Abstract
Inflammatory signaling may play a role in high-fat diet (HFD)-related circadian clock disturbances that contribute to systemic metabolic dysregulation. Therefore, palmitate, the prevalent proinflammatory saturated fatty acid (SFA) in HFD and the anti-inflammatory, poly-unsaturated fatty acid (PUFA), docosahexaenoic acid (DHA), were analyzed for effects on circadian timekeeping and inflammatory responses in peripheral clocks. Prolonged palmitate, but not DHA, exposure increased the period of fibroblast Bmal1-dLuc rhythms. Acute palmitate treatment produced phase shifts of the Bmal1-dLuc rhythm that were larger in amplitude as compared to DHA. These phase-shifting effects were time-dependent and contemporaneous with rhythmic changes in palmitate-induced inflammatory responses. Fibroblast and differentiated adipocyte clocks exhibited cell-specific differences in the time-dependent nature of palmitate-induced shifts and inflammation. DHA and other inhibitors of inflammatory signaling (AICAR, cardamonin) repressed palmitate-induced proinflammatory responses and phase shifts of the fibroblast clock, suggesting that SFA-mediated inflammatory signaling may feed back to modulate circadian timekeeping in peripheral clocks.
Keywords: Circadian clocks, Bmal1, Fatty acids, Inflammation, Cytokines, AMPK, NF-κB, AMP-activated protein kinase (AMPK), Cardamonin, Aminoimidazole-4-Carboxamide Riboside (AICAR), Fibroblasts, Adipocytes
Highlights
-
•
The saturated fatty acid (SFA) palmitate differentially modulates the circadian timekeeping mechanism in peripheral clocks;
-
•
Palmitate induces time-dependent phase shifts that coincide with its rhythmic induction of inflammatory signaling;
-
•
Time-dependent nature of the palmitate-induced phase shifts and inflammatory signaling is cell specific;
-
•
Inhibitors of inflammatory signaling repress the proinflammatory and phase shifting effects of palmitate;
-
•
Inflammatory signaling plays a role in the mechanism by which palmitate alters circadian timekeeping in peripheral clocks.
Circadian or 24-hour clocks throughout the body mediate the local temporal coordination of tissue- or cell-specific processes necessary for normal inflammatory responses and metabolic homeostasis. Dysregulation of peripheral clocks and their timekeeping function contribute to obesity-related metabolic disorders (e.g., type 2 diabetes). Our data unveil a novel mechanism by which mutual interactions between peripheral clocks and inflammatory signaling pathways dysregulate circadian timekeeping, and exacerbate proinflammatory responses to saturated fatty acids. These studies will guide the development of chronotherapeutic drug and/or dietary omega-3 fatty acid treatments for managing and preventing metabolic disorders and other inflammation-related pathologies (e.g., cardiovascular disease, stroke, arthritis).
1. Introduction
Over-nutrition, especially through the consumption of a HFD, is a critical factor in the rapidly increasing incidence of obesity. HFD-induced obesity is associated with systemic insulin resistance and the corresponding development of metabolic disorders such as type 2 diabetes, cardiovascular disease and non-alcoholic fatty liver disease (Angulo, 2002, Hossain et al., 2007, Jensen et al., 2008). Increasing evidence indicates that chronic low-grade inflammation in peripheral tissues contributes to HFD-mediated obesity and insulin resistance. HFD increases plasma levels of saturated fatty acids, which can trigger proinflammatory responses through the induction of NF-ĸB and JNK signaling. Thus, the precise modulation of free fatty acid levels and inflammatory signaling are vital for the maintenance of metabolic homeostasis.
Cell-autonomous circadian clocks in peripheral tissues are involved in the regulation of inflammatory responses and metabolic homeostasis. Endogenous clocks in immune cells provide for the circadian control of their abundance in the circulation, production of inflammatory factors, and functional responses to inflammatory challenge (Keller et al., 2009, Lange et al., 2010). Tissue- and cell-specific clocks also provide for the local coordination of circadian rhythms in the metabolism of fatty acids (Stenvers et al., 2012). For example, circulating levels of free fatty acids and adipose tissue expression of genes mediating lipolysis and fatty acid biosynthesis or transport are characterized by clock-controlled rhythmicity (Shostak et al., 2013). Moreover, genetic or environmental disruption of circadian clock function has been shown to potentiate inflammatory responses and to produce obesity and other metabolic disorders (Turek et al., 2005, Marcheva et al., 2010, Gibbs et al., 2012, Paschos et al., 2012). Our recent findings demonstrate that circadian clock disruption in bone marrow cells exacerbates HFD-induced tissue inflammation, adiposity, and systemic insulin dysregulation (Xu et al., 2014).
While the hierarchy of circadian clocks throughout the body is clearly involved in regulating inflammatory and metabolic processes, this interaction is not strictly unidirectional as inflammatory signals and fatty acid metabolism have been conversely implicated in the feedback modulation of the circadian clock mechanism. In this regard, the cytokines, TNF-α and IL-1β induce phase delays and HFD increases the free-running period of the activity rhythm in mice (Kohsaka et al., 2007, Leone et al., 2012, Xu et al., 2014). Thus, mutual interactions between circadian clocks and key mediators of inflammation may be important in maintaining metabolic homeostasis and in linking clock dysregulation and metabolic phenotypes in diet-associated obesity. Because inflammatory responses and corresponding metabolic disturbances in diet-induced obesity are differentially engaged by specific types of fatty acids found in HFDs, we compared the differential effects of palmitate, the prevalent proinflammatory SFA in HFD versus the anti-inflammatory, PUFA DHA on circadian clock function. The extent of the coupling between diet-mediated inflammatory responses and alterations in fundamental clock properties was examined by analyzing the phase-shifting effects of acute palmitate and DHA treatment at different times throughout the circadian cycle and then determining their coincidence with fatty acid-mediated induction of inflammatory signaling. To examine the role of inflammatory signaling in the mechanism by which SFAs modulate clock properties, experiments were also conducted to determine whether inhibition of proinflammatory responses blocks palmitate-induced phase shifts of peripheral circadian clocks.
2. Materials and Methods
2.1. Cell Culture
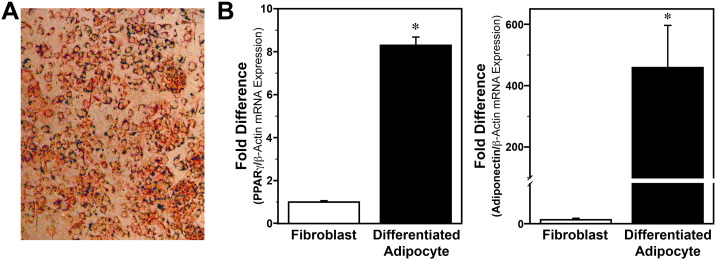
Bmal1-dLuc fibroblasts (Dr. Andrew Liu, University of Memphis, Memphis, TN; Ramanathan et al., 2012) were propagated on 60 mm dishes in Dulbecco's Modified Eagle Medium (DMEM; HyClone) containing 292 μg/ml l-glutamine, 10% Fetal Bovine Serum (FBS), 100 units/ml penicillin, and 100 μg/ml streptomycin and maintained at 37 °C and 5% CO2. Medium was replaced every 48 h and cultures were split 1:4 every 3 days. As established previously (Huo et al., 2010, Huo et al., 2012), adipocytes were differentiated from Bmal1-dLuc fibroblasts maintained in DMEM containing 10 μg/ml insulin, 1 μM dexamethasone, and 0.5 mM 3-isobutyl-1-methylxanthine for 48 h, and then incubated for 4 additional days in medium supplemented with 10 μg/ml insulin. Following differentiation, adipocytes were maintained for 2 days in normal growth medium prior to experimentation. Cell differentiation into adipocytes was verified by positive staining with Oil Red O (Fig. S1A) and by upregulated expression of PPARγ and adiponectin (Fig. S1B). While our analysis of these phenotypic markers indicates that Bmal1-dLuc fibroblasts exhibit many adipocyte-like properties following differentiation, it is unclear whether the cells are fully differentiated into mature adipocytes, thus warranting their subsequent designation as ‘differentiated adipocytes’.
Fig. S1.
Characterization of adipocyte phenotypes following differentiation of Bmal1-dLuc fibroblasts. (A) Representative microscopic image illustrating the cytoplasmic accumulation of lipids in Bmal1-dLuc cells stained with Oil Red O staining after differentiation using the methods of Huo et al., 2010, Huo et al., 2012. (B) Real-time PCR determinations (mean ± SEM) of PPARγ and adiponectin mRNA expression in Bmal1-dLuc fibroblast and differentiated adipocyte cultures. PPARγ (left panel) and adiponectin (right panel) mRNA signal were normalized to b-actin mRNA levels in each sample and the fold difference was determined by adjusting these values in relation to the average of undifferentiated fibroblast cultures, which was set at 1. The relative levels of PPARγ and adiponectin mRNA in differentiated adipocyte cultures were increased by 8-fold and 450-fold, respectively and were significantly greater (p < 0.05) than that found in undifferentiated Bmal1-dLuc fibroblasts.
2.2. Fatty Acid/Drug Preparation and Treatment
Palmitate (Sigma) and DHA (Nu-Chek-Prep, Inc.) were dissolved in ethanol and then diluted (1:5.4 ratio) with 10% BSA (fatty acid-free and low endotoxin) diluted in 0.1 M phosphate-buffered saline (PBS). Palmitate and DHA treatment in these studies was based on physiological concentrations that have been previously observed in vivo or used for in vitro studies (Ajuwon and Spurlock, 2005, Han et al., 2010, Puri et al., 2009, Weldon et al., 2007). Controls for fatty acid treatment contained BSA diluted in PBS with an equivalent ratio of ethanol.
AICAR (Tocris) or cardamonin (Tocris) were dissolved in DMSO and then diluted 1:400 and 1:10000 in culture medium to achieve final concentrations of 500 μM or 5 μM, respectively. Vehicle controls for AICAR and cardamonin treatment consisted of cultures in which an equivalent amount of DMSO was added to the medium.
2.3. Effect of Prolonged Fatty Acid Treatment on Circadian Period
Bmal1-dLuc fibroblasts were plated onto 35 mm dishes and ≈ 24 h later treated with either BSA (10% in PBS with EtOH), palmitate (150 μM), or DHA (150 μM) for 48 h. Following fatty acid treatment, cultures were rinsed and then maintained in recording media for 6–7 days during real-time analysis of Bmal1-dLuc bioluminescence.
2.4. Time-dependent Variation in the Phase Shifting and Proinflammatory Effects of Acute Fatty Acid Treatment
Bmal1-dLuc fibroblast cultures on 35 mm dishes were exposed for 2 h to medium containing 15 μM forskolin to facilitate circadian oscillation synchronization across cultures (Menger et al., 2007) and then treated for 4 h with BSA (10% in PBS with EtOH), palmitate (250 μM) or DHA (250 μM) at 6 h intervals throughout the circadian cycle. Cultures were subjected to control or fatty acid treatments at 6, 12, 18 or 24 h after forskolin administration and then placed in recording media for bioluminescence analysis of treatment-induced phase shifts of Bmal1-dLuc oscillations.
