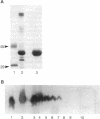
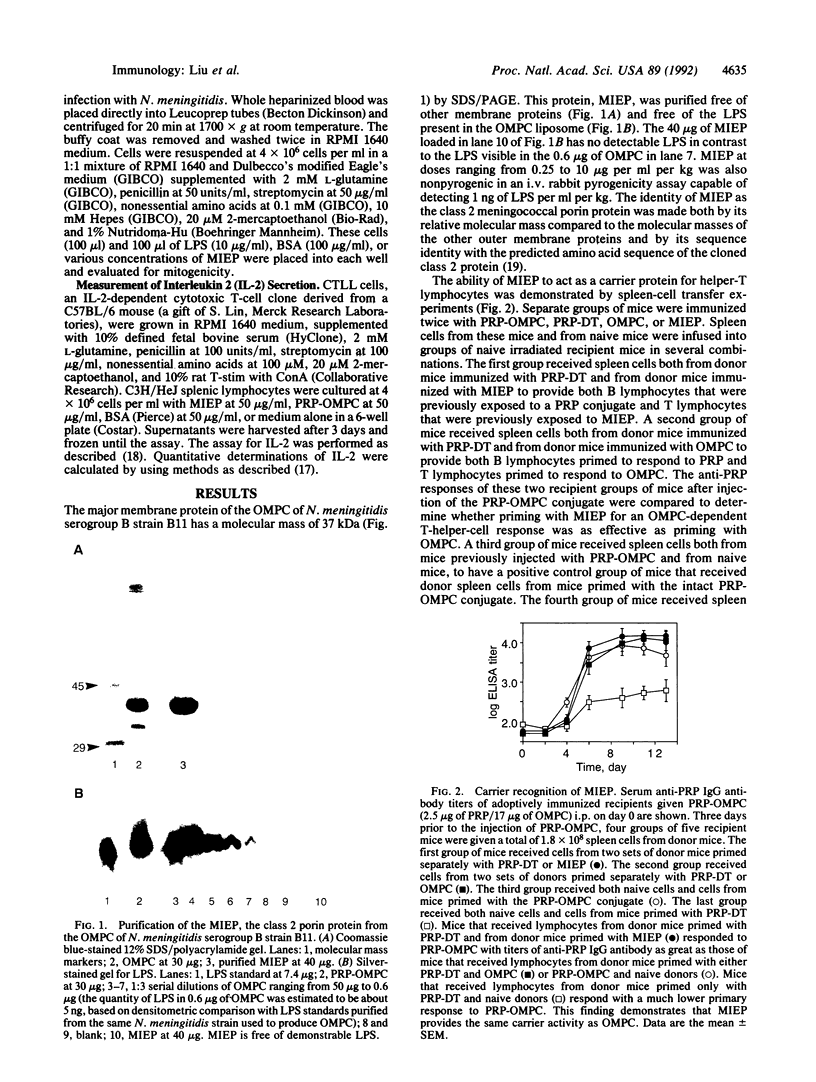
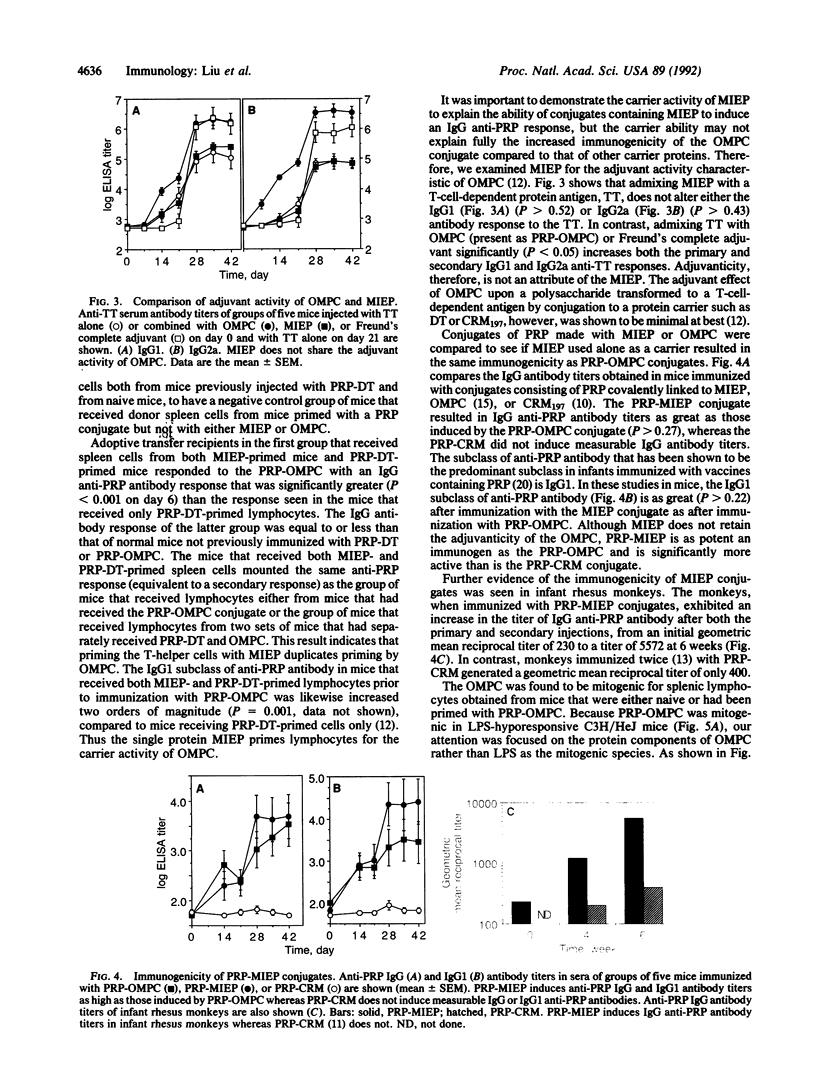
Abstract
Protein carriers vary in their ability to increase the immunogenicity of poorly immunogenic or T-lymphocyte-independent antigens. We examined one such carrier, the outer membrane protein complex derived from Neisseria meningitidis serogroup B strain B11, in an attempt to determine why this outer membrane protein complex was more immunogenic in young infants and in relevant animal models than two other carriers used in conjugates made with Haemophilus influenzae type b polysaccharide, a T-cell-independent antigen. A single protein of the outer membrane protein complex, the class 2 porin protein, was purified and shown to function as a T-helper lymphocyte carrier protein. Unexpectedly, it was also found to have mitogenic activity for lymphocytes that was not due to lipopolysaccharide. This mitogenic activity appears to date to be unique to this carrier protein of the carrier proteins tested and may contribute to the ability of the H. influenzae type b conjugate vaccine made with the outer membrane protein complex to generate IgG anti-polysaccharide antibody responses in mice and infant monkeys and protective immune responses in infants less than 6 months of age.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahonkhai V. I., Lukacs L. J., Jonas L. C., Matthews H., Vella P. P., Ellis R. W., Staub J. M., Dolan K. T., Rusk C. M., Calandra G. B. Haemophilus influenzae type b conjugate vaccine (meningococcal protein conjugate) (PedvaxHIB): clinical evaluation. Pediatrics. 1990 Apr;85(4 Pt 2):676–681. [PubMed] [Google Scholar]
- Anderson P. W., Pichichero M. E., Stein E. C., Porcelli S., Betts R. F., Connuck D. M., Korones D., Insel R. A., Zahradnik J. M., Eby R. Effect of oligosaccharide chain length, exposed terminal group, and hapten loading on the antibody response of human adults and infants to vaccines consisting of Haemophilus influenzae type b capsular antigen unterminally coupled to the diphtheria protein CRM197. J Immunol. 1989 Apr 1;142(7):2464–2468. [PubMed] [Google Scholar]
