinylatedfibronectin fragment containing the seventh to tenth Type III repeats (FnIII7-10) of human fibronectin was expressed in JM109 cells and purified by affinity chromatography as previously described (Petrie et al., 2006). A biotin tag was coupled to the cysteines in the GFOGER peptide using the EZ-link Maleimide-PEG-Biotin kit according to the manufacturer’s instructions. The synthetic peptides and fibronectin fragment were then conjugated to agarose hydrogels with the heterobifunctionalsulfo-SANPAH crosslinker(Dodla and Bellamkonda, 2006). Briefly, the primary amines on the ligands were reacted with the NHS-ester group of the sulfo-SANPAH in PBS at room temperature for 4 hours in the dark with a 10-fold molar excess of the crosslinker. Seaprepagarose solutions were prepared in Ca++/Mg++-free PBS, autoclaved, and cooled to 37º C. One part peptide/sulfo-SANPAH solution was combined with three parts 4% agarose and mixed thoroughly to yield a 3% agarose solution. The mixture was exposed to 365 nm ultraviolet light for 3 minutes to activate the photoreactive groups of the sulfo-SANPAH and conjugate the peptide to CH groups in the agarose. The agarose was allowed to gel at 4º C for 20 minutes and washed four times with fivefold excess PBS over 3 days to remove the unbound peptide and crosslinker. Controls were prepared as stated previously but without addition of the sulfo-SANPAH. Final ligand densities in the agarose gels were determined by dot blot detection of biotinylated ligands (Fig 1). Gels were digested with agarase (4 U/gel) at 45º C for 4 hours and blotted onto nitrocellulose (n = 3/condition). Membranes were probed with alkaline phosphatase-conjugated antibiotin antibodies and developed with the ECF substrate and Fuji Image Analyzer (Fuji, Tokyo, Japan). For an input concentration of 8 μM (400 μg/mL) FnIII7-10, the conjugation efficiency was approximately 30%, resulting in a final density of 2.4 μM (Fig 1C). For an input density of 37 μM (200 μg/mL) GFOGER, the conjugation efficiency was approximately 20%, resulting in a final density of 7.4 μM (Fig 1C). Low levels (less than 5%) of the ligands were present in gels without the sulfo-SANPAH crosslinker. For reference, the conjugation efficiencies for thefibronectin- and collagen-mimetic ligands were slightly higher than those previously reported for laminin-1 (Dodla and Bellamkonda, 2006).
Figure 1.
Conjugation of FnIII7-10 and GFOGER ligands to agarose hydrogels. (a) FnIII7-10 and (b) GFOGER densities in the agarose gels were determined by dot blot detection of the biotin tag after agarase digestion. The initial concentration of FnIII7-10 and GFOGER were 400 μg/mL (8 mM) and 200 μg/mL (37 mM), respectively, and the standards were diluted from the ligand stocks. Control gels were prepared with the same initial ligand density but without the sulfo-SANPAH crosslinker. (c) Conjugation efficiency for the FnIII7-10 and GFOGER modified gels was quantified by measuring the dot blot intensities relative to the initial densities. For the concentrations examined, there was a linear relationship between biotin intensity and ligand concentration. Data represent the mean ± SEM, n = 3 gels per condition.
Cell seeding and gel culture
Bone marrow was harvested from the tibiae and femora of an immature calf as previously described (Connelly et al., 2007). The adherent BMSCs were expanded three times in low-glucose DMEM supplemented with 10% fetal bovine serum (FBS) and 1 ng/mL bFGF. The 3% modified agarose gels were melted at 45º C and cooled to 37º C. BMSCs were then seeded into cylindrical (4 mm diameter) gels by resuspending the cells in the melted agarose at a density of 10e6 cells/mL and casting the cell suspension into custom molds. The gels were cooled at 4º C for 30 minutes and transferred to fresh culture medium. The basal, chemically defined medium consisted of high-glucose DMEM, 1% ITS+ Premix, 1% NEAA, 1% antibiotic/antimycotic, and 50 μg/mL ascorbate. The chondrogenic medium was basal medium supplemented with 10 ng/ml TGF-β1 and 100 nM dexamethasone. The FBS-supplemented medium consisted of DMEM, 10% FBS, 1% NEAA, 1% antibiotic/antimycotic, and 50 μg/mL ascorbate. BMSC seeded gels were cultured up to 8 days under the specified conditions and media were changed every 2 days.