For parallel analyses of inflammatory responses to acute fatty acid treatment, confluent cultures of Bmal1-dLuc fibroblasts on 6-well plates were exposed for 2 h to 15 μM forskolin and then 6, 12, 18 or 24 h later treated with BSA, palmitate (250 μM) or DHA (250 μM) for 4 h. After treatment, cells were rinsed, collected, frozen in liquid nitrogen and stored at − 80 °C for subsequent analyses of NF-ĸB activation or cytokine mRNA expression.
2.5. Effect of Inflammatory Signaling Inhibitors on Fatty Acid-induced Phase Shifts of the Circadian Clock
Real-time analysis of cells transfected with an inducible NF-kB-responsive GFP construct was used to test whether treatment with DHA, AICAR, or cardamonin, a chalcone with anti-inflammatory activity (Ahmad et al., 2006), modulates palmitate-induced inflammatory signaling when its phase-shifting effects are maximal. GFP-reported NF-ĸB activity was quantified in cells that were treated with: 1) DHA (50 μM) for 12 h in advance and during exposure to palmitate (250 μM) for 4 h; or 2) AICAR (500 μM) or cardamonin (5 μM) in conjunction with palmitate (250 μM) administration for 4 h. Effects of these anti-inflammatory treatments on peak phase-shifting responses of the Bmal1-dLuc rhythm were examined in parallel cultures that were similarly treated with DHA, AICAR or cardamonin relative to palmitate exposure at hour 12. Following treatment, cultures were placed in recording media for bioluminescence analysis of treatment effects on palmitate-induced phase shifts of Bmal1-dLuc oscillations.
2.6. Protein Extraction and Western Blot analysis of NF-ĸB Activation
Bmal1-dLuc fibroblasts were lysed in mammalian protein extraction reagent (Pierce Biotechnology) containing protease and phosphatase inhibitor cocktail (Thermo Scientific). Protein content in cell homogenates was measured using the bicinchoninic acid method. NF-ĸB and phospho-NF-ĸB in cell lysates (10–30 μg protein/lane) were assessed by Western blot analysis using 10% Tris-Glycine gels as described previously (Shende et al., 2013). Membranes were probed with antibodies against phospho-NF-ĸB (p-p65; Cell Signaling Technology) or NF-ĸB (p-65; Cell Signaling Technology) followed by incubation with horse-radish peroxidase-conjugated goat anti-rabbit IgG (Biorad). Densitometric analyses of size-appropriate immunoreactive bands were performed using NIH ImageJ software. To control for inter-sample differences in protein content, p-NF-ĸB signal intensity was normalized to NF-ĸB values in each sample.
2.7. Real-time Reporter Assay of NF-ĸB Activity
The Cignal NF-ĸB Reporter Assay Kit (Qiagen) was used to quantify treatment-induced changes in the transcriptional regulatory activity of NF-ĸB. Cultures of Bmal1-dLuc fibroblasts (70–80% confluent) on opaque 96-well plates were transfected for 48 h with an inducible NF-kB-responsive GFP reporter construct (Qiagen) complexed with Lipofectamine® 3000 (Invitrogen). Approximately 24 h after transfection, cells were exposed to medium containing 15 μM forskolin and 12 h later treated with BSA or palmitate for 4 h. Experimental groups included cultures treated with: 1) DHA (50 μM) for 12 h in advance and during exposure to palmitate; or 2) AICAR (500 μM) or cardamonin (5 μM) in conjunction with palmitate administration. NF-ĸB signaling activity was assessed through quantitative measurement of GFP fluorescence and then compared relative to negative controls.
2.8. RNA Extractions and Real-time PCR
Total RNA was extracted from all cell lysates and relative quantification of interleukin-6 (IL-6) mRNA abundance was performed using SYBR-Green PCR technology [Applied Biosystems Inc. (ABI)] as described previously (Farnell et al., 2011, Xu et al., 2014). For each sample, real-time PCR analysis of IL-6 mRNA was performed on duplicate aliquots using the cDNA equivalent of 1 ng of total RNA. To control for differences in sample RNA content, β-actin mRNA was amplified with the cDNA equivalent of 1 ng total RNA from the same samples. Consistent with our previous studies (Xu et al., 2014), β-actin mRNA showed no sign of circadian variation. The comparative CT method was utilized to calculate the relative abundance for a given cytokine mRNA by normalization to corresponding β-actin levels in each sample and to a calibrator consisting of pooled cDNA from multiple samples.
2.9. Real-time Analysis of Bmal1-dLuc Rhythms
Prior to bioluminescence analysis, growth medium was removed and cultures were placed in DMEM recording medium containing 1 μM forskolin, 25 mM HEPES, 292 μg/ml l-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin and 100 μM luciferin (Promega). Cultures were sealed airtight with sterile glass coverslips, and sterile silicon grease. The temporal patterns of Bmal1-dLuc bioluminescence were analyzed using an automated 32-channel luminometer (LumiCycle; Actimetrics) housed in a standard culture incubator at 35 °C. Bioluminescence from individual cultures was continuously recorded for ~ 70 s at intervals of 10 min. Rhythm parameters were determined from baseline-subtracted data using the damped sine fit and Levenberg–Marquardt algorithm. The amplitude of phase shifts in response to fatty acid treatment was determined by measuring the time difference between the peaks of the Bmal1-dLuc rhythms during the third cycle in BSA or BSA/vehicle (DMSO) control and experimental treatment groups.
2.10. Statistical Analysis
Independent t-tests were performed to determine the significance of treatment effects (DHA, palmitate, AICAR, or cardamonin relative to vehicle controls) on NF-κB activity, IL-6 expression and palmitate-induced phase shifts. In each case, the α-value was set at 0.05. Time-dependent differences in phase-shifting effects of DHA or palmitate on the Bmal1-dLuc rhythm were first analyzed by one-way analysis of variance (ANOVA). Paired comparisons between peak phase-shifting responses and the corresponding minima were analyzed post-hoc for statistical differences using the Student Newman–Keuls sequential range test. The α-value was set at 0.05 for these post-hoc analyses.
3. Results
3.1. Effect of Prolonged Fatty Acid Treatment on Circadian Period
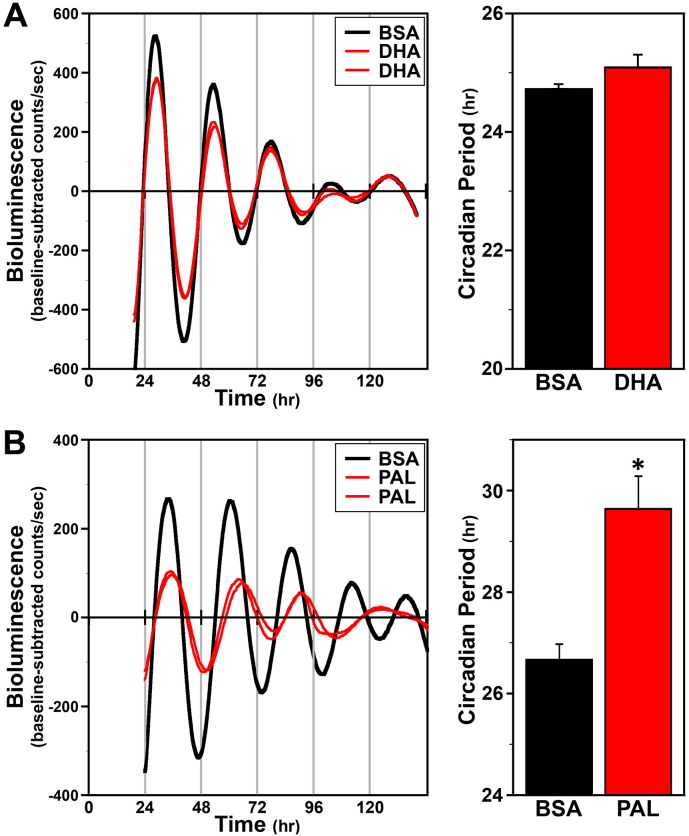
Given that HFD feeding induces pronounced alterations in circadian timekeeping in peripheral clocks (Xu et al., 2014), we first determined whether prolonged treatment with palmitate, the prevalent SFA in HFD, similarly affects clock gene oscillations in Bmal1-dLuc fibroblasts in vitro. All BSA-, DHA- and palmitate-treated fibroblast cultures exhibited circadian rhythms of Bmal1-dLuc bioluminescence that persisted at least 4–5 cycles (Fig. 1). The period of the Bmal1-dLuc rhythms in cultures exposed to DHA for 48 h was greater (by ≈ 0.4 h) but not significantly different (p = 0.14) relative to that observed in BSA-treated controls (Fig. 1A). In contrast, palmitate-treated fibroblast cultures were distinguished by Bmal1-dLuc rhythms in which the period was significantly increased (p > 0.05) by ≈ 3 h in comparison to BSA controls (Fig. 1B). Palmitate treatment also had a distinct effect in reducing rhythm amplitude to ≈ 40% of control values.
Fig. 1.
Effects of prolonged PUFA and SFA treatment on ensemble Bmal1-dLuc rhythms in cultured fibroblasts. Individual recordings of ensemble bioluminescence (expressed as detrended baseline-subtracted counts per second) from representative cultures of Bmal1-dLuc fibroblasts treated with: (A) BSA (n = 6) or 150 μM DHA (n = 8), and (B) BSA (n = 6) or 150 μM palmitate (n = 8). Bar graphs depict comparisons of the circadian period (mean + SEM) of the Bmal1-dLuc rhythms in BSA control and DHA- or palmitate-treated fibroblasts. Asterisk indicates that the period of the Bmal1-dLuc rhythms in palmitate-treated cultures was significantly greater (p < 0.05) than that in BSA controls.