- Avery O. T., Goebel W. F. CHEMO-IMMUNOLOGICAL STUDIES ON CONJUGATED CARBOHYDRATE-PROTEINS : II. IMMUNOLOGICAL SPECIFICITY OF SYNTHETIC SUGAR-PROTEIN ANTIGENS. J Exp Med. 1929 Sep 30;50(4):533–550. doi: 10.1084/jem.50.4.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly J. J., Deck R. R., Liu M. A. Immunogenicity of a Haemophilus influenzae polysaccharide-Neisseria meningitidis outer membrane protein complex conjugate vaccine. J Immunol. 1990 Nov 1;145(9):3071–3079. [PubMed] [Google Scholar]
- Einhorn M. S., Weinberg G. A., Anderson E. L., Granoff P. D., Granoff D. M. Immunogenicity in infants of Haemophilus influenzae type B polysaccharide in a conjugate vaccine with Neisseria meningitidis outer-membrane protein. Lancet. 1986 Aug 9;2(8502):299–302. doi: 10.1016/s0140-6736(86)90001-2. [DOI] [PubMed] [Google Scholar]
- Feldman S., Gigliotti F., Shenep J. L., Roberson P. K., Lott L. Risk of Haemophilus influenzae type b disease in children with cancer and response of immunocompromised leukemic children to a conjugate vaccine. J Infect Dis. 1990 May;161(5):926–931. doi: 10.1093/infdis/161.5.926. [DOI] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Kotb M., Majumdar G., Tomai M., Beachey E. H. Accessory cell-independent stimulation of human T cells by streptococcal M protein superantigen. J Immunol. 1990 Sep 1;145(5):1332–1336. [PubMed] [Google Scholar]
- Liu M. A., Liu T. Effect of recombinant soluble CD4 on human peripheral blood lymphocyte responses in vitro. J Clin Invest. 1988 Dec;82(6):2176–2180. doi: 10.1172/JCI113842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore D. V., Johnson C. L., Phipps D. C., Popejoy L. A., Eby R., Smith D. H. Safety and immunologic response to Haemophilus influenzae type b oligosaccharide-CRM197 conjugate vaccine in 1- to 6-month-old infants. Pediatrics. 1990 Mar;85(3):331–337. [PubMed] [Google Scholar]
- Marrack P., Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990 May 11;248(4956):705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- Moxon E. R., Kroll J. S. The role of bacterial polysaccharide capsules as virulence factors. Curr Top Microbiol Immunol. 1990;150:65–85. doi: 10.1007/978-3-642-74694-9_4. [DOI] [PubMed] [Google Scholar]