Ligand functionality
To examine the functionality of the ligands in the 3D hydrogels, BMSCs were seeded into agarose gels functionalized with the RGD peptide (0, 50, and 100 μM), FnIII7-10 fragment (0, 0.5, and 1 μM), GFOGER peptide (0, 3.75, 15 μM), or nonadhesive RGE peptide (100 μM). Cells in gels were cultured for 24 hours in basal medium and examined for changes in cell morphology. Agarose gels were fixed with 10% neutral-buffered formalin for 30 minutes at 4º C and rinsed in PBS. Portions of the fixed gels were blocked with 5% FBS and permeabilized with 1% Triton-X100. Vinculin localization was examined by staining with the antivinculin antibody (10 μg/mL) for 90 minutes at room temperature and secondary detection was performed with the antimouse FITC-labeled antibody (10 μg/mL) for 90 minutes at room temperature. The F-actin cytoskeleton was visualized by staining with AlexaFluor594-phalloidin for 90 minutes at room temperature, and DNA was labeled with Hoechst dye. The agarose gels were imaged with a Zeiss 510 laser scanning confocal microscope (Zeiss, Heidelberg, Germany). Fluorescence images of the F-actin cytoskeleton from two different gels (three images per gel) were analyzed with Scion Image software (Frederick, MD). The area (A) and perimeter (P) for each individual cell were used to calculate the circularity (circularity = 4πA/P2) as a quantitative measure of cell spreading with a lower circularity indicating a greater degree of spreading (Connelly et al., 2008a).
Effects of ligands on BMSC differentiation
To investigate the effects of these ligand-functionalized gels on chondrogenic differentiation, BMSCs were first seeded into RGE (100 μM), RGD (100 μM), FnIII7-10 (1 μM), or GFOGER (15 μM) modified agarose, cultured for 8 days in basal or chondrogenic medium, and examined for changes in matrix synthesis rates and accumulation (n = 5/group). To determine the lineage-specific effects of the gels, BMSCs were again seeded into RGE, RGD, FnIII7-10, or GFOGER agarose with the same ligand densities as the chondrogenic experiment, cultured for 8 days in chondrogenic or serum supplemented medium, and examined for changes in chondrocytic and osteoblastic gene expression (n = 4/group).
During the final 24 hours of culture, 5 μCi/mL 35S-sodium sulfate and 10 μCi/mL 3H-proline were included in the culture medium to measure sulfated glycosaminoglycan (sGAG) and protein synthesis, respectively. At the end of the culture period, radiolabel incorporation was quenched with four sequential 30-minute washes in PBS plus 0.8 mM sodium sulfate and 1.6 mM L-proline at 4° C. The agarose gel constructs were weighed wet, lyophilized, and sequentially digested with Proteinase K (1 mg/80 mg sample) at 60º C overnight and agarase (4 U/construct) at 45º C for 4 hours. Radiolabel contents were measured using a liquid scintillation counter. The sGAG contents were measured using the 1,9-dimethylmethylene blue assay (Farndale et al., 1982), and the DNA contents were measured using the Hoechst dye assay (Kim et al., 1988). Total collagen content was measured using the hydroxyproline assay (Woessner, 1961).
RNA was isolated from the agarose gels using the Tri-spin method (Chomczynski and Sacchi, 1987). Gels were immediately dissociated in lysis buffer containing 2-mercaptoethanol. RNA was extracted from the gel using the Trizol reagent and chloroform and precipitated with 100% isoproponol. The RNA was further purified using the QiagenRNeasy kit according to the manufacturer’s protocol. Total RNA (1 μg) was reverse-transcribed to cDNA using the Promega AMV reverse transcriptase kit. Gene expression was measured by real-time reverse transcription–polymerase chain reaction (PCR) using the SybrGreen master mix and custom primers for bovine collagen II, aggrecan, collagen I (Brodkin et al., 2004), and osteocalcin(Bosnakovski et al., 2005). The PCR reactions and detection were performed with an ABI Prism 7700 (Applied Biosystems, Forest City, CA) and the transcript number relative to total RNA was calculated based on standards of known concentrations run on the same plate.