3.2. Time-dependent Variation in the Phase Shifting and Proinflammatory Effects of Acute Fatty Acid Treatment
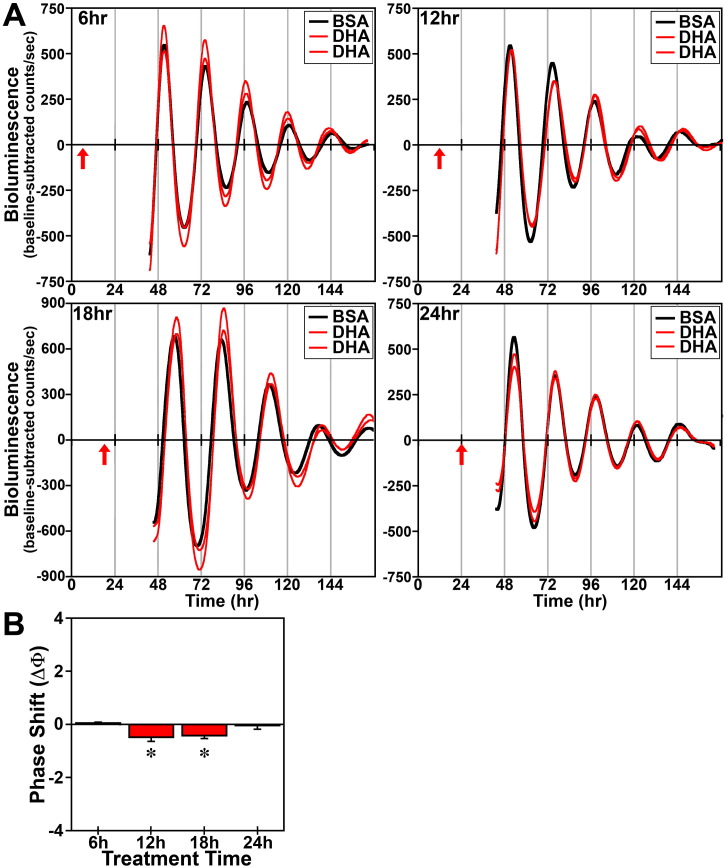
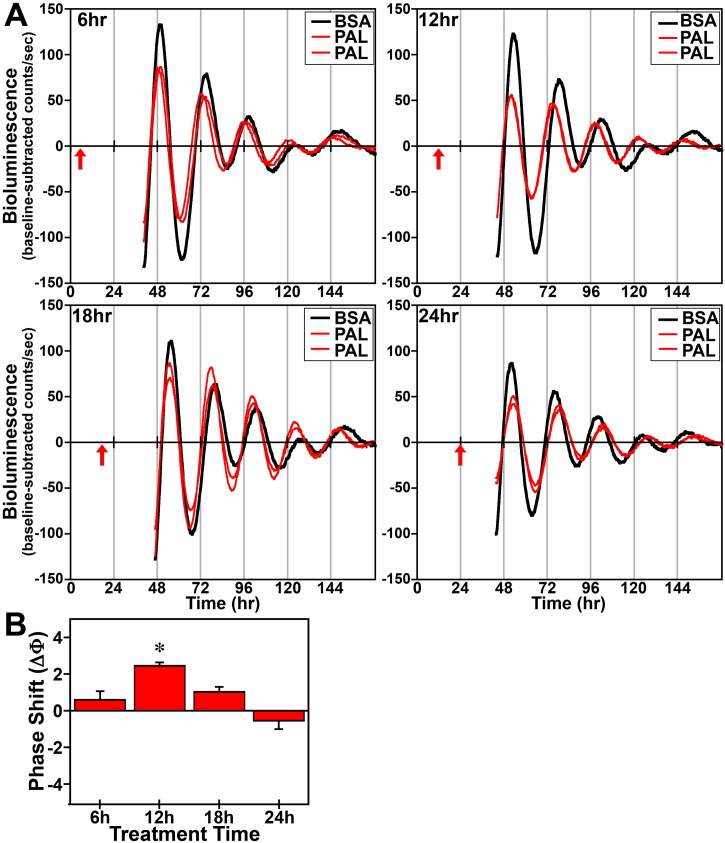
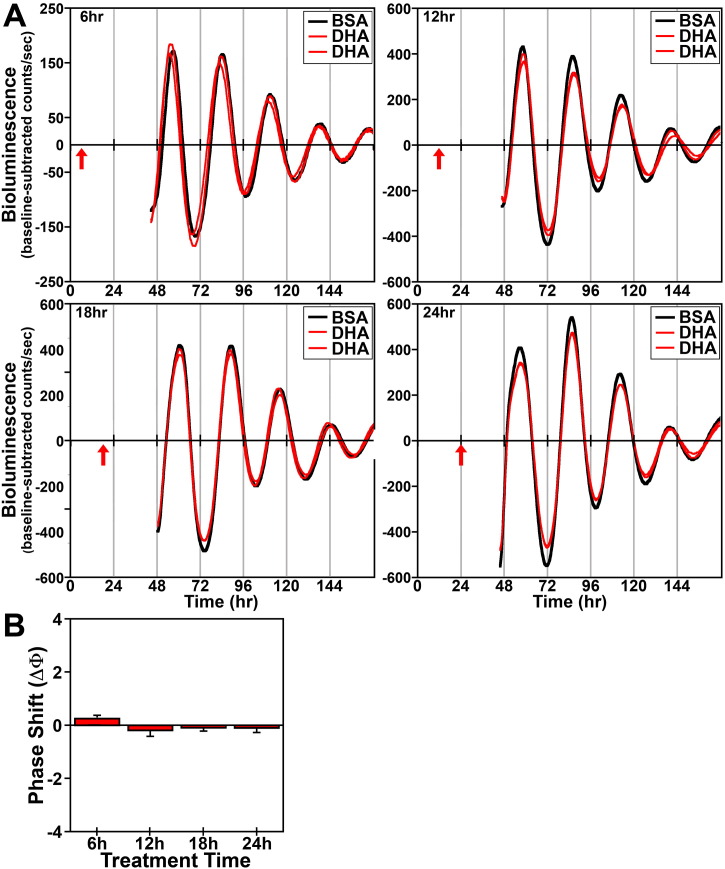
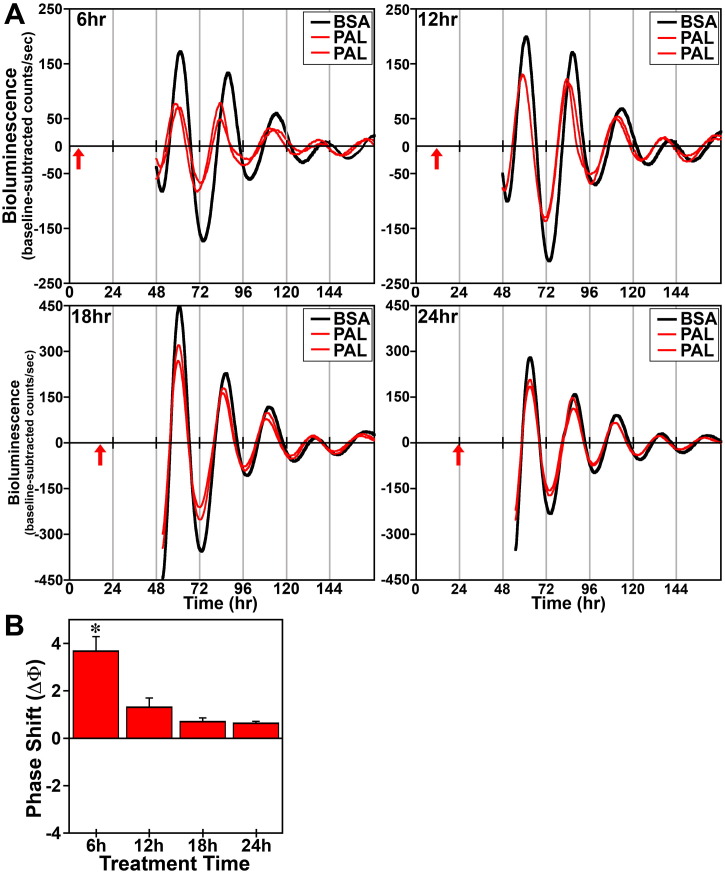
In response to acute (4 h) treatment at hours 6, 12, 18 and 24 with 250 μM DHA or palmitate, the period of the Bmal1-dLuc rhythms in cultures of undifferentiated fibroblasts was not significantly different from that observed in experiment-matched BSA controls. Phase shifting analysis revealed that DHA had negligible effects on the Bmal1-dLuc rhythm at hours 6 and 24 whereas treatment at hours 12 and 18 induced small phase delays of about 0.5 h (Fig. 2). Following bioluminescence analysis, no differences in cell viability were evident in cultures treated with 250 μM DHA. Similar to DHA, acute palmitate treatment shifted the phase of fibroblast Bmal1-dLuc rhythms in a time-dependent manner, but the directionality of these shifts was different and their amplitude was much larger (Fig. 3). At hours 12 and 18 when DHA induced small phase delays, palmitate produced the opposite effect: phase advances of fibroblast Bmal1-dLuc rhythms. Peak phase-shifting responses to palmitate were observed at hour 12 when treatment induced large phase advances of ≈ 2.5 h. Palmitate treatment at hour 18 also produced clear phase advances of the Bmal1-dLuc rhythm although the amplitude (≈ 1 h) was reduced relative to its phase-shifting effects at hour 12. Palmitate-induced phase shifts were minimal at hours 6 and 24, with treatment inducing small advances and delays of ≈ 0.5 h respectively at these timepoints. Unlike prolonged exposure, acute treatment with DHA or palmitate had little or no consistent effect on the amplitude of the Bmal1-dLuc rhythm.
Fig. 2.
Phase-shifting effects of acute DHA treatment on ensemble Bmal1-dLuc rhythms in cultured fibroblasts. (A) Representative recordings of ensemble bioluminescence from individual cultures of Bmal1-dLuc fibroblasts treated for 4 h with BSA (n = 4) or 250 μM DHA (n = 8) at hours 6, 12, 18 and 24. Red arrows indicate time of BSA or DHA treatment. (B) Time-dependent phase shifts of fibroblast Bmal1-dLuc rhythms in response to DHA treatment for 4 h. Mean (+ SEM) phase shifts (ΔΦ) in hours are plotted as a function of the timing of DHA treatment (at hours 6, 12, 18 and 24). Phase delays of the fibroblast Bmal1-dLuc rhythm are indicated by negative values and advances are denoted by positive values. Asterisks indicate that phase-shifting responses to DHA at hours 12 and 18 were significantly greater (p < 0.05) than those observed during the corresponding minima.
Fig. 3.
Phase-shifting effects of acute palmitate (PAL) treatment on ensemble Bmal1-dLuc rhythms in cultured fibroblasts. (A) Representative recordings of ensemble bioluminescence from individual cultures of Bmal1-dLuc fibroblasts treated for 4 h with BSA (n = 5–6) or 250 μM PAL (n = 5–6) at hours 6, 12, 18 and 24. Red arrows indicate time of BSA or PAL treatment. (B) Time-dependent phase shifts (mean ± SEM) of fibroblast Bmal1-dLuc rhythms in response to PAL treatment at hours 6, 12, 18 and 24. The asterisk indicates that peak phase-shifting responses to PAL at hour 12 were significantly greater (p < 0.05) than those observed during the corresponding minima.
To determine whether DHA or palmitate phase shift clock gene rhythms in other peripheral cell types and whether the time-dependent nature of phase-shifting responses differs among peripheral circadian clocks, parallel analysis was performed on adipocytes differentiated from Bmal1-dLuc fibroblasts. Irrespective of treatment time, DHA had little or no phase-shifting effects on the Bmal1-dLuc rhythm in differentiated adipocytes (Fig. 4). DHA produced small phase advances (≈ 0.25 h) at hour 6 and phase delays (≈ 0.2 h) at hour 12 whereas phase-shifting effects of this PUFA were marginal (< 0.1 h) at hours 18 and 24. Similar to its effects on undifferentiated fibroblasts, acute palmitate treatment shifted the phase of adipocyte Bmal1-dLuc rhythms (Fig. 5). However, the time-dependent variation in adipocyte phase-shifting responses to palmitate was markedly different from the pattern in undifferentiated fibroblasts. Palmitate-induced phase shifts in differentiated adipocytes were maximal at hour 6, when treatment had negligible effects on the phase of undifferentiated fibroblast Bmal1-dLuc rhythms. At hour 6, palmitate advanced the phase of adipocyte Bmal1-dLuc rhythms by ≈ 4 h. In differentiated adipocyte cultures, palmitate also induced small phase advances (≈ 1.3 h) at hour 12 but had minimal effects on the Bmal1-dLuc rhythm at hours 18 and 24, producing phase delays of less than 0.7 h. Bmal1-dLuc rhythms in differentiated adipocytes showed no clear signs of DHA- or palmitate-induced changes in amplitude.
