Abstract
The regulation of cellular signaling in vivo has been a challenging task owing to the lack of effective methods for tunable control of the amplitude, location, and duration of cell-signaling events at a deep-tissue level. In this issue of ACS Nano, an intriguing paper by Ambrosone et al. demonstrates that deep-tissue-penetrating near-infrared (NIR) light can be used to control the Wnt/β-catenin-signaling pathway in a single-cell organism (Hydra) by utilizing microcapsules that contain plasmonic gold nanoparticles. In parallel, in recent work, we proposed upconversion nanoparticles (UCNPs) as NIR-light-activatable “wireless” optogenetic tools, and we showed their ability to modulate cell signaling pathways in both mammalian cells and mice. We believe that these interesting NIR-light-responsive nanotechnologies will open new avenues for both basic research and clinical applications.
Cellular signaling pathways are present in all living organisms and govern a wide variety of essential physiological processes, including tissue homeostasis, organism development, tissue repair, and immunity. The amplitude and frequency of cell signaling regulate basic cellular activities and coordinate cell actions in living organisms. In fact, cells rely on these vital communications to perceive and to respond to changes in their microenvironment. Failure and/or errors in cellular signaling often lead to severe health consequences, such as birth defects, cancers, diabetes, and immune diseases. Thus, strategies that can manipulate cell signaling will open up new avenues for elucidating cell functions and, potentially, for the treatment of diseases as well as other exciting possibilities.
As a highly orthogonal external stimulus, light enjoys wide usage in materials science, chemistry, biology, and drug-delivery systems because it possesses the unique ability to manipulate cell-signaling systems precisely. In particular, light can act as a noninvasive tool to monitor and to regulate the spatiotemporal dynamics of cell signaling in living systems. Past attempts to control cellular signaling included covalently attaching photolabile chemical groups to the signaling molecules that are necessary for cellular functions.1 Upon exposure to light, these photolabile groups would cleave and dissociate, thereby “uncaging” the molecules to recover their normal cellular functions. However, it is difficult to deliver these “caged” small molecules to specific cells and organisms, and these caged small molecules respond only to ultraviolet light. Excessive use of such short-wavelength light, particularly over a significant area of tissue, can have a phototoxic effect on living systems. Moreover, the depth of tissue penetration using shorter-wavelength light is also quite limited.
Recently, cell signaling control was made easier through the development of optogenetic tools.2 Such optical modulation is a particularly powerful method to study and to dissect cellular functions, gene expression, and protein interaction because of its high spatiotemporal resolution.3,4 In this approach, the genetically encoded light-sensitive proteins are expressed in cells and act like “small hatches”. When light at specific wavelengths shines on these optogenetic constructs, the “hatches” open or shut, and the cell signaling can then be tuned in a spatiotemporal manner. Compared to caged small molecules, these light-sensitive proteins show rapid reversibility. However, optogenetic tools typically rely on visible light (e.g., blue or yellow), which has a quite shallow depth of tissue penetration (<1 mm). Therefore, until now, current protocols have required invasive fiber-optic probes to deliver the visible light into organs and animal tissues. Fiber-optic probes have inherent limitations in animals, including tissue damage, inflammation, and insertion site infection, as well as the necessity of constraining the animal with fiber-optic tethers.