Data analysis
All data are presented as the mean ± SEM of representative experiments. Gene expression levels were transformed by optimal Box-Cox transformation to improve normality (Sokal and Rohlf, 1995). The effects of ligands in the presence or absence of chondrogenic supplements on matrix synthesis rates and sGAG accumulation were examined using two factor (ligand, medium) general linear models with Tukey’s test for pairwise comparisons. The effects of ligands on chondrocytic and osteoblastic gene expression in chondrogenic or serum-supplemented medium were examined using two factor (ligand, medium) general linear models with Tukey’s test for pairwise comparisons. Significance was at p<0.05.
Results
Integrin adhesive ligands alter BMSC morphology in 3D agarose gels
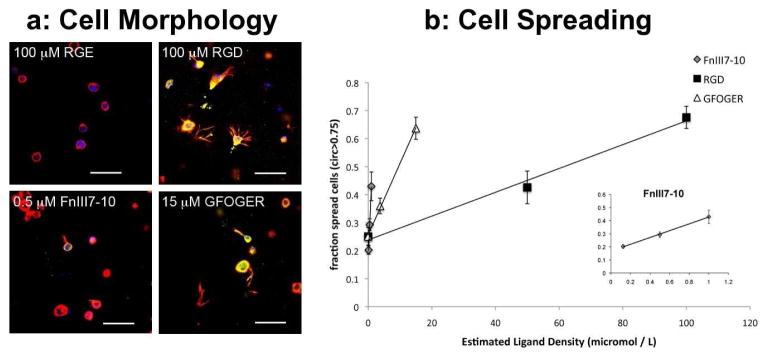
Like the linear RGD peptide, the recombinant fibronectin fragment FnIII7-10 and collagen-mimetic peptide containing the GFOGER sequence retained biologic functionality after conjugation to the agarose. After 24 hours, BMSCs in the non-adhesive RGE hydrogels maintained a rounded morphology. In contrast, cells in hydrogels conjugated with RGD, FnIII7-10, and GFOGER demonstrated marked changes in morphology characterized by large F-actin projections and positive staining for vinculin (Fig 2A), consistent with integrin-mediated adhesion to the hydrogel. For each of the adhesive molecules, there was a monotonic increase in the number of spread cells with increasing ligand density (Fig 2B). On a molar basis, FNIII7-10 was the most effective at inducing cell spreading, whereas RGD was the least effective. Similar changes in cell morphology at 24 hours were observed for 100 μM RGD, 1 μM FnIII7-10, and 15μM GFOGER.
Figure 2.
Effects of ligand-modified agarose on bone marrow stromal cell (BMSC) morphology. (a) BMSC morphology was examined 24 hours after seeding into functionalized gels by immunofluorescence detection of the F-actin cytoskeleton (red), vinculin (green), and DNA (blue). Agarose gels were modified with 100 μM RGE, 100 μM RGD, 0.5 μM FnIII7-10, and 15 μM GFOGER (final densities). Scale bars are 50μm. (b) Three-dimensional cell morphology was quantified by measuring the fraction of spread cells (circularity greater than 0.75) over a range of ligand densities for RGD, FnIII7-10 (inset), and GFOGER. Data represent the mean ± SEM, n = 6 (Original magnification, ×10 images per condition).
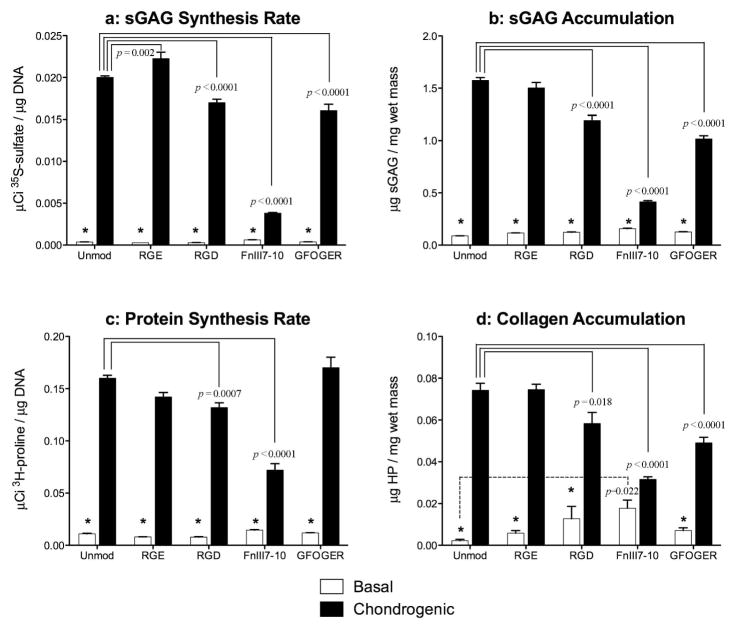
Interactions with FnIII7-10 and GFOGER inhibit matrix production during BMSC chondrogenesis
At concentrations inducing comparable degrees of cell spreading at 24 hours, all three adhesive ligands inhibited stimulation of ECM production by chondrogenic medium. Compared to basal medium, chondrogenic medium stimulated sGAG synthesis on day 8(as measured by 35S-sulfate incorporation) and sGAG accumulation within the agarose gels over the 8 day culture (both p<0.0001). Compared with unmodified gels, these responses were both inhibited by the presence of RGD, FnIII7-10 or GFOGER (all p<0.0001) (Fig 3A-B). The chondrogenic medium also stimulated overall protein synthesis