Fig. 4.
Phase-shifting effects of acute DHA treatment on ensemble Bmal1-dLuc rhythms in cultures of differentiated adipocytes. (A) Representative recordings of ensemble bioluminescence from individual cultures of differentiated Bmal1-dLuc adipocytes treated for 4 h with BSA (n = 4) or 250 μM DHA (n = 5–10) at hours 6, 12, 18 and 24. Red arrows indicate the approximate time at which cultures were exposed to BSA or DHA. (B) Time-dependent phase shifts (mean ± SEM) of adipocyte Bmal1-dLuc rhythms in response to DHA treatment at hours 6, 12, 18 and 24.
Fig. 5.
Phase-shifting effects of acute palmitate (PAL) treatment on ensemble Bmal1-dLuc rhythms in cultures of differentiated adipocytes. (A) Representative recordings of ensemble bioluminescence from individual cultures of differentiated Bmal1-dLuc adipocytes treated for 4 h with BSA (n = 6) or 250 μM PAL (n = 6) at hours 6, 12, 18 and 24. Red arrows indicate time of BSA or PAL treatment. (B) Time-dependent phase shifts (mean ± SEM) of differentiated adipocyte Bmal1-dLuc rhythms in response to PAL treatment at hours 6, 12, 18 and 24. The asterisk indicates that peak phase-shifting responses to PAL at hour 6 were significantly greater (p < 0.05) than those observed during the corresponding minima.
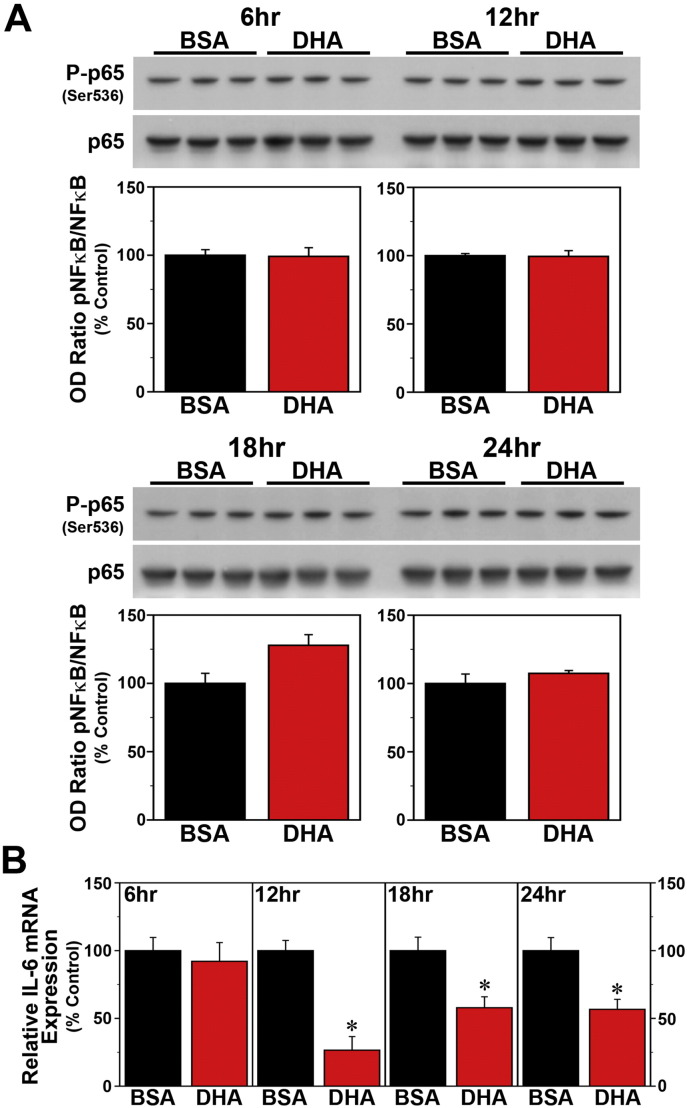
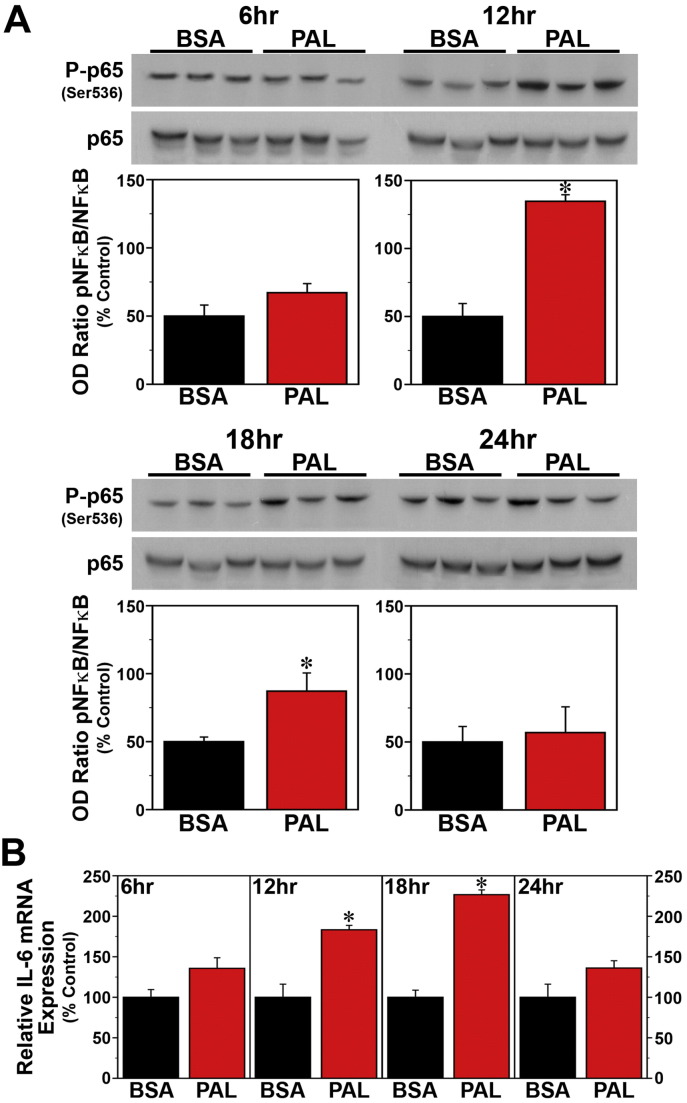
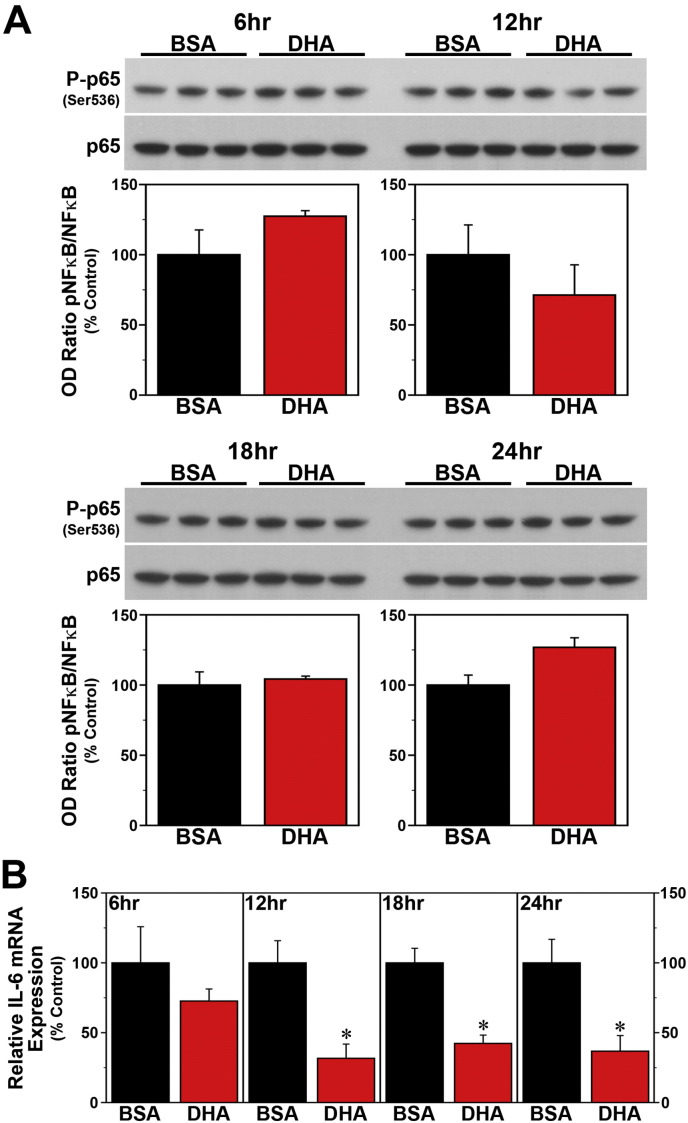
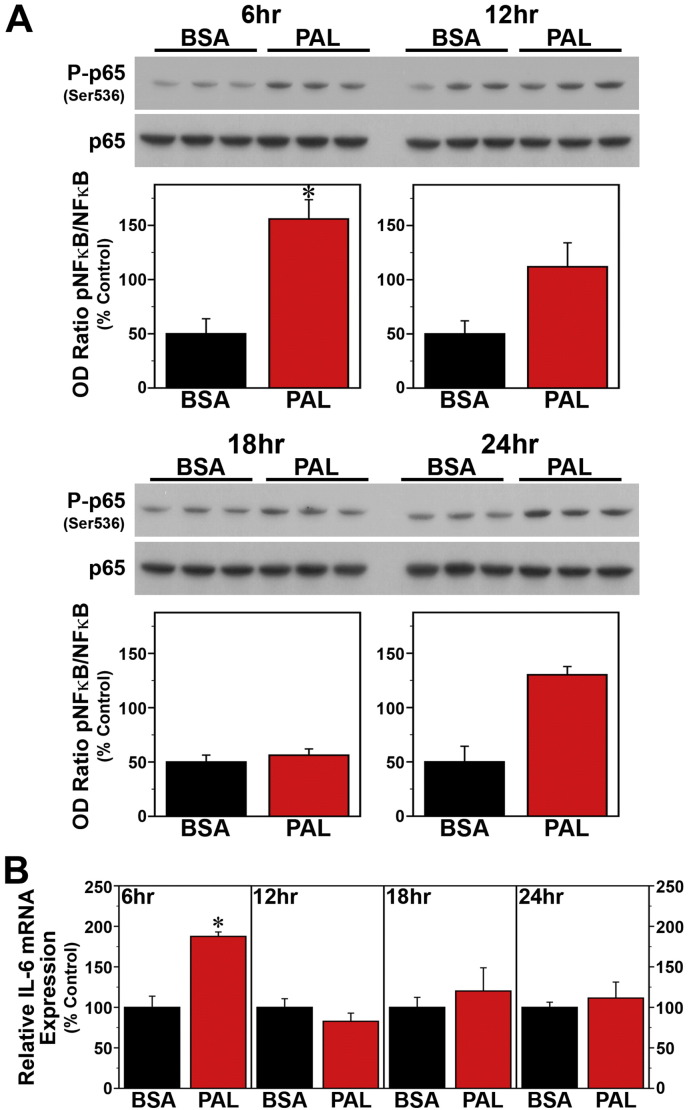
Since SFAs trigger NF-κB-mediated signaling that leads to the induction of proinflammatory cytokines (Ajuwon and Spurlock, 2005, Maloney et al., 2009, Wang et al., 2012), we next determined whether the effects of palmitate on these key mediators of fatty acid-induced inflammation are both time-dependent and coincident with the temporal variation in the phase-shifting responses of the clock mechanism to this SFA. Consistent with previous reports (Novak et al., 2003, Gladine et al., 2014), acute DHA treatment had no effect on NF-κB activation but modulated expression of the proinflammatory cytokine IL-6 in Bmal1-dLuc fibroblasts. In cultures treated with DHA at hours 6, 12, 18 or 24, levels of NF-κB phosphorylation (Fig. 6A) were not significantly different from those in time-matched BSA controls. However, DHA administration at hours 12, 18 and 24 induced significant decreases (p < 0.05) in IL-6 mRNA expression (≈ 40–75%) relative to BSA-treated fibroblasts (Fig. 6B). In contrast to DHA, palmitate induced both NF-κB signaling and IL-6 mRNA expression in Bmal1-dLuc fibroblasts in a time-dependent manner. Relative to BSA-treated cultures, palmitate induced significant increases (p < 0.05) in NF-ĸB phosphorylation at hours 12 and 18 but not at hours 6 and 24 (Fig. 7A), coinciding with the temporal variation in the amplitude of phase-shifting responses to this SFA. Palmitate-induced IL-6 expression in Bmal1-dLuc fibroblasts provided further evidence of the close temporal coincidence between proinflammatory and phase-shifting responses to treatment; in palmitate-treated cultures, IL-6 mRNA expression was significantly increased (p < 0.05) by 180–225% at hours 12 and 18 relative to BSA controls but this inductive effect was blunted (≈ 135%) at hours 6 and 24 (Fig. 7B).