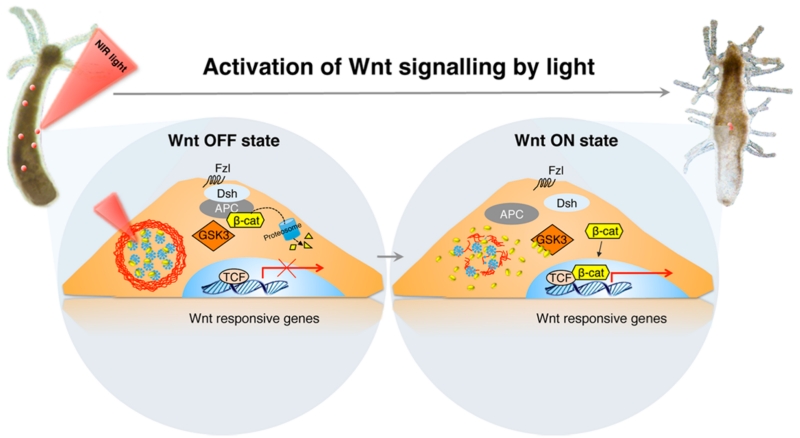
In contrast to visible light, the near-infrared range (~700–1000 nm) has less tissue scattering and blood absorption and less photodamage for living organisms, and most importantly, it has much deeper tissue penetration than visible light.5,6 Thus, it has been considered to be a “tissue-transmissible window” for biological tissues.7 In this issue of ACS Nano, Ambrosone et al. utilized the photothermal effect of plasmonic gold nanoparticles and developed an NIR-triggered drug-release system that can elegantly control the activation of the Wnt/β-catenin (β-cat)-signaling pathway in Hydra.8 More specifically, the Wnt/β-cat-signaling pathway is one of the fundamental cell-signaling mechanisms that directs cell proliferation, cell polarity, and cell fate determination during embryonic development and tissue homeostasis.
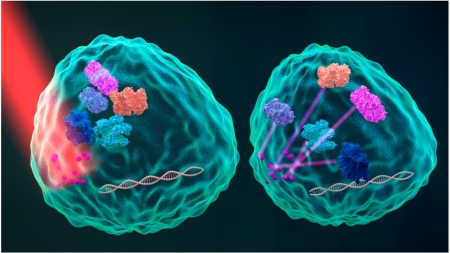
In their study, Ambrosone et al. designed a temperature-sensitive microcapsule that contains gold nanoparticles. The Wnt/β-catenin-signaling pathway modulator, alsterpaullone (ALP), was then enclosed in the microcapsules (Figure 1). Upon NIR light irradiation, gold nanoparticles in the microcapsule can efficiently transform the energy of NIR light into local heat, breaking the temperature-sensitive multilayer polyelectrolyte assembly, which leads to ALP release from the microcapsules. The ALP, in turn, binds to glycogen synthase kinase-3β (GSK-3β) by competing with the adenosine triphosphate (ATP). As the kinase of GSK3β controls β-cat phosphorylation for proteasome degradation, its inhibition causes better accumulation of β-cat in the nucleus, where it interacts with members of the TCF/LEF transcription factor family. This ensures efficient transcriptional activation of a variety of downstream genes. In this study, Hydra was used as a single-cell animal model because Wnt ligands are involved in critical Hydra development (e.g., maintenance of body axis polarity). The forced activation of the Wnt pathway induces the appearance of ectopic tentacles along the body column, enabling fast functional readouts of optical regulation bioactivity. In particular, this method of NIR-triggered drug release has been shown to release ALP at precisely the 3 μm2 area on the Hydra body column. This technique assured local activation of the Wnt pathway as a consequence of high ALP release in a confined area, which may offer the further ability to exploit the Wnt/β-cat-signaling pathway in a higher spatiotemporal manner in single-cell analysis.
Figure 1.
Schematic of Wnt/β-catenin-signaling activation by NIR photoactivated ALP release. The gold nanoparticle assembled microcapsules effectively transform NIR light into heat, leading to the disruption of microcapsules and resulting in ALP release. The ALP can bind to the GSK3β, resulting in nuclei accumulation of β-cat, down-regulating gene expression. Reprinted from ref 8. Copyright 2016 American Chemical Society.
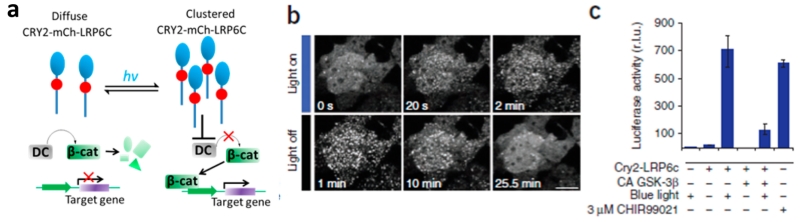
The optical regulation of the Wnt/β-cat-signaling pathway not only has been presented in the Hydra but has made significant progress in its usage in mammalian cells, as well. Bugaj et al. reported on an optogenetic method that is based on Arabidopsis thaliana cryptochrome 2 (CRY2) for rapid and reversible protein oligomerization in response to blue light (Figure 2).9 This method can efficiently regulate the target-gene transcription by photoactivating the Wnt/β-cat-signaling pathway. However, as mentioned previously, the existing optogenetic constructs are limited by their inability to extend spectral sensitivity into the near-infrared tissue-transmissible range.