on day 8(as measured by 3H-proline incorporation)and collagen accumulation over the 8 day culture (both p<0.0001). Compared with unmodified gels, interactions with RGD (p=0.0007) or FnIII7-10 (p<0.0001) inhibited protein synthesis, and interaction with RGD (p=0.018), FnIII7-10 (p<0.0001), or GFOGER (p<0.0001) inhibited collagen accumulation (Fig 3C-D). The RGE peptide did not substantially alter matrix synthesis rates or accumulation, as only the sGAG synthesis rate was significantly different from unmodified gels (p=0.002). The presence of FnIII7-10 substantially increased (p=0.022) collagen deposition in basal medium (Fig 3D).
Figure 3.
Regulation of matrix accumulation by ligand-modified agarose over 8 days of culture in basal or chondrogenic medium (10 ng/mL TGF-β1 and 100 nM dexamethasone). (a) Proteoglycan synthesis as measured by incorporation of 35S-sulfate over the final 24 hoursof culture (Day 7–8) for unmodified and functionalized agarose with 100 μM RGE, 100 μM RGD, 1 μM FnIII7-10, or 15 μM GFOGER final ligand densities. (b) Total sGAG accumulation in the gels after the 8-day culture period as measured by the DMMB assay. (C) Protein synthesis as measured by incorporation of 3H-proline over the final 24 hours of culture; and (d) total collagen accumulation as measured by assaying for hydroxyproline (HP) content. Data represent the mean ± SEM, n = 4–6 gels/condition. Significance vs. chondrogenic for a given gel is indicated by * (p<0.0001) or + (p<0.001).
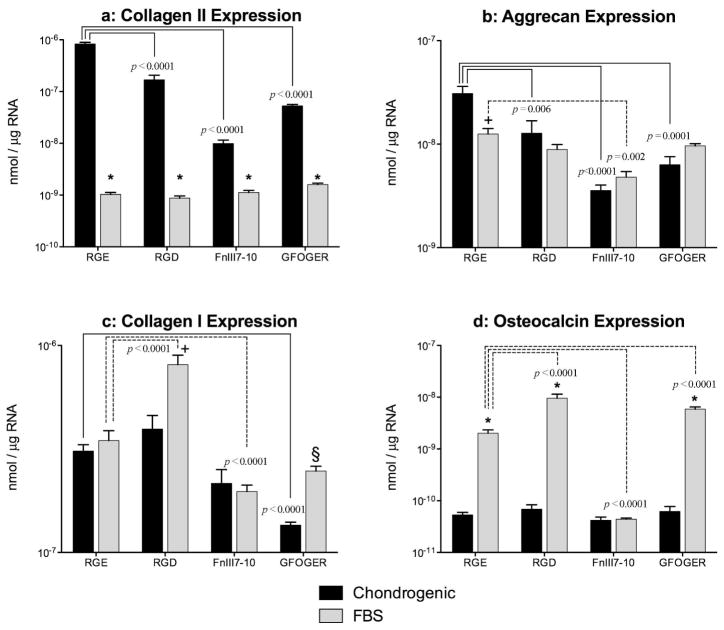
Interactions with functionalized hydrogels influence lineage-specific gene expression in a context dependent manner
The effects of the adhesive ligands on BMSC gene expression differed depending on the biochemical cues present in the culture medium. After 8 days of culture in the presence of the chondrogenic stimuli TGF-β1 and dexamethasone, interactions with RGD, FnIII7-10 or GFOGER all inhibited mRNA levels(Fig 4A–B)of the chondrocytic genes Type II collagen (all p<0.0001) and aggrecan (p=0.0064, p<0.0001 and p=0.0001, respectively). In contrast, expression of collagen II was substantially lower after 8 days of culture in 10% FBS-supplemented medium than in chondrogenic medium for all gel groups (all p<0.0001) and was not significantly affected by the presence of any of the adhesive ligands. Inserum-supplemented medium, expression of aggrecan was significantly lower than in chondrogenic medium for RGE-modified gels only (p=0.009), and was inhibited by interactions with FnIII7-10 (p=0.0018). Interactions with the RGD motif stimulated Type I collagen expression (Fig 4C) did not significantly differ between chondrogenic and serum-supplemented medium in gels modified with RGE or FnIII7-10, but was significantly greater in serum-supplemented medium in gels modified with RGD (p=0.002) or GFOGER (p=0.025). Relative to RGE-modified gels, collagen I expression was stimulated only by interactions with RGD in serum-supplemented medium (p<0.0001), and was inhibited by interactions with FnIII7-10 in serum-supplemented medium ( and GFOGER in chondrogenic medium (both p<0.0001). Expression of the osteogenic marker osteocalcin (Fig 4D)was substantially higher in serum-supplemented medium compared with the chondrogenic medium in gels modified with RGE, RGD or GFOGER (all p<0.0001), and did not significantly vary among ligands in chondrogenic medium. In serum-supplemented medium, osteocalcin expression was enhanced by RGD (p<0.0001) or GFOGER (p=0.0011) interactions and inhibited by FnIII7-10 interactions (p<0.0001).