Fig. 6.
Time-dependent effects of acute DHA treatment on inflammatory signaling in Bmal1-dLuc fibroblasts. (A) Representative Western blot and densitometric analyses of NF-κB-mediated inflammatory signaling in fibroblast cultures treated for 4 h with BSA (n = 3) or 250 μM DHA (n = 3) at hours 6, 12, 18 and 24. Bar graphs depict the ratios of P-p65/p65 immunoreactive signal that were adjusted in relation to control (BSA) values (% Control). (B) Real-time PCR determinations (mean ± SEM) of IL-6 mRNA expression in parallel groups of BSA- and DHA-treated fibroblast cultures. Plotted values correspond to the ratio of IL-6 mRNA signal normalized to β-actin mRNA levels in each sample and adjusted relative to the averages of time-matched BSA controls, which were set at 100%. Asterisks denote treatment times in which the relative expression of IL-6 mRNA in DHA-treated fibroblasts was significantly decreased (p < 0.05) in comparison with that found in BSA controls.
Fig. 7.
Time-dependent effects of acute palmitate (PAL) treatment on inflammatory signaling in Bmal1-dLuc fibroblasts. (A) Representative Western blot and densitometric quantification (mean ± SEM) of P-p65/p65 immunoreactive signal (% Control) in fibroblast cultures treated for 4 h with BSA (n = 3) or 250 μM PAL (n = 3) at hours 6, 12, 18 and 24. (B) IL-6 mRNA expression (mean ± SEM) in parallel groups of BSA- and PAL-treated fibroblast cultures. Asterisks denote treatment times in which NF-κB phosphorylation or the relative expression of IL-6 mRNA in PAL-treated fibroblasts were significantly increased (p < 0.05) compared to that found in control cultures.
The effects of acute DHA treatment on NF-κB activation and IL-6 expression in differentiated adipocytes were similar to those observed in undifferentiated fibroblasts. Following administration at hours 6, 12, 18 and 24, DHA-treated adipocyte cultures and time-matched BSA controls exhibited no significant differences in levels of NF-κB phosphorylation (Fig. 8A). However, IL-6 mRNA expression was significantly decreased (p < 0.05) by ≈ 60–70% in response to DHA administration at hours 12, 18 and 24 relative to that observed in BSA-treated adipocytes (Fig. 8B). At hour 6, IL-6 mRNA expression in DHA-treated cultures was decreased but not significantly different in comparison to time-matched controls. In differentiated adipocytes, palmitate treatment produced time-dependent increases in both NF-κB activation and IL-6 mRNA expression. Peak induction of NF-ĸB phosphorylation was observed in response to palmitate administration at hour 6 (Fig. 9A), when phase-shifting responses to this SFA were maximal in differentiated adipocytes. Relative to time-matched BSA controls, palmitate induced significant increases (p < 0.05) in NF-ĸB activation not only at hour 6 but also at hour 24. NF-κB phosphorylation in palmitate-treated adipocyte cultures was moderately elevated at hour 12 but unchanged at hour 18 in comparison with the levels observed in BSA controls. The effects of palmitate on IL-6 expression in differentiated adipocytes closely coincided with the timing of peak phase-shifting responses to this SFA. Palmitate had no significant effect on IL-6 mRNA levels following administration at hours 12, 18 and 24 (Fig. 9B) but significantly increased (p < 0.05) expression of this proinflammatory cytokine (≈ 188%) at hour 6, when treatment induced maximal phase advances of adipocyte Bmal1-dLuc rhythms.
Fig. 8.
Time-dependent effects of acute DHA treatment on inflammatory signaling in differentiated Bmal1-dLuc adipocytes. (A) Representative Western blot and densitometric quantification (mean ± SEM) of P-p65/p65 immunoreactive signal (% Control) in differentiated adipocyte cultures treated for 4 h with BSA (n = 3) or 250 μM DHA (n = 3) at hours 6, 12, 18 and 24. (B) Determinations (mean ± SEM) of IL-6 mRNA expression in parallel groups of BSA- and DHA-treated differentiated adipocyte cultures. Asterisks denote treatment times in which the relative expression of IL-6 mRNA in DHA-treated differentiated adipocytes was significantly decreased (p < 0.05) in comparison with that found in BSA controls.
Fig. 9.
Time-dependent effects of acute palmitate (PAL) treatment on inflammatory signaling in differentiated Bmal1-dLuc adipocytes. (A) Representative Western blot and densitometric quantification (mean ± SEM) of P-p65/p65 immunoreactive signal (% Control) in differentiated adipocyte cultures treated for 4 h with BSA (n = 3) or 250 μM PAL (n = 3) at hours 6, 12, 18 and 24. (B) IL-6 mRNA expression (mean ± SEM) in parallel groups of BSA- and PAL-treated differentiated adipocyte cultures. Asterisks denote treatment times in which NF-κB phosphorylation or the relative expression of IL-6 mRNA in PAL-treated differentiated adipocytes were significantly increased (p < 0.05) compared to that found in control cultures.
3.3. Effect of Inhibiting Inflammatory Signaling on Fatty Acid-induced Phase Shifts of the Circadian Clock
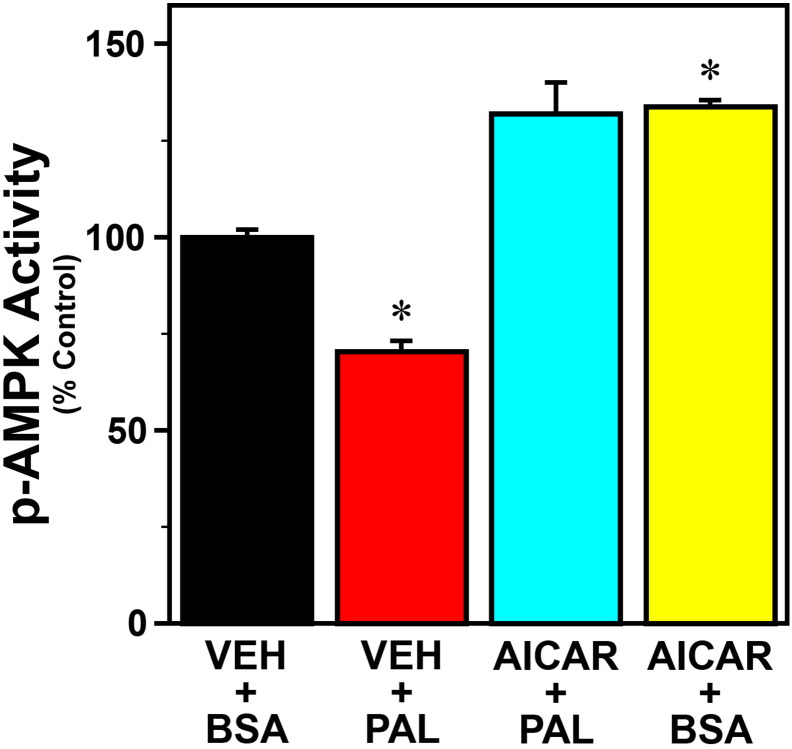
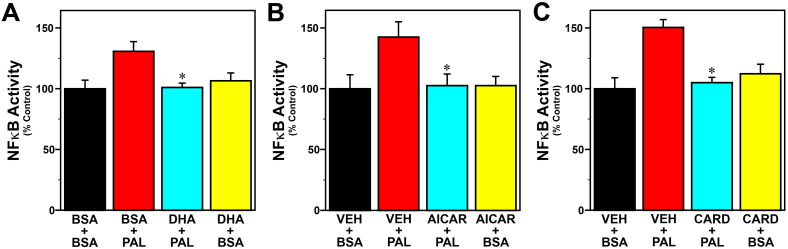
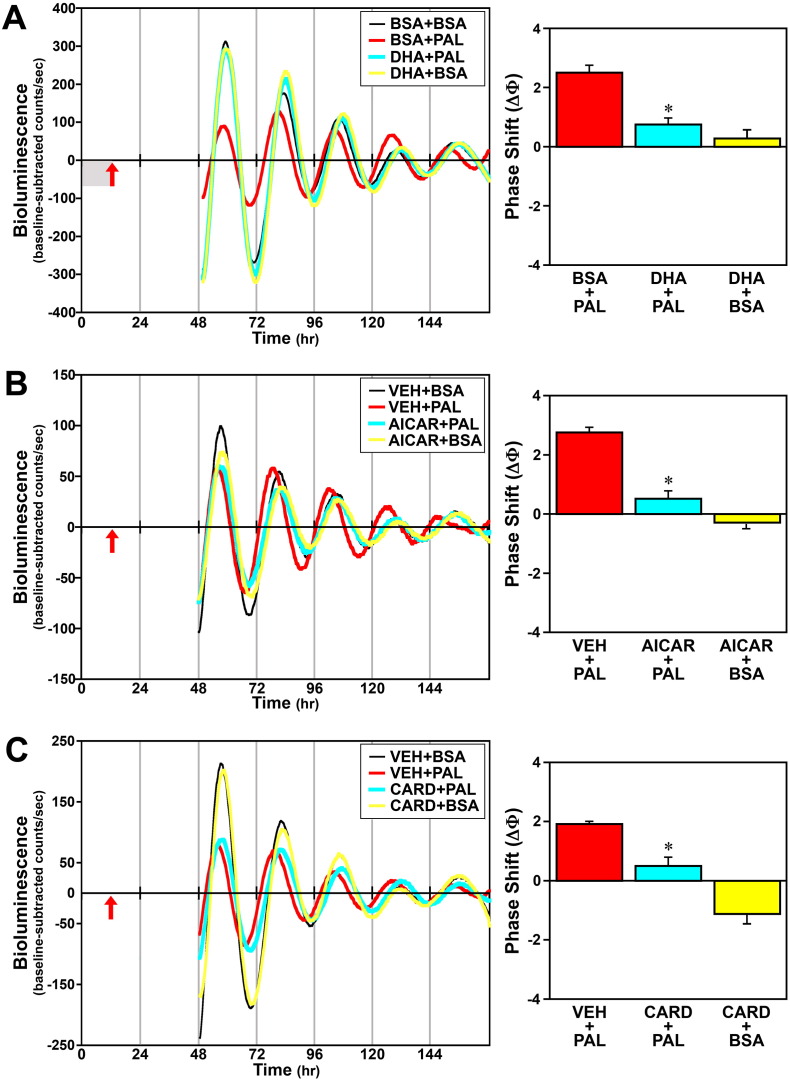
To examine the role of inflammatory signaling in the mechanism by which palmitate modulates circadian clock function, we next determined whether the omega-3 fatty acid DHA and other inhibitors of inflammation abate peak inflammatory and phase-shifting responses to this SFA at hour 12 in Bmal1-dLuc fibroblasts. Because AMPK plays a role in regulating fatty acid oxidation and inhibiting NF-ĸB-mediated inflammatory responses (Salminen et al., 2011), the AMPK activator, AICAR, was utilized in parallel to inhibit palmitate-induced inflammatory signaling. Consistent with previous reports on AMPK regulation in response to palmitate and other SFAs (Sun et al., 2008, Lindholm et al., 2013), phospho-AMPK activity was significantly decreased (p < 0.05) in Bmal1-dLuc fibroblasts treated with palmitate and was significantly increased (p < 0.05) following exposure to AICAR alone (Fig. S2). Palmitate administration at hour 12 induced a 35–40% increase in GFP-reported NF-κB activation in Bmal1-dLuc fibroblasts, but treatment with DHA (Fig. 10A), AICAR (Fig. 10B) or cardamonin (Fig. 10C) significantly inhibited (p < 0.05) the inductive effects of this SFA on NF-κB such that signaling activity was comparable to the basal levels found in vehicle/BSA controls. Treatment with DHA, AICAR or cardamonin similarly inhibited the phase-shifting responses of Bmal1-dLuc fibroblasts to palmitate. In DHA-treated cultures, palmitate-induced phase shifts of the Bmal1-dLuc rhythm were significantly decreased (p < 0.05) relative to those observed in controls (Fig. 11A). Administration of palmitate alone produced 2.5-hour phase advances in vehicle-pretreated controls whereas the amplitude of these shifts was reduced by 70% following pretreatment with DHA. Little or no phase-shifting effect was observed in response to DHA alone (+ BSA at hour 12). Similar to the effects of DHA, AICAR significantly decreased (p < 0.05) the phase-shifting responses of fibroblast Bmal1-dLuc rhythms to palmitate administration at hour 12 (Fig. 11B). Once again, palmitate alone induced large phase advances of ≈ 2.8 h in vehicle-pretreated cultures. In comparison, the amplitude of these palmitate-induced phase shifts was abated by ≈ 80% in AICAR-treated fibroblasts. Phase-shifting effects of AICAR alone were minimal as only small advances of the Bmal1-dLuc rhythm (< 0.5 h) were observed in cultures treated with BSA and AICAR at hour 12. Concurrent treatment with cardamonin, an anti-inflammatory chalconoid that inhibits LPS-stimulated NF-κB signaling (Chow et al., 2012), also had a significant effect (p < 0.05) in repressing palmitate-induced phase shifts of fibroblast Bmal1-dLuc rhythms at hour 12. Palmitate induced phase advances of ≈ 2 h in vehicle-treated cultures but only advanced Bmal1-dLuc rhythms by 0.5 h or less in cultures concurrently treated with cardamonin (Fig. 11C). Treatment with cardamonin alone (+ BSA at hour 12) induced small phase delays (≈ 1 h).