Figure 2.
Blue-light-mediated CRY2 regulation of Wnt/β-cat-signaling pathways by protein oligomerization. (a) Schematic of the photoactivation strategy. Upon light activation, the clustering of the LRP6c domain inhibits the destruction complex (DC), relieving β-catenin inhibition and allowing its translocation to the nucleus and regulation of target-gene transcription to occur. (b) CRY2-LRP6c retains the ability to cluster reversibly in HEK 293T cells. Scale bar, 20 μm. (c) Light-induced β-catenin transcriptional activity assayed in HEK 293T cells carrying CRY2-LRP6c and a luciferase reporter shows strong β-catenin activity after 16 h exposure to blue light pulses (500 ms pulse every 10 s). The light-induced signal was attenuated in the presence of the constitutively active (CA) β-catenin pathway inhibitor GSK-3β and was comparable to that observed with a small-molecule pathway agonist CHIR99021 (3 μM). Reprinted with permission from ref 9. Copyright 2013 Nature Publishing Group.
To overcome this roadblock, we recently proposed a wireless optogenetic strategy based on upconversion nanoparticles (UCNPs), which have the key advantage of unnatural inverse excitation and emission profiles.10 These UCNPs are excited using low-power, tissue-penetrating, near-infrared radiation that is efficiently converted to a higher energy output emission at shorter wavelengths, including visible light. Because UCNPs can be illuminated by NIR sources that are located outside of the animal body, they act as “nano-antennae”. Thus, such in vivo light delivery vehicles can be used to activate optogenetic constructs in deep tissue without the need for implanted fiber optics.
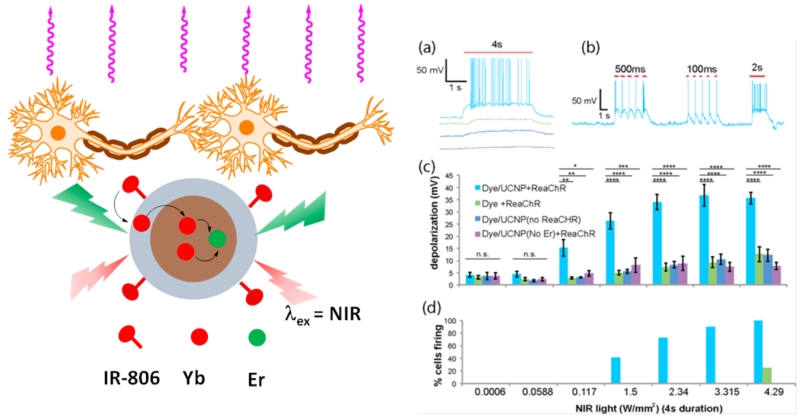
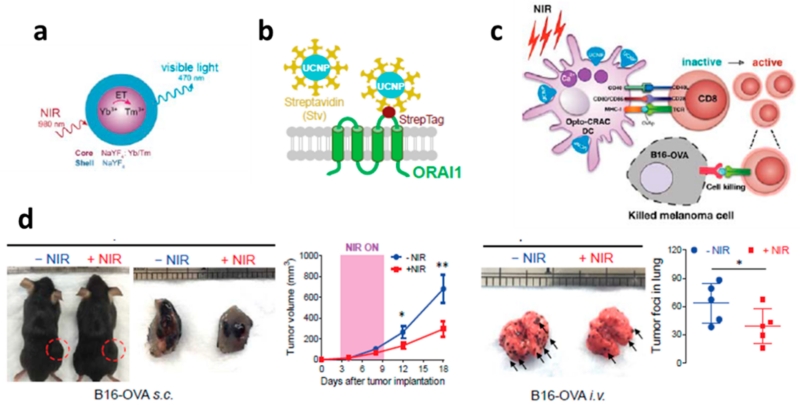
In this regard, the Han research group synergized two effective ways (i.e., dye sensitizing and shell enhancing) to improve upconversion efficiency, and they reported a dye-sensitized core/active shell upconversion nanoparticle that possesses broadened and enhanced upconversion luminescence (Figure 3).11 Further, because these UCNPs have emission in the visible spectrum that can overlap with activation wavelengths of optogenetic opsins (e.g., ReaChR), Han and his colleagues demonstrated that ReaChR opsin-expressed hippocampal neurons can be spatiotemporally activated by such dye-sensitized core/active shell UCNPs when excited at 800 nm. This work showed that the optogenetic operation windows are shifted from visible light to the deep-tissue-penetrable NIR region. Since this discovery, the Han and Zhou research groups have further