Figure 4.
Effects of ligand modified agarose on lineage-specific gene expression. Levels of mRNA for the chondrocyte genes (a) Type II collagen and (b) aggrecan were measured by real-time polymerase chain reaction after 8 days of culture in RGE, RGD, FnIII7-10, or GFOGER modified agarose. Gels were cultured in chondrogenic medium or basal medium supplemented with 10% FBS. Expression of (c) Type I collagen and the osteogenic marker (d) osteocalcin were also measured at Day 8. Data represent the mean ± SEM, n = 4–6 gels/condition, and are presented on a logarithmic scale. Significance vs. chondrogenic for a given gel is indicated by * (p<0.0001), + (p<0.001) or § (p<0.05).
Discussion
Our data demonstrate that ECM-mimetic ligands with complex secondary and tertiary structures can be coupled to agarose hydrogels and retain their biologic activity. Similar to short, RGD containing oligopeptides(Connelly et al., 2008a), GFOGER and FnIII7-10 promoted density-dependent changes in the three-dimensional morphology of BMSCs. On stimulation with the chondrogenic factors TGF-β1 and dexamethasone, the presence each of the adhesive ligands inhibited matrix synthesis and chondrocytic gene expression relative to nonadhesive controls. Several studies show adhesion to short RGD peptides inhibits the chondrogenesis of BMSCs (Connelly et al., 2007; Salinas and Anseth, 2008) and that this effect is mediated by the actin cytoskeleton (Connelly et al., 2008a). Our data provide additional support for the role of the cytoskeleton and suggest that cell shape, more so than the specific integrin ligand, is a key regulator of chondrogenesis. Similarly, surface chemistry has less of an effect on human MSC chondrogenesis than overall morphology and cell aggregation (Phillips et al., 2009).
In contrast, the RGD and GFOGER ligands increased expression of the osteoblastic gene, osteocalcin, in the presence of 10% FBS, whereas the FnIII7-10 inhibited this response. Taken together, these findings indicate that the specific responses of BMSCs to cues supplied by engineered three-dimensional matrices depend on the biochemical environment. Although cell shape and cytoskeletal tension also control osteogenic versus adipogenic differentiation (Engler et al., 2006; McBeath et al., 2004), our findings and others further suggest that osteogenic differentiation may depend on the specific ECM and integrins involved (Keselowsky et al., 2005). It is interesting to note that the RGD peptide, but not GFOGER or FnIII7-10, increased collagen I expression in the serum supplemented medium. These results raise the intriguing possibility that different components of the ECM may regulate different aspects of osteoblastic differentiation.