Fig. S2.
Effects of palmitate (PAL) and AICAR treatment on phospho-AMPK activity in Bmal1-dLuc fibroblasts. Using an ELISA assay for phospho-AMPKα (Invitrogen), p-AMPK activity was analyzed in cultures treated with AICAR (500 μM) in conjunction with palmitate (250 μM) administration. Effects of treatment with PAL (VEH + PAL) or AICAR (AICAR + BSA) alone on p-AMPK activity were also tested. Control cultures were treated with vehicle (DMSO) and BSA (VEH + BSA). Plotted values denote determinations of colorimetric signal intensity (mean ± SEM) in each treatment group (n = 4) that were normalized to the average signal for controls, which was arbitrarily set at 100%. Phospho-AMPK activity was significantly decreased (p < 0.05) in Bmal1-dLuc fibroblasts treated with PAL (VEH + PAL) and was significantly increased (p < 0.05) in cultures treated with AICAR alone (AICAR + BSA) relative to that found in controls (VEH + BSA).
Fig. 10.
Effects of DHA (A), AICAR (B) or cardamonin (C) treatment on NF-κB-regulated inflammatory responses of Bmal1-dLuc fibroblasts to palmitate (PAL; 250 μM). Bar graphs depict real-time fluorometric analysis of cells transfected with an inducible NF-κB-responsive GFP construct that were treated with DHA (50 μM), AICAR (500 μM) or cardamonin (5 μM) in advance of and/or during palmitate (250 μM) administration for 4 h at hour 12. Effects of treatment with DHA, AICAR or cardamonin alone (DHA + BSA, AICAR + BSA, CARD + BSA) on NF-κB activity were also tested. Control cultures were treated with BSA or vehicle (DMSO) and BSA (VEH + BSA). Plotted values denote determinations of GRP signal intensity (mean ± SEM) in each treatment group (n = 5) that were normalized to the average signal for controls, which was arbitrarily set at 100%. Asterisks indicate that palmitate-induced NF-κB activity was significantly decreased (p < 0.05) in Bmal1-dLuc fibroblasts treated with DHA (DHA + PAL), AICAR (AICAR + PAL) or cardamonin (CARD + PAL) in comparison with those exposed to palmitate alone (BSA + PAL or VEH + PAL).
Fig. 11.
Effects of DHA (A), AICAR (B) or cardamonin (C, CARD) treatment on the phase-shifting responses of Bmal1-dLuc fibroblasts to palmitate (PAL). Representative recordings of ensemble bioluminescence from individual fibroblast cultures treated with either vehicle (VEH; DMSO), DHA (50 μM) for 12 h in advance or AICAR (500 μM) or cardamonin (5 μM) for 4 h in conjunction with PAL administration (250 μM) for 4 h at hour 12. Phase-shifting effects of these anti-inflammatory treatments alone were also tested in BSA control cultures treated with DHA for 12 h, or with AICAR or cardamonin for 4 h. Specific experimental groups include: BSA + BSA or VEH + BSA (n = 15), BSA + PAL or VEH + PAL (n = 18), DHA + PAL (n = 6), DHA + BSA (n = 6), AICAR + PAL (n = 10), AICAR + BSA (n = 8), CARD + PAL (n = 6) and CARD + BSA (n = 6). Red arrows denote time of palmitate administration after 12 h pretreatment with DHA (adjacent shaded area in A) or during AICAR (B) or cardamonin treatment (C). Bar graphs depict the mean (± SEM) phase shifts (ΔΦ) in hours as a function of treatment group. Asterisks indicate that palmitate-induced phase shifts were significantly decreased (p < 0.05) in Bmal1-dLuc fibroblasts treated with DHA (DHA + PAL), AICAR (AICAR + PAL) or cardamonin (CARD + PAL) in comparison with those observed in controls (VEH + PAL).
4. Discussion
While circadian clocks throughout the body clearly play a role in regulating metabolic pathways and their homeostatic function, increasing evidence for diet-induced alterations in clock gene oscillations and behavioral rhythms suggests that nutrient metabolism may, in turn, feed back on the clock mechanism and modulate fundamental properties of circadian rhythms. HFD has been shown to alter circadian timekeeping function although its effects vary greatly among clocks in the brain and peripheral tissues. For example, HFD had no effect on core clock gene oscillations in the hypothalamus and only produced small increases in the free-running period of the SCN-regulated rhythm of locomotor activity (Kohsaka et al., 2007, Xu et al., 2014). Consistent with the effects of HFD on circadian clocks in the brain, palmitate alters clock gene expression and the amplitude of their rhythmic profiles in a hypothalamic cell line and this effect is not accompanied by a corresponding induction of inflammatory cytokines such as IL-6 (Fick et al., 2011, Greco et al., 2014). In contrast, HFD-induced dysregulation of circadian timekeeping is more robust in peripheral clocks; in HFD-fed mice, clock gene oscillations are distinguished by severe damping in both liver and fat tissue (Kohsaka et al., 2007) and by circadian period increases of up to 9 h in adipose tissue and bone marrow-derived macrophages (Xu et al., 2014). Similar to the latter effect of HFD, prolonged treatment with palmitate induced large increases in the period of fibroblast Bmal1-dLuc rhythms, suggesting that this proinflammatory SFA is a key mediator of HFD-induced modulation of peripheral circadian clocks.
Importantly, our findings indicate that the effects of HFD and its major constituent, palmitate, on circadian clock properties are not limited to changes in period and rhythm amplitude, but include differential phase shifts of clock gene oscillations in peripheral tissues. Previous studies have established that phase-shifting responses to HFD again differ among peripheral and central circadian clocks. In this regard, HFD treatment for 7 days has been shown to shift Per2 oscillations, producing large advances in the liver and small delays in the spleen but no effect on the phase of SCN rhythms (Pendergast et al., 2013). In the present study, 4-hour exposure of peripheral cell types to palmitate similarly induced marked phase shifts of clock gene oscillations that varied in amplitude depending on the time of treatment. It is noteworthy that the time-dependent nature of palmitate-mediated phase shifts of the Bmal1 rhythm was cell-specific; the phase responses of undifferentiated fibroblasts to this proinflammatory SFA were maximal at hour 12 and negligible at hour 6 when palmitate-induced phase advances were at peak amplitude in differentiated adipocytes. Because the omega-3 fatty acid DHA had little or no phase-shifting effects on clock gene oscillations in both fibroblasts and differentiated adipocytes, these findings collectively suggest that SFAs may differentially reset the clock mechanism in some but not all cells within a given tissue, thereby altering the local coordination of circadian timekeeping among individual cellular clocks.