- Murakami K., Gotschlich E. C., Seiff M. E. Cloning and characterization of the structural gene for the class 2 protein of Neisseria meningitidis. Infect Immun. 1989 Aug;57(8):2318–2323. doi: 10.1128/iai.57.8.2318-2323.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkelä P. H., Eskola J., Peltola H., Takala A. K., Käyhty H. Clinical experience with Haemophilus influenzae type b conjugate vaccines. Pediatrics. 1990 Apr;85(4 Pt 2):651–653. [PubMed] [Google Scholar]
- Schneerson R., Barrera O., Sutton A., Robbins J. B. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J Exp Med. 1980 Aug 1;152(2):361–376. doi: 10.1084/jem.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro E. D., Murphy T. V., Wald E. R., Brady C. A. The protective efficacy of Haemophilus b polysaccharide vaccine. JAMA. 1988 Sep 9;260(10):1419–1422. [PubMed] [Google Scholar]
- Siber G. R., Weitzman S. A., Aisenberg A. C. Antibody response of patients with Hodgkin's disease to protein and polysaccharide antigens. Rev Infect Dis. 1981 Mar-Apr;3 (Suppl):S144–S159. doi: 10.1093/clinids/3.supplement_1.s144. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E., Rivera E., Hochstein H. D. Measurements of lipopolysaccharide (endotoxin) in meningococcal protein and polysaccharide preparations for vaccine usage. J Biol Stand. 1989 Jul;17(3):249–258. doi: 10.1016/0092-1157(89)90017-6. [DOI] [PubMed] [Google Scholar]
- Vella P. P., Ellis R. W. Immunogenicity of Haemophilus influenzae type b conjugate vaccines in infant rhesus monkeys. Pediatr Res. 1991 Jan;29(1):10–13. doi: 10.1203/00006450-199101000-00003. [DOI] [PubMed] [Google Scholar]
- Vella P. P., Staub J. M., Armstrong J., Dolan K. T., Rusk C. M., Szymanski S., Greer W. E., Marburg S., Kniskern P. J., Schofield T. L. Immunogenicity of a new Haemophilus influenzae type b conjugate vaccine (meningococcal protein conjugate) (PedvaxHIB). Pediatrics. 1990 Apr;85(4 Pt 2):668–675. [PubMed] [Google Scholar]
- Weinberg G. A., Granoff D. M. Polysaccharide-protein conjugate vaccines for the prevention of Haemophilus influenzae type b disease. J Pediatr. 1988 Oct;113(4):621–631. doi: 10.1016/s0022-3476(88)80369-x. [DOI] [PubMed] [Google Scholar]