When coupled to biomaterial surfaces, the FnIII7-10 and GFOGER ligands enhance the osteoblastic differentiation of rat MSCs in vitro and the osseointegration of titanium screws in vivo (Petrie et al., 2008; Reyes et al., 2007). Although the effects of the GFOGER modified gels on osteocalcin expression in the present study are consistent with these findings, the inhibitory effects of FnIII7-10 appear somewhat contradictory. Such differences may reflect variations in the specific media formulation, which have the potential to alter cellular responses to biomaterials. For example, the presence of a phosphate source, which was absent in our culture medium, may impact the extent of osteogenesis. Alternatively, altered presentation of the FnIII7-10 between two-dimensional and three-dimensional may influence the activity and integrin specificity of the ligand. Much work is clearly still required to understand the complex and context-dependent roles of cell-ECM interactions in differentiation, and new insights in this area could lead to improved biomaterials and regenerative therapies.
Although functionalizing hydrogels with ECM mimetic ligands may be detrimental for in vitro chondrogenesis, this strategy may be advantageous for other tissue engineering applications. Adhesion to RGD peptides can enhance initial cell viability within gels, and the incorporation of enzymatically cleavable tethers allows these ligands to be removed during later stages of differentiation (Kloxin et al., 2009; Salinas and Anseth, 2008). Patterning of adhesive ligands may also allow for undifferentiated progenitors to be retained within specified regions of an engineered tissue. Finally, graded levels of bioactive molecules could be a useful strategy for engineered heterogeneous tissues or interfaces such as fibrocartilage or osteochondral replacements, where controlling the extent of the chondrogenic response to initial biochemical cues may allow the development of tissues with desired spatial patterns of phenotypic heterogeneity.
Acknowledgments
This work was supported primarily by funding (MEL) from the Georgia Tech/Emory Center (GTEC) for the Engineering of Living Tissues, an ERC Program of the National Science Foundation under Award Number EEC-9731643 and was also supported by the National Institutes of Health through R01EB004496 (AJG).
References
- Aota S, Nomizu M, Yamada KM. The short amino acid sequence Pro-His-Ser-Arg-Asn in human fibronectin enhances cell-adhesive function. J Biol Chem. 1994;269:24756–24761. [PubMed] [Google Scholar]
- Bang OS, Kim EJ, Chung JG, Lee SR, Park TK, Kang SS. Association of focal adhesion kinase with fibronectin and paxillin is required for precartilage condensation of chick mesenchymal cells. Biochem Biophys Res Commun. 2000;278:522–529. doi: 10.1006/bbrc.2000.3831. [DOI] [PubMed] [Google Scholar]
- Benoit DS, Schwartz MP, Durney AR, Anseth KS. Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nat Mater. 2008;7:816–823. doi: 10.1038/nmat2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- Bosnakovski D, Mizuno M, Kim G, Ishiguro T, Okumura M, Iwanaga T, Kadosawa T, Fujinaga T. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells in pellet cultural system. Exp Hematol. 2004;32:502–509. doi: 10.1016/j.exphem.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Bosnakovski D, Mizuno M, Kim G, Takagi S, Okumura M, Fujinaga T. Isolation and multilineage differentiation of bovine bone marrow mesenchymal stem cells. Cell Tissue Res. 2005;319:243–253. doi: 10.1007/s00441-004-1012-5. [DOI] [PubMed] [Google Scholar]
- Bosnakovski D, Mizuno M, Kim G, Takagi S, Okumura M, Fujinaga T. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnol Bioeng. 2006;93:1152–1163. doi: 10.1002/bit.20828. [DOI] [PubMed] [Google Scholar]
- Brodkin KR, Garcia AJ, Levenston ME. Chondrocyte phenotypes on different extracellular matrix monolayers. Biomaterials. 2004;25:5929–5938. doi: 10.1016/j.biomaterials.2004.01.044. [DOI] [PubMed] [Google Scholar]
- Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- Caterson EJ, Nesti LJ, Li WJ, Danielson KG, Albert TJ, Vaccaro AR, Tuan RS. Three-dimensional cartilage formation by bone marrow-derived cells seeded in polylactide/alginate amalgam. J Biomed Mater Res. 2001;57:394–403. doi: 10.1002/1097-4636(20011205)57:3<394::aid-jbm1182>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Connelly JT, Garcia AJ, Levenston ME. Inhibition of in vitro chondrogenesis in RGD-modified three-dimensional alginate gels. Biomaterials. 2007;28:1071–1083. doi: 10.1016/j.biomaterials.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Connelly JT, Garcia AJ, Levenston ME. Interactions between integrin ligand density and cytoskeletal integrity regulate BMSC chondrogenesis. J Cell Physiol. 2008a;217:145–154. doi: 10.1002/jcp.21484. [DOI] [PubMed] [Google Scholar]