utilized UCNPs to develop an NIR-stimulable optogenetic system (termed “Opto-CRAC”) to manipulate Ca2+/NFAT (the nuclear factor of activated T cells) signaling through one of the most Ca2+-selective ion channels, the Ca2+ release-activated Ca2+ (CRAC) channel in mammalian cells and mice.12 This platform is based on a CRAC channel with genetically built-in visible blue light sensitivity that is subsequently paired with cell-targeted lanthanide-doped upconversion nanoparticles, enabling the local conversion of input NIR light to visible light. The Opto-CRAC tool was demonstrated to be able to control remotely and reversibly the Ca2+ influx, nuclear translocation of a master transcriptional factor, the nuclear factor of activated T cells (NFAT); to fine-tune NFAT-dependent gene expression; and to modulate immune-cell activation. More specifically, when the cells were exposed to NIR light, the calcium ion gates in the membranes opened, and when the light was turned off, the gates closed. Consequently, Ca2+ influx through CRAC channels activates calcineurin, a downstream Ca2+-dependent phosphatase that, in turn, dephosphorylates the master transcriptional regulator NFAT and then results in NFAT nuclear translocation.13 The NFAT then cooperates with the activator protein 1 (AP-1) to turn on a number of genes (e.g., IL-2 and IFN-γ) that are responsible for immune responses. Further, we reported that UCNP upconverted emissions can, in fact, conduct in vivo optogenetic immunomodulation, which is capable of instructing immune cells to attack melanoma tumors in mice for therapeutic purposes. In this study, dendritic cells were genetically engineered with a light-sensitive calcium-gating protein. Mice were injected with these light-sensitive dendritic cells and the UCNP conjugates and then were exposed to NIR light. We found that NIR light can actually regulate Ca2+/NFAT signaling and selectively activate immune responses to boost tumor antigen presentation through engineered dendritic cells. This then activated CD8+ T cells and expanded the cytotoxic T lymphocyte population to aid tumor cell killing in vivo. Thus, we observed that NIR light therapy effectively suppresses melanoma growth and metastasis to the lung in mice (Figure 4). Indeed, this work explores development toward UCNP-based optogenetic therapy.
Figure 3.
Left panel is a schematic showing dye-sensitized core/active shell upconversion nanoparticles. Right panel show the NIR light activation of ReaChR in cultured hippocampal neurons. Reprinted from ref 11. Copyright 2016 American Chemical Society.
Figure 4.