How HFD and palmitate modulate circadian timekeeping is unknown. The present evidence for the close coincidence between the time-dependent variation in inflammatory and phase-shifting responses to palmitate raises the possibility that mutual interactions between peripheral circadian clocks and inflammatory signaling pathways may play a key role in the underlying mechanism. In both Bmal1-dLuc fibroblasts and differentiated adipocytes, the maxima and minima for palmitate-induced inflammatory signaling were contemporaneous with the equivalent variation in the amplitude of the phase shifts induced by this SFA. Thus, the signaling cascades through which HFD and SFAs induce inflammation in peripheral tissues may govern the modulatory effects of palmitate on circadian timekeeping. The induction of inflammatory signaling by palmitate and other SFAs is mediated through activation of NF-κB, ultimately leading to the secretion of proinflammatory cytokines in various peripheral tissues (Weigert et al., 2004, Ajuwon and Spurlock, 2005, Jové et al., 2006). In addition, the nutrient sensor, AMP-activated protein kinase (AMPK), is involved in coupling fatty acid metabolism to inflammatory responses. Palmitate and other SFAs decrease AMPK activity (Sun et al., 2008, Lindholm et al., 2013) and correspondingly their inductive effects on NF-κB activation and the expression of proinflammatory cytokines are repressed by AMPK activators (Yang et al., 2010, Green et al., 2011). The role of inflammatory signaling in SFA-mediated phase regulation of peripheral circadian clocks is directly supported by the present findings that the anti-inflammatory inhibitor of NF-ĸB signaling, cardamonin, and the AMPK activator, AICAR, suppress peak induction of both inflammatory and phase-shifting responses to palmitate at hour 12. Because DHA represses inflammation-induced NF-ĸB signaling and cytokine expression via activation of AMPK (Xue et al., 2012), the observed inhibition of palmitate-induced inflammation and phase shifts following pretreatment with this PUFA also underscores the importance of clock-gated inflammatory signaling in the mechanism by which palmitate alters circadian timekeeping. In conjunction with evidence for the circadian regulation of AMPK activity (Um et al., 2011) and inflammatory responses of the NF-ĸB pathway (Spengler et al., 2012) in different tissues, these observations indicate that mutual interactions between peripheral clocks and inflammatory signaling mediate the time-dependent variation in the extent to which SFA overload triggers inflammation and in turn, leads to the feedback modulation of circadian timekeeping.
The endoplasmic reticulum (ER) stress pathway may also be involved in the palmitate-mediated regulation of circadian clock properties. Recent evidence supports a link between the circadian clock and ER homeostasis involving mutual regulation of each other (Pluquet et al., 2014). Moreover, SFAs such as palmitate induce ER stress via multiple mechanisms (Milanski et al., 2009, Baldwin et al., 2012, Ariyama et al., 2010) whereas the omega-3 fatty acid DHA attenuates ER stress (Kolar et al., 2011). Because ER stress has been linked to inflammation in metabolic disorders (Cao et al., 2016), further studies are needed to determine whether ER stress plays a role in palmitate-mediated modulation of circadian timekeeping in parallel to, or perhaps in concert with, the inductive effects of this SFA on inflammatory signaling.
The association between circadian clock dysfunction and metabolic disorders is largely based on studies that involve the complete disruption of rhythmicity using genetic or environmental approaches. However, the present data have novel implications for how differential and cell-specific modulation, rather than complete malfunction, of peripheral circadian clocks may be germane factors in the amplification of proinflammatory responses to HFD and SFA excess that are closely linked to systemic metabolic dysregulation. In both fibroblasts and differentiated adipocytes, palmitate-mediated inflammatory signaling is gated rhythmically and at times of peak induction, provides feedback that modulates the phase of the core clock mechanism. Because the phase-shifting responses to palmitate appear to differ among peripheral cell types, this feedback modulation is likely to disrupt the local coordination of circadian timekeeping among cell-autonomous clocks within a given tissue, thus further potentiating SFA-induced inflammation that contributes to systemic insulin resistance. The palmitate-induced inflammatory signals directly responsible for the feedback modulation of circadian phase remain to be determined, but future studies are warranted to examine the role of proinflammatory cytokines such as IL-6 in mediating the phase-shifting effects of this SFA. Finally, these interactions between peripheral circadian clocks and pathways linking fatty acid metabolism to tissue inflammation suggest that chronotherapeutic strategies using DHA and/or metformin, an AMPK agonist exploited in the treatment of hyperglycemia in type II diabetes (Akbar, 2003), may be critical in the management of metabolic diseases associated with fatty acid overload.
The following are the supplementary data related to this article.
Disclosure Statement
The authors have no conflicts of interest to disclose.
Author Contributions
Conceptualization, S.-M.K., N.N., R.S.C. and D.J.E.; Methodology, S.-M.K., N.N., R.S.C. and D.J.E.; Investigation, S.-M.K., N.N., R.S.C. and D.J.E.; Writing — Original Draft, S.-M.K., N.N., R.S.C. and D.J.E.; Writing — Review & Editing, S.-M.K., N.N., R.S.C. and D.J.E.; Funding Acquisition, R.S.C. and D.J.E.; Resources, R.S.C. and D.J.E.; Supervision, R.S.C. and D.J.E.
Acknowledgments
This study was supported by the Center for Translational Environmental Health Research P30ES023512 (R.S.C.) and NIH grant R35CA197707 (R.S.C.).
Contributor Information
Robert S. Chapkin, Email: r-chapkin@tamu.edu.
David J. Earnest, Email: dearnest@medicine.tamhsc.edu.
References
- Ahmad S., Israf D.A., Lajis N.H., Shaari K., Mohamed H., Wahab A.A., Ariffin K.T., Hoo W.Y., Aziz N.A., Kadir A.A., Sulaiman M.R., Somchit M.N. Cardamonin, inhibits pro-inflammatory mediators in activated RAW 264.7 cells and whole blood. Eur. J. Pharmacol. 2006;538:188–194. doi: 10.1016/j.ejphar.2006.03.070. [DOI] [PubMed] [Google Scholar]
- Ajuwon K.M., Spurlock M.E. Palmitate activates the NF-κB transcription factor and induces IL-6 and TNFα expression in 3T3-L1 adipocytes. J. Nutr. 2005;135:1841–1846. doi: 10.1093/jn/135.8.1841. [DOI] [PubMed] [Google Scholar]
- Akbar D.H. Effect of metformin and sulfonylurea on C-reactive protein level in well-controlled type 2 diabetes with metabolic syndrome. Endocrine. 2003;20:215–218. doi: 10.1385/ENDO:20:3:215. [DOI] [PubMed] [Google Scholar]
- Angulo P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- Ariyama H., Kono N., Matsuda S., Inoue T., Arai H. Decrease in membrane phospholipid unsaturation induces unfolded protein response. J. Biol. Chem. 2010;285:22027–22035. doi: 10.1074/jbc.M110.126870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin A.C., Green C.D., Olson L.K., Moxley M.A., Corbett J.A. A role for aberrant protein palmitoylation in FFA-induced ER stress and β-cell death. Am. J. Physiol. Endocrinol. Metab. 2012;302:E1390–E1398. doi: 10.1152/ajpendo.00519.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S.S., Luo K.L., Shi L. Endoplasmic reticulum stress interacts with inflammation in human diseases. J. Cell. Physiol. 2016;231:288–294. doi: 10.1002/jcp.25098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow Y.L., Lee K.-H., Vidyadaran S., Lajis N.H., Akhtar M.N., Israf D.A., Syahida A. Cardamonin from Alpinia rafflesiana inhibits inflammatory responses in IFN-©/LPS-stimulated BV2 microglia via NF-|B signalling pathway. Int. Immunopharmacol. 2012;12:657–665. doi: 10.1016/j.intimp.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Farnell Y.F., Shende V.R., Neuendorff N., Allen G.C., Earnest D.J. Immortalized cell lines for real-time analysis of circadian pacemaker and peripheral oscillator properties. Eur. J. Neurosci. 2011;33:1533–1540. doi: 10.1111/j.1460-9568.2011.07629.x. [DOI] [PubMed] [Google Scholar]
- Fick L.J., Fick G.H., Belsham D.D. Palmitate alters the rhythmic expression of molecular clock genes and orexigenic neuropeptide Y mRNA levels within immortalized, hypothalamic neurons. Biochem. Biophys. Res. Commun. 2011;413:414–419. doi: 10.1016/j.bbrc.2011.08.103. [DOI] [PubMed] [Google Scholar]
- Gibbs J.E., Blaikley J., Beesley S., Matthews L., Simpson K.D., Boyce S.H., Farrow S.N., Else K.J., Singh D., Ray D.W., Loudon A.S. The nuclear receptor REV-ERBalpha mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc. Natl. Acad. Sci. U. S. A. 2012;109:582–587. doi: 10.1073/pnas.1106750109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladine C., Zmojdzian M., Joumard-Cubizolles L., Verny M.-A., Comte B., Mazur A. The omega-3 fatty acid docosahexaenoic acid favorably modulates the inflammatory pathways and macrophage polarization within aorta of LDLR−/− mice. Genes Nutr. 2014;9:424. doi: 10.1007/s12263-014-0424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco J.A., Oosterman J.E., Belsham D.D. Differential effects of omega-3 fatty acid docosahexaenoic acid and palmitate on the circadian transcriptional profile of clock genes in immortalized hypothalamic neurons. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014;307:R1049–R1060. doi: 10.1152/ajpregu.00100.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green C.J., Macrae K., Fogarty S., Hardie D.G., Sakamoto K., Hundal H.S. Counter-modulation of fatty acid-induced pro-inflammatory nuclear factor kappaB signalling in rat skeletal muscle cells by AMP-activated protein kinase. Biochem. J. 2011;435:463–474. doi: 10.1042/BJ20101517. [DOI] [PubMed] [Google Scholar]