- Connelly JT, Wilson CG, Levenston ME. Characterization of proteoglycan production and processing by chondrocytes and BMSCs in tissue engineered constructs. Osteoarthritis Cartilage. 2008b;16:1092–1100. doi: 10.1016/j.joca.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodla MC, Bellamkonda RV. Anisotropic scaffolds facilitate enhanced neurite extension in vitro. J Biomed Mater Res A. 2006;78:213–221. doi: 10.1002/jbm.a.30747. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Farndale RW, Sayers CA, Barrett AJ. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9:247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- Keselowsky BG, Collard DM, Garcia AJ. Integrin binding specificity regulates biomaterial surface chemistry effects on cell differentiation. Proc Natl Acad Sci U S A. 2005;102:5953–5957. doi: 10.1073/pnas.0407356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Sah RL, Doong JY, Grodzinsky AJ. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal Biochem. 1988;174:168–176. doi: 10.1016/0003-2697(88)90532-5. [DOI] [PubMed] [Google Scholar]
- Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science. 2009;324:59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauck RL, Yuan X, Tuan RS. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthritis Cartilage. 2006;14:179–189. doi: 10.1016/j.joca.2005.09.002. [DOI] [PubMed] [Google Scholar]
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- Petrie TA, Capadona JR, Reyes CD, Garcia AJ. Integrin specificity and enhanced cellular activities associated with surfaces presenting a recombinant fibronectin fragment compared to RGD supports. Biomaterials. 2006;27:5459–5470. doi: 10.1016/j.biomaterials.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Petrie TA, Raynor JE, Reyes CD, Burns KL, Collard DM, Garcia AJ. The effect of integrin-specific bioactive coatings on tissue healing and implant osseointegration. Biomaterials. 2008;29:2849–2857. doi: 10.1016/j.biomaterials.2008.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JE, Petrie TA, Creighton FP, Garcia AJ. Human mesenchymal stem cell differentiation on self-assembled monolayers presenting different surface chemistries. Acta Biomater. 2009 doi: 10.1016/j.actbio.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Reyes CD, Garcia AJ. Engineering integrin-specific surfaces with a triple-helical collagen-mimetic peptide. J Biomed Mater Res A. 2003;65:511–523. doi: 10.1002/jbm.a.10550. [DOI] [PubMed] [Google Scholar]
- Reyes CD, Petrie TA, Burns KL, Schwartz Z, Garcia AJ. Biomolecular surface coating to enhance orthopaedic tissue healing and integration. Biomaterials. 2007;28:3228–3235. doi: 10.1016/j.biomaterials.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas CN, Anseth KS. The enhancement of chondrogenic differentiation of human mesenchymal stem cells by enzymatically regulated RGD functionalities. Biomaterials. 2008;29:2370–2377. doi: 10.1016/j.biomaterials.2008.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Temenoff JS, Bowden GC, Zygourakis K, Farach-Carson MC, Yaszemski MJ, Mikos AG. Osteogenic differentiation of rat bone marrow stromal cells cultured on Arg-Gly-Asp modified hydrogels without dexamethasone and beta-glycerol phosphate. Biomaterials. 2005;26:3645–3654. doi: 10.1016/j.biomaterials.2004.09.050. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry : the principles and practice of statistics in biological research. W.H. Freeman; New York: 1995. [Google Scholar]
- Varghese S, Hwang NS, Canver AC, Theprungsirikul P, Lin DW, Elisseeff J. Chondroitin sulfate based niches for chondrogenic differentiation of mesenchymal stem cells. Matrix Biol. 2008;27:12–21. doi: 10.1016/j.matbio.2007.07.002. [DOI] [PubMed] [Google Scholar]
- White DG, Hershey HP, Moss JJ, Daniels H, Tuan RS, Bennett VD. Functional analysis of fibronectin isoforms in chondrogenesis: Full-length recombinant mesenchymal fibronectin reduces spreading and promotes condensation and chondrogenesis of limb mesenchymal cells. Differentiation. 2003;71:251–261. doi: 10.1046/j.1432-0436.2003.7104502.x. [DOI] [PubMed] [Google Scholar]
- Woessner JF., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]