(a) Schematic illustration of the core/shell structure and the energy transfer between lanthanide ions in the NaYF4:Yb,Tm/NaYF4 core/shell upconversion nanoparticles. (b) Schematic showing the interaction between streptavidin-coated upconversion nanoparticles (UCNPs-Stv) and the engineered ORAI1 Ca2+ channel that harbors a streptavidin-binding tag (StrepTag) in the second extracellular loop. (c) Scheme showing that an NIR-stimulated Ca2+ influx in Opto-CRAC DCs prompts immature DC maturation and OVA antigen cross-presentation in order to activate and to boost antitumor immune responses that are mediated by OT-1 CD8 T cells (cytotoxic T lymphocytes, CTLs). This action results in sensitizing tumor cells to OVA-specific, CTL-mediated killing in the B16-OVA melanoma model. The OVA peptide (OVAp, 257SIINFEKL264) is used here as a surrogate tumor antigen. (d) Tumor-inoculated sites (left) were isolated from tumor-bearing mice (n = 5) shielded or exposed to NIR, and tumor sizes (mm3) were measured at the indicated time points, as shown in the growth curve (right) after tumor implantation. Representative lungs with melanoma metastases (left) were isolated from tumor-bearing mice that were shielded or exposed to NIR. The histogram represents the counted numbers of visible pigmented tumor foci (denoted by the arrows) with pulmonary melanoma metastases on the surface of lungs (right panel; n = 5 mice). Reprinted with permission from ref 12. Copyright 2015 eLife Sciences Publications.
OUTLOOK AND FURTHER CHALLENGES
Near-infrared-light-responsive nanomaterials have unique optical properties that enable noninvasive “remote control” activation and suppression of signal-transduction cascades in vivo. Among other exciting possibilities, we anticipate that these novel tools will provide opportunities to modulate the cell-signaling cascades while interfering minimally with host physiology. These powerful tools offer opportunities to address the underlying mechanisms of cell signaling and its correlations with pathologies where cell signaling is aberrantly regulated. In addition, these tools would enable the exploration of potential noninvasive therapeutic approaches.
The impact of these new nanomaterials is also potentially broad, extendeding to many clinical applications, from regenerative medicine to anticancer strategies. For example, plasmonic nanoparticle capsules would enable the loaded drugs to be released at a particular tissue part by an NIR source located outside the body, without the potential off-targeting complications. Likewise, UCNPs as wireless optogenetic tools activated by an external NIR source might also be used to regulate numerous cell functions via implanted optogenetic-engineered cells.
Along with the exciting promise of these nanomaterials come significant challenges. For example, while the release of biologically active molecules has been demonstrated, the quantitative drug release from plasmonic nanoparticle capsules is technically difficult. In addition, the required NIR laser is often also associated with photothermal heating that may damage cells and tissues. Moreover, the photothermal efficiency of plasmonic gold nanoparticle capsules and the quantum efficiency of currently used UCNPs are still suboptimal for satisfying practical clinical applications. Their potential in vivo toxicity needs to be resolved through future research.
Therefore, novel NIR-responsive materials that are more biocompatible and less toxic will need to be developed in the future. Gold NPs can be synthesized in different shapes and with distinct plasmon resonance peaks. Innovative strategies that utilize alternative sensitizers,14 host matrices,6 or dye antennae15 have been designed in order to improve the quantum efficiency greatly and to engineer the excitation wavelengths of upconversion nanoparticles. As a result, such advances would provide the additional new opportunity of an orthogonal regulator of cell-signaling cascades.
Overall, although they are in an early stage of development, NIR-light-responsive nanomaterials could transform the way researchers interrogate and intervene cell signaling with high spatiotemporal resolution, from the level of single cells up to entire animals. These new approaches will also enable scientists of diverse interests to understand and to probe the physiological functions of cell signaling pathways in previously inaccessible areas. Ultimately, these approaches will lead to important new insights and therapies that are relevant to clinical practice.