- Han C.Y., Kargi A.Y., Omer M., Chan C.K., Wabitsch M., O'Brien K.D., Wight T.N., Chait A. Differential effect of saturated and unsaturated free fatty acids on the generation of monocyte adhesion and chemotactic factors by adipocytes: dissociation of adipocyte hypertrophy from inflammation. Diabetes. 2010;59:386–396. doi: 10.2337/db09-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain P., Kawar B., El Nahas M. Obesity and diabetes in the developing world — a growing challenge. N. Engl. J. Med. 2007;356:213–215. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- Huo Y., Guo X., Li H., Wang H., Zhang W., Wang Y., Zhou H., Gao Z., Telang S., Chesney J., Chen Y.E., Ye J., Chapkin R.S., Wu C. Disruption of inducible 6-phosphofructo-2-kinase ameliorates diet-induced adiposity but exacerbates systemic insulin resistance and adipose tissue inflammatory response. J. Biol. Chem. 2010;285:3713–3721. doi: 10.1074/jbc.M109.058446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo Y., Guo X., Li H., Xu H., Halim V., Zhang W., Wang H., Fan Y.-Y., Ong K.T., Woo S.-L., Chapkin R.S., Mashek D.G., Chen Y., Dong H., Lu F., Wei L., Wu C. Targeted overexpression of inducible 6-phosphofructo-2-kinase in adipose tissue increases fat deposition but protects against diet-induced insulin resistance and inflammatory responses. J. Biol. Chem. 2012;287:21492–21500. doi: 10.1074/jbc.M112.370379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen M.K., Chiuve S.E., Rimm E.B., Dethlefsen C., Tjønneland A., Joensen A.M., Overvad K. Obesity, behavioral lifestyle factors, and risk of acute coronary events. Circulation. 2008;117:3062–3069. doi: 10.1161/CIRCULATIONAHA.107.759951. [DOI] [PubMed] [Google Scholar]
- Jové M., Planavila A., Sánchez R.M., Merlos M., Laguna J.C., Vázquez-Carrera M. Palmitate induces tumor necrosis factor-alpha expression in C2C12 skeletal muscle cells by a mechanism involving protein kinase C and nuclear factor-kappaB activation. Endocrinology. 2006;147:552–561. doi: 10.1210/en.2005-0440. [DOI] [PubMed] [Google Scholar]
- Keller M., Mazuch J., Abraham U., Eom G.D., Herzog E.D., Volk H.D., Kramer A., Maier B. A circadian clock in macrophages controls inflammatory immune responses. Proc. Natl. Acad. Sci. U. S. A. 2009;106:21407–21412. doi: 10.1073/pnas.0906361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohsaka A., Laposky A.D., Ramsey K.M., Estrada C., Joshu C., Kobayashi Y., Turek F.W., Bass J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6:414–421. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Kolar S., Barhoumi R., Jones C.K., Wesley J., Lupton J.R., Fan Y.-Y., Chapkin R.S. Interactive effects of fatty acid and butyrate-induced mitochondrial Ca2 + loading and apoptosis in colonocytes. Cancer. 2011;117:5294–5303. doi: 10.1002/cncr.26205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange T., Dimitrov S., Born J. Effects of sleep and circadian rhythm on the human immune system. Ann. N. Y. Acad. Sci. 2010;1193:48–59. doi: 10.1111/j.1749-6632.2009.05300.x. [DOI] [PubMed] [Google Scholar]
- Leone M.J., Marpegan L., Duhart J.M., Golombek D.A. Role of proinflammatory cytokines on lipopolysaccharide-induced phase shifts in locomotor activity circadian rhythm. Chronobiol. Int. 2012;29:715–723. doi: 10.3109/07420528.2012.682681. [DOI] [PubMed] [Google Scholar]
- Lindholm C.R., Ertel R.L., Bauwens J.D., Schmuck E.G., Mulligan J.D., Saupe K.W. A high-fat diet decreases AMPK activity in multiple tissues in the absence of hyperglycemia or systemic inflammation in rats. J. Physiol. Biochem. 2013;69:165–175. doi: 10.1007/s13105-012-0199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney E., Sweet I.R., Hockenbery D.M., Pham M., Rizzo N.O., Tateya S., Handa P., Schwartz M.W., Kim F. Activation of NF-kappaB by palmitate in endothelial cells: a key role for NADPH oxidase-derived superoxide in response to TLR4 activation. Arterioscler. Thromb. Vasc. Biol. 2009;29:1370–1375. doi: 10.1161/ATVBAHA.109.188813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcheva B., Ramsey K.M., Buhr E.D., Kobayashi Y., Su H., Ko C.H., Ivanova G., Omura C., Mo S., Vitaterna M.H., Lopez J.P., Philipson L.H., Bradfield C.A., Crosby S.D., JeBailey L., Wang X., Takahashi J.S., Bass J. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–631. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menger G.J., Allen G.C., Neuendorff N., Nahm S.-.S., Thomas T., Cassone V.M., Earnest D.J. Circadian profiling of the transcriptome in NIH/3T3 fibroblasts: comparison with rhythmic gene expression in SCN2.2 cells and the rat SCN. Physiol. Genomics. 2007;29:280–289. doi: 10.1152/physiolgenomics.00199.2006. [DOI] [PubMed] [Google Scholar]
- Milanski M., Degasperi G., Coope A., Morari J., Denis R., Cintra D.E., Tsukumo D.M.L., Anhe G., Amaral M.E., Takahashi H.K., Curi R., Oliveira H.C., Carvalheira J.B.C., Bordin S., Saad M.J., Velloso L.A. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J. Neurosci. 2009;29:359–370. doi: 10.1523/JNEUROSCI.2760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak T.E., Babcock T.A., Jho D.H., Helton W.S., Espat N.J. NF-κB inhibition by ω − 3 fatty acids modulates LPS-stimulated macrophage TNF-α transcription. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003;284:L84–L89. doi: 10.1152/ajplung.00077.2002. [DOI] [PubMed] [Google Scholar]
- Paschos G.K., Ibrahim S., Song W.-L., Kunieda T., Grant G., Reyes T.M., Bradfield C.A., Vaughan C.H., Eiden M., Masoodi M., Griffin J.L., Wang F., Lawson J.A., Fitzgerald G.A. Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat. Med. 2012;18:1768–1777. doi: 10.1038/nm.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendergast J.S., Branecky K.L., Yang W., Ellacott K.L., Niswender K.D., Yamazaki S. High-fat diet acutely affects circadian organisation and eating behavior. Eur. J. Neurosci. 2013;37:1350–1356. doi: 10.1111/ejn.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluquet O., Dejeans N., Chevet E. Watching the clock: endoplasmic reticulum-mediated control of circadian rhythms in cancer. Ann. Med. 2014;46:233–243. doi: 10.3109/07853890.2013.874664. [DOI] [PubMed] [Google Scholar]
- Puri P., Wiest M.M., Cheung O., Mirshahi F., Sargeant C., Min H.-K., Contos M.J., Sterling R.K., Fuchs M., Zhou H., Watkins S.M., Sanyal A.J. The plasma lipidomic signature of nonalcoholic steatohepatitis. Hepatology. 2009;50:1827–1838. doi: 10.1002/hep.23229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan C., Khan S.K., Kathale N.D., Xu H., Liu A.C. Monitoring cell-autonomous circadian clock rhythms of gene expression using luciferase bioluminescence reporters. J. Vis. Exp. 2012;67:4234. doi: 10.3791/4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A., Hyttinen J.M., Kaarniranta K. AMP-activated protein kinase inhibits NF-kappaB signaling and inflammation: impact on healthspan and lifespan. J. Mol. Med. 2011;89:667–676. doi: 10.1007/s00109-011-0748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shende V.R., Neuendorff N., Earnest D.J. Role of miR-142-3p in the post-transcriptional regulation of the clock gene Bmal1 in the mouse SCN. PLoS One. 2013;8:e65300. doi: 10.1371/journal.pone.0065300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shostak A., Meyer-Kovac J., Oster H. Circadian regulation of lipid mobilization in white adipose tissues. Diabetes. 2013;62:2195–2203. doi: 10.2337/db12-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spengler M.L., Kuropatwinski K.K., Comas M., Gasparian A.V., Fedtsova N., Gleiberman A.S., Gitlin I.I., Artemicheva N.M., Deluca K.A., Gudkov A.V., Antoch M.P. Core circadian protein CLOCK is a positive regulator of NF-κB-mediated transcription. Proc. Natl. Acad. Sci. U. S. A. 2012;109:2457–2465. doi: 10.1073/pnas.1206274109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenvers D.J., Jonkers C.F., Fliers E., Bisschop P.H., Kalsbeek A. Nutrition and the circadian timing system. Prog. Brain Res. 2012;199:359–376. doi: 10.1016/B978-0-444-59427-3.00020-4. [DOI] [PubMed] [Google Scholar]
- Sun Y., Ren M., Gao G.Q., Gong B., Xin W., Guo H., Zhang X.J., Gao L., Zhao J.J. Chronic palmitate exposure inhibits AMPKalpha and decreases glucose-stimulated insulin secretion from beta-cells: modulation by fenofibrate. Acta Pharmacol. Sin. 2008;29:443–450. doi: 10.1111/j.1745-7254.2008.00717.x. [DOI] [PubMed] [Google Scholar]
- Turek F.W., Joshu C., Kohsaka A., Lin E., Ivanova G., McDearmon E., Laposky A., Losee-Olson S., Easton A., Jensen D.R., Eckel R.H., Takahashi J.S., Bass J. Obesity and metabolic syndrome in circadian clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um J.H., Pendergast J.S., Springer D.A., Foretz M., Viollet B., Brown A., Kim M.K., Yamazaki S., Chung J.H. AMPK regulates circadian rhythms in a tissue- and isoform-specific manner. PLoS One. 2011;6:e18450. doi: 10.1371/journal.pone.0018450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Liu D., Wang F., Liu S., Zhao S., Ling E.A., Hao A. Saturated fatty acids activate microglia via Toll-like receptor 4/NF-kappaB signaling. Br. J. Nutr. 2012;107:229–241. doi: 10.1017/S0007114511002868. [DOI] [PubMed] [Google Scholar]
- Weigert C., Brodbeck K., Staiger H., Kausch C., Machicao F., Haring H.U., Schleicher E.D. Palmitate, but not unsaturated fatty acids, induces the expression of interleukin-6 in human myotubes through proteasome-dependent activation of nuclear factor-kappaB. J. Biol. Chem. 2004;279:23942–23952. doi: 10.1074/jbc.M312692200. [DOI] [PubMed] [Google Scholar]
- Weldon S.M., Mullen A.C., Loscher C.E., Hurley L.A., Roche H.M. Docosahexaenoic acid induces an anti-inflammatory profile in lipopolysaccharide-stimulated human THP-1 macrophages more effectively than eicosapentaenoic acid. J. Nutr. Biochem. 2007;18:250–258. doi: 10.1016/j.jnutbio.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Xu H., Li H., Woo S.-L., Kim S.-M., Neuendorff N., Guo X., Guo T., Qi T., Ji J.-Y., Alaniz R.C., Earnest D.J., Wu C. Myeloid cell-specific disruption of period 1 and period 2 exacerbates diet-induced inflammation and insulin resistance. J. Biol. Chem. 2014;289:16374–16388. doi: 10.1074/jbc.M113.539601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue B., Yang Z., Wang X., Shi H. Omega-3 polyunsaturated fatty acids antagonize macrophage inflammation via activation of AMPK/SIRT1 pathway. PLoS One. 2012;7:e45990. doi: 10.1371/journal.pone.0045990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Kahn B.B., Shi H., Xue B.Z. Macrophage alpha1 AMP-activated protein kinase (α1AMPK) antagonizes fatty acid-induced inflammation through SIRT1. J. Biol. Chem. 2010;285:19051–19059. doi: 10.1074/jbc.M110.123620. [DOI] [PMC free article] [PubMed] [Google Scholar]