ACKNOWLEDGMENTS
This research was supported by National Institutes of Health R01MH103133 to G.H., R01GM112003 to Y.Z., Human Frontier Science Program RGY-0090/2014 to G.H., and the Special Fellow Award from the Leukemia and Lymphoma Society LLS 3013-12 to Y.Z.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Ellis-Davies GC. Caged Compounds: Photorelease Technology for Control of Cellular Chemistry and Physiology. Nat. Methods. 2007;4:619–628. doi: 10.1038/nmeth1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Zhang K, Cui B. Optogenetic Control of Intracellular Signaling Pathways. Trends Biotechnol. 2015;33:92–100. doi: 10.1016/j.tibtech.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Fenno L, Yizhar O, Deisseroth K. The Development and Application of Optogenetics. Annu. Rev. Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Deisseroth K. Optogenetics. Nat. Methods. 2011;8:26–29. doi: 10.1038/nmeth.f.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Wu X, Chen G, Shen J, Li Z, Zhang Y, Han G. Upconversion Nanoparticles: A Versatile Solution to Multiscale Biological Imaging. Bioconjugate Chem. 2015;26:166–175. doi: 10.1021/bc5003967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Punjabi A, Wu X, Tokatli-Apollon A, El-Rifai M, Lee H, Zhang Y, Wang C, Liu Z, Chan EM, Duan C, Han G. Amplifying the Red-Emission of Upconverting Nanoparticles for Biocompatible Clinically Used Prodrug-Induced Photodynamic Therapy. ACS Nano. 2014;8:10621–10630. doi: 10.1021/nn505051d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Shi Kam NW, O’Connell M, Wisdom JA, Dai HJ. Carbon Nanotubes as Multifunctional Biological Transporters and Near-Infrared Agents for Selective Cancer Cell Destruction. Proc. Natl. Acad. Sci. U. S. A. 2005;102:11600–11605. doi: 10.1073/pnas.0502680102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Ambrosone A, Marchesano V, Carregal-Romero S, Intartaglia D, Parak WJ, Tortiglione C. Control of Wnt/β-Catenin Signaling Pathway in Vivo via Light Responsive Capsules. ACS Nano. 2016 doi: 10.1021/acsnano.5b07817. DOI: 10.1021/acsnano.5b07817. [DOI] [PubMed] [Google Scholar]
- (9).Bugaj LJ, Choksi AT, Mesuda CK, Kane RS, Schaffer DV. Optogenetic Protein Clustering and Signaling Activation in Mammalian Cells. Nat. Methods. 2013;10:249–252. doi: 10.1038/nmeth.2360. [DOI] [PubMed] [Google Scholar]
- (10).Shen J, Zhao L, Han G. Lanthanide-Doped Upconverting Luminescent Nanoparticle Platforms for Optical Imaging-Guided Drug Delivery and Therapy. Adv. Drug Delivery Rev. 2013;65:744–755. doi: 10.1016/j.addr.2012.05.007. [DOI] [PubMed] [Google Scholar]
- (11).Wu X, Zhang YW, Takle K, Bilsel O, Li ZJ, Lee H, Zhang ZJ, Li DS, Fan W, Duan CY, Chan EM, Lois C, Xiang Y, Han G. Dye-Sensitized Core/Active Shell Upconversion Nanoparticles for Optogenetics and Bioimaging Applications. ACS Nano. 2016;10:1060–1066. doi: 10.1021/acsnano.5b06383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).He L, Zhang YW, Ma GL, Tan P, Li ZJ, Zang SB, Wu X, Jing J, Fang SH, Zhou LJ, Wang YJ, Huang Y, Hogan PG, Han G, Zhou YB. Near-Infrared Photoactivatable Control of Ca2+ Signaling and Optogenetic Immunomodulation. eLife. 2015;4:e10024. doi: 10.7554/eLife.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Muller MR, Rao A. NFAT, Immunity and Cancer: A Transcription Factor Comes of Age. Nat. Rev. Immunol. 2010;10:645–656. doi: 10.1038/nri2818. [DOI] [PubMed] [Google Scholar]
- (14).Shen J, Chen GY, Vu AM, Fan W, Bilsel OS, Chang CC, Han G. Engineering the Upconversion Nanoparticle Excitation Wavelength: Cascade Sensitization of Tri-doped Upconversion Colloidal Nanoparticles at 800 nm. Adv. Opt. Mater. 2013;1:644–650. [Google Scholar]
- (15).Wu X, Lee H, Bilsel O, Zhang YW, Li ZJ, Chen T, Liu Y, Duan CY, Shen J, Punjabi A, Han G. Tailoring Dye-Sensitized Upconversion Nanoparticle Excitation Bands Towards Excitation Wavelength Selective Imaging. Nanoscale. 2015;7:18424–18428. doi: 10.1039/c5nr05437k. [DOI] [PMC free article] [PubMed] [Google